Abstract
Study Objectives:
Poor sleep may play a role in insulin resistance and diabetes risk. Yet few studies of sleep and insulin resistance have focused on the important developmental period of adolescence. To address this gap, we examined the association of sleep and insulin resistance in healthy adolescents.
Design:
Cross-sectional.
Setting:
Community setting in one high school.
Participants:
245 (137 African Americans, 116 males) high school students.
Measurements and Results:
Participants provided a fasting blood draw and kept a sleep log and wore a wrist actigraph for one week during the school year. Participants' families were from low to middle class based on family Hollingshead scores. Total sleep time across the week averaged 7.4 h by diary and 6.4 h by actigraph; homeostatic model assessment of insulin resistance ([HOMA-IR] unadjusted) averaged 4.13. Linear regression analyses adjusted for age, race, gender, body mass index, and waist circumference showed that the shorter the sleep, the higher the HOMA-IR, primarily due to sleep duration during the week. No evidence was found for long sleep being associated with elevated HOMA-IR. Fragmented sleep was not associated with HOMA-IR but was associated with glucose levels.
Conclusions:
Reduced sleep duration is associated with HOMA-IR in adolescence. Long sleep duration is not associated. Interventions to extend sleep duration may reduce diabetes risk in youth.
Citation:
Matthews KA; Dahl RE; Owens JF; Lee L; Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. SLEEP 2012;35(10):1353-1358.
Keywords: Sleep duration, insulin resistance, diabetes, adolescence, race
INTRODUCTION
An epidemic of obesity is occurring among children and adolescents.1 In consequence, the associated problems of insulin resistance, elevated blood pressure (BP), and diabetes are also increasing in prevalence.2,3 This epidemic is setting the stage for long-term chronic health problems for the current generation of youth as they enter into young adulthood and mid-life.4,5
Among the multitude of potentially modifiable factors relevant to these high-stakes health concerns, there are compelling reasons to include a focus on sleep in adolescence. First, growing evidence links sleep and metabolic regulation broadly, and more specifically, to insulin resistance and diabetes risk in adulthood. Self-reported usual sleep duration, both short and long, are associated with insulin resistance and diabetes.6–8 Experimental evidence shows that acute sleep restriction appears to increase insulin resistance.9
Second, adolescence represents a period of dramatic developmental changes that create unique vulnerabilities that impact both sleep and metabolic regulation. Pubertal maturation activates a period of intense growth and physical development and changes in sleep and circadian regulation.10 In addition to these biological changes, adolescence is a time of vulnerability due to social changes that impact sleep and activity patterns. Adolescents stay up later at night for a variety of reasons,11,12 including social activities, media use, school activities, homework, part-time jobs, and the 24/7 lifestyle now typical of many Americans. Yet most adolescents must negotiate the demands of early school start times, resulting in the observation that up to 87% of US adolescents do not receive what is considered to be sufficient sleep on school nights.13 Moreover, a great deal of the catch-up sleep (on weekends and holidays) occurs at a different circadian phase—introducing jet lag-like circadian shifts.
Given the large numbers of adolescents obtaining relatively short sleep on school nights, evidence linking short sleep and insulin resistance in adults, and the increasing risk for obesity-related diseases in the current generation of adolescents, it is surprising how few studies have investigated the association between sleep and insulin resistance and diabetes risk directly in adolescents. Among obese adolescents and children, there has been no consistent association between sleep duration and indicators of insulin resistance in several studies.14–16 In a large study of children and adolescents aged 6 to 18 years not selected for obesity, insulin resistance was associated with short self-reported sleep in girls but not boys; adjustment for waist circumference attenuated the association in girls.17 In the Cleveland Sleep and Health Study of 387 black and white adolescents, short and long sleep duration measured by actigraphy was associated with age-adjusted insulin resistance as based on the homeostatic model assessment of insulin resistance (HOMA-IR).18 Covariate-adjusted analyses showed that the association between short sleep duration and insulin resistance was largely attenuated by central adiposity; the association with long sleep duration remained significant in the fully adjusted model.
