Abstract
Study Objectives:
To interact with the robotic Phoenix Mars Lander (PML) spacecraft, mission personnel were required to work on a Mars day (24.65 h) for 78 days. This alien schedule presents a challenge to Earth-bound circadian physiology and a potential risk to workplace performance and safety. We evaluated the acceptability, feasibility, and effectiveness of a fatigue management program to facilitate synchronization with the Mars day and alleviate circadian misalignment, sleep loss, and fatigue.
Design:
Operational field study.
Setting:
PML Science Operations Center.
Participants:
Scientific and technical personnel supporting PML mission.
Interventions:
Sleep and fatigue education was offered to all support personnel. A subset (n = 19) were offered a short-wavelength (blue) light panel to aid alertness and mitigate/reduce circadian desynchrony. They were assessed using a daily sleep/work diary, continuous wrist actigraphy, and regular performance tests. Subjects also completed 48-h urine collections biweekly for assessment of the circadian 6-sulphatoxymelatonin rhythm.
Measurements and Results:
Most participants (87%) exhibited a circadian period consistent with adaptation to a Mars day. When synchronized, main sleep duration was 5.98 ± 0.94 h, but fell to 4.91 ± 1.22 h when misaligned (P < 0.001). Self-reported levels of fatigue and sleepiness also significantly increased when work was scheduled at an inappropriate circadian phase (P < 0.001). Prolonged wakefulness (≥ 21 h) was associated with a decline in performance and alertness (P < 0.03 and P < 0.0001, respectively).
Conclusions:
The ability of the participants to adapt successfully to the Mars day suggests that future missions should utilize a similar circadian rhythm and fatigue management program to reduce the risk of sleepiness-related errors that jeopardize personnel safety and health during critical missions.
Citation:
Barger LK; Sullivan JP; Vincent AS; Fiedler ER; McKenna LM; Flynn-Evans EE; Gilliland K; Sipes WE; Smith PH; Brainard GC; Lockley SW. Learning to live on a Mars day: fatigue countermeasures during the Phoenix Mars Lander mission. SLEEP 2012;35(10):1423-1435.
Keywords: Shift work, performance, sleep, circadian, light
INTRODUCTION
Space exploration presents physiological challenges for engineers, scientists and mission controllers. The Phoenix Mars Lander (PML) mission aimed to investigate the Martian arctic soils for history of water and potential for habitability.1 In order to maximize personnel and communication efficiency as well as science output, mission control personnel were required to communicate with the solar powered Lander based on a Mars, rather than an Earth, day that lasts 24.65 h, almost 40 min longer per day than on Earth. Unfortunately for this mission, the human circadian system, which governs the daily timing of sleep, performance, and alertness, among many other functions, has evolved to synchronize strictly to a 24-h Earth day. Although early reports stated that the period of the human circadian pacemaker was close to 25 h (and therefore close to a Mars day),2 more careful assessment of the circadian pacemaker in humans shows that the intrinsic circadian period is much closer to 24 h (24.2 h on average).3,4 Synchronizing the circadian system to a 24.65-h Mars day, and therefore the physiology, metabolism, and behavior it controls, requires resetting the clock later (phase delay) by 39 min per day, the equivalent of 2 time zones westward every 3 days. While this challenge appears modest, repeated failure to entrain to the Mars day over several days or weeks would soon result in desynchrony between the circadian system and the sleep-wake schedule, causing disrupted sleep, impaired cognition, and poor performance.5–7 These problems were indeed experienced by the Jet Propulsion Laboratory (JPL) personnel and visiting scientists during previous Mars robotic missions (the Mars Pathfinder and Mars MER missions) who found the work schedule demands of a Mars day extremely challenging.8,9 Those supporting the Sojourner Rover during the Mars Pathfinder Mission abandoned the Mars day schedule after only one month of the almost 3-month mission,10 and many of the 250 MER controllers also found the schedule very difficult to maintain, with 82% reporting increased fatigue, sleepiness, and irritability, as well decreased levels of concentration and energy.11 Clearly, without appropriate circadian rhythm and fatigue management countermeasures, maintaining daily life on the Mars day presents a significant challenge.
The physiological consequences of suboptimal adaptation to a Mars day are well documented. Laboratory simulations have shown that the near 24-h intrinsic circadian period (τ) in humans cannot entrain to the Mars day without specific intervention. Wright and colleagues reported that 100% (6/6) of subjects failed to entrain to the Mars day when exposed to dim light conditions during wake episodes,6,12 resulting in disturbed sleep and endocrine physiology, and impairment of alertness, mood, vigilance, cognitive function, and learning.6,12 Increasing the light during wake episodes to ∼100 lux, similar to typical indoor room light, was also insufficient to maintain appropriate entrainment.7 More sophisticated photic countermeasures can facilitate entrainment to a Mars day, however, if the circadian phase response curve (PRC) to light is considered. The PRC predicts that evening light delays the circadian pacemaker (the direction required for most people to synchronize to a 24.65-h day), whereas morning light advances the clock.13 Gronfier and colleagues reported that two 45-min evening pulses of bright white fluorescent light (∼9,500 lux) could delay the intrinsic circadian period by 1 h per day, which would be sufficient for most individuals to entrain to the Mars day.7 The same effect, enhancing a phase delay shift of the circadian pacemaker, can be achieved using less light if subjects are exposed to very dim light (∼1.8 lux) for the first half of scheduled wakefulness and moderately bright light (∼450 lux) for the remainder of the day, designed to enhance a phase delay shift of the circadian pacemaker.5
In August 2007, the Phoenix Mars Lander launched from Kennedy Space Center and landed inside the Arctic Circle on Mars on May 25, 2008.1 A core of visiting scientists, University of Arizona personnel, and NASA JPL personnel worked around the clock on a Mars day schedule at the Science Operations Center (SOC) in Tucson, AZ, in support of the mission. The mission provided an opportunity to investigate the acceptability, feasibility, and effectiveness of a photic countermeasure as part of a fatigue management program in an operational environment, to alleviate circadian misalignment, sleep deficiency, sleepiness, decreased alertness, and performance impairment associated with working on a Mars day.
MATERIALS AND METHODS
Education and Recruitment
We provided 3 open educational sessions to the scientists, engineers, and support personnel working on the Phoenix Mars Lander project in the week prior to the mission. During those planned 1-h sessions, we provided information on sleep, circadian rhythms and appropriate countermeasures (e.g., photic [light] exposure, napping, use of caffeine) to facilitate entrainment to a Mars day schedule to maximize performance and alertness during work hours and maximize sleep during time off. We also invited those in attendance to volunteer to participate in the intervention study being conducted during the 78 days when personnel were required to live and work on the Mars day. The Partners Human Research Committee and the Committee for Protection of Human Subjects at NASA Johnson Space Center approved the procedures for the protocol.
Subjects
Twenty subjects volunteered to participate and provided written informed consent. One withdrew before data collection began. All were scientists or engineers working in support of the Phoenix Mars Lander at the SOC in Tucson, AZ, and 6 were local residents. Ophthalmological examinations were conducted pre- and post-mission to ensure subject safety. Subjects were asked to complete pre- and post-mission questionnaires to gather demographic information and subjective comments regarding work on the Mars day schedule.
Photic Countermeasure
The Science Operations Center (SOC) has typical office lighting although external windows in the work areas were blacked out. Access to windows was not restricted elsewhere in the building. At the start of the study, 16 participants were provided a portable light box to place at their work stations; the other 3 subjects indicated that the light box was not compatible with their job duties (e.g., required to move about the SOC during the working day). Participants were instructed to turn the light box on during their work shifts at the SOC. The light units consisted of an array of 276 blue LEDs mounted behind a plastic lens diffuser, housed within 50 × 60 cm panels (Apollo Health, American Fork, UT). Short-wavelength blue light was chosen for this operation because it had been shown that circadian resetting, melatonin suppression, and the alerting effects of light are short-wavelength sensitive in healthy human subjects14–17 via stimulation of a novel non-rod, non-cone, melanopsin-based photoreception system located in specialized ganglion cells in the mammalian eye.18,19 Each panel was mounted on an adjustable stand, and a 50-cm piece of string was attached to each light box to remind volunteers of the correct viewing distance. The light boxes emitted narrow-band short wavelength blue visible light (± 27 nm half-peak bandwidth) with a peak (λmax) of 468 nm. The mean irradiance 50 cm from the light box was adjusted on-site at the SOC to an average of 505 ± 24 μW/cm2 (1.04 × 1015 photons/cm2/s) with an International Light Radiometer/Photometer (Newburyport, MA), a photon density known to produce robust circadian resetting and alerting effects.14,17,20–22 Although photopic lux is not an appropriate measure of light for non-visual circadian, neuroendocrine, and neurobehavioral responses, the nominal lux (for qualitative comparison with other photic countermeasures) was approximately 377 lux measured 50 cm from the source. This measure underestimates the “active” stimulus for a narrow band light source with a short wavelength (blue) peak as the photopic light sensor is tuned to a peak sensitivity of 555 nm.14–17 A Motionlogger-L ([ML] Ambulatory Monitoring, Inc., Ardsley, NY) with a battery-powered light sensor capable of detecting ambient light levels in the range of 0 to 4000 lux was attached to each light box as a means of verifying when it was turned on and off.
