Abstract
Study Objective:
We compared sleep problems during pregnancy and sleep dissatisfaction 18 months after pregnancy in pregnant women with binge eating disorder (BED) symptoms and pregnant women without an eating disorder.
Design:
Norwegian Mother and Child Cohort Study (MoBa).
Patients or Participants:
Data were gathered from 72,435 women. A total of 1,495 (2.1%) women reported having BED symptoms both before and during pregnancy; 921 (1.3%) reported pre-pregnancy BED symptoms that remitted during pregnancy; 1,235 (1.7%) reported incident BED symptoms during pregnancy; and 68,784 (95.0%) reported no eating disorder symptoms before or during pregnancy (referent).
Measurements and Results:
Questionnaires were collected at 3 time points, with a median completion time of 17.1 weeks gestation, 30.1 weeks gestation, and 18.7 months after childbirth. We collected information on demographics, eating disorder status before and during pregnancy, sleep problems during the first 18 weeks of pregnancy, hours of sleep during the third trimester, and sleep satisfaction 18 months after childbirth. All BED symptom groups were significantly more likely to report sleep problems during the first 18 weeks of pregnancy than the referent (adjusted odds ratio [OR] = 1.26-1.42, false discovery rate [FDR] P < 0.05). In the third trimester, women with incident BED symptoms during pregnancy were more likely to report more hours of sleep than the referent (adjusted OR = 1.49, FDR P < 0.01). All BED symptom groups had higher odds of reporting more dissatisfaction with sleep 18 months after childbirth (adjusted ORs = 1.28-1.47, FDR P < 0.01).
Conclusions:
BED before or during pregnancy is associated with sleeping problems during pregnancy and dissatisfaction with sleep 18 months after childbirth. Health care professionals should inquire about BED during pregnancy as it may be associated with sleep disturbances, in addition to the hallmark eating concerns.
Citation:
Ulman TF; Von Holle A; Torgersen L; Stoltenberg C; Reichborn-Kjennerud T; Bulik CM. Sleep disturbances and binge eating disorder symptoms during and after pregnancy. SLEEP 2012;35(10):1403-1411.
Keywords: Binge eating disorder, sleep, pregnancy, eating disorders, post-partum, MoBa, The Norwegian Mother and Child Cohort Study
INTRODUCTION
Binge eating disorder (BED) is poised to be an independent diagnostic category in the Diagnostic and Statistical Manual Fifth Edition. BED is characterized by binge eating (eating an unusually large amount of food in a discrete period of time and feeling out of control) in the absence of the regular compensatory behaviors characteristic of bulimia nervosa.1 As such, BED is often, but not always, seen in individuals who are overweight or obese.2 Estimates of binge eating behavior in the population range from 2% to 5%.2,3
Previously, we have shown that for some individuals, pregnancy represents a window of remission for BED.4 For a considerable number of women, however, pregnancy appears to be a window of risk for incident BED.4 Although this is not entirely surprising given the impact that pregnancy-related hormones can have on appetite and mood,5,6 it nonetheless remains concerning because of the potential impact of binge eating on the developing fetus, including higher weight babies and increased likelihood of Cesarean section.7,8
A variety of eating disorder presentations—especially those marked by binge eating—have been shown to be associated with sleep disturbances.9 Although we know of no study that has examined sleep specifically in BED, there are several lines of evidence to suggest that sleep may be disrupted in individuals with the disorder. Individuals with BED show increased morning levels of cortisol, and symptom severity is positively related to cortisol production.10,11 Cortisol is a hormone that plays a critical role in the sleep/wake cycle.12 The daily cycle of caloric intake has been shown to differ in BED compared with body mass index (BMI) matched controls, with fewer calories consumed in the midday and more in the evening.13 Further evidence of a relationship between BED and sleep disturbances emerges from twin studies, which reveal shared genetic factors between binge eating and night eating—a syndrome which clearly involves a disruption in both eating and sleeping circadian rhythms.14
Four lines of evidence converged to result in our current hypothesis. First, as noted above, BED often continues or has its onset during pregnancy.4 Second, eating disorders marked by binge eating appear to be associated with sleep disturbances.9 Third, BED is commonly associated with obesity, which itself is being increasingly recognized as having reciprocal adverse associations with sleep disturbances.15 Fourth, pregnancy and the period after childbirth are known to profoundly affect sleep.16,17
Based on these observations, we applied existing data from a large national cohort study of pregnant women, The Norwegian Mother and Child Cohort Study, to explore patterns of sleep and sleep satisfaction both during and after pregnancy in women with BED symptoms relative to women with no eating disorder both during and after pregnancy. We hypothesized that women with BED symptoms would report greater sleep difficulties and lower sleep satisfaction during pregnancy and after childbirth, and that these differences would be independent of the effects of BMI on sleep domains.
METHODS
Participants
The data collection was conducted as part of the Norwegian Mother and Child Cohort Study (MoBa) at the Norwegian Institute of Public Health.18 The study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill, the Regional Committee for Medical Research Ethics in South-Eastern Norway, and the Norwegian National Data Inspectorate.
MoBa is a prospective population-based pregnancy cohort study. Participants were recruited from all over Norway from 1999-2008; 38.5% of invited women consented to participate. The cohort now includes 108,000 children, 90,700 mothers, and 71,500 fathers. Blood samples were obtained from both parents during pregnancy and from mothers and children (umbilical cord) at birth. Follow-up is conducted by questionnaires at regular intervals and by linkage to national health registries. Pregnant women were recruited through a postal invitation after registering for a routine prenatal ultrasound at about 17 weeks' gestation. To date, 8 waves of assessment have been initiated following the mother and child from the beginning of pregnancy to the child's seventh year. Data collection is ongoing. Thus, sample sizes for this study decrease across questionnaires for cohorts who have finished completion of all surveys. Full text of questionnaire 1, 3, and 5 can be viewed at http://www.fhi.no/dokumenter/1f32a49514.pdf, http://www.fhi.no/dokumenter/7b6b32b0cd.pdf, and http://www.fhi.no/dokumenter/2640dd4bcc.pdf, respectively.