The primary objective of the present paper is to evaluate the associations between actigraphy and diary measures of sleep duration and sleep fragmentation and insulin resistance in healthy black and white adolescents. This work extends the literature in several important ways. First, it provides a test of the association of insulin resistance and sleep characteristics, separately by school night vs. weekend, in addition to the full week. Second, it addresses whether central adiposity attenuates the association with short sleep but not with longer sleep, as reported in the Cleveland Health and Sleep Study. Third, it explicitly addresses whether the associations vary by race.
METHODS
Participants
We recruited 250 adolescents between the ages of 14 and 19 years from a single public high school within 10 miles of downtown Pittsburgh, PA. The high school served a diverse population, both in terms of ethnicity and socioeconomic status. Approval of the research project was obtained from the superintendent of the school district and the principal of the high school, as well as the University of Pittsburgh Institutional Review Board. Participants were recruited from health or gym classes for “Pittsburgh Project Pressure,” an adolescent health study designed to measure stress, sleep, and risk factors for cardiovascular disease. Participants, and in the case of students under the age of 18, a parent or legal guardian, provided written informed consent prior to any research procedures. Exclusionary criteria included being treated for cardiovascular or kidney disease, medication use for emotional or psychological disorders, diabetes or blood pressure medication, and use of any medication known to affect the cardiovascular system or sleep. Obesity was not an exclusionary criterion unless it prevented getting resting blood pressure measures with the appropriate-sized cuff. Sixteen students who were screened were ineligible to participate due to taking exclusionary medication, and 7 students who signed consent did not actively enroll in the study. The final sample was 47% male and 44% non-black (primarily Caucasian, with two Hispanics).
Measures
Sleep
The Mini-Mitter Actiwatch model AW-16 (Phillips Respironics, Bend, OR) was used to collect actigraphy data continuously over 7 days and nights. Actigraphs were configured to collect data over a 1-min epoch. Stored data were downloaded into the Actiware software program (version 5.57) for processing and analysis. The medium threshold (default) was selected to detect nocturnal sleep periods ≥ 3 h in duration based upon sleep onset and offset using the 10-min criterion. Sleep periods occurring within 30 min of the major nocturnal sleep interval (either 30 min prior to sleeping or after waking) that were ≥ 15 min in duration were combined with the major sleep interval (i.e., if a 6-h sleep interval was detected from 00:00-06:00, and a 20-min sleep interval was detected beginning at 23:30, the 20-min interval was combined with the major sleep interval. The new major rest interval would become 23:30-06:00). All subsequent sleep variables were then calculated from data within these set rest periods. Total sleep time was calculated as the time spent asleep between initial sleep onset and final sleep offset, excluding periods of wakefulness throughout the night. Sleep fragmentation, a measure of restlessness, was calculated as ([%1-min intervals of movement during sleep + % 1-min intervals of immobility] divided by total 1-min immobility intervals). The actiwatch has been widely used in research studies and has been validated against polysomnography measures in clinic.19,20 A diary measure of total sleep time was calculated based on the time participants estimated they were in bed trying to go to sleep to the time they awakened in the morning minus self-reported sleep latency and time awake during the night. The nights prior to vacation days during the school week were considered to be weekends and Sunday night was considered to be a school night. Sleep data were averaged across the entire week, school nights only, and weekend/non-school nights only.
Insulin Resistance and Glucose
Morning fasting blood samples were obtained and stored on ice for transport. Then serum was separated by refrigerated centrifuge, aliquoted, and stored at −80°C until assay. Samples were assayed in the Heinz Lipid Laboratory at the Graduate School of Public Health, University of Pittsburgh. In this laboratory duplicate samples, standards and control sera are included in each run. Serum glucose was quantitatively determined by an enzymatic determination utilizing coupled enzyme reactions.21 The laboratory coefficient of variation (CV) between runs is 1.8%. Insulin was measured using an RIA procedure developed by Linco Research Inc (Linco Research, St. Charles, Missouri). An insulin-antibody complex was precipitated, and after centrifugation the supernatant was decanted and the pellets counted. The limit of sensitivity was 2 microU/mL to 200 microU/mL in a linear fashion. The lab CV for insulin is 10%. Standards, blanks, quality controls, and a control pool were run simultaneously with all samples. The HOMA-IR was calculated as the product of the fasting glucose and insulin divided by the constant 22.5.22 Because insulin was highly correlated with the HOMA-IR, we report results for glucose and the HOMA-IR.