Sleep
Each participant was issued a handheld Palm Tungsten E2 Personal Digital Assistant (PDA; Palm, Inc., Sunnyvale, CA) on which to complete a daily sleep/work log. The questions were similar to those used in the Harvard Work Hours, Health, and Safety Group study of medical interns,23 where there was 95% agreement between sleep self-reported in the diary and polysomnographically recorded sleep epochs.23 The daily diary also included questions about subjective fatigue and the use of fatigue countermeasures, such as napping and caffeine.
Each participant wore the ML on his or her wrist on work days and on days off; the ML continuously recorded gross motor movement using a precision piezoelectric bimorph-ceramic cantilevered beam.24 Sleep was estimated for each day using the Action-W version 2.0 software (Ambulatory Monitoring, Inc., Ardsley, NY; UCSD algorithm with rescoring). Out of a possible 1,482 days (19 subjects × 78 days), we collected 1,314 days of actigraphy and 996 daily logs. Actigraphy loss was also due, in some instances, to equipment malfunction and subject error (e.g., forgetting to wear the device).
Light Exposure
The wrist-worn ML also included a light sensor that continuously recorded ambient light (lux). To verify individual light box exposures, the light levels recorded by the ML attached to the light box were compared to the light levels recorded by the ML worn by the individual at the times the individual reported being at work. Prior measures taken 50 cm from the light box (the approximate working distance from the light box) gave an average light level of 377 lux. The volunteer was therefore deemed to be exposed to the light box when he or she reported being at work (sleep/work diary), the light box surface-mounted ML was > 2000 lux and the individual's ML recorded ≥ 375 lux.
Performance
Performance tasks included the psychomotor vigilance task (PVT) and the Automated Neuropsychological Assessment Metrics (ANAM). The PVT detects changes in basic neurobehavioral performance involving vigilant attention, response speed, and impulsivity. The 10-min version of the PVT has been extensively validated in ground-based laboratory studies to detect cognitive deficits caused by a variety of factors, including restricted sleep, sleep/wake shifts, motion sickness, and residual sedation from sleep medications.25–27 In the study reported here, participants were asked to complete a 5-min version of the PVT at least twice per day: at the beginning and end of each shift if at work, and during the morning and evening of their days off. This 5-min PVT has been previously validated.28–31
The ANAM is a library of computer based tests that measure cognitive processing efficiency. The ANAM evolved from computerized test batteries developed by the Department of Defense and correlates well with traditional neuropsychological measures.32–35 Selected tests from the ANAM36 were used to construct the Performance Assessment Work Station (PAWS), a computer-based test battery specifically designed for the assessment of cognitive performance in space or space-related operational environments.37 PAWS was successfully used to explore cognitive deficits associated with bedrest38 and during microgravity.39 In addition, ANAM tests were used to construct the NASA Spaceflight Cognitive Assessment Tool for Windows (WinSCAT), a computer test battery designed to give astronauts an objective and automated means of assessing their cognitive functioning during space flight. The ANAM battery constructed for use in this study provided measures of simple reaction time (SRT) and nonverbal memory (M2S), as well as self-reported states of mood and sleepiness. For the mood and sleepiness scales, a scale of numbered blocks ranging from 0 to 6, with “0” having the verbal anchor “Not at all,” the midpoint “3” labeled “Somewhat,” and “6” labeled “Very much” was utilized. The volunteer was presented a series of adjectives, each adjective contributing to one of the mood categories, and was instructed to select the box/number that best represented the current state with respect to the presented adjective. Two computers in the study investigators' office in the Phoenix SOC were dedicated to ANAM testing. Volunteers were asked to come to that office to complete the ANAM battery at the beginning and end of each shift. ANAM batteries were not completed on non-work days.
Of a possible 2,964 PVT tests and 1976 ANAM tests (assuming a 4/2 work schedule with no travel), we collected 1435 and 744 tests, respectively. Some data were missed due to participant travel during the mission. All subjects completed ≥ 1 PVT test; the mean number of PVT tests competed per subject was 75.5 ± 52.0. All 19 subjects completed at least one ANAM battery; the average number of sessions completed per subject was 41.3 ± 33.1 sessions out of approximately 100, if 2 sessions were completed every work day. ANAM and PVT data that did not have corresponding time since awakening (i.e., no actigraphy data recorded for that day) or circadian phase (i.e., could not be estimated from available aMT6s data) were excluded from analysis.
Circadian Phase
In order to determine if the participants' circadian pacemaker was able to adapt to the Mars day, we obtained a reliable measure of circadian phase throughout the mission by assaying urine samples for 6-sulphatoxymelatonin (aMT6s). Volunteers collected sequential 4-hourly urine samples (8-hourly overnight) for 48 h approximately every 2 weeks. These collections were scheduled at the convenience of the participant and may have occurred on work or non-work days. Collection duration and urine volume were measured, and a 5-mL sample saved and frozen (−20°C) until assayed. This method has been used successfully in prior studies of shiftworkers on North Sea oil rigs,40–42 in shiftworking nurses,43,44 in shiftworkers living in Antarctica,45,46 and in many clinical populations and experimental protocols.47–49
Urinary aMT6s concentrations were measured by radioimmunoassay using the methods of Aldhous and Arendt.50 Antiserum was supplied by Stockgrand, Ltd., University of Surrey (Guildford, UK). The intraassay coefficients of variation were 11.2, 7.3, and 9.4% at 4.2, 12.6, and 24.6 ng/mL (n = 21, each), respectively. The interassay coefficients of variation were 9.1, 6.2, and 8.3 at 4.4, 13.0, and 25.3 ng/mL (n = 10, each), respectively. Cosinor analysis of aMT6s values was used to determine acrophase (peak) time.51 Regression analysis of significant (α set 0.10; 97% were P < 0.05) acrophases then determined observed period.48
When urine collection procedures were compromised (e.g., collection error), analysis methods were modified. In 17 of the 894 (1.9%) samples used to calculate acrophase time, the subject failed to record urine volume. In these cases, the mean urine volume for the 48-h collection period was substituted. The first data point for one subject in one 48-h collection period was removed prior to cosinor analysis due to the subject including the first morning void in the first sample of that collection period. In three 48-h urine collection periods in 3 separate subjects, the 24-h profile with no collection errors was double-plotted to provide a 48-h sequence for analysis. Finally, in two 48-h urine collections in one subject and one collection in a separate subject, one void was discarded by the subject and no sample was obtained. The subsequent sample was used for the missed collection interval in each of these 3 instances.
We were able to determine observed circadian period via linear regression in 15/19 subjects who had ≥ 3 valid phase assessments during the course of the study. Of a possible 133 collections (19 subjects, each having a maximum of 7 collection periods), 77 sets of 48-h urine samples were collected, yielding valid aMT6s phase assessments with significant cosinor-derived acrophase (4.5 ± 1.2 per subject). The observed period in 2 subjects was segmented before and after transmeridan travel. Urine samples were not usable from one subject.
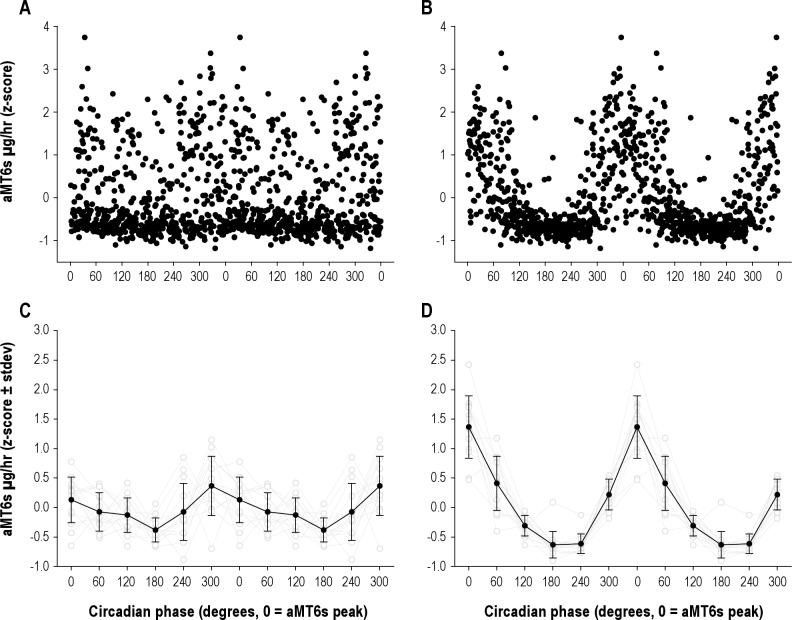
In many cases, the best fit regression line could have approximated a 24.65-h observed period or one closer to 24 h, assuming, respectively, that the subjects were or were not adapted to the Mars day. Subjects' raw aMT6s data were folded at 24 h (Figure 1A) and 24.65 h (Figure 1B). Figure 1D depicts the rhythmicity associated 24.65 h, as compared with the 24-h plot (Figure 1C), demonstrating the more likely 24.65-h observed period in this instance. Visual inspection of the rhythmicity of these plots revealed the best fit in cases of 2 potential observed periods.