Our analyses are based on version 5 of the quality-assured data files released in 2010. The analysis population for this report included MoBa participants who: (a) did not complete an early pilot version of Questionnaire 1 (n = 2,605), (b) had valid values for self-reported age, weight, and height, (c) returned Questionnaire 1 before delivery, and (d) had a singleton birth. If a woman enrolled in MoBa more than once (due to additional pregnancies), only the first pregnancy that occurred during the course of MoBa data collection was included. Of the initial 103,474 mother-child records, 81,322 (79%) met the 5 criteria above. Of the 81,322 records, there were 3,651 women with self-reported broadly defined BED (defined below) before and/or during pregnancy and no missing values across those 2 time points and 68,784 women with no reported eating disorder symptoms and no missing values at either time point, leading to a final sample size of 72,435. Respondents from the sample completed Questionnaire 1 at a median of 17.1 weeks gestation (n = 70,362, interquartile range [IQR] 15.9-18.9 weeks). Questionnaire 3 was completed at a median of 30.1 weeks gestation (n = 65,599, IQR 29.7-31.1 months). Questionnaire 5 was completed at a median of 18.7 months postpartum (n = 45,189, IQR 18.4-19.2 months).
Measures
Items from Questionnaires 1, 4, and 5, as well as the maternal birth registry (MBR) provided information on demographics, eating disorder status, and patterns of maternal sleep. Demographic information and other characteristics for the sample were gathered from the MBR and Questionnaires 1 and 4 from items including maternal age, self-reported height and weight before pregnancy, gestational age of baby at birth, sex of child, parity, combined parental income, smoking status during pregnancy, mother's education, total number of live births, maternal anxiety and depression during pregnancy, and maternal BMI before pregnancy. The measure in Questionnaire 1 for anxiety and depression (SCL-5) was taken from the Hopkins Symptom Checklist-25 and included 5 items, which were averaged to form the score. This measure, as used in MoBa and eating disorders, is further described by Knopf Berg et al.19
Eating disorders were assessed on Questionnaire 1 from items addressing DSM-IV criteria for anorexia nervosa, bulimia nervosa, and eating disorder not otherwise specified. The eating disorder related questions have been used previously in research on BED in several studies.4,20–23 As in these previous studies, a diagnostic algorithm was established to identify broadly defined BED (at least weekly binge eating episodes without the presence of compensatory behaviors). In this paper, we refer to this symptom cluster as “BED symptoms” for clarity of presentation (see footnote following manuscript). The symptom of binge eating was assessed with questions addressing both eating an unusually large amount of food and feeling out of control. BED symptoms were assessed for a 6-month interval prior to pregnancy and around 18 weeks gestation. Respondents were classified into 4 groups: BED symptoms before and during pregnancy (n = 1,495), BED symptoms before pregnancy that remitted during pregnancy (n = 921), incident BED symptoms during pregnancy (1,235), and no reported eating disorder symptoms before or during pregnancy (n = 68,784). There were 182 women who reported BED symptoms either before or during pregnancy but were missing a response at the other time point and were thus excluded from the analyses, as they were unable to be correctly classified.
Maternal sleep was assessed at 3 time points. In Questionnaire 1, mothers were asked if they experienced sleeping problems during weeks 0-4, 5-8, 9-12, and 13+ of pregnancy (response option “yes/no”). In Questionnaire 3, mothers were asked how many hours of sleep per day they currently get with the following response options: < 4 h, 4-5 h, 6-7 h, 8-9 h, or > 10 hours. In Questionnaire 5, around 18 months after the birth, mothers were asked “How satisfied are you with your sleep?” with the following response options: “very satisfied,” “satisfied,” “neither satisfied nor dissatisfied,” “dissatisfied,” or “very dissatisfied.”
Statistical Analyses
We described sample characteristics by BED symptom status with means and standard deviations for continuous variables and frequencies for categorical variables. To test differences in descriptive variables by BED symptom status we used Kruskal-Wallis statistic for continuous variables and a χ2 test for categorical variables. A logistic regression model was used to estimate odds of reporting any sleep problems by BED symptom status at 4 time points spanning the first trimester of pregnancy and time beyond 13 weeks of pregnancy. Time was a covariate in the logistic regression, and generalized estimating equations (GEE) were used to estimate the covariance structure for time. There was no evidence of an interaction between time and BED symptom status, so it was omitted from the model. Odds were adjusted with and without confounders. Confounders in all analyses included gestational age of baby at birth, sex of child, parity, combined parental income, smoking status during pregnancy, mother's education, maternal anxiety and depression during pregnancy, maternal age, and maternal BMI, with the exception of the logistic regression described above.
Lastly, we used a proportional odds model to estimate the odds of ordinal responses for questions relating to sleep for mothers in the third trimester and at 18 months following the child's birth. If the proportional odds model assumptions did not hold, evaluated with a proportional odds score statistic, then a generalized logit model was used instead. Both models assess the odds of giving a particular response in a designated referent group, which for this study is mothers with no eating disorder before or during pregnancy, and compares these odds to the odds of giving such a response in the comparison group. A significant difference would indicate that the comparison group is either significantly more or less likely to report the response than the referent group. Proportional odds models estimate one odds ratio (OR) corresponding to a summary of ORs for all cut points cumulated over an ordinal response.24 If the ORs for each of the cut points are significantly different from one another, then assumptions for the proportional odds model are not met. The generalized logit model estimates separate ORs for each level of the response versus a referent. Odds ratios were estimated for each BED symptom group with and without confounders, using the appropriate statistical model.
The effects of confounders on the predictor, BED symptom status, were assessed in separate analyses. The percent change in the BED symptom effect was measured after singly adding each confounder to each model. Addition of the anxiety and depression variable resulted in an approximately 20% change in the BED status effect size when predicting sleep problems in the first trimester. When predicting satisfaction with sleep at 18 months after childbirth, the anxiety and depression variable was the only one to exceed a 10% change in BED (effect size approximately 15%). Variables including anxiety and depression, sex of child, maternal education, maternal age, and BMI resulted in ≥ 10% change in the BED symptom status effect size after being added in the model predicting maternal hours of sleep in the third trimester. All other confounders resulted in a BED symptom status effect size change < 10% when added to with BED symptom status as the only predictor. All confounders were left in the model as they were determined a priori.
In response to review, we conducted a post hoc evaluation of the interaction between anxiety and depression and BED symptom status. This evaluation was done for each of the regression models predicting sleep outcomes. With one exception, the addition of the interaction term between anxiety and depression and BED symptom status did not result in a statistically significant type 3 statistic, which provided no evidence that the inclusion of the interaction terms resulted in a model that differed from the model without the interaction term via a likelihood ratio statistic. For the “sleep problems” outcome in the second trimester, the type 3 statistic was significant (P < 0.03), and the interaction term between anxiety and depression and BED symptom status was retained in the model. For the other models we did not retain the interaction term. Inclusion of an interaction term in this context complicates interpretation of effects. Thus, when reporting odds ratios for BED symptom groups, inclusion of the interaction term as specified above necessitates specifying the anxiety and depression scale value at which the BED symptom group differences occur. For representation of effect sizes in the tables, we selected the mode of the anxiety and depression scale for the entire sample, which was 1 with a frequency of 50.0%.