Covariates
Age, sex, and race/ethnicity were determined by participant self-report. Height was measured using a stadiometer, and weight was measured on a Tanita digital scale to one-tenth of a pound. Participants were asked to remove shoes, socks, coats, and any other excess clothing and to empty their pockets before being weighed. Body mass index (BMI) was calculated using the National Heart, Lung, and Blood Institute on-line calculator. Waist circumference was measured by research staff using a cloth tape measure placed at the point of the natural bend of the waist under clothing after 2 forced exhalations. Participants reported number of days during the past 7 that they were physically active for ≥ 60 min per day on a question that is part of the Centers for Disease Control Youth Risk Behavior Scale. Pubertal status was assessed based on self-reported changes in secondary sex characteristics.23 Pubertal status in our sample was categorized as follows: for girls, 2 midpubertal, 75 advanced pubertal, and 50 postpubertal (with 2 missing); for boys, 1 prepubertal, 2 beginning pubertal, 34 midpubertal, 68 advanced pubertal, and 9 postpubertal (with 2 missing). Because of the sample distributions, girls were classified into 2 groups: mid- or advanced pubertal and postpubertal; boys were classified into 3 groups: pre- to midpubertal, advanced pubertal, and postpubertal.
Procedure
The parents or legal guardian of a student expressing interest in the study were contacted for a phone interview to determine participant eligibility. After obtaining signed informed consent from the parent/guardian and/or the student, parents were interviewed regarding household socioeconomic status and family history. Eligible students were scheduled to start the protocol on a school day. Study staff met the student in a school administrative suite to review the protocol and obtain anthropometric measures. Participants wore the actigraph on their nondominant wrist continuously over the 7 days and nights of the study. They were also instructed to hit an event marker on the face of the actigraph when they tried to go to sleep at night and when they finally awoke in the morning. In addition, they answered several questions about their sleep in the handheld computer each morning and evening. A fasting venous blood draw occurred on a school day morning, typically between 08:00 and 10:00. Additional study procedures that are not relevant to the current analyses included ambulatory blood pressure measures, completion of a battery of psychosocial questionnaires, and a brief (< 10-min) semi-structured interview. After completion of the protocol, participants were compensated $100, and a follow-up report of the student's BP, sleep, anthropometric measures, and glucose and lipid levels was sent to the student and his or her parent/guardian.
Analysis
Data were checked for normality and outliers based on probability plots, box plots and skewness, and kurtosis statistics. HOMA-IR was log transformed due to skewness. Five participants were excluded from the analytic sample: 1 was missing sleep data due to equipment malfunction; 2 had BMI values that fell over +4 standard deviations from the mean; and 3 did not have the blood draw (one of whom also had elevated BMI). Preliminary analyses showed that self-reported physical activity was not related to HOMA-IR and glucose, independent of BMI, so it was not considered further. Preliminary analysis stratified by gender showed that pubertal status was unrelated to HOMA-IR but was related to fasting glucose in boys only. Hence, follow-up analyses adjusted for pubertal status in the analysis of fasting glucose. We first examined the distributions of glucose, HOMA-IR, BMI, and waist circumference by race and gender using analysis of variance. Then we examined by linear regression the association between sleep duration and fragmentation and glucose and HOMA-IR, with adjustments for age, race, gender, BMI, and waist circumference. For these regressions, waist circumference was standardized within males and females separately because of gender differences in body shape; BMI was residualized for waist circumference z-scores and standardized based on the entire sample because of the high colinearity between BMI and waist circumference in both males and females. Because studies show nonlinear associations between sleep duration and glucose and diabetes risk, we then categorized the sample into quartiles based on sample distribution and examined whether the relationships appeared nonlinear. The final step was a formal statistical test for nonlinearity.
