Abstract
Study Objectives:
Obese patients develop obstructive sleep apnea syndrome (OSAS), at least in part because of a narrowed upper airway. However, many obese adolescents do not develop OSAS, despite having a presumably narrower airway. The reasons for this phenomenon are unclear. The authors hypothesized that obese controls have a compensatory neuromuscular response to subatmospheric pressure loads during sleep, making them less likely to develop upper airway collapse.
Design:
Patients underwent pressure-flow measurements during sleep while wearing intraoral electrodes to measure genioglossal electromyography (EMGgg). Two techniques were applied to decrease nasal pressure (PN) to subatmospheric levels, resulting in an activated and relatively hypotonic upper airway.
Setting:
Sleep laboratory.
Participants:
There were 35 obese patients with OSAS, 28 obese controls, and 43 lean controls.
Results:
In the activated state, the two control groups had a flatter slope of the pressure-flow relationship and a more negative critical closing pressure (less collapsible) than the OSAS group. In the hypotonic state, the lean controls had a flatter slope of the pressure-flow relationship than the OSAS and obese control groups. In the activated state, the slope of EMGgg versus PN was greater in the obese control group than in the OSAS or lean control groups (P = 0.002 and P = 0.028, respectively); there were no differences in the hypotonic state.
Conclusions:
Obese controls have vigorous upper airway neuromuscular responses during sleep. Upper airway reflexes normally decline during adolescent development. It is speculated that obese adolescents without OSAS maintain protective upper airway reflexes during adolescent development, whereas those who go on to develop OSAS do not.
Citation:
Huang J; Pinto SJ; Yuan H; Katz ES; Karamessinis LR; Bradford RM; Gallagher PR; Hannigan JT; Nixon T; Ward MB; Lee YN; Marcus CL. Upper airway collapsibility and genioglossus activity in adolescents during sleep. SLEEP 2012;35(10):1345-1352.
Keywords: EMG, obstructive sleep apnea syndrome, Pcrit, puberty
INTRODUCTION
Upper airway patency is affected by both structural and neuromuscular factors. Imbalances between these factors results in increased upper airway collapsibility, leading to obstructive sleep apnea syndrome (OSAS). In young children, adenotonsillar hypertrophy is the major contributing structural factor,1 whereas in adolescents, obesity plays a prominent role.2,3 Obesity-related OSAS in adolescents has become a major health problem, concomitant with the increased prevalence of obesity in this age group (currently 17% in the United States).4 However, many obese adolescents do not develop OSAS. The reasons why some obese adolescents develop OSAS whereas others do not are not understood.
Prepubertal children have active upper airway reflexes during sleep in response to stimuli such as subatmospheric pressure and hypercapnia, presumably to compensate for a relatively smaller upper airway.5 It has been shown that these reflexes decline during adolescence, albeit with much individual variability.5 We hypothesized that obese adolescents without OSAS have robust compensatory neuromuscular response to subatmospheric pressure loads during sleep, making them less likely to develop upper airway collapse, whereas those with OSAS have attenuated protective upper airway reflexes during sleep. Some of the results of these studies have been previously reported in the form of an abstract.6,7
METHODS
Obese adolescents with OSAS, obese adolescent controls without OSAS, and lean controls were studied. Baseline polysomnography was performed to evaluate for OSAS. On a separate night, changes in airflow and surface genioglossal electromyographic (EMG) activity (EMGgg) in response to decreases in upper airway pressure were measured during nonrapid eye movement (NREM). The Institutional Review Board at the Children's Hospital of Philadelphia approved the study. Informed consent was obtained from the parents/legal guardians of the patients, and assent from the patients.
Study Group
Three groups of individuals age 12-16 yr were studied: obese OSAS, obese control, and lean control. The obese OSAS group was recruited from the Sleep Center at Children's Hospital of Philadelphia. Those in the obese and lean control groups were asymptomatic adolescents without a history of snoring, recruited from the general community by means of advertisements. Exclusion criteria included prior adenotonsillectomy, major medical illnesses, and medications affecting the central nervous system. Tanner staging was self-reported by the patients.8 Weight was measured on a calibrated digital electronic scale (Scale Tronix, Carol Stream, IL). Height was measured with a stadiometer (Holtain, Crymych, UK). Obesity was defined as body mass index (BMI) ≥ 95th percentile for age, race, and sex,9 and lean controls were defined as having a BMI < 85th percentile.9 All patients underwent baseline polysomnography using standard pediatric techniques10 as previously described for our laboratory.11 OSAS was defined as having an apnea- hypopnea index (AHI) ≥ 5/hr, and controls were only included if they had an AHI < 1.5/hr.12–15
Awake EMGgg
Intraoral surface EMG signals were obtained from dental mouthpieces positioned beneath the tongue and customized for each patient.5,6,16–19 The EMG signal was derived from a number of upper airway muscles, presumably primarily from the genioglossus. The signal was first referenced to electrical zero. Then a maximal tongue thrust maneuver was performed several times to obtain maximal EMGgg during wakefulness (EMGggMax).16,20 EMGgg activity was also recorded for 30 sec during quiet, nasal breathing when the patients were awake and in the supine position in bed.
Pressure-Flow and EMGgg Measurements During Sleep
Pressure-flow relationships were measured during a second overnight polysomnogram, using previously published techniques.5,11,18,21,22 Routine polysomnographic measurements were obtained. In addition, the patient wore a continuous positive airway pressure mask (Philips Respironics, Murrysville, PA). Airflow was measured with a heated pneumotachometer (Hans Rudolph, Inc., Kansas City, MO) and pressure transducer (ADInstruments, Colorado Springs, CO) attached to the mask. Nasal pressure (PN) was measured at the mask with a differential pressure transducer (Validyne Engineering Corp., Northridge, CA) referenced to atmosphere. Intraoral surface EMG signals were obtained simultaneously from dental mouthpieces.5,6,16–19
Signals were acquired on a PowerLab system (ADInstruments) and simultaneously displayed on a Rembrandt polysomnography system (Embla, Denver, CO). PN was altered in either a positive or subatmospheric direction, using a device provided by Philips Respironics.11,22,23 A toggle switch allowed the patient to be switched rapidly between positive and negative nasal pressure, ranging from −25 to +30 cm H2O.