Inclusion of the interaction term consistently revealed a negative association between BED symptom status and anxiety and depression for each of the BED symptom groups compared with the referent. For a one-unit increase in the anxiety and depression measure, the effect for the group of women with BED symptoms before pregnancy that remitted during pregnancy, BED symptoms before and during pregnancy, and incident BED symptoms during pregnancy declined by 26.7% (95% CI = −46.1% - −0.4%), 0.6% (95% CI = −21.3% - 25.6%), and 30.5% (95% CI = −46.7% - −9.4%), respectively. For example, at the mode for anxiety and depression (1), the OR for sleep problems was 1.42 (1.15-1.75) for the group of women with incident BED symptoms during pregnancy versus referent. Given a one-unit increase in the anxiety and depression score, at a level of 2, the OR for sleep problems declined to 0.99 (0.81-1.20) for the same group of women versus the referent, which represents a 30.5% decline in the OR of reporting sleep problems for a one-unit increase in the anxiety and depression score. In sum, as the measure for anxiety and depression increases, the difference in the outcome declines for BED symptoms versus the referent.
All differences were considered significant at an α level of 0.05, and all estimates were adjusted within one family using the Benjamini-Hochberg false discovery rate (FDR) method.25 All analyses were conducted using the SAS/STAT software, version 9.2 of the SAS System for Windows.26
RESULTS
Demographic information for the sample is presented in Table 1. Of the 72,435 participants, 1,495 (2.1%) mothers reported BED symptoms both before and during pregnancy, 921 (1.3%) reported pre-pregnancy BED symptoms that remitted during pregnancy, 1,235 (1.7%) reported incident BED symptoms during pregnancy, and 68,784 (95.0%) reported no eating disorder symptoms before or during pregnancy (referent group). Variables pertaining to maternal sleep are presented as frequencies and percentages in Table 2. For the entire sample, 2.9% reported sleeping problems in weeks 0-4, 6.3% in weeks 5-8, 8.7% in weeks 9-12, and 10.3% in weeks 13+. In the third trimester, 10.3% slept > 10 h, 57.4% slept 8-9 h, 29.4% slept 6-7 h, 2.7% slept 4-5 h, and 0.3% slept < 4 hours. Eighteen months after pregnancy, 3.4% were very dissatisfied with their sleep, 14.4% were dissatisfied with their sleep, 14.1% were neither satisfied nor dissatisfied with their sleep, 45.5% were satisfied with their sleep, and 22.7% were very satisfied with their sleep.
Table 1.
Population characteristics, number (%)
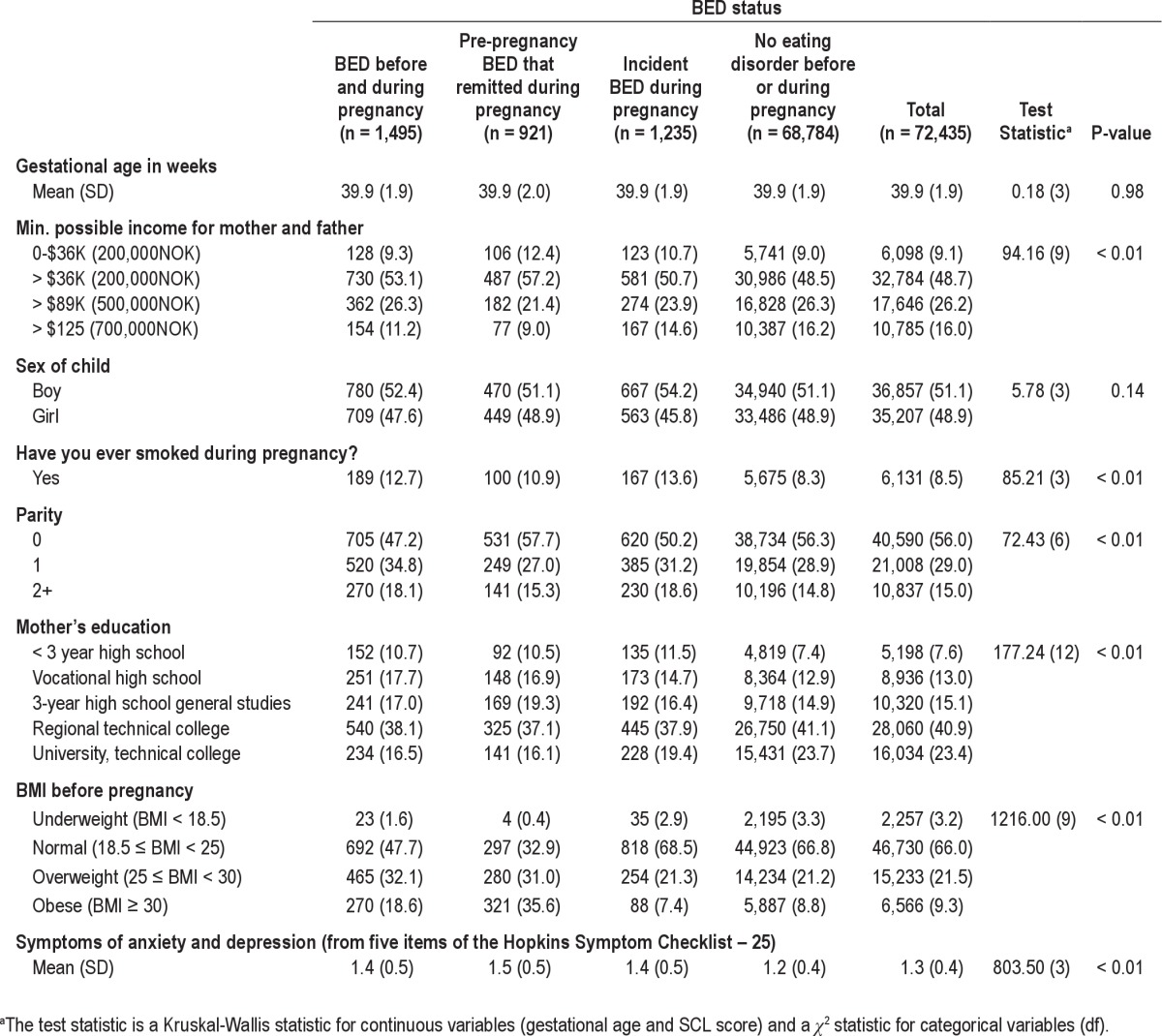
Table 2.