RESULTS
The analytic sample was composed of 245 adolescents (116 males, 137 blacks), with a mean age of 15.7 years (SD 1.3). The Hollingshead score, which takes into account parental/guardian education and occupational prestige,24,25 averaged 30.3 (SD 11.5), and ranged from 10 to 54, indicating a low- to middle-class status group. Of the sample, 60% were raised in a single parent household and 80% in a household with ≥ 1 parent/guardian employed. Almost half of the sample was overweight or obese by conventional adult categories: 62 had BMIs from 25-29.9, 55 had BMIs ≥ 30.
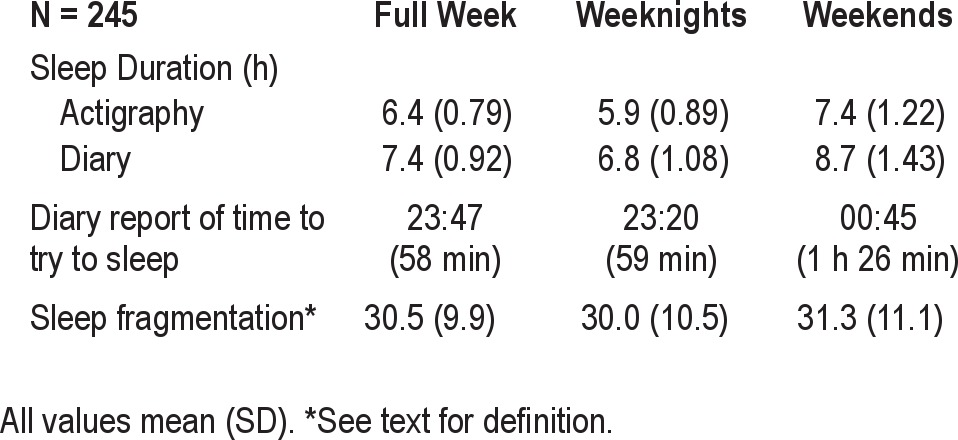
Sleep duration based on actigraphy averaged 6.4 h over the week, with school days significantly lower than weekends, P < 0.001 (Table 1). There was no relationship between time spent asleep during the weekday and weekend, P = 0.12. Sleep duration ranged overall, weekday, and weekend from 4.3 to 9.2, 3.4 to 8.6, and 3.5 to 10.9, respectively. On average, students tried to go to sleep about an hour later on the weekend, relative to weekday. Self-reported and actigraphy-assessed sleep duration were substantially associated (r values > 0.59, P values < 0.001), although self-reported values were higher than actigraphy-measured sleep duration, all P values < 0.001. Sleep fragmentation during the week tended to be lower than during the weekend, P ≤ 0.08, and the 2 were highly correlated, r = 0.64, P < 0.001.
Table 1.
Sample sleep characteristics
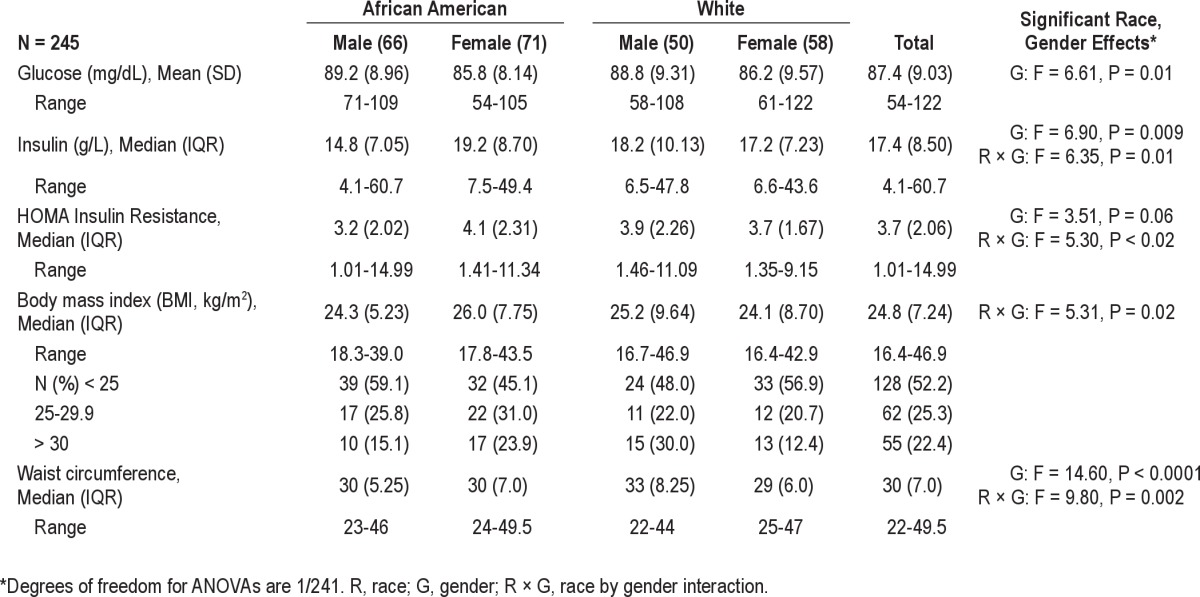
Males had higher glucose levels compared to females (Table 2). The HOMA-IR and insulin values varied by race and gender, with the lowest insulin and HOMA-IR apparent in black males. BMI and waist circumference also varied by race and gender, with higher BMI in the black females and white males, and higher waist circumference in males, especially white males.