Two techniques were applied to decrease the nasal pressure below the level of the holding pressure, resulting in an activated and relatively hypotonic upper airway, respectively.5,11,18,19,23,24 For both techniques, the holding pressure, i.e., the PN just above the pressure at which flow limitation first became discernible, was determined.11 For the activated technique, the run was initiated at the holding pressure. PN was then decreased in 2 cm H2O steps every 5 breaths until the patient had an arousal or obstructive apnea. This technique results in graded upper airway activation.5,11,18,22,25 For the hypotonic technique, PN was acutely decreased by 2 cm H2O from the holding pressure for 5 breaths, following which it was rapidly returned to the holding pressure. PN was dropped repeatedly to incrementally lower levels, with a return each time to the holding pressure, until either arousal or obstructive apnea occurred. This technique results in an upper airway with minimal activation during the first 3 breaths of negative pressure, and most of the changes in EMG activation were appreciated after the first 3 breaths.5,18,19 Multiple trials using either of the two techniques in random order were performed during stable NREM sleep. For each technique, the trial reaching the lowest PN before arousal/apnea was selected for data analysis.
Data Analysis
The maximal EMGgg activity during tongue thrust maneuvers was assumed to represent maximal EMGgg activity (EMGggMax). Inspiratory EMGgg signals were rectified, and filtered at low frequency of 10 Hz and at high frequency of 100 Hz.10,11,16,17,19,26 The signals were then moving-time averaged with a time constant of 200 ms.11,19 The area under the curve (AUC) of the inspiratory EMGgg moving-time average was calculated and corrected for inspiratory time (TI), i.e., inspiratory EMGgg AUC divided by TI. This AUC of the EMGgg reflects total tonic and phasic inspiratory EMGgg activity. The AUC of the EMGgg corrected for TI represents the average inspiratory EMGgg activity within one second. EMGgg activity during awake, quiet, nasal breathing was presented as a percentage of the EMGggMax. Baseline EMGgg during NREM sleep was defined as the average EMGgg inspiratory activity over 5 breaths at the holding pressure prior to the pressure challenge. Baseline EMGgg during NREM sleep then was compared to EMGggMax and EMGgg during wakefulness. EMGgg activity during pressure challenges was presented as a percentage of baseline EMGgg during NREM sleep. Changes in EMGgg in response to decrements in nasal pressure were presented as the slope of the EMGgg versus nasal pressure (slope of EMGgg-PN).11,17 Note that a more negative slope of EMGgg-PN corresponds to more prominent genioglossal activation.
Pressure-flow curves were constructed based on analysis of flow-limited breaths, and the slope of the pressure-flow curve (SPF) as well as the critical closing pressure (Pcrit) were calculated.11,18,21,27 Pcrit was defined as the extrapolated nasal pressure at which flow reached zero. Because many adolescents were able to maintain airflow even at markedly negative pressure, Pcrit could not always be determined without extreme extrapolation.5,11,18,21,22 This study used −25 cm H2O to represent Pcrit when the extrapolated Pcrit was < −25 cm H2O, as −25 cm H2O was the lowest pressure our device could provide.5,11,18,21,22 The SPF is the ratio of changes in airflow to changes in nasal pressure, and is used to represent the conductance of the upper airway. A high SPF corresponds to an upper airway with low conductance and high collapsibility. 21 We used both Pcrit and SPF to characterize the flow response to changes in PN.
Statistical Analysis
Statistical analysis was performed with SPSS software version 17.0 for Windows (SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov test was used to test for normality. Categorical data were compared using the chi-square test. Noncategorical data were presented as median and range, because most of the data were not normally distributed. Kruskal-Wallis nonparametric tests were used to examine differences among the three groups, with a P value of 0.05. Mann-Whitney nonparametric tests were used to examine pairwise differences between groups. The P value for pairwise comparisons was 0.05.
RESULTS
Study Group
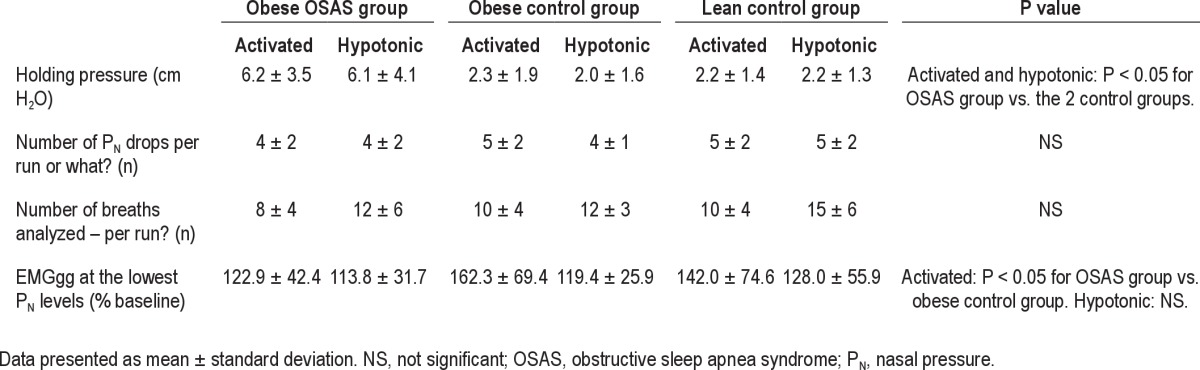
Thirty-five obese patients with OSAS, 28 obese controls, and 43 lean controls were studied. Patient characteristics are shown in Table 1. As planned, those in the obese OSAS group had a significantly higher AHI than those in the control groups, and the obese OSAS and obese control groups had a significantly higher BMI z-score than the lean controls. There was no significant difference in BMI z-score between the two obese groups. Data on holding pressure levels, numbers of pressure drops, breaths analyzed, and EMGgg values at the lowest pressure levels are reported in Table 2. As expected, the holding pressure was higher in the obese OSAS group than in the two control groups in both the activated and hypotonic states. There was no difference in the number of PN drops or breaths analyzed among the three groups. Those in the obese OSAS group had a significantly lower EMGgg at the lowest PN compared with obese controls in the activated state. No difference in EMGgg at the lowest PN was found in the hypotonic state.