Variables pertaining to maternal sleep, frequency (%)
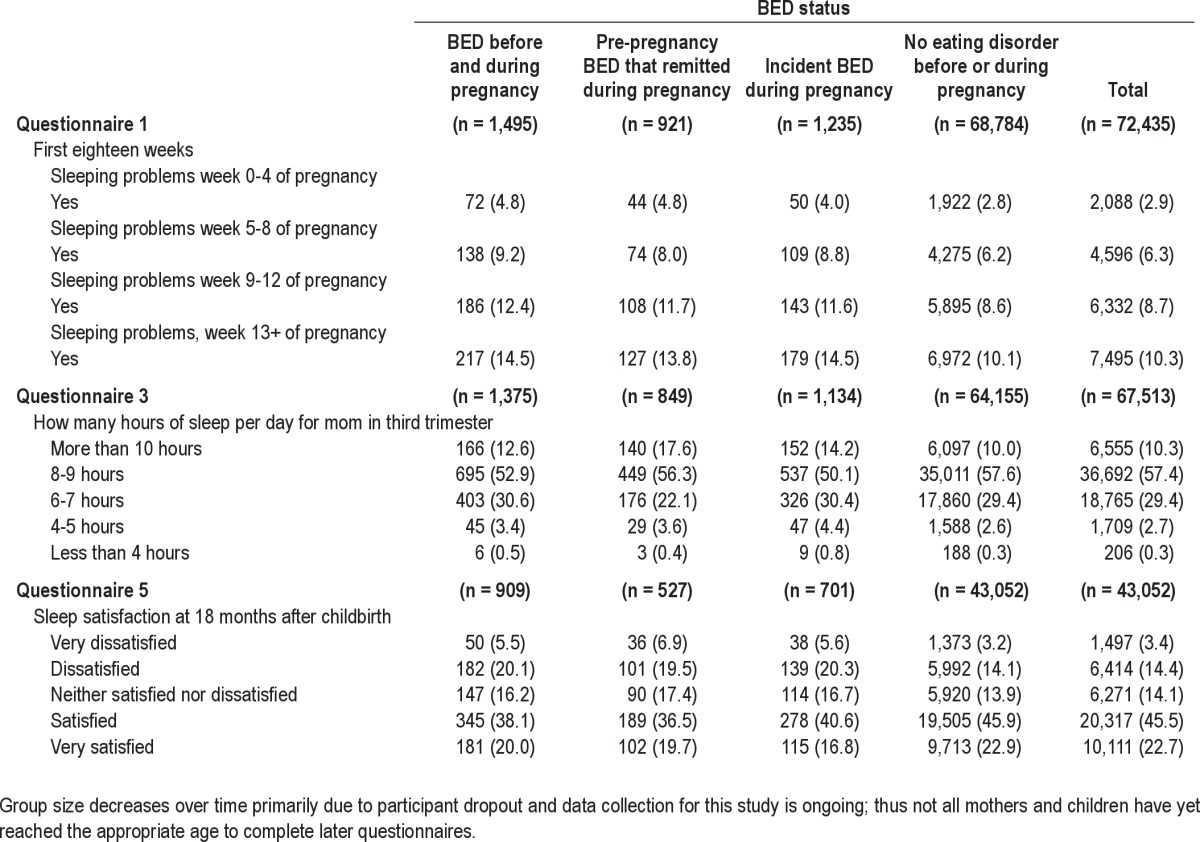
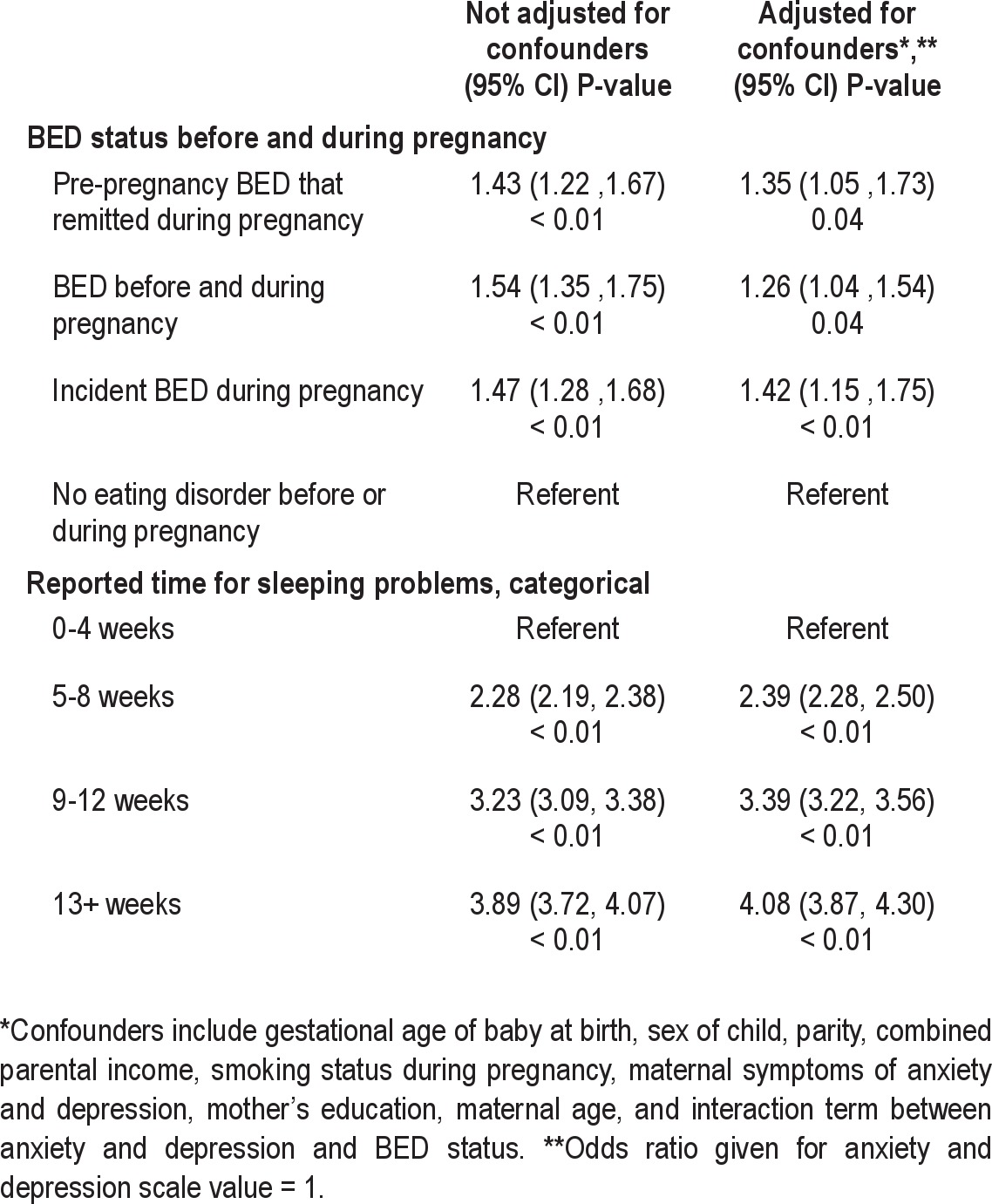
Table 3 shows the ORs for reporting any sleep problems in the first questionnaire, a dichotomous response; ORs were significantly higher for all groups with BED symptoms either before or during pregnancy than for the referent group, and these differences remained significant after adjusting for confounders. After adjusting for confounders, women with BED symptoms before and during pregnancy, women with pre-pregnancy BED symptoms that remitted during pregnancy, or women with incident BED symptoms during pregnancy were 26% (FDR P < 0.05), 35% (FDR P < 0.05), and 42% (FDR P < 0.01) more likely, respectively, to report sleeping problems than the referent group. Because there is an interaction term between BED symptoms and maternal anxiety and depression (SCL-5), in the final adjusted model, the OR is reported at SCL-5 = 1. The odds of reporting sleeping problems increased throughout the first 18 weeks of pregnancy, with no significant interaction between the groups and time.
Table 3.
Odds ratios for reporting sleep problems in mothers in the first 18 weeks of pregnancy
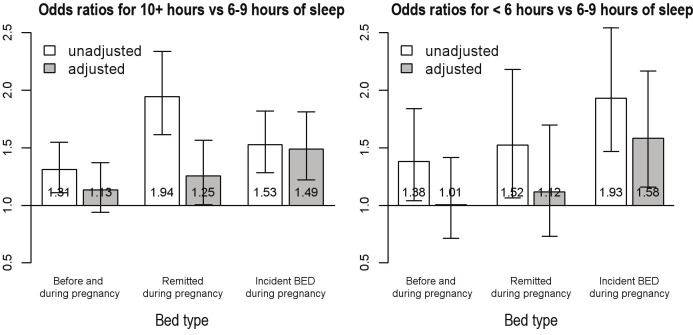
The OR of reporting > or < 6-9 h of sleep during the third trimester by BED symptom group are shown in Figure 1. The proportional odds model assumptions were not met, and a generalized logit model was used with 2 ORs reported: one with > 10 h versus 6-9 h and the other with < 6 h of sleep versus 6-9 hours. Women with BED symptoms before and during pregnancy, women with pre-pregnancy BED symptoms that remitted during pregnancy, or women with incident BED symptoms during pregnancy were 31% (FDR P < 0.01), 94% (FDR P < 0.01), and 53% (FDR P < 0.01) more likely, respectively, to report more (> 10) hours of sleep than the referent group. After adjusting for confounders the odds of > 10 h of sleep versus 6-9 h was still higher for those with BED symptoms at some point during pregnancy, but no longer significant with the exception of women with incident BED