Table 2.
Unadjusted fasting glucose and insulin, and weight characteristics of sample
Linear regression analyses showed that the HOMA-IR was higher among shorter sleepers based on the entire week, whether measured by actigraphy or diary (Table 3). This effect was primarily due to the school night sleep patterns, with no relationship occurring with weekend sleep patterns. In this model for the entire week, higher waist circumference z-score (B = 0.314, P = 0.017, residualized BMI z-score (B = 0.273, P = 0.04), and being female (B = 0.165, P = 0.001) were also associated with higher HOMA-IR. An interaction between sleep duration across the week and gender was significant, P = 0.05, such that the association between short sleep and HOMA-IR was stronger in males than females. Diary reports of later times trying to go to sleep were also related to the HOMA-IR. There were no relationships with fragmentation, P values > 0.36, and no significant interactions by race. There was no evidence of a curvilinear relationship, i.e., no significant quadratic effect. To illustrate, Figure 1 presents the mean unadjusted HOMA-IR scores in 4 approximately equal-sized groups of participants based on the sample distribution.
Table 3.
Linear regression models* predicting Log HOMA-IR index
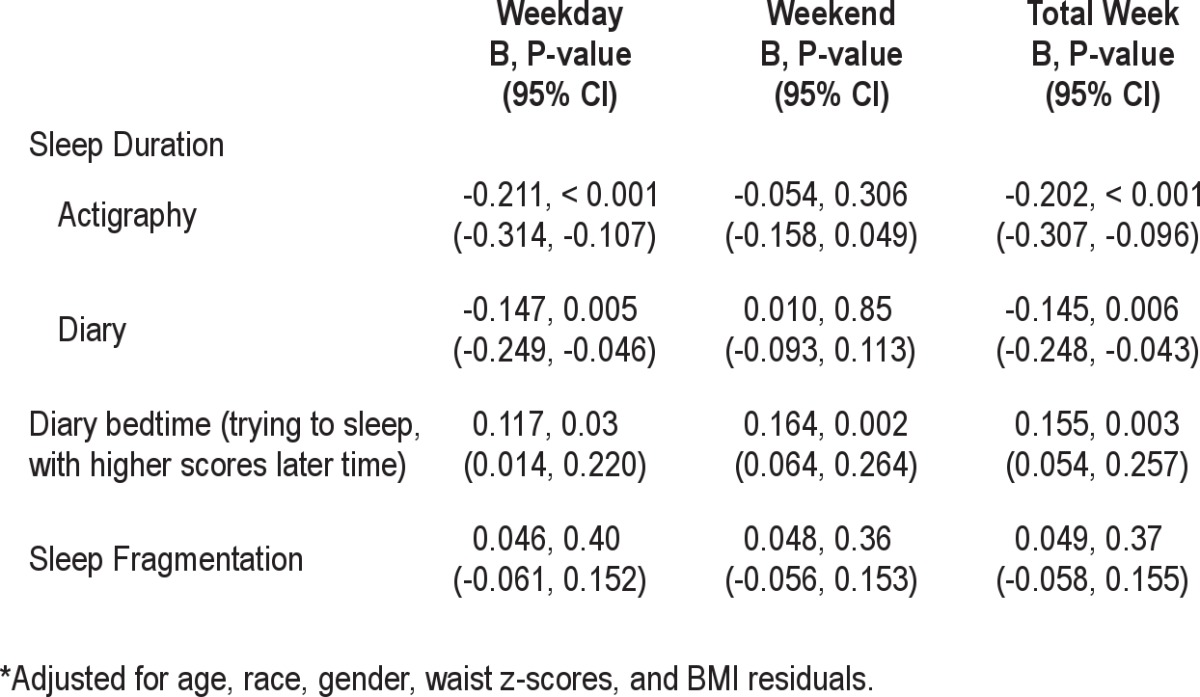
Figure 1.