Table 1.
Demographic and polysomnographic data
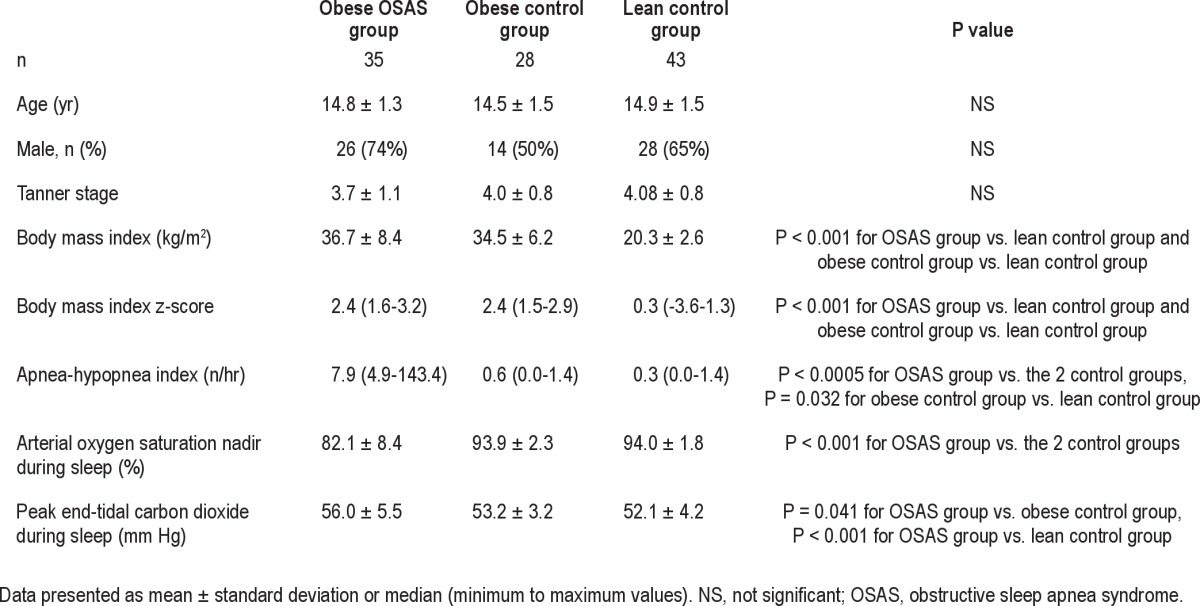
Table 2.
Technical details of the negative pressure challenges
Slope of Pressure-Flow Curve
Typical examples of pressure-flow runs from an obese control patient are shown in Figure 1, and data in Table 3. Within the obese OSAS group, no difference in SPF was observed under activated or hypotonic conditions. In contrast, both control groups had a steeper SPF (indicating a more collapsible upper airway) in the hypotonic compared with the activated state.
Figure 1.
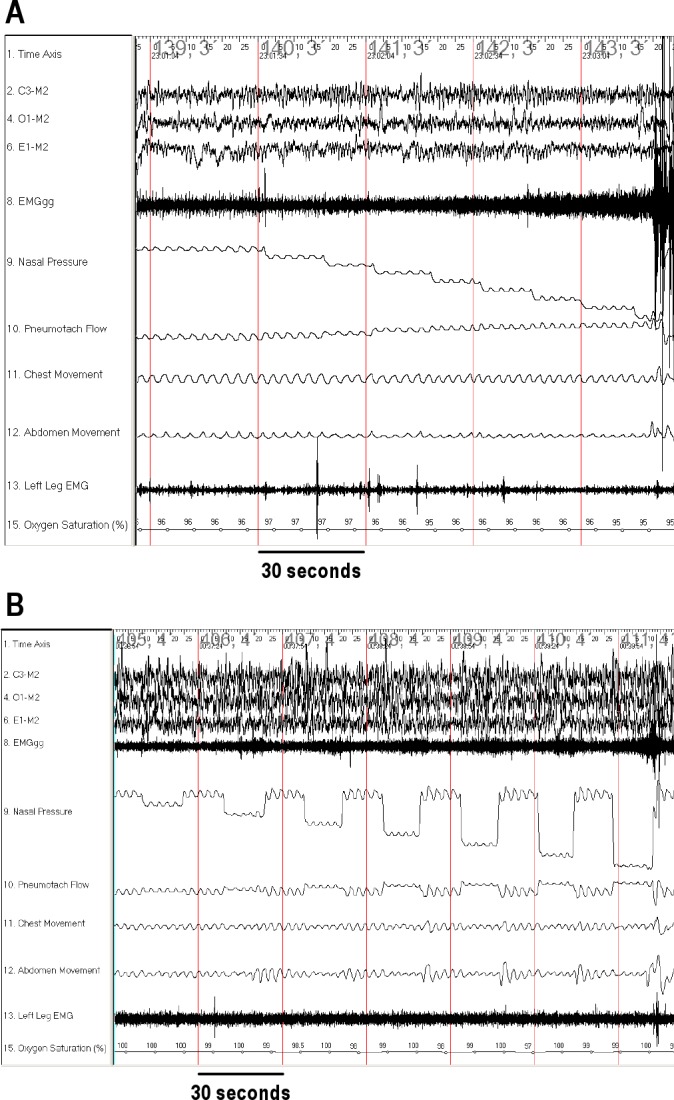
Airflow and EMGgg responses to subatmospheric pressure challenges in an obese control using the activated and hypotonic techniques. Typical examples of airflow and EMGgg changes during pressure-flow runs in slow wave sleep are shown for an obese control patient. During inspiration, airflow and nasal pressure signal tracings have a negative deflection. The baseline of the airflow tracing moves upwards during the pressure drops due to the change in bias flow. (A) Activated technique. The gradual decrease in nasal was associated with a gradual increase in EMGgg and maintenance of inspiratory flow. (B) Hypotonic technique. Increases in EMGgg were observed approximately 3 breaths after each pressure drop. Note that EMGgg is calculated for the hypotonic technique using only the first 3 breaths.