Figure 1.
Thirteen subjects' aMT6s data were double plotted, assuming both no adaptation to the Mars day and assuming they did adapt. The assumption that there was no adaptation (A, C) resulted in an arrhythmic pattern for aMT6s with respect to circadian phase, suggesting that the data are not consistent with entrainment to a 24-h day. When the data are plotted according to the Mars day (B, D), a very robust aMT6s circadian rhythm was observed, suggesting that the data are consistent with synchronization to a 24.65-h day. These data support the interpretation that the aMT6s rhythm exhibited a period closer to 24.6 h than 24 h during the study.
Modeling
In addition to cosinor analysis, circadian phase was also estimated with a mathematical model. Sleep-wake (binary) and light exposure data (averaged in 1-h bins) collected from the MLs were used as input in a model of the effect of light on the circadian pacemaker that predicts core body temperature minimum as a marker of circadian timing (Circadian Performance Simulation Software [CPSS], version 1.2).52–55 The model's phase and amplitude predictions have been experimentally correlated with established circadian markers.56–60
Analysis
To facilitate analysis by circadian phase and hours of wakefulness, outcome measures were assigned a time since wake (hours) and circadian phase (degrees). Beginning with the wake time from the main sleep episode, hours of wakefulness were assigned in eight 3-h bins. For circadian analysis, 0 degrees was assigned to the fitted daily acrophase of the aMT6s rhythm, and each circadian day was divided into four 90 degree (∼6-h) bins. To estimate daily circadian phase, point-to-point lines were drawn between aMT6s acrophases and the acrophase interpolated on days without urine collections. In the case of missing acrophase data, the line was extended 3 days beyond last known acrophase (performance or mood data collected beyond those 3 days was excluded). The duration of each circadian degree was adjusted based on the observed period between each fitted acrophase. Sleep duration, performance and mood data were analyzed according to these circadian and wake duration bins.61,62
Statistics
A mixed model analysis of variance was employed to determine the effects of time since awakening and circadian phase for all sleep, performance and mood variables (SAS Institute, Cary, NC, version 9.1.3). A Pearson correlation coefficient determined the association among observed periods estimated from aMT6s, the mathematical models, and between observed period and exposure to blue light. A Wilcoxon 2-sample test was used to compare categorical answers on the questionnaire. All errors are reported as standard deviation of the mean unless otherwise noted.
RESULTS
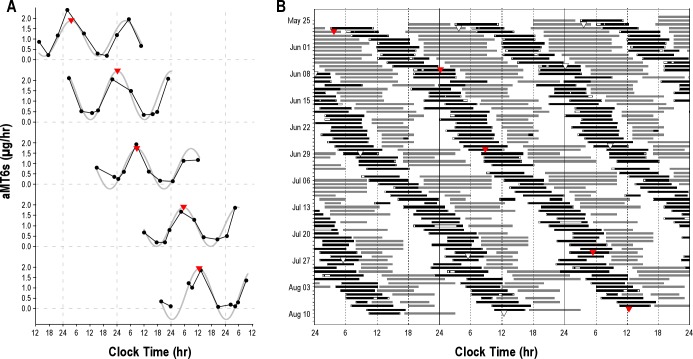
Detailed studies were conducted in a subset (n = 19, 6F, mean age ± SD = 36.8 ± 9.7 years), of ∼13% of the approximately 150 ground crew mission personnel. The first Mars day began at ∼17:00 when the Phoenix spacecraft landed on Mars. Two main shifts provided coverage, with each shift scheduled to start ∼39 min later each day, although satellite communications necessitated variations in the schedule (Figure 2). Personnel were generally required to work 4-days-on/2-days-off for 78 days, before reverting to an Earth schedule on August 11, 2008. The average reported work shift duration was 8.6 ± 2.6 h, with ∼30% of shifts lasting ≥ 10 hours.
Figure 2.
(A) Cosinor analysis (gray fitted curve) of aMT6s values (μg/h, filled circles) were used to determine the acrophase (red triangles) for each 48-hour urine collection. White triangles show the acrophase on Earth time collection. (B) Subjects reported work (gray bars) and sleep (black bars) times in a daily diary. This representative subject (#07) worked on a Mars day schedule from May 25 to August 11, 2008. Subsequent acrophases are triple plotted and regression analysis was used to determine the observed period.
Circadian Period
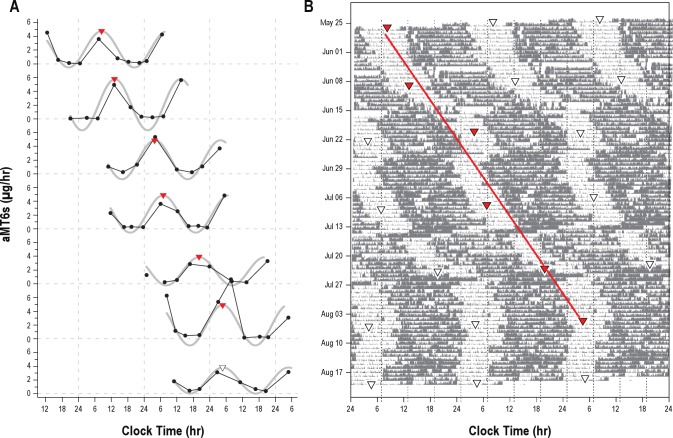
Most of the participants (13/15, 87%) exhibited circadian rhythms in urinary 6-sulfatoxymelatonin (aMT6s) that were synchronized to the Mars day (τ range, 24.58-24.83h; mean τ ± SD = 24.70 ± 0.07h; Table 1, Figures 2 and 3). Two of these subjects (#09 and #10) traveled to Europe in the middle of the mission and, while both were adapted to the Mars day prior to travel (τ = 24.78 h and 24.83 h), they were unable to re-adapt upon their return (τ = 24.08 and 24.37 h, respectively). Two subjects remained entrained to Earth time (τ = 23.98 and 23.93 h); one had a very erratic travel schedule throughout the mission (#06), and the other (#19) did not appear to adhere to a Mars day work schedule. Four subjects had insufficient urine data for assessment.
Table 1.
Observed circadian period ± 95% confidence intervals calculated from aMT6s rhythms and estimated using the Kronauer-Jewett model, as implemented by CPSS (Version 1.2)
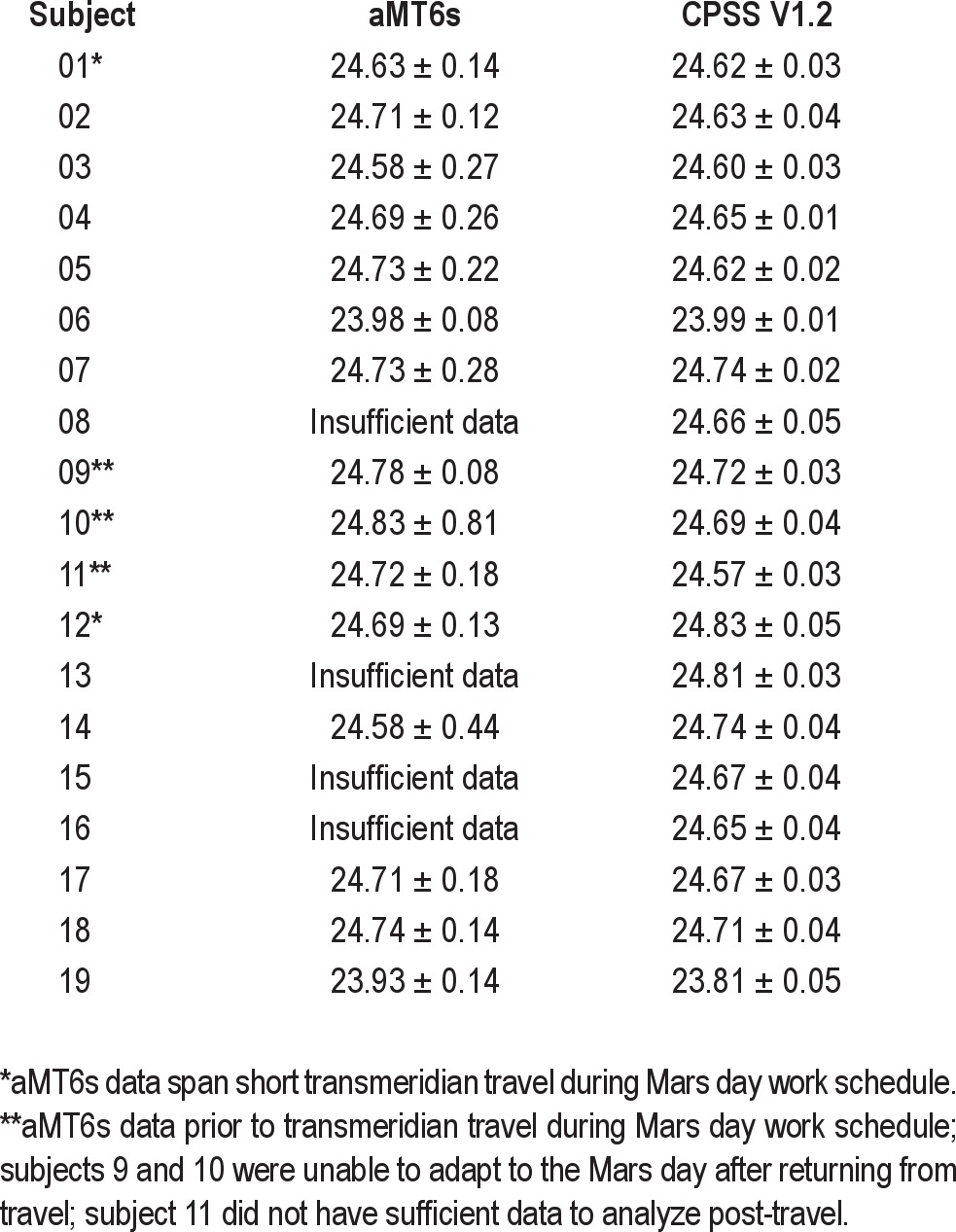
Figure 3.