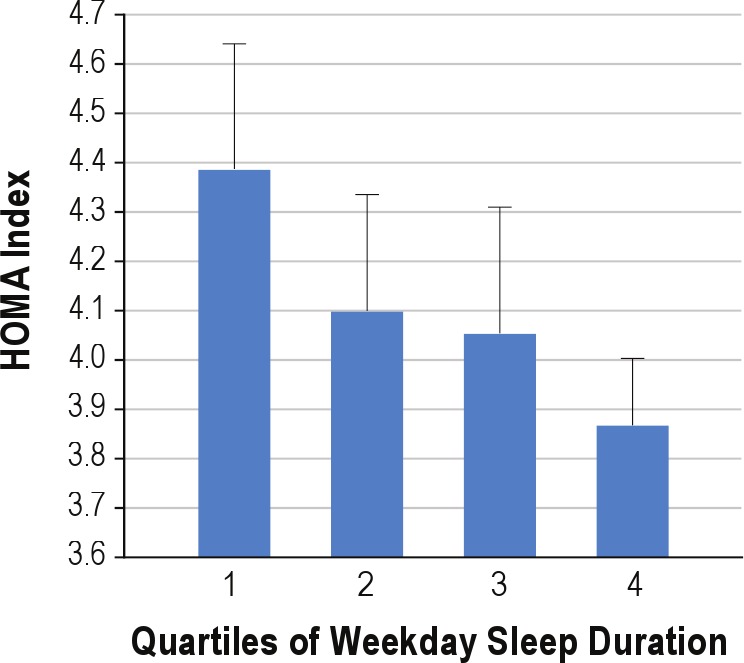
Unadjusted means (SEM) of HOMA Index across quartiles of weekday sleep duration in sample. Quartiles are based on approximately equally sized groups based on mean actigraph sleep duration during the week where 1 = 3.4–5.41, 2 = 5.41–6.00; 3 = 6.00–6.48; and 4 = 6.48–8.6 hours.
Linear regressions showed that higher glucose levels were associated with greater sleep fragmentation overall (B = 0.140, P = 0.035), during school nights (B = 0.138, P = 0.037), and trending on weekends (B = 0.122, P = 0.06). In these models, only being male was related to higher glucose levels (B = 0.152, P = 0.017. Neither the tests for curvilinear relationships nor the race/or gender by fragmentation interactions were significant. There were no relationships of glucose with sleep duration by either actigraphy, P values > 0.25, or self-report, P values > 0.48. Repeating the analysis for males, adjusting for standard covariates plus pubertal status, showed a similar positive association between fasting glucose and fragmentation, P = 0.013.
DISCUSSION
This study showed that higher insulin resistance is associated with shorter sleep duration assessed by actigraphy or diary among black and white healthy adolescents. These associations were independent of race, age, gender, waist circumference, and body mass index, and were obtained for measures of weekday sleep, not weekend sleep. The associations were stronger in males than in females. Based on the present regression model, we estimate that for adolescents who received 6 hours of sleep of night, one additional hour of sleep would have changed their HOMA-IR from 3.179 to 2.896. Glucose levels were higher among those with fragmented sleep, especially during the week. This is a novel finding and is consistent with some of the acute sleep restriction studies in adults.9 However, fragmentation was unrelated to HOMA-IR; note that the HOMA-IR is primarily determined by insulin levels. To our knowledge, our study is the only one in healthy adolescents that shows a relationship between shorter sleep and insulin resistance that is independent of adiposity.