during pregnancy (OR = 1.49, FDR P < 0.01). Likewise, women with BED symptoms before and during pregnancy, women with pre-pregnancy BED symptoms that remitted during pregnancy, or incident BED symptoms during pregnancy were 38% (FDR P < 0.05), 52% (FDR P < 0.05), and 93% (FDR P < 0.01) more likely, respectively, to report fewer hours of sleep (< 6 h) than the referent. After adjusting for all confounders, the OR of less sleep remained significant only for the group with incident BED symptoms during pregnancy (OR = 1.58, FDR P < 0.01).
Figure 1.
Odds ratios (ORs) for maternal hours of sleep during the third trimester shown unadjusted and adjusted for gestational age of baby at birth, sex of child, parity, combined parental income, smoking status during pregnancy, mother's education, anxiety and depression during pregnancy, maternal age, and maternal BMI before pregnancy. Referent group is those with no reported eating disorder symptoms before or during pregnancy.
The likelihood of dissatisfaction with sleep at 18 months after childbirth in women with BED symptoms is reported in Figure 2. The proportional odds assumptions were met for this response variable, and the ORs represent being less satisfied with sleep versus more satisfied. All 3 BED symptom groups were more likely to report sleep dissatisfaction than the referent group. Women with BED symptoms before and during pregnancy, women with pre-pregnancy BED symptoms that remitted during pregnancy, and women with incident BED symptoms during pregnancy had, respectively, 60% (FDR P < 0.01), 72% (FDR P < 0.01), and 64% (FDR P < 0.01) higher odds of reporting less satisfaction with sleep than the referent group. After adjusting for all confounders, these estimates remain elevated and significant with 28% (FDR P < 0.01), 47% (FDR P < 0.01), and 40% (FDR P < 0.01) higher odds of being dissatisfied with sleep compared to the referent group for women with BED symptoms before and during pregnancy, women pre-pregnancy BED symptoms that remitted during pregnancy, and women with incident BED symptoms during pregnancy, respectively.
Figure 2.