(A) aMT6s circadian rhythm data and (B) activity (gray bars) from a single subject (#01) demonstrate the methodology used in analysis of circadian physiology. Data are plotted as in Figure 2.
We also predicted circadian period from simulations using experimental sleep-wake and light-dark cycle data as input to a mathematical model of the effects of light on the human circadian pacemaker.63 The mathematical model-based simulations were in agreement with the aMT6s acrophases, which showed that the majority of participants (17/19; 89%) were adapted to a Mars day (Table 1). The observed aMT6s circadian period was highly correlated with the model predictions (r2 = 0.86, P < 0.001).
Sleep
For sleep episodes recorded by actigraphy, subjects slept an average of 6.2 ± 0.9 h per Mars day during their main sleep period, representing only 25% of each “day” (Figure 4). Subjects exhibited chronic sleep deficiency, with 50% of sleep episodes lasting ≤ 6 h, and only 23% lasting ≥ 7 hours.26,64 Sleep duration was dependent on the circadian phase at which sleep was initiated (F = 16.96, P < 0.001; Figure 5). In those sleep periods when circadian phase was available, main sleep duration was 5.98 ± 0.94 h when synchronized but fell to 4.91 ± 1.22 h when misaligned (P < 0.001), a decrease of 18% when sleep was initiated during the biological day (Figure 5). Seventeen of the 19 subjects used naps as a fatigue countermeasure, as was recommended in the pre-mission education. Each nap lasted 1.3 ± 0.6 h, and participants used this countermeasure on an average of 6.2 ± 5.0 days.
Figure 4.
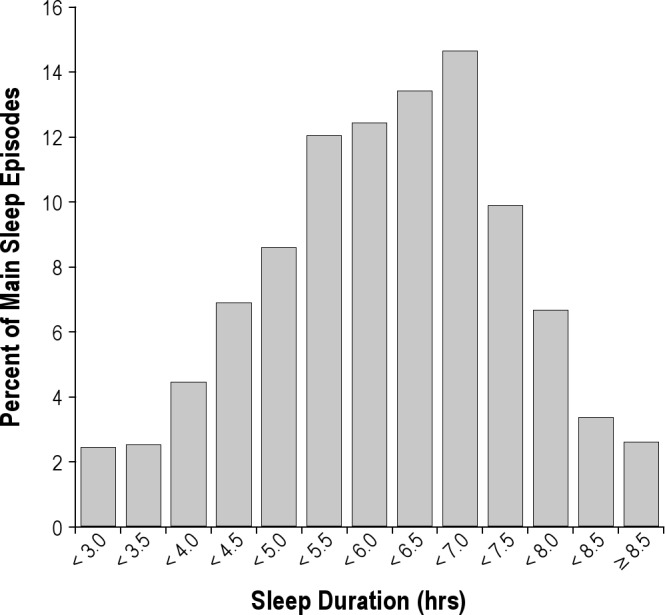
The daily mean total sleep time for all actigraphy-estimated sleep was 6.2 ± 0.9 hours. Six percent of days had sleep duration > 8 h, and 9% of days had sleep duration of < 4 hours. Forty percent of days had sleep duration of 4 to 6 h, and 45% had sleep duration between 6 and 8 hours.
Figure 5.
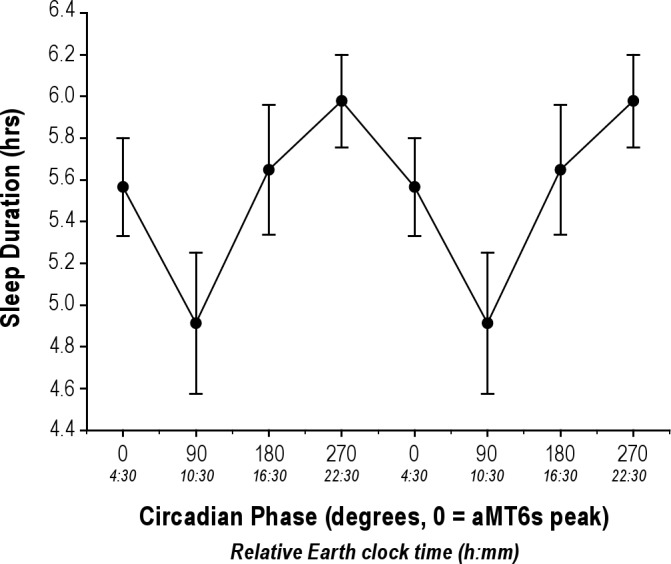
The duration of sleep during the main sleep episode was dependent on the circadian phase at which sleep was initiated. Actigraph-determined sleep (± SEM) varied significantly by circadian phase (P < 0.001). Sleep initiated outside of the biological night was shorter than when sleep occurred at a normal circadian phase. Only sleep data that had a circadian phase associated with it could be included in these analyses, n = 18 subjects.
Alertness and Performance
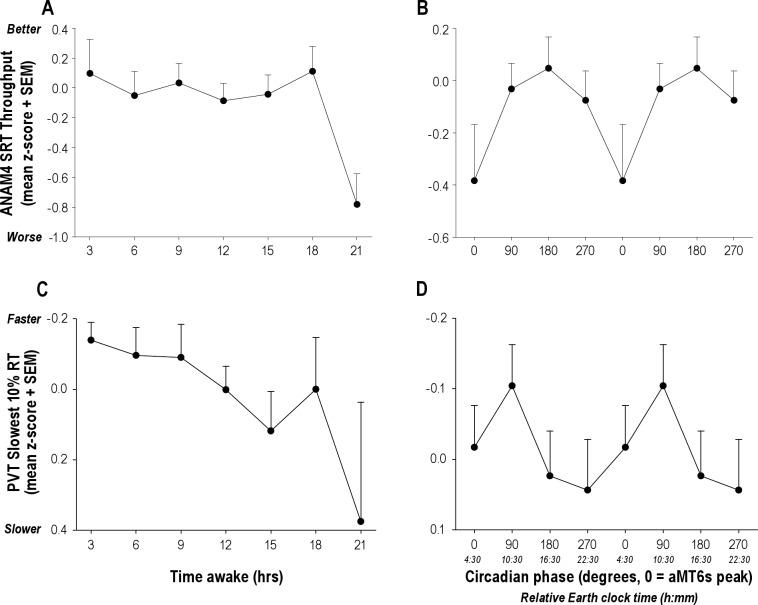
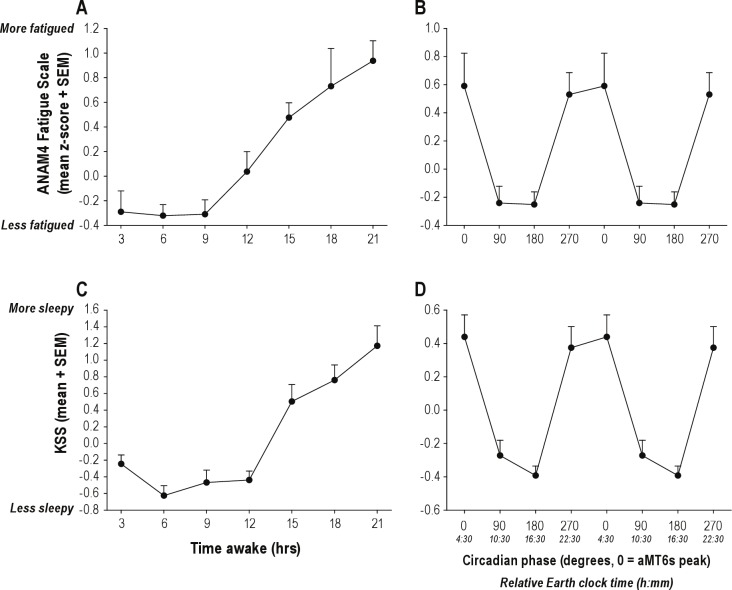
Performance on the nonverbal memory (M2S) and serial reaction time (SRT) tasks deteriorated with increased time awake (F6,128 = 2.42, P = 0.030; F6,135 = 2.35, P = 0.024, respectively; Figure 6) as did the response time of the slowest 10% of PVT trials (F6,1161 = 2.76, P = 0.012; Figure 6). Self-reported ratings for anxiety, sleepiness and vigor also worsened with increased time awake (F6,127 = 2.37, P = 0.04;F6,170 = 13.97, P < 0.001; F6,131 = 8.21, P < 0.0001, respectively). Self-reported Karolinska Sleepiness Scale (KSS) ratings were dependent on both time since awakening (F6,1178 = 30.58, P < 0.0001) and circadian phase (F3,1178 = 6.81, P = 0.0001), as were self-reported ratings of fatigue (F17,120 = 1.84, P = 0.03; [interaction]; Figure 7). Sleepiness and fatigue were greater with increased time awake and when assessed during the biological night.