Our results differ from those obtained in the Cleveland Sleep and Health Study18 in a number of respects. The effect of short sleep on insulin resistance was not eliminated by controls for adiposity in our study but was in the Cleveland Study. Furthermore, we did not observe a curvilinear relationship between sleep duration and insulin resistance. These differences may be due to the differences in the characteristics of our samples. Pittsburgh Project Pressure had 31.9% with a BMI ≥ 30 or ≥ 95th percentile based on age and gender,26 whereas the Cleveland Sleep and Health Study had 18.7% meeting these criteria. Given higher levels of obesity, it is not surprising that our HOMA-IR values were also higher than those observed in the Cleveland sample—4.13 vs. 1.95. In NHANES, obese (BMI ≥ 95 percentile) adolescents had higher HOMA-IR values than those considered to be normal weight (BMI < 85th percentile), Ms = 4.93 vs. 2.30, respectively, values in line with our sample.27 Pittsburgh students also slept less (based on actigraphy) during the week than those in the Cleveland Study, Ms = 6.4 vs. 7.6 h, and too few could be considered to be long sleepers to detect a relationship between long sleep and insulin resistance. The association between long sleep and insulin resistance or diabetes risk obtained in other studies is perplexing. As is oft noted, it may be due to premorbid health conditions and depression, resulting in excessive fatigue and long sleep. Furthermore, it is difficult to identify which mechanisms would account for the long sleep relationship with insulin resistance and diabetes.
On the other hand, multiple mechanisms may be important to explain the association between short sleep and increased insulin resistance. One pathway may be through sleep and/or circadian disruptions of the hormones that regulate food intake, i.e., leptin, an appetite-suppressing hormone, and ghrelin, an appetite-stimulating hormone. A review of 17 acute sleep restriction studies, however, shows inconsistent patterns of changes in these hormones, perhaps because the vast majority of participants were normal weight or lean and methodological differences among studies.9 Furthermore, this pathway is most likely related to the increased obesity associated with short sleep,28 and our findings are independent of obesity. Another potential pathway is through the autonomic nervous system, with sympathetic activation inhibiting and parasympathetic activation stimulating insulin release. Sleep loss may lead to lowered parasympathetic activity at night, allowing enhanced cardiac sympathetic activity.29–31 Sleep loss also results in increased evening cortisol, which is associated with hyperinsulinemia, especially in conjunction with central adiposity.32 Sleep loss can lead to insulin resistance through dysregulation of adipokines, independent of obesity. In an adult sample, total sleep time was inversely associated with leptin and visfatin levels, independent of age, race, gender, obesity, hypertension, diabetes, and obstructive sleep disorders.33 Finally, during the deeper stages of sleep, cerebral glucose metabolism (or utilization) is decreased, and loss of deep sleep may affect total body glucose regulation.34
Although not the purpose of this paper, our data indicate how little sleep healthy adolescents from a contemporary urban sample obtain during the school week. The amount of sleep is not near the optimal 9 hours recommended for adolescents, whether measured by actigraphy or diary. Also it is noteworthy that prospectively collected diary measures of sleep duration were statistically related to insulin resistance. This suggests that in clinical settings where objective measures of sleep are not feasible, diary measures may be useful. We should note though that our measures were collected on hand-held computers and were time-stamped to encourage on-time reporting.
Our study has a number of limitations as well as strengths. First, it used a cross-sectional design, such that the direction of the effect could be that heightened insulin resistance leads to reduced sleep, or that insulin resistance and sleep duration are caused by a third factor. Second, the sample is unique in that it is composed of low- to middle-class participants from a single urban community that is served by one high school. Thus, findings cannot be generalized to adolescents from a broader range of socioeconomic strata. Third, it did not measure sleep disordered breathing, which co-occurs with obesity. We did conduct a brief interview with parents on their observations of their children's symptoms of sleep disordered breathing. Only one adolescent had such symptoms, which were reviewed by one of the coauthors, a pediatric sleep expert (RD). Our effects were independent of central obesity, which also suggests that sleep disordered breathing may not be a substantial confounder of our results. Regarding strengths, the sample was healthy overall, without any known psychiatric, sleep, or metabolic disorders, and free of medications that might have influenced sleep or insulin resistance. It was a community-based sample as opposed to clinic-based, and had a substantial number of African American participants. Having the combination of diary and actigraphy measures for a full week allowed for a convergence of findings across sleep indicators.