Table 3.
Upper airway dynamics, ventilatory and EMG data
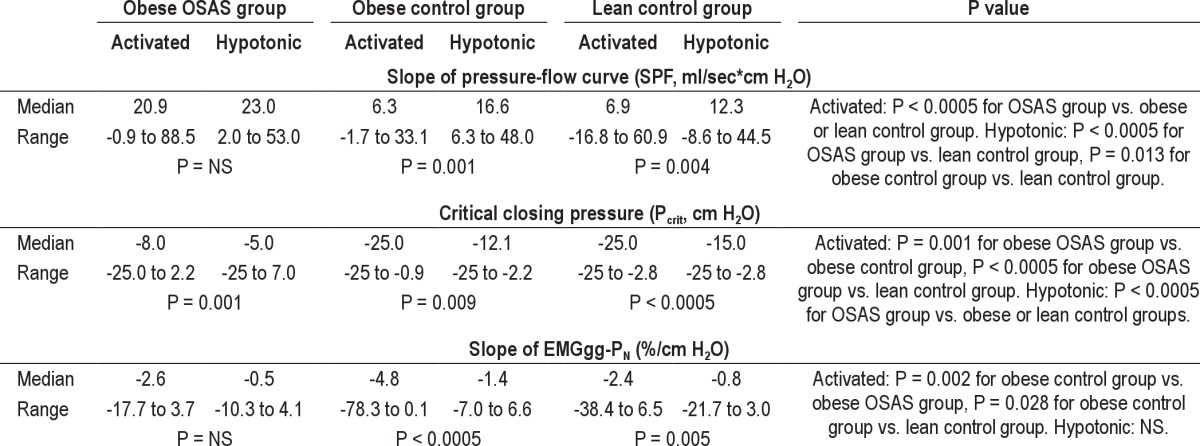
Significant differences in activated SPF were observed among the three groups. Activated SPF was significantly higher (more collapsible) in the obese OSAS group compared with either obese or lean controls. No difference in activated SPF was observed between the two control groups.
Significant differences in hypotonic SPF were observed among the three groups. Hypotonic SPF was significantly higher in the obese OSAS and obese control groups compared with the lean control groups; no difference was observed between the two obese groups (i.e., obese OSAS and obese controls).
Critical Closing Pressure
Within each of the three groups, significant difference in Pcrit was observed using the activated or the hypotonic techniques (Table 3).
Significant differences in activated Pcrit were observed among the three groups. The obese OSAS group had a significantly more positive Pcrit compared with the two control groups. No difference in activated Pcrit was observed between the two control groups.
Similar to activated Pcrit, significant differences in hypotonic Pcrit were found among the three groups. Obese OSAS had a significantly more positive Pcrit compared with obese control and lean controls. No difference in hypotonic Pcrit was observed between the obese and lean control groups.
EMGgg During Wakefulness
Presented as a percentage of the EMGggMax, the median, minimum, and maximum values of awake EMGgg for the obese OSAS, obese control, and lean control groups were 4.7% (2.5-7.3), 1.3% (0.7-6.2), and 1.4% (0.7-3.1), respectively. The obese OSAS group had significantly higher awake EMGgg compared with either control groups (P < 0.001 for both comparisons). No difference between the two control groups was observed.
Baseline EMGgg During NREM Sleep
Presented as a percentage of EMGggMax, the median, minimum, and maximum values of baseline EMGgg during NREM sleep for the obese OSAS, obese, and lean control groups were 1.8% (0.2-4.0), 0.6% (0.4-1.5), and 0.4% (0.1-2.0), respectively. Those in the obese OSAS group had significantly higher baseline EMGgg during NREM sleep compared with the obese and lean control groups (P < 0.001 for both comparisons). Those in the obese control group had higher baseline EMGgg compared with those in the lean control group (P = 0.017). Presented as a percentage of awake EMGgg, the median, minimum, and maximum values of baseline EMGgg during NREM sleep for the three groups were 44% (4-73), 52% (25-94), and 45% (7-90), respectively. No difference among the three groups was observed.
Slope of EMGgg-PN
Data on the slope of the EMGgg-PN relationship are shown in Table 3 and Figure 2. Within the obese OSAS group, no difference in the slope of EMGgg-PN was observed in the activated versus the hypotonic state. However, both control groups had a significantly more negative slope of EMGgg-PN in the activated compared with the hypotonic state.
Figure 2.