Figure 6.
Performance was dependent on the number of continuous hours awake. The slowest 10% response times (mean + SEM) significantly increased (A; P = 0.012) and throughput on the ANAM SRT decreased (C; P = 0.024) as a function of time awake. Both performance measures also exhibited evidence of circadian modulation, with a trend for decreased performance during biological night (B and D).
Figure 7.
Subjective sleepiness and fatigue ratings were dependent on the number of continuous hours awake and circadian phase. Self-reported sleepiness significantly increased with time awake on both the KSS (A; P < 0.0001) and the ANAM Fatigue scale (C; P = 0.0001). Both measures also exhibited a robust circadian rhythm, with highest sleepiness reported when subjects were awake during the biological night (B, P = 0.0001; and D, P = 0.0003).
Short-Wavelength (Blue) Light Exposure
The use of the light boxes varied by participant; some switched it on when at work almost all the time, some switched it on sporadically, while others started and then discontinued use. Across the 78 days of Mars days, subjects were nominally scheduled to work ∼52 days on a 4on/2off schedule; the light boxes were switched on an average of 25.3 ± 12.4 days of the 78-day mission. On those days, it was switched on for an average of 4.0 ± 1.8 hours. The subjects' average exposure to the light box, calculated from ML light exposure data, was slightly less in number of days (21.1 ± 10.5 days) and hours per day (2.2 ± 1.1 h). Short-wavelength light exposure duration may be underestimated, as light exposures could not be quantified on days when actigraphy was not collected. Additionally, the light sensor was part of the ML worn on the wrist; it was thus not a perfect measure of light exposure to the eye and may have further underestimated exposure.
There was no significant association between an individual's exposure to the light box and the observed circadian period. Two of the 3 participants who did not receive a light box adapted to the Mars day work schedule, according to aMT6s-estimated observed period (#02 and #03). We were unable to analyze the urine collected from the third participant who did not receive a light box (#08), but the subject did appear to adapt to the Mars day work schedule according to the circadian model estimates, based on wrist light exposure and actigraphy-estimated sleep-wake timing (Table 1).
Questionnaires
According to the sleep/work log, volunteers reported 2.16 ± 0.35 servings of caffeine per day (1 serving = 1 cup of coffee/tea or 1 caffeinated soda) which trended slightly higher on work (2.23 ± 0.36 servings) compared to non-work days (1.91 ± 0.36 servings; P = 0.06). Volunteers also completed a post-mission questionnaire (response rate 74%, [14/19]). Most (64%) reported that working on a Mars day was “somewhat easy” or “very easy,” 29% reported it to be “somewhat difficult.” Fatigue was reported “strongly increased” or “moderately increased” for 64% of respondents. Twenty-eight additional support personnel (out of the 52 present when surveys were distributed; 54% response rate) who did not volunteer for the full study also completed the questionnaire, and 75% reported that fatigue “strongly increased” or “moderately increased” during Mars day operations. Of those individuals, 42% reported it was “somewhat difficult” to work on a Mars day and 8% reported it was “very difficult.” Thus, in this limited sample, those who did not volunteer for the fatigue management program reported greater difficulty working on the Mars day than the study volunteers (Z = −2.01, P = 0.044).
DISCUSSION
The ground operations were linked with to the 24.65-hour Mars day in order to optimize the work of the solar powered lander, and to maximize the Lander time and the amount of science that can be conducted in the Martian environment that was limited by the imminent Martian winter. That is, the Lander is solar powered and operations on Mars could only occur during the daylight hours or shortly thereafter. After the data were transmitted to Earth each day; they then had to be assessed before scientific and operational decisions could be made, prioritized, programmed, and uploaded to the spacecraft to maximize scientific operations. Additionally, the expertise in each Lander system was limited, such that there were not enough personnel qualified to accomplish the work in a typical terrestrial shift schedule.
Adapting the human sleep-wake and performance cycle to a 24.65-h Mars day represents a substantial physiological challenge, with individuals requiring as much as an hour per day delay in their intrinsic circadian pacemaker. While successful countermeasures have been simulated previously in the laboratory, the Mars Phoenix Lander mission provided an opportunity to test the acceptability, feasibility, and effectiveness of a fatigue management program to facilitate synchronization with the Mars day in an operational environment. Eighty-seven percent of the subjects who participated in our study, as part of the Mars Phoenix Lander fatigue management program, were able to synchronize to a Mars day schedule as assessed using both physiological measures of circadian phase and mathematical modeling. As expected, when subjects' work and sleep schedules were appropriately synchronized with the circadian pacemaker, they exhibited longer sleep duration, better alertness, and reduced fatigue. Overall, daily sleep duration averaged just over 6 hours across the study, not unexpectedly reflecting sleep deficiency in modern society.65,66
Prior missions have demonstrated that working Mars day schedules without appropriate countermeasures can cause severe problems with sleep, performance, and compliance. Reports from the earlier Mars Pathfinder missions that did not employ dedicated circadian and sleep countermeasures indicated less success in adaptation to the Mars day schedule than our current study. Based on NASA surveys of 24 Mars Pathfinder veterans, those supporting the Sojourner Rover indicated that fatigue significantly affected their performance at work to the extent that they discontinued work on the Mars day schedule after only one month and described the schedule as “broken.”8 JPL managers described the scientists' and engineers' discontinuation of the Mars day schedule as a “rebellion.”10
The 2004 MER mission personnel appeared more successful than those supporting the Pathfinder in adapting to a Mars day. Nine subjects participated in the objective measurement of sleep during the mission using actigraphy, and 22 subjects completed questionnaires. Actigraphy-based sleep/wake period reportedly increased from 24.08 h during a 2-week baseline Earth schedule to 24.84 h during the approximately 90-day Mars day schedule.9 Although these data suggest that the circadian systems of these participants were able to adjust to the Mars day schedule, the sleep-wake cycle is a relatively poor measure of circadian phase,67 especially when risk of desynchrony is high.43,68 Despite the apparent entrainment, the survey responses indicated that fatigue and stress problems were greater than in the previous Pathfinder mission.9 Notably, in our current study, Phoenix support personnel who did not participate in the fatigue management program reported nearly double the rate of difficulty in working on a Mars day (50% versus 29%, respectively) and more fatigue (75% versus 64%, respectively) than those who participated in our program.
Performance data were not reported in the MER technical report, although Bass and colleagues reported one MER team member was injured after a series of Mars time shifts when he mistakenly walked into a wall and another reported falling asleep at the onramp to the freeway.11 A previous two-week “Mars analog” study (but conducted on Earth time) in four subjects did not show decrements associated with time awake in PVT performance or subjective sleepiness (KSS). The authors attributed this result to the high motivation of the crew,69 although motivation has limited ability to override circadian and homeostatic regulation of alertness and performance and is, in fact, subject to these influences itself.70 Seven crewmembers at the Flashline Mars Arctic Research Station on Devon Island, Canada, lived on a Mars day for 37 days. O'Griofa and colleagues reported improved alertness and reduced fatigue from diary data and improved decision speed in performance testing.71 Objective cardiopulmonary monitoring of sleep was conducted on four of the crewmembers and showed no statistical differences between pre-mission and the Mars day.71 These data, collected relative to the sleep-wake cycle of the study participants, are difficult to interpret. As demonstrated in our current study, although the average circadian period may be synchronized to the Mars day, subjects' sleep-wake and work schedules sometimes occur at an abnormal circadian phase, resulting in reduced sleep and poorer alertness and performance. Quantifying these important distinctions is not possible in studies that do not measure a marker of endogenous circadian phase in addition to sleep. Although the measurement of circadian phase can be challenging in operational settings and melatonin's sensitivity to light may be considered a potential confounder in the analysis of aMT6s results, the method has been successfully used in a multitude of field studies (See supplemental matierals for additional information on light as a potential confounding factor in interpreting aMT6s data).40–49 We would recommend inclusion of a circadian marker in all future analog studies and spaceflight missions in order to interpret sleep, alertness, and performance data most accurately.