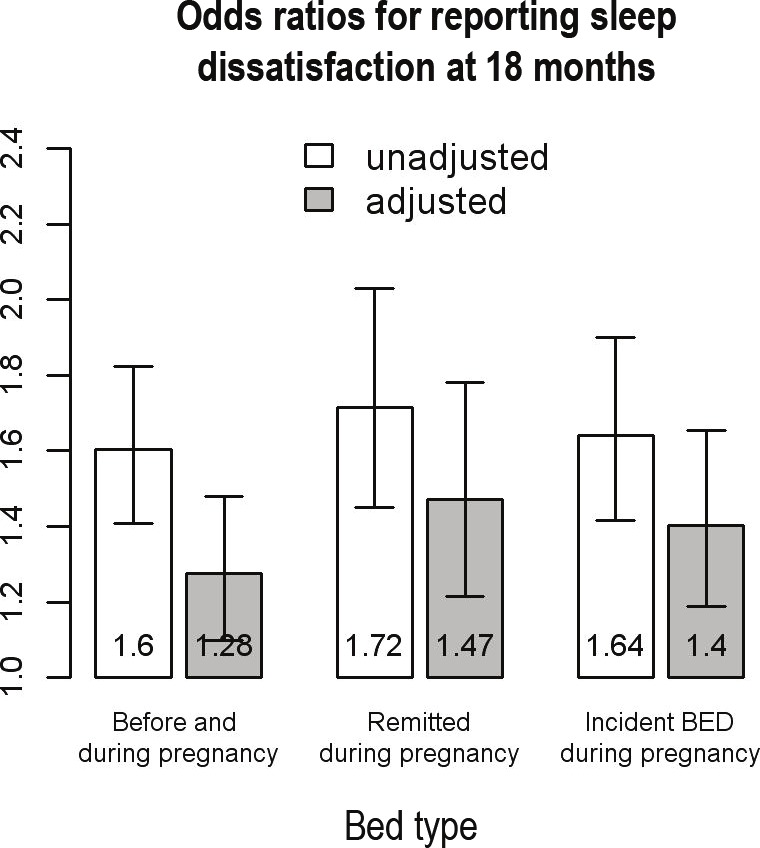
Odds ratios (ORs) for maternal sleep dissatisfaction eighteen months after childbirth, shown unadjusted and adjusted for the gestational age of baby at birth, sex of child, parity, combined parental income, smoking status during pregnancy, mother's education, anxiety and depression during pregnancy, maternal age, and maternal BMI before pregnancy. Referent group is those with no reported eating disorder symptoms before or during pregnancy.
DISCUSSION
To our knowledge, this is the first study to examine sleep and BED symptoms during pregnancy. Women with BED symptoms before and during pregnancy reported more self-reported sleep problems than a referent group of women with no reported eating disorder symptoms, as well as increased sleep dissatisfaction eighteen months after childbirth.
All women, regardless of eating disorder status, reported increasing sleep problems in the interval spanning the first eighteen weeks of pregnancy. This is consistent with the fact that women experience changes in their sleep patterns by 11-12 weeks gestation, with increased hours of sleep but less deep sleep and more nighttime awakenings.16,17 Women with BED symptoms before and during pregnancy experienced more sleep problems than the referent group during the first eighteen weeks of pregnancy. Our nonspecific sleep assessment precluded the precise characterization of the nature of sleep problems experienced, but we can report preliminarily that general sleep problems are more common during pregnancy in women with BED symptoms than women without eating disorders.
Of the three time periods assessed, the analysis of sleep outcomes in the interval spanning the first and second trimester included an interaction term between anxiety and depression and BED symptom status. This interaction term indicates that the positive association between BED symptom groups as a predictor of the likelihood of reporting sleep problems differs across anxiety and depression values. In fact, weaker associations between BED symptom groups and the likelihood of reporting sleep problems exist for all three groups as anxiety and depression scores increase. An opposite direction of effect would be expected if there were a synergistic relationship between BED symptoms and the effect of anxiety and depression on sleep. A change in anxiety and depression levels may reflect an underlying biological or psychological effect that dampens the association between BED symptoms groups and sleep problems.
Duration of sleep also differed across groups during the third trimester of pregnancy. All BED symptom groups reported higher odds of sleeping more than 6-9 hours during the third trimester compared with the referent. After controlling for confounders, this difference remained significant only for women with incident BED symptoms during pregnancy.
Incident BED during pregnancy in the MoBa sample is associated with symptoms of anxiety and depression, low life satisfaction, low self-esteem, low partner relationship satisfaction, negative health behaviors, low social support, a history of sexual or physical abuse, and pregnancy-related weight concerns.27 The increased likelihood of hypersomnia during pregnancy in women with BED symptoms before and during pregnancy may be attributable to increased rates of anxiety and atypical depression. Although we controlled for depression, the measure used was a screening tool that selected for its ease in administration. A more nuanced diagnostic approach would be better able to characterize depression. The relationship among anxiety, depression, sleep, and eating is complex. One in four women with BED reports current comorbid anxiety disorders28; nearly two-thirds of individuals with anxiety disorders report poor sleep29; one-fifth to one-fourth of depressed individuals exhibit atypical depressive features, including increased appetite and hypersomnia30–32; and women with BED with comorbid depression are over twice as likely to have atypical than typical depressive features.30
Eighteen months after giving birth, women with BED symptoms before or during pregnancy reported more dissatisfaction with their sleep than the referent group, even after controlling for confounders. We know very little about the trajectory of normalization of sleep, with most studies truncated at six months postpartum and therefore not capturing returns to baseline sleep patterns.16,17,33,34 The most common reason for sleep disturbances during the postpartum period are the infant's sleep and feeding cycle.35 Child sleep becomes an increasingly less likely culprit for maternal sleep dissatisfaction as babies mature. Most infants are sleeping through the night by 18 months,36 suggesting that the sleep problems observed in this cohort may reflect processes both associated with and independent of pregnancy.
Women with incident BED in the MoBa experience a general “matrix of disadvantage.”27 As such, stress may be a uniting factor underlying both incident binge eating and sleep dissatisfaction. Stress plays a critical role in animal models of binge eating,37,38 and stress in humans is associated with binge eating in both laboratory and naturalistic settings.11,39–41 Cortisol may be the common biological mechanism influencing the emergence of both binge eating and sleep difficulties during pregnancy. Cortisol, a steroid hormone produced in the adrenal gland and part of the hypothalamic-pituitary-adrenal (HPA) axis, is secreted in a diurnal pattern with highest levels early in the morning.42 During pregnancy, trait anxiety and a history of child abuse are related to lower baseline awakening levels of cortisol.43,44 In women with BED, binge eating severity is positively associated with cortisol levels.10 Short sleep duration and high sleep disturbance are also associated with elevated evening cortisol secretion.12 Interestingly, binge eating behaviors occur most frequently in the evening hours,13 and patterns of cortisol secretion may shift in anticipation of this regular food intake pattern even when food is not always ingested.45–47 Stress may underlie the association between BED, sleep, and cortisol during pregnancy, and stress itself has been shown to shift the circadian rhythm in animal models of stress during pregnancy.48
Ghrelin and leptin, hormones that regulate satiety cues, may also be implicated. Ghrelin levels are lower in fasting and post-meal conditions in women with BED compared with BMI matched controls,49 and leptin levels are increased in women with BED compared with women with anorexia nervosa and normal weight women with bulimia nervosa.50 Both of these hormones influence sleep. A positive correlation between feeding, ghrelin levels, and wake activity has been observed in rats51; in humans, sleep deprivation alters plasma concentrations of these hormones52,53 and sleep cycle shifts also shift secretion of these hormones.54 The role of appetite hormones is worthy of further studies of sleep and binge eating during pregnancy.