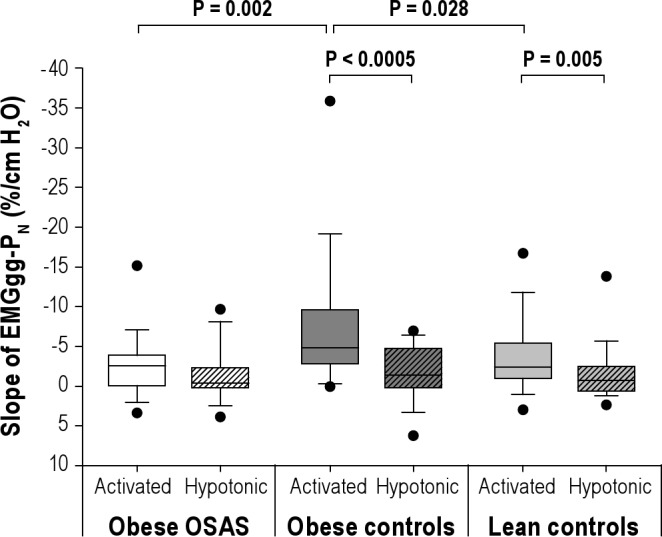
Genioglossal EMG responses to subatmospheric pressure drops using the activated and hypotonic techniques. Slopes of the EMGgg versus nasal pressure (slope of EMGgg-PN) from obese OSAS, obese control, and lean control groups under the activated and hypotonic states are shown. The box represents the interquartile range that contains 50% of the values. The line across the box indicates the median. The whiskers above and below the box indicate the 90th and 10th percentiles, respectively. The dots above and below the whiskers indicate the 95th and 5th percentiles, respectively. In the two control groups, increase in EMGgg in response to decrease in PN was significantly higher under the activated state compared with hypotonic state. Using activated state, the obese control group had a stronger EMGgg response compared with the obese OSAS and lean control groups.
Among the three groups, significant differences in the slope of EMGgg-PN were observed in the activated state only. Those in the obese control group had a significantly higher activated slope of EMGgg-PN compared with the obese OSAS and lean control groups. There was no difference in the hypotonic slope of EMGgg-PN among the three groups.
Effect of Sex on Upper Airway Collapsibility and Genioglossal Activity
Within the OSAS group, males had a higher SPF (more collapsible upper airway) than females in the activated state (Table 4). No difference in hypotonic SPF was observed. No difference in Pcrit or slope of EMGgg-PN in either the activated or hypotonic state was observed between males and females.
Table 4.
Effect of Sex in Upper Airway Collapsibility and Genioglossus Activity Data
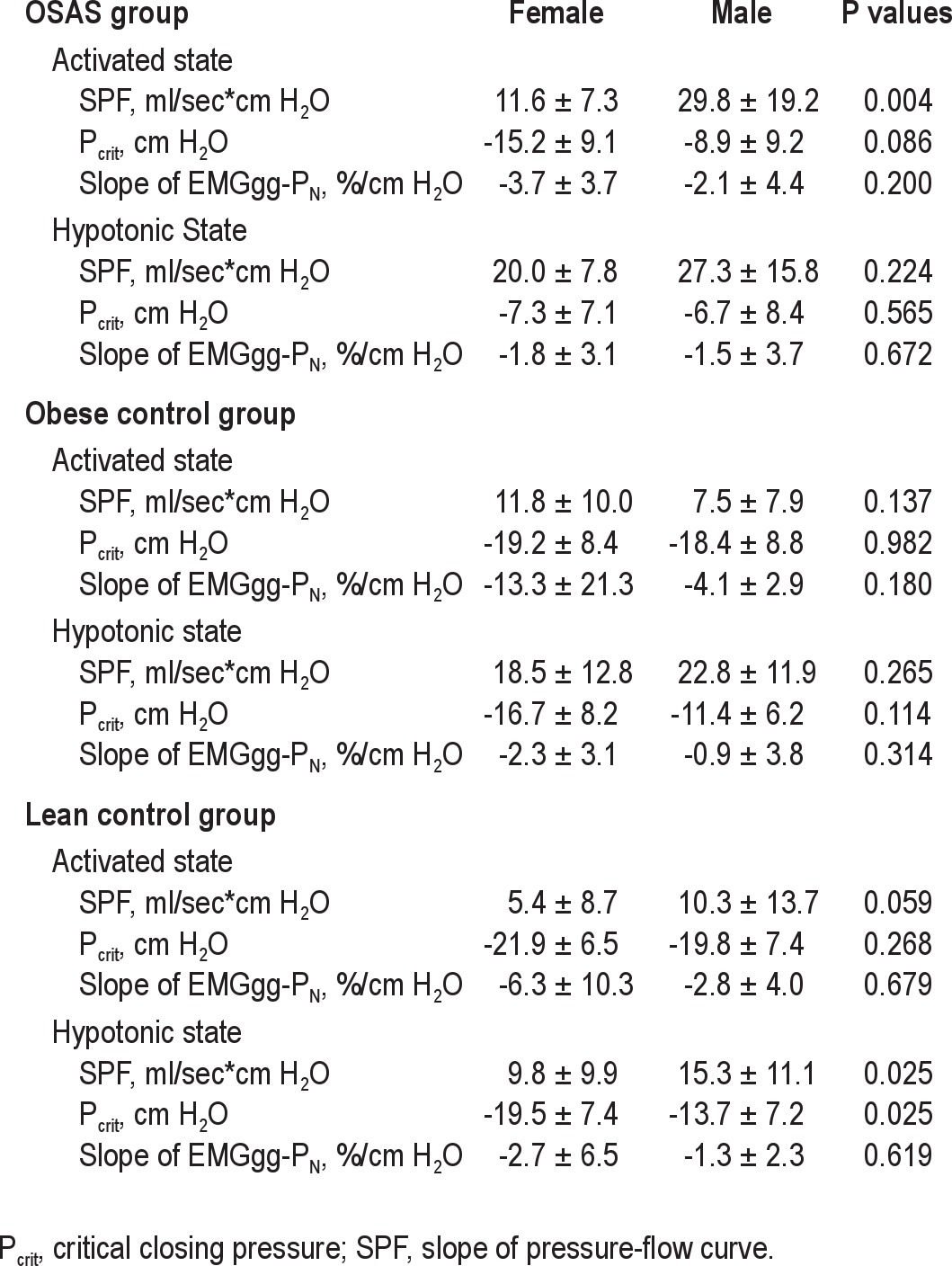
Within the obese control group, no difference in SPF, Pcrit, or slope of EMGgg-PN in either the activated or hypotonic state was found between males and females.