The consequence of misalignment between sleep and circadian phase on alertness and performance were illustrated in the current study. The time course and pattern of alertness and performance are regulated by the interaction of two oscillatory processes—the circadian clock and a homeostatic hourglass regulator—as described in the two-component model of sleep-wake regulation.61,72–74 The circadian system regulates the daily pattern of sleep propensity, such that alertness and performance are worst during the biological night, when sleep usually occurs. The homeostatic component predicts alertness and performance based on the duration of wake and sleep; performance worsens with increasing time awake and improves after greater time asleep. These factors interact in a nonlinear manner75 and are further exacerbated by chronic sleep deficiency.76 Despite the operational setting and large number of factors that could affect alertness and performance in the workplace (e.g., unlimited access to caffeine, light, mission anxiety and other potential confounders), we were able to detect significant changes in alertness and fatigue with respect to both time awake and circadian phase. When subjects worked after an extended time awake (≥ 21 h), or worked during the biological night (Figures 6, 7), alertness and fatigue worsened, as predicted.73,77 Significant circadian variations were not observed in all performance metrics, most likely due to study power, although performance was slowest when wake time occurred during the biological night as predicted from prior laboratory6,62,78–80 and field studies61,81 of circadian misalignment. The biological principles that underlie alertness and performance are therefore apparent in an uncontrolled operational setting, even among such highly trained and highly motivated professionals.
The success of manned and unmanned space missions depends on the ability of the crew and the support team to be alert and maintain high levels of cognitive function while operating complex, highly technical equipment, regardless of whether they are in-flight or part of mission control. Human error is a common cause of accidents and injuries,82 and even relatively simple mistakes can jeopardize critical mission operations that have often taken decades to plan, at a cost of hundreds of millions of dollars. The risks to sleepiness and performance conferred by unusual work schedules have been extensively documented.9–11,83,84 Our current data show that the risk of making a sleepiness-related error is not random and is predicted by sleep and circadian principles, and therefore may be prevented with the application of appropriate countermeasures. Such risk mitigation should be considered an integral part of future mission planning, particularly when there is a risk of circadian desynchrony due to working on a non–24-hour schedule in space and other environments, such as submarines85 and polar regions.45,86
A major component of our planned intervention was a timed photic countermeasure, provided via a portable blue light box. Placing the light box at the work station was only feasible for 84% (16/19) of the participants in the study, and many jobs required them to move about the SOC frequently away from this light stimulus. Light box use was highly variable among participants, averaging just over two hours of blue light exposure per day. Although the majority of volunteers successfully adapted to the Mars day schedule as measured by aMT6s analysis, there was no significant relationship between the number of hours of light box exposure and the observed circadian period. Outside of the SOC, natural light was also freely available in the Tucson summer which could enhance or inhibit appropriate adaptation, depending on the circadian phase of exposure.13 We provided education sessions to explain these circadian principles and gave generic advice when to seek and when to avoid light exposure. Given that two of the three who were not provided with a light box also adapted to the Mars day, the effects of all environmental light exposure (measured with the Motionlogger-L) was evaluated with a mathematical model, which demonstrated that appropriate exposure to external light may have provided sufficient entraining stimulus. In future missions, a more integrated lighting countermeasure that could benefit the entire ground crew would be preferable, for example by retrofitting existing fixtures with white-appearing, blue-enriched lamps87 or developing new state-of-the-art programmable smart lighting systems.88
Environmental factors other than light may also have contributed to the adaptation to the Mars day schedule, given that knowledge of time of day, exercise, living routine, social communication, and group habitation can influence the human circadian system, albeit weakly.89 Bass and colleagues suggested that personnel who temporarily locate to the mission operations center have less difficulty working on the Mars day schedule,11 possibly due to lack of competing family and personal commitments that continued on an Earth schedule. The Phoenix Mars Lander mission was the first Mars robotic mission based outside the NASA JPL, perhaps lessening the number of workers living with their families; approximately two-thirds of our participants did not permanently live in Tucson. Future studies should consider the influence of these social pressures and account for them in study designs. Operational constraints prevented us from implementing a randomized control study design. As such, we cannot rule out that outcomes were not influenced by a study or placebo effect.90 It is unlikely, however, that adaptation to the Mars day results were under this influence. Circadian adaptation and melatonin regulation (assessed in this study by measurement of urinary aMT6s) have been shown to be quite resistant to placebo effects.91,92 Additionally, we cannot determine the relative contribution of each aspect of the fatigue management program (e.g., education, photic countermeasure) on adaptation to the Mars day. Laboratory studies have shown that scheduling alone, without sufficient light, is inadequate for adaptation to a Martian schedule.6,7
NASA has an ambitious Mars exploration program. The Mars rover, Curiosity, landed on Mars in August 2012, and both unmanned and manned missions are in the planning stages.84,93,94 Thus, the challenges of living and working on a Mars day will require additional investigation and resources. In the near-term, we would recommend development of an extensive education and integrated countermeasures program for forthcoming missions. An ongoing circadian rhythm and fatigue management program that would continually monitor individual performance and provide rapid feedback and countermeasure adjustment as a mission progresses is also recommended. Our study demonstrates that it is possible to collect precise circadian phase and sleep data during a high-tempo, dynamic, and operational mission environment. These data are critically important for the identification and mitigation of performance and sleepiness-related risk. Missions of this importance and cost demand a dedicated, integrated fatigue management program with appropriate countermeasures to prevent avoidable and potentially catastrophic fatigue-related error.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Barger has received research support from Cephalon, Inc. Dr. Gilliland reports that he served as the director of a research center that received a subcontract from a private company. He reports receiving royalty payments for intellectual property associated with the ANAM test. Dr. Gilliland reports that he served as the director of a research center that received a subcontract from a private company. He reports receiving royalty payments for intellectual property associated with the ANAM test. Dr. Lockley receives consulting fees from Naturebright, Sound Oasis, Thomas Jefferson University, and Wyle Integrated Science and Engineering. He has received equipment from ResMed, Inc., Philips Lighting and Bionetics Corporation. He has received Phoenix Mars Lander monetary gifts from Swinburne University and Optalert, Pty. He reports receiving research support from Alcon, Inc., Apollo Lighting, Biological Illumination, Philips Lighting, Philips Respironics, and Vanda Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the research participants who volunteered for this study; Joseph T. Hull, PhD, Jeffrey Tarpy and Amy Hallal for technical support; Stuart F. Quan, MD, for providing ophthalmology contacts at the University of Arizona; Rene Mercer, who arranged all the ophthalmological appointments and all of the physicians at University Physicians HealthCare, Department of Ophthalmology, University of Arizona, and Elizabeth Klerman, MD, PhD, for her thoughtful comments on the manuscript.
We offer a special note of thanks to Carla Bitter, for giving up her office for the Fatigue Countermeasures Team; and Frankie L. Kolb (Administrative Associate to Dr. Peter Smith), Tisha Saltzman (Senior Business Manager) and the rest of the staff of the Science Operations Center. The blue light emitting diode panels were donated by Kent Savage and Scott Wheelhouse, Apollo Health (American Fork, UT), since purchased by Philips Healthcare.
The study presented here was primarily supported by a grant from the National Aeronautics and Space Administration (NNX08AD66A). During the performance of the study, Drs. Barger, Brainard, and Lockley were supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. The Phoenix Mars Lander mission was supported by NASA contract NNH04CC16C.
SUPPLEMENTAL MATERIAL
Is Light a Confounding Factor in Interpretation of aMT6a Data?