Limitations to our study must be considered. First, although the items used to create eating disorder categories have been widely used,4,–23,27 they were self-reported and some were retrospective. Cohort studies yield large sample sizes, but the logistic necessity of self-report may yield less accurate information and yield higher prevalence of BED than face-to-face diagnostic interviews.55 The prevalence of BED (herein referred to as BED symptoms) in this sample is, however, somewhat lower than that reported in another population-based sample.56 Second, BED symptoms were defined to include episodes of binge eating once a week, which differs from the current DSM-IV criterion of twice a week, but is consistent with the proposed DSM-V criteria (http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=372).57–60 Third, although a 38.5% response rate is typical for large population-based studies, MoBa participants differ from the general Norwegian population of pregnant women by having a lower prevalence of preterm births (7.2% vs. 7.7%) and lower birthweights (4.6% vs. 5.1%). Participants are more educated (61% attending some form of college compared with 49% in the general population between the ages of 25 and 29 and 46% between the ages 30 and 39). Fourth, the cross sectional design of these analyses does not allow for conclusions about patterns of onset between BED symptoms and sleep disturbances. Fifth, the MoBa study was designed to cover a large range of topics related to pregnancy and child outcomes rather than designed specifically to address questions of sleep in pregnancy and the postpartum period.
Our results highlight research questions that can be translated into clinical investigations to further elucidate the role of stress and appetite on sleep during pregnancy. The pattern of onset between BED symptoms and sleep disturbances, using more precise sleep assessments is worthy of study. Concurrent assessment of eating and appetite, stress, and biological indices (e.g., cortisol, ghrelin, and other appetite hormones) would enable a more comprehensive understanding of how these factors may interact to influence binge eating and sleep during and after pregnancy. The assessment of infant/child outcomes could also shed light on long-term effects of maternal appetite and sleep dysregulation during pregnancy.
These findings further support our recommendation for comprehensive mental health screening during pregnancy.4 BED symptoms during pregnancy are associated not only with appetite dysregulation but also with sleep disturbances. Effective detection may assist with appropriate referrals and interventions that may mitigate any potential untoward effects on both mother and baby.
DISCLOSURE STATEMENT
This was not an industry-supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (grant no. N01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10). We are grateful to all the participating families in Norway who take part in this ongoing cohort study. T. Frances Ulman was supported by National Institute of Mental Health grant T32MH076694. Work for this study was performed at University of North Carolina at Chapel Hill and the Norwegian Institute of Public Health.
FOOTNOTE
N.B. The label of BED symptoms in this paper is at the request of reviewers. There are no differences between the participants identified as having “BED symptoms” in this publication and all other papers reporting “BED” in the MoBa sample.4,7,8,19,27
REFERENCES
- 1.Arlington, VA: American Psychiatric Association; [cited 16 August 2011]. Binge Eating Disorder Proposed Revision of Diagnostic Criteria. updated 31 January 2011; http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=372. [Google Scholar]
- 2.Hudson JI, Hiripi E, Pope HG, Jr., Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preti A, Girolamo G, Vilagut G, et al. The epidemiology of eating disorders in six European countries: results of the ESEMeD-WMH project. J Psychiatr Res. 2009;43:1125–32. doi: 10.1016/j.jpsychires.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Bulik CM, Von Holle A, Hamer R, et al. Patterns of remission, continuation and incidence of broadly defined eating disorders during early pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Psychol Med. 2007;37:1109–18. doi: 10.1017/S0033291707000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:766–76. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol. 2010;22:805–17. doi: 10.1111/j.1365-2826.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- 7.Bulik CM, Von Holle A, Siega-Riz AM, et al. Birth outcomes in women with eating disorders in the Norwegian Mother and Child cohort study (MoBa) Int J Eat Disord. 2009;42:9–18. doi: 10.1002/eat.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siega-Riz AM, Haugen M, Meltzer HM, et al. Nutrient and food group intakes of women with and without bulimia nervosa and binge eating disorder during pregnancy. Am J Clin Nutr. 2008;87:1346–55. doi: 10.1093/ajcn/87.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim KR, Jung YC, Shin MY, Namkoong K, Kim JK, Lee JH. Sleep disturbance in women with eating disorder: prevalence and clinical characteristics. Psychiatry Res. 2010;176:88–90. doi: 10.1016/j.psychres.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho WF, Moreira RO, Spagnol C, Appolinario JC. Does binge eating disorder alter cortisol secretion in obese women? Eat Behav. 2007;8:59–64. doi: 10.1016/j.eatbeh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–81. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 12.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–9. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond NC, Neumeyer B, Warren CS, Lee SS, Peterson CB. Energy intake patterns in obese women with binge eating disorder. Obesity Res. 2003;11:869–79. doi: 10.1038/oby.2003.120. [DOI] [PubMed] [Google Scholar]
- 14.Root TL, Thornton LM, Lindroos AK, et al. Shared and unique genetic and environmental influences on binge eating and night eating: a Swedish twin study. Eat Behav. 2010;11:92–8. doi: 10.1016/j.eatbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, Ware JC. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers sleep. Sleep Med. 2002;3:37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 18.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 19.Knoph Berg C, Torgersen L, Von Holle A, Hamer RM, Bulik CM, Reichborn-Kjennerud T. Factors associated with binge eating disorder in pregnancy. Int J Eat Disord. 44:124–33. doi: 10.1002/eat.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health Twin Panel: a description of the sample and program of research. Twin Res. 2002;5:415–23. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 21.Reichborn-Kjennerud T, Bulik CM, Kendler KS, et al. Gender differences in binge-eating: a population-based twin study. Acta Neurol Scand. 2003;108:196–202. doi: 10.1034/j.1600-0447.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 22.Reichborn-Kjennerud T, Bulik CM, Sullivan PF, Tambs K, Harris JR. Psychiatric and medical symptoms in binge eating in the absence of compensatory behaviors. Obesity Res. 2004;12:1445–54. doi: 10.1038/oby.2004.181. [DOI] [PubMed] [Google Scholar]