Within the lean control group, males had a higher SPF and more positive Pcrit (more collapsible upper airway) than females in the hypotonic state. No difference in SPF or Pcrit in the activated state was found. No difference in slope of EMGgg-PN was found.
Overall, although in some groups and conditions males had a more collapsible upper airway than females, there was no difference in the slope of EMGgg- PN between males and females in any of the three groups.
DISCUSSION
There were two major findings in our study. First, obese and lean adolescents without OSAS had a less collapsible upper airway than obese adolescents with OSAS in the activated state. Obese controls had a more prominent increase in genioglossal EMG activity compared with patients in the OSAS and lean control groups. Second, lean controls had a less collapsible upper airway than the two obese groups (obese control and obese OSAS groups) in the hypotonic state. These findings suggest that obese adolescents without OSAS have active upper airway neuromuscular responses that maintain upper airway patency during sleep.
Importance of the Adolescent Phase of Development
Adolescence, the period of transition from childhood to adulthood, is a period of development known to be associated with major sleep issues, yet which remains virtually unstudied. Adolescence is a critical time of transition from child to adult, characterized not only by changes in sexual development but also in somatic growth and cortical processing.28 Nevertheless, OSAS in adolescents remains virtually unstudied. As children progress through adolescence, the upper airway becomes increasingly more collapsible, with attenuation of protective upper airway reflexes.22 Nevertheless, despite these physiologic changes, most healthy, nonobese adolescents have very few obstructive apneas during sleep, similar to younger children.22,29,30 Little is known about the pathophysiology of OSAS in obese adolescents.
Measurements of Upper Airway Collapsibility
Extensive studies in children and adults have described the dynamic properties of the upper airway during sleep by evaluating the slope of the pressure-flow curve as well as Pcrit.5,11,18,21,22,31,32 With this technique, positive nasal pressure suppresses upper airway tone,33,34 whereas subatmospheric nasal pressure results in increased upper airway tone.35 Thus, sequentially lowering PN to subatmospheric levels results in an activated upper airway. However, in children the effect of subatmospheric pressure is minimal in the first three breaths.5,19 Therefore, maintaining positive nasal pressure and then acutely decreasing negative pressure for 1-3 breaths results in a relatively hypotonic airway. This hypotonic technique primarily tests upper airway structure, whereas the activated technique tests the combination of upper airway anatomy and neuromotor function.5,18,21,24
As described in our previous work,23 patients with OSAS had a more positive holding pressure, and positive airway pressure reduces EMGgg activity.17 However, we believe that difference in upper airway responses between OSAS and controls cannot be explained by the differences in holding pressure or the negative pressure applied, but rather, by different upper airway collapsibility between patients with OSAS and controls.23
Upper Airway Collapsibility in Subjects With OSAS
In the current study, we used activated and hypotonic techniques to investigate upper airway collapsibility and neuromuscular responses to subatmospheric pressure challenges among three adolescent groups: obese OSAS, obese control, and lean control. As anticipated, obese patients with OSAS had a significantly more collapsible upper airway than either obese or lean controls.
Effects of Structural Factors on Upper Airway Collapsibility
Measurements made using the hypotonic technique primarily reflect the effects of upper airway structural factors. The hypotonic SPF (although not the hypotonic Pcrit) was similar among the two obese groups (OSAS and obese control), suggesting that upper airway adipose tissue affects upper airway function.36
Effects of Neuromuscular Factors on Upper Airway Collapsibility
Measurements made using the activated technique reflect the combined effects of upper airway structural factors and neuromuscular function during sleep, and the difference between the activated and hypotonic states in an individual represents upper airway reflex activity.18 In the current study, obese adolescents with OSAS had a steeper activated SPF and more positive Pcrit than either control group. In addition, in contrast to either the lean or obese control groups, the obese OSAS group failed to show a difference in SPF in the activated versus the hypotonic state, indicating decreased upper airway reflex activity during sleep.
Previous studies used both peak EMGgg activity16,37 and AUC of EMGgg 17,19 during inspiration to present EMGgg activity. However, even after being moving-time averaged, peak EMG activity may still be nonrepresentative because occasional brief muscle transients may occur. Therefore, this study used AUC of the EMGgg during inspiration rather than the peak EMGgg value during that time period. Furthermore, because TI increased in response to negative pressure challenges23 the AUC of the inspiratory EMGgg can increase due to an increase in TI without much change in the EMGgg. Therefore, we used AUC of the inspiratory EMGgg divided by TI to present total tonic and phasic inspiratory EMGgg activity.
During wakefulness, the EMGgg activity, as a percentage of maximal voluntary EMGgg activity, was higher in the obese OSAS group than the two control groups. This is similar to findings in children and adults,16,20 and suggests that across the age spectrum, patients with OSAS compensate during wakefulness for a narrower upper airway. In addition, we found that the obese OSAS group had higher EMGgg activity during stable, nonobstructed sleep at holding pressure than the control groups. However, presented as a percentage of the baseline EMGgg during NREM sleep, the EMGgg at the lowest pressure levels in the activated state was lower in the obese OSAS group than in the obese control group. Thus, it is possible that the obese OSAS group did not further increase EMGgg in response to subatmospheric loads because they were already functioning at the upper limits of EMG contraction during sleep.