Suppression of melatonin by light is a potential confounding factor in interpreting the urinary 6-sulfatoxymelatonin (aMT6s) data. We examined whether circadian amplitude changed systematically during the study as might be expected if the light-dark cycle was affecting melatonin level. First, we examined whether there was a systematic change across the study. As shown in Figure S1, no simple systematic change was apparent. Subjects began the study at different circadian phases (n = 14, initial 48-h collection period within 5/24-28/2008, 5.6 am ± 1.9 h, range 3.7-9.7 am, median 4.8 am; the majority of participants were not local Tucson residents).
aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score) plotted across duration of study. Each symbol represents a different subject (symbols consistent with Figures S2 and S4)
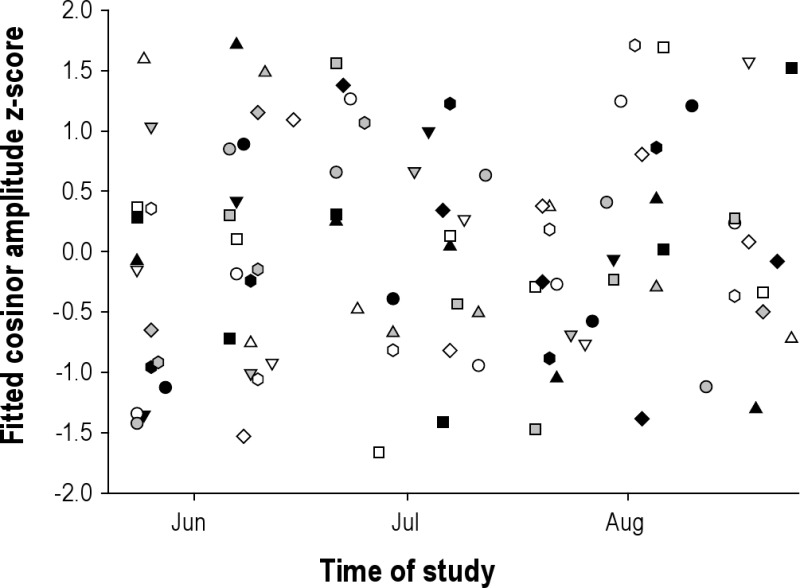
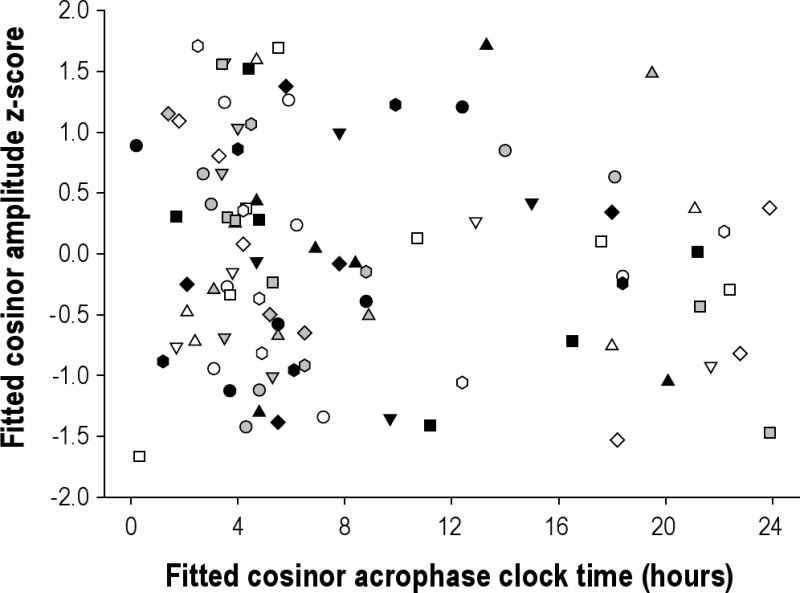
We then examined whether the amplitude varied with the timing of the melatonin peak (acrophase); if light was suppressing melatonin, one might expect that the melatonin amplitude would be lower when melatonin peaked in the daytime. As shown in Figure S2, there was no evidence of reduced amplitude during daytime peaks when controlled for inter-individual differences in melatonin level.
aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score) plotted across time of day of fitted acrophase. Each symbol represents a different subject (symbols consistent with Figures S1 and S4).
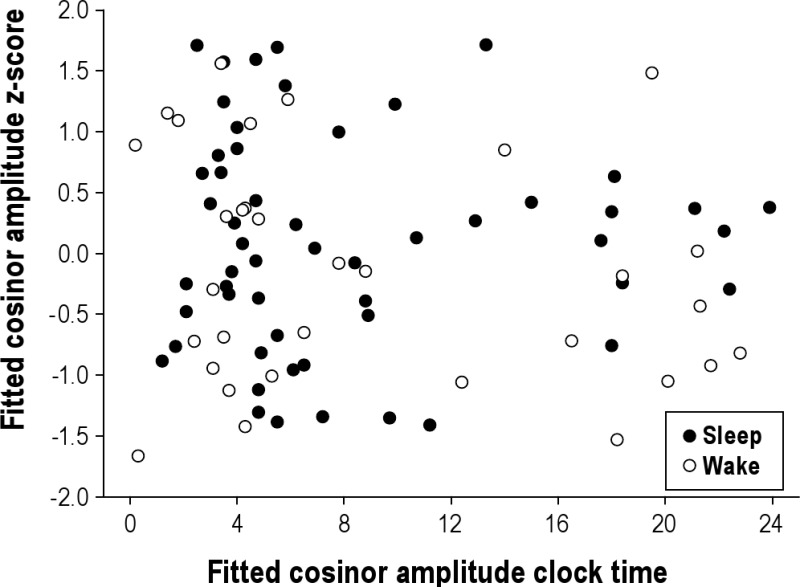
Given that the Mars day work schedule was not aligned with the 24-h light-dark cycle, the relevance of examining amplitude with respect to day and night could be questioned. The next step, therefore, was to examine whether the amplitude changed as a function of whether it occurred during sleep episodes, in the dark, or a wake episode, when it would be more likely to be light. If light was systematically suppressing melatonin, one might hypothesize lower amplitudes when the peak time occurred during wake episodes as compared to sleep episodes. As shown in Figure S3, there was again no systematic change in amplitude with respect to the light-dark cycle.
aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score, 8 acrophases excluded due to missing actigraphy to categorize sleep/wake). Closed circles indicate acrophases that occurred during sleep and open circles indicate acrophases that occurred during wake. There was no difference in amplitude when the peak occurred during wake or sleep (unpaired Student's t, P = 0.25).
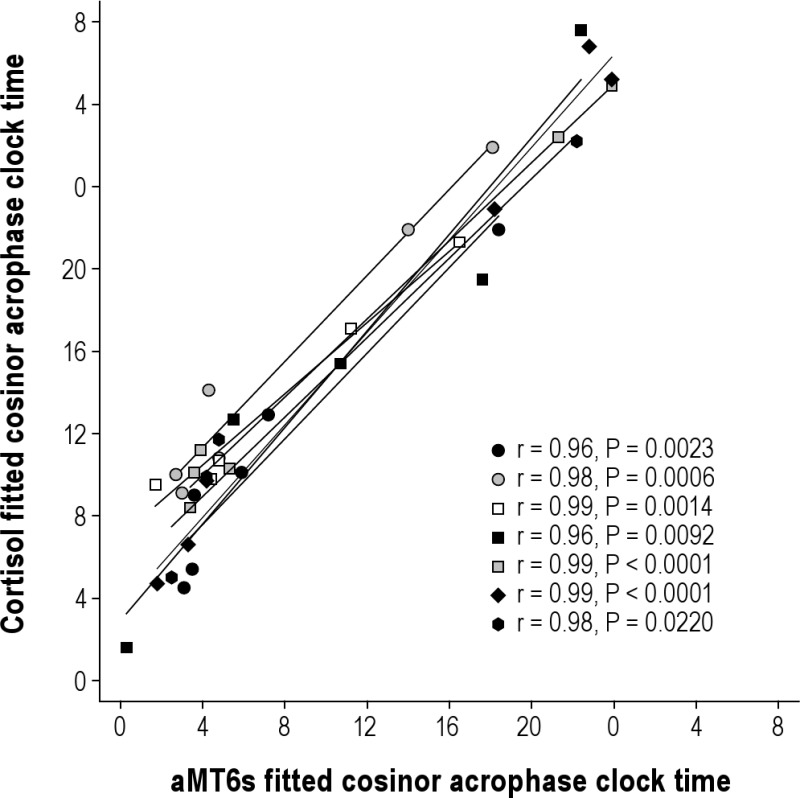
In a subset of 7 subjects, we assayed the same urine samples for cortisol, which is much less confounded by light, Figure S4. The acrophases for aMT6s and cortisol were significantly correlated (average Pearson r ± STDEV, 0.98 ± 0.01) in these sighted subjects, as we have previously reported for these urinary markers in the blind.1 Cortisol data were not reported in this manuscript because financial restrictions only allowed analysis of a subset of participants.
aMT6s acrophase times vs. cortisol acrophase times for 7 subjects. Each symbol represents a different subject (symbols consistent with Figures S1 and S2).
Additionally, this method has been used successfully to assess circadian phase in multiple real-world studies, including studies of shiftworking nurses, in shiftworkers on North Sea oil rigs, and in shiftworkers living in Antarctica. It has also been used to assess melatonin levels and circadian rhythms in children, including patients with autism, blindness, and epilepsy and in many other clinical populations and experimental protocols.3–23
Furthermore, we plotted the aMT6s data assuming no adaptation to the Mars day (i.e., on a ∼24-h scale) and assuming that they did adapt (i.e., on a ∼24.65-h scale). As illustrated in Figure 1, the assumption that there was no adaptation (Figure 1, Panels A, C) resulted in an arrhythmic pattern for aMT6s with respect to circadian phase, suggesting that the data are not consistent with entrainment to a 24-h day. When the data are plotted according to the Mars day (Figure 1, Panels B, D), a very robust aMT6s circadian rhythm was observed, suggesting the data are consistent with synchronization to a 24.65-h day.