In summary, we found that short sleep during the school week was associated with elevated insulin resistance in adolescents, independent of age, race, gender, and adiposity. In general, the participants received inadequate amounts of sleep, relative to the recommended amount of 9 hours. To the extent that short sleep leads to elevated insulin resistance, interventions designed to extend sleep in short sleepers may be beneficial for metabolic health in adolescence and beyond.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (HL025767).
ABBREVIATIONS
- BP
blood pressure
- HOMA-IR
homeostatic model assessment of insulin resistance
- BMI
body mass index
- CV
coefficient of variation
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;303:242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Ford ES, Zhao G, Mokdad AH. Associations of health risk factors and chronic illnesses with life dissatisfaction among U.S. adults: the Behavioral Risk Factor Surveillance System, 2006. Prev Med. 2009;49:253–9. doi: 10.1016/j.ypmed.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Rosner B, Cook N, Portman R, Daniels S, Falkner B. Blood pressure differences by ethnic group among United States children and adolescents. Hypertension. 2009;54:502–8. doi: 10.1161/HYPERTENSIONAHA.109.134049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rademacher ER, Jacobs DR, Jr., Moran A, Steinberger J, Prineas RJ, Sinaiko A. Relation of blood pressure and body mass index during childhood to cardiovascular risk factor levels in young adults. J Hypertens. 2009;27:1766–74. doi: 10.1097/HJH.0b013e32832e8cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AS, Mulder C, Twisk JW, van MW, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9:474–88. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 7.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carskadon MA. Maturation of processes regulating sleep in adolescents. In: Marcus C, editor. Sleep in children: Developmental changes in sleep patterns. New York: Informa Healthcare; 2008. pp. 95–114. [Google Scholar]
- 11.Olds T, Blunden S, Petkov J, Forchino F. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010;14:371–8. doi: 10.1016/j.smrv.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Teens and Sleep. National Sleep Foundation. Sleep in America Poll. Available from http://www.sleepfoundation.org/sites/default/files/2006_sumary_of_findings.pdf.
- 14.Flint J, Kothare SV, Zihlif M, et al. Association between inadequate sleep and insulin resistance in obese children. J Pediatr. 2007;150:364–9. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 15.Sung V, Beebe DW, Vandyke R, et al. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. Sleep. 2011;34:891–8. doi: 10.5665/SLEEP.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–7. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitze B, Bosy-Westphal A, Bielfeldt F, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr. 2009;63:739–46. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158:617–23. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–96. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 20.Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27:158–65. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- 21.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20:586–90. [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 23.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 24.Barratt W. Unpublished manuscript. Indiana State University; 2006. The Barratt simplified measure of social status (BMSS) measuring SES. Available from http://wbarratt.instate.edu/socialclall/barratt_simplified_measure_of_social_status.pdf. [Google Scholar]
- 25.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 26.Kuczmarski RD, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development, 2002. Report No.11. [PubMed] [Google Scholar]
- 27.Lee JM, Okumura MJ, Davis MM, Herman WH, Gurney JG. Prevalence and determinants of insulin resistance among U.S. adolescents: a population-based study. Diabetes Care. 2006;29:2427–32. doi: 10.2337/dc06-0709. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 29.Hall M, Vasko R, Buysse D, et al. Acute stress affects heart rate variability during sleep. Psychosom Med. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- 30.Spiegelhalder K, Fuchs L, Ladwig J, et al. Heart rate and heart rate variability in subjectively reported insomnia. J Sleep Res. 2011;20:137–45. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- 31.Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54:369–76. [PubMed] [Google Scholar]
- 32.Broussard J, Brady MJ. The impact of sleep disturbances on adipocyte function and lipid metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24:763–73. doi: 10.1016/j.beem.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–52. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]