The most notable finding in this study was that the obese control group had significantly more prominent genioglossal activation in response to subatmospheric pressure during sleep than either obese patients with OSAS or lean controls. Obese individuals are predisposed to OSAS in part because of narrowed airways secondary to adipose tissue encroachment.38 However, in some obese adolescents, the upper airway narrowing can be counterbalanced by increased upper airway muscle activation, thereby protecting the individual from upper airway collapse.
Effect of Sex on Upper Airway Collapsibility and EMGgg Activity
As described in the literature, the prevalence of OSAS is equal in prepubertal boys and girls.39 Although there have been no population-based studies on the prevalence of OSAS in adolescents, a recent review of 85 articles concluded that the prevalence of sleep disordered breathing in boys is 50-100% higher than that in girls.40 Similarly, the prevalence is higher in men than in premenopausal women.41 The prevalence of OSAS in postmenopausal women increases, but is still significantly lower than that in men after controlling for age and BMI.42 Our previous work has shown that the SPF is higher in nonobese male adolescents without OSAS than in their female counterparts, suggesting a more collapsible upper airway in normal male adolescents. This phenomenon suggests that sex differences in upper airway collapsibility and hence in the development of OSAS may begin during adolescence. However, in the current study, the EMG responses to upper airway negative pressure challenges were similar in females and males, suggesting that factors other than neuromotor activation may play an important role in upper airway collapsibility. Those factors may include the effect of hormones on upper airway structure as well as differences in upper airway length and size,43 rather than the effect of hormones on ventilatory drive.44,45
The Role of Obesity in the Pathophysiology of OSAS in Children and Adolescents
The relationship between obesity and OSAS in the pediatric population has been investigated by different groups. It is well known that in young children, adenotonsillar size is correlated with the severity of OSAS.46,47 When both preadolescents and adolescents were studied, both tonsil size and BMI z-score were related to the severity of OSAS.48 Furthermore, studies have demonstrated that the prevalence and severity of OSAS are higher in obese children and adolescents compared with their lean counterparts, but a strong correlation between BMI and the severity of OSAS was not found.2,48,49 Moreover, at given levels of AHI, obese children and adolescents had a lesser magnitude of adenotonsillar hypertrophy compared with lean counterparts with OSAS.50 These studies suggest that obesity becomes an increasingly more important contributing factor to the pathogenesis of OSAS as children are entering adolescence. However, no previous study has focused solely on the adolescent population, including both lean and obese controls.
To better understand the neuromuscular factors contributing to upper airway patency, the role of the genioglossus muscle in the maintenance of upper airway patency has been investigated. Previous studies have demonstrated that children with OSAS have increased EMGgg activity during wakefulness and sleep compared with controls, in order to overcome the anatomic abnormalities and maintain upper airway patency.16 Children with OSAS have a larger decline in EMGgg activity from wakefulness to sleep onset compared with controls,16 and the reduction in EMGgg activity is temporally associated with obstructive events.17 In contrast, most normal children and adolescents are able to sustain minute ventilation when challenged by upper airway subatmospheric pressure by increasing EMGgg activity and respiratory rate,19 indicating that these children and adolescents have active upper airway neuromuscular reflexes during sleep.
It has previously been shown that healthy prepubertal children have active upper airway reflexes during sleep in response to factors such as subatmospheric pressure and hypercapnia.5 These reflexes decline during adolescence and are lowest in adults.5,22 However, there is substantial individual variability in the rate of reflex decline during adolescence.22 Both children and adults with OSAS have blunted upper airway reflexes during sleep compared with age-matched controls.18,51
The current study suggests that neuromuscular factors differ more than structural factors in obese adolescents with OSAS compared with obese adolescents of a similar BMI without OSAS, as shown by a similar hypotonic SPF between groups. However, obese adolescents without OSAS have increased upper airway reflexes compared with their obese counterparts with OSAS. One possible explanation is that the obese control groups have not yet undergone the developmental, age-related decline in upper airway reflexes. It can be speculated that, with time, the obese control group will lose their upper airway reflexes and develop OSAS as they enter adulthood. During adulthood, obesity is more directly related to upper airway patency during sleep, and weight gain or loss can predict changes in AHI.52
Study Limitations
This study has several limitations. Surface EMG electrodes rather than needle EMG electrodes were used to measure EMGgg activity, so that it is possible that subtle EMG activation at relatively more positive pressure levels was not detected. Pharyngeal pressure was not measured due to poor tolerance of the procedure. Flow limitation was instead determined by the characteristic waveform pattern.5,18,22,23,27,53. To facilitate sleep in these young patients during this intensive protocol, body position during negative pressure challenges was not restricted. Another limitation of this study was the cross-sectional design. Future studies, following obese children through adolescence into adulthood, would be necessary to confirm these theories.
CONCLUSION
Obese adolescents with OSAS manifest blunted upper airway neuromuscular compensatory responses to decreases in upper airway pressure during sleep. In contrast, obese adolescents without OSAS manifest active upper airway reflexes during sleep, which help maintain upper airway patency.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Marcus has received research support from Philips Respironics. The Pcrit machine used in this study was loaned to her laboratory by Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by NIH grants R01 HL58585 and UL1 RR024134. Philips Respironics, Inc. provided the airway pressure device. The authors thank all of the Children's Hospital of Philadelphia sleep laboratory technologists who helped conduct this study. We are grateful to the children and their families for their enthusiastic participation in this study.