- 23.Reichborn-Kjennerud T, Bulik CM, Tambs K, Harris JR. Genetic and environmental influences on binge eating in the absence of compensatory behaviors: a population-based twin study. Int J Eat Disord. 2004;36:307–14. doi: 10.1002/eat.20047. [DOI] [PubMed] [Google Scholar]
- 24.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. Cary, NC: SAS Institute; 2001. [Google Scholar]
- 25.Benjamini YH, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statis Soc B. 1995;57:289–300. [Google Scholar]
- 26.SAS. Institute Inc. SAS/STAT 9.2 User's Guide. Cary, NC: SAS Institute Inc; 2008. [Google Scholar]
- 27.Knoph Berg C, TL, Von Holle A, Hamer RM, Bulik CM, Reichborn-Kjennerud T. Factors associated with binge eating disorder in pregnancy. Int J Eat Disord. 2011;44:124–33. doi: 10.1002/eat.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hilbert A, Pike KM, Wilfley DE, Fairburn CG, Dohm FA, Striegel-Moore RH. Clarifying boundaries of binge eating disorder and psychiatric comorbidity: A latent structure analysis. Behav Res Ther. 49:202–11. doi: 10.1016/j.brat.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsawh HJ, Stein MB, Belik SL, Jacobi F, Sareen J. Relationship of anxiety disorders, sleep quality, and functional impairment in a community sample. J Psychiatr Res. 2009;43:926–33. doi: 10.1016/j.jpsychires.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Angst J, Gamma A, Sellaro R, Zhang H, Merikangas K. Toward validation of atypical depression in the community: results of the Zurich cohort study. J Affect Disord. 2002;72:125–38. doi: 10.1016/s0165-0327(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 31.Horwath E, Johnson J, Weissman MM, Hornig CD. The validity of major depression with atypical features based on a community study. J Affect Disord. 1992;26:117–25. doi: 10.1016/0165-0327(92)90043-6. [DOI] [PubMed] [Google Scholar]
- 32.Levitan RD, Lesage A, Parikh SV, Goering P, Kennedy SH. Reversed neurovegetative symptoms of depression: a community study of Ontario. Am J Psychiatry. 1997;154:934–40. doi: 10.1176/ajp.154.7.934. [DOI] [PubMed] [Google Scholar]
- 33.Cottrell L, Karraker KH. Correlates of nap taking in mothers of young infants. J Sleep Res. 2002;11:209–12. doi: 10.1046/j.1365-2869.2002.00305.x. [DOI] [PubMed] [Google Scholar]
- 34.Thomas KA, Foreman SW. Infant sleep and feeding pattern: effects on maternal sleep. J Midwifery Womens Health. 2005;50:399–404. doi: 10.1016/j.jmwh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Hunter LP, Rychnovsky JD, Yount SM. A selective review of maternal sleep characteristics in the postpartum period. J Obstet Gynecol Neonatal Nurs. 2009;38:60–8. doi: 10.1111/j.1552-6909.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- 36.Chou Y. Survey of sleep in infants and young children in northern Taiwan. Sleep Biol Rhythms. 2007;5:40–9. [Google Scholar]
- 37.Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav. 2007;91:424–31. doi: 10.1016/j.physbeh.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 39.Freeman LM, Gil KM. Daily stress, coping, and dietary restraint in binge eating. Int J Eat Disord. 2004;36:204–12. doi: 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- 40.Harrington EF, Crowther JH, Henrickson HC, Mickelson KD. The relationships among trauma, stress, ethnicity, and binge eating. Cultur Divers Ethnic Minor Psychol. 2006;12:212–29. doi: 10.1037/1099-9809.12.2.212. [DOI] [PubMed] [Google Scholar]
- 41.Wolff GE, Crosby RD, Roberts JA, Wittrock DA. Differences in daily stress, mood, coping, and eating behavior in binge eating and nonbinge eating college women. Addict Behav. 2000;25:205–16. doi: 10.1016/s0306-4603(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 42.Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–49. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- 43.Pluess M, Bolten M, Pirke KM, Hellhammer D. Maternal trait anxiety, emotional distress, and salivary cortisol in pregnancy. Biol Psychol. 2010;83:169–75. doi: 10.1016/j.biopsycho.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–20. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 46.Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–9. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2007;19:127–37. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- 48.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–27. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 49.Geliebter A, Gluck ME, Hashim SA. Plasma ghrelin concentrations are lower in binge-eating disorder. J Nutr. 2005;135:1326–30. doi: 10.1093/jn/135.5.1326. [DOI] [PubMed] [Google Scholar]
- 50.Monteleone P, Di Lieto A, Tortorella A, Longobardi N, Maj M. Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry Res. 2000;94:121–9. doi: 10.1016/s0165-1781(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 51.Tolle V, Bassant MH, Zizzari P, et al. Ultradian rhythmicity of ghrelin secretion in relation with GH, feeding behavior, and sleep-wake patterns in rats. Endocrinology. 2002;143:1353–61. doi: 10.1210/endo.143.4.8712. [DOI] [PubMed] [Google Scholar]
- 52.Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am J Physiol. 2004;286:E963–7. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- 53.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 54.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–9. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 55.Goldfein JA, Devlin MJ, Kamenetz C. Eating Disorder Examination-Questionnaire with and without instruction to assess binge eating in patients with binge eating disorder. Int J Eat Disord. 2005;37:107–11. doi: 10.1002/eat.20075. [DOI] [PubMed] [Google Scholar]
- 56.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Latner JD, Clyne C. The diagnostic validity of the criteria for binge eating disorder. Int J Eat Disord. 2008;41:1–14. doi: 10.1002/eat.20465. [DOI] [PubMed] [Google Scholar]
- 58.Sullivan PF, Bulik CM, Kendler KS. Genetic epidemiology of binging and vomiting. Br J Psychiatry. 1998;173:75–9. doi: 10.1192/bjp.173.1.75. [DOI] [PubMed] [Google Scholar]
- 59.Walsh BT. DSM-V from the perspective of the DSM-IV experience. Int J Eat Disord. 2007;40(Suppl):S3–7. doi: 10.1002/eat.20397. [DOI] [PubMed] [Google Scholar]
- 60.Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. Int J Eat Disord. 2003;34(Suppl):S96–106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]