REFERENCES
- 1.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:E69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–83. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Marcus CL, Fernandes Do Prado LB, Lutz J, et al. Developmental changes in upper airway dynamics. J Appl Physiol. 2004;97:98–108. doi: 10.1152/japplphysiol.00462.2003. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Pinto SJ, Katz ES, et al. Genioglossus activity in obese, non-snoring adolescents during sleep. Sleep. 2009;32:A169. [Google Scholar]
- 7.Huang J, Pinto SJ, Katz ES, et al. Upper airway collapsibility and genioglossus activity in obese adolescents with OSAS and obese and normal weight controls during sleep. Am.J.Respir.Crit.Care Med. 2010:A5390. 181. [Google Scholar]
- 8.Schlossberger NM, Turner RA, Irwin CE., Jr Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13:109–13. doi: 10.1016/1054-139x(92)90075-m. [DOI] [PubMed] [Google Scholar]
- 9.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U. S. children 5 to 17 years of age. J Pediatr. 1998;132:211–22. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 10.The American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Pinto SJ, Allen JL, et al. Upper airway genioglossal activity in children with sickle cell disease. Sleep. 2011;34:773–8. doi: 10.5665/SLEEP.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 13.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 14.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 15.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 16.Katz ES, White DP. Genioglossus activity in children with obstructive sleep apnea during wakefulness and sleep onset. Am J Respir Crit Care Med. 2003;168:664–70. doi: 10.1164/rccm.200301-092OC. [DOI] [PubMed] [Google Scholar]
- 17.Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:553–60. doi: 10.1164/rccm.200403-262OC. [DOI] [PubMed] [Google Scholar]
- 18.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 19.Katz ES, Marcus CL, White DP. Influence of airway pressure on genioglossus activity during sleep in normal children. Am J Respir Crit Care Med. 2006;173:902–9. doi: 10.1164/rccm.200509-1450OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism) J Clin Invest. 1992;89:1571–9. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626–33. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 22.Bandla P, Huang J, Karamessinis L, et al. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–41. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Karamessinis LR, Pepe ME, et al. Upper airway collapsibility during REM sleep in children with the obstructive sleep apnea syndrome. Sleep. 2009;32:1173–81. doi: 10.1093/sleep/32.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AR, O'Donnell CP, Baron J, et al. The hypotonic upper airway in obstructive sleep apnea - Role of structures and neuromuscular activity. American Journal of Respiratory and Critical Care Medicine. 1998;157:1051–7. doi: 10.1164/ajrccm.157.4.9706067. [DOI] [PubMed] [Google Scholar]
- 25.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol. 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redfern MS, Hughes RE, Chaffin DB. High-Pass Filtering to Remove Electrocardiographic Interference from Torso Emg Recordings. Clinical Biomechanics. 1993;8:44–8. doi: 10.1016/S0268-0033(05)80009-9. [DOI] [PubMed] [Google Scholar]
- 27.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918–24. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 28.Chugani HT. Biological basis of emotions: brain systems and brain development. Pediatrics. 1998;102:1225–9. [PubMed] [Google Scholar]
- 29.Acebo C, Millman RP, Rosenberg C, Cavallo A, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Chest. 1996;109:664–72. doi: 10.1378/chest.109.3.664. [DOI] [PubMed] [Google Scholar]
- 30.Tapia IE, Karamessinis L, Bandla P, et al. Polysomnographic values in children undergoing puberty: Pediatric vs. adult respiratory rules in adolescents. Sleep. 2008;31:1737–44. doi: 10.1093/sleep/31.12.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64:789–95. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 32.Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002;121:1531–40. doi: 10.1378/chest.121.5.1531. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport DM, Garay SM, Goldring RM. Nasal CPAP in obstructive sleep apnea: mechanisms of action. Bull Eur Physiopathol Respir. 1983;19:616–20. [PubMed] [Google Scholar]
- 34.Strohl KP, Redline S. Nasal CPAP therapy, upper airway muscle activation, and obstructive sleep apnea. Am Rev Respir Dis. 1986;134:555–8. doi: 10.1164/arrd.1986.134.3.555. [DOI] [PubMed] [Google Scholar]
- 35.Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–53. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- 36.Kim C, Huang J, Gallagher PR, et al. Relationship between upper airway collapsibility and the size of upper airway soft tissues in obese adolescents with OSAS and obese controls. American Journal of Respiratory and Critical Care Medicine. 2011:A5331. 183. [Google Scholar]
- 37.Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–62. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 38.Horner RL, Mohiaddin RH, Lowell DG, et al. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2:613–22. [PubMed] [Google Scholar]
- 39.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 40.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 42.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 43.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Sexual influence on the control of breathing. J Appl Physiol. 1983;54:874–9. doi: 10.1152/jappl.1983.54.4.874. [DOI] [PubMed] [Google Scholar]
- 45.Dutton K, Blanksby BA, Morton AR. CO2 sensitivity changes during the menstrual cycle. J Appl Physiol. 1989;67:517–22. doi: 10.1152/jappl.1989.67.2.517. [DOI] [PubMed] [Google Scholar]
- 46.Arens R, McDonough JM, Costarino AT, et al. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 47.Fregosi RF, Quan SF, Kaemingk KL, et al. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol. 2003;95:2030–8. doi: 10.1152/japplphysiol.00293.2003. [DOI] [PubMed] [Google Scholar]
- 48.Lam YY, Chan EY, Ng DK, et al. The correlation among obesity, apnea-hypopnea index, and tonsil size in children. Chest. 2006;130:1751–6. doi: 10.1378/chest.130.6.1751. [DOI] [PubMed] [Google Scholar]
- 49.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–8. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dayyat E, Kheirandish-Gozal L, Sans CO, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137–44. doi: 10.1378/chest.08-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–56. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 52.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 53.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150:475–80. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]