Finally, it is well established from highly controlled laboratory studies conducted in dim light that sleep duration and alertness change in a predictable manner with respect to circadian melatonin rhythm, such that sleep is longest when the sleep episode coincides with the biological night, indicated by the melatonin peak, and shortest when sleep occurs during the biological day, when no melatonin is produced.2 As illustrated in Figure 4, these predicted relationships persist in the current data.
Thus, although that suppression of melatonin by light is a potential confounder in interpreting the aMT6s data, we do not believe it is a factor in this study.
REFERENCES
- 1.Skene DJ, Lockley SW, James K, Arendt J. Correlation between urinary cortisol and 6-sulphatoxymelatonin rhythms in field studies of blind subjects. Clin Endocrinol. 1999;50:715–9. doi: 10.1046/j.1365-2265.1999.00714.x. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 3.Benhaberou-Brun D, Lambert C, Dumont M. Association between melatonin secretion and daytime sleep complaints in night nurses. Sleep. 1999;22:877–85. doi: 10.1093/sleep/22.7.877. [DOI] [PubMed] [Google Scholar]
- 4.Dumont CP. Procedures nurses use to remove central venous catheters and complications they observe: a pilot study. Am J Crit Care. 2001;10:151–5. [PubMed] [Google Scholar]
- 5.Marie Hansen A, Garde AH, Hansen J. Diurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiol Int. 2006;23:1203–15. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 6.Barnes RG, Forbes MJ, Arendt J. Shift type and season affect adaptation of the 6-sulphatoxymelatonin rhythm in offshore oil rig workers. Neurosci Lett. 1998;252:179–82. doi: 10.1016/s0304-3940(98)00585-0. [DOI] [PubMed] [Google Scholar]
- 7.Barnes RG, Deacon SJ, Forbes MJ, Arendt J. Adaptation of the 6-sulphatoxymelatonin rhythm in shiftworkers on offshore oil installations during a 2-week 12-h night shift. Neurosci Lett. 1998;241:9–12. doi: 10.1016/s0304-3940(97)00965-8. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs M, Hampton S, Morgan L, Arendt J. Adaptation of the circadian rhythm of 6-sulphatoxymelatonin to a shift schedule of seven nights followed by seven days in offshore oil installation workers. Neurosci Lett. 2002;325:91–4. doi: 10.1016/s0304-3940(02)00247-1. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs M, Hampton S, Morgan L, Arendt J. Predicting circadian response to abrupt phase shift: 6-sulphatoxymelatonin rhythms in rotating shift workers offshore. J Biol Rhythms. 2007;22:368–70. doi: 10.1177/0748730407302843. [DOI] [PubMed] [Google Scholar]
- 10.Thorne H, Hampton S, Morgan L, Skene DJ, Arendt J. Differences in sleep, light, and circadian phase in offshore 18:00-06:00 h and 19:00-07:00 h shift workers. Chronobiol Int. 2008;25:225–35. doi: 10.1080/07420520802106850. [DOI] [PubMed] [Google Scholar]
- 11.Midwinter MJ, Arendt J. Adaptation of the melatonin rhythm in human subjects following night-shift work in Antarctica. Neurosci Lett. 1991;122:195–8. doi: 10.1016/0304-3940(91)90856-o. [DOI] [PubMed] [Google Scholar]
- 12.Ross JK, Arendt J, Horne J, Haston W. Night-shift work in Antarctica: sleep characteristics and bright light treatment. Physiol Behav. 1995;57:1169–74. doi: 10.1016/0031-9384(95)00018-e. [DOI] [PubMed] [Google Scholar]
- 13.Bearn J, Franey C, Arendt J, Checkley SA. A study of the effects of desipramine treatment alone and in combination with L-triiodothyronine on 6-sulphatoxymelatonin excretion in depressed patients. Br J Psychiatry. 1989;155:341–7. doi: 10.1192/bjp.155.3.341. [DOI] [PubMed] [Google Scholar]
- 14.Deacon SJ, Arendt J. Phase-shifts in melatonin, 6-sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clin Endocrinol (Oxf) 1994;40:413–20. doi: 10.1111/j.1365-2265.1994.tb03940.x. [DOI] [PubMed] [Google Scholar]
- 15.Deacon S, English J, Tate J, Arendt J. Atenolol facilitates light-induced phase shifts in humans. Neurosci Lett. 1998;242:53–6. doi: 10.1016/s0304-3940(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 16.Skene DJ, Vivien-Roels B, Sparks DL, et al. Daily variation in the concentration of melatonin and 5-methoxytryptophol in the human pineal gland: effect of age and Alzheimer's disease. Brain Res. 1990;528:170–4. doi: 10.1016/0006-8993(90)90214-v. [DOI] [PubMed] [Google Scholar]
- 17.Griefahn B, Bröde P, Remer T, Blaszkewicz M. Excretion of 6-hydroxymelatonin sulfate (6-OHMS) in siblings during childhood and adolescence. Neuroendocrinology. 2003;78:241–3. doi: 10.1159/000074444. [DOI] [PubMed] [Google Scholar]
- 18.Potocki L, Glaze D, Tan DX, et al. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med Genet. 2000;37:428–33. doi: 10.1136/jmg.37.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol Psychiatry. 2005;57:134–8. doi: 10.1016/j.biopsych.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Mulder EJ, Anderson GM, Kemperman RF, Oosterloo-Duinkerken A, Minderaa RB, Kema IP. Urinary excretion of 5-hydroxyindoleacetic acid, serotonin and 6-sulphatoxymelatonin in normoserotonemic and hyperserotonemic autistic individuals. Neuropsychobiology. 2010;61:27–32. doi: 10.1159/000258640. [DOI] [PubMed] [Google Scholar]
- 21.Tzischinsky O, Skene D, Epstein R, Lavie P. Circadian rhythms in 6-sulphatoxymelatonin and nocturnal sleep in blind children. Chronobiol Int. 1991;8:168–75. doi: 10.3109/07420529109063923. [DOI] [PubMed] [Google Scholar]
- 22.Uberos J, Augustin-Morales MC, Molina Carballo A, Florido J, Narbona E, Muñoz-Hoyos A. Normalization of the sleep-wake pattern and melatonin and 6-sulphatoxy-melatonin levels after a therapeutic trial with melatonin in children with severe epilepsy. J Pineal Res. 2011;50:192–6. doi: 10.1111/j.1600-079X.2010.00828.x. [DOI] [PubMed] [Google Scholar]
- 23.Bazil CW, Castro LH, Walczak TS. Reduction of rapid eye movement sleep by diurnal and nocturnal seizures in temporal lobe epilepsy. Arch Neurol. 2000;57:363–8. doi: 10.1001/archneur.57.3.363. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Smith P, Tamppari L, Arvidson R, et al. H2O at the Phoenix landing site. Science. 2009;325:58–61. doi: 10.1126/science.1172339. [DOI] [PubMed] [Google Scholar]
- 2.Aschoff J, Wever R. The circadian system of man. In: Aschoff J, editor. Biological rhythms: handbook of behavioral neurobiology. New York: Plenum Press; 1981. pp. 311–31. [Google Scholar]
- 3.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 4.Duffy JF, Cain SW, Chang AM, et al. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Nat Acad Sci. 2011;108:15602–8. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheer FA, Wright KP, Jr., Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE. 2007;2:e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright KP, Jr., Hughes R, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc Natl Acad Sci U S A. 2001;98:14027–32. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronfier C, Wright KP, Jr., Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24h days. Proc Natl Acad Sci U S A. 2007;104:9081–6. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parke B, Shafto M, Trimble J, Wales R. Mars Pathfinder: Fatigue/Stress Questionnaire Report: NASA Ames Research Center. 2001 [Google Scholar]
- 9.DeRoshia C, Colletti L, Mallis M. The effects of the Mars Exploration Rovers (MER) work schedule regime on locomotor activity circadian rhythms, sleep and fatigue, 2006. Technical Report no. 214560 [Google Scholar]
- 10.Czeisler CA, Carskadon MA, Gronfier C, Roth T, Mallis MM, Wright KP. Consultation Report: Mars Exploration Rover Surface Operations Human Factors Workshop. Arcadia, CA: California Institute of Technology; 2001. [Google Scholar]
- 11.Bass DS, Wales RC, Shalin VL Choosing Mars time: analysis of the Mars Exploration Rover experience. Institute of Electrical and Electronic Engineers Aerospace Conference 2004 [Google Scholar]
- 12.Wright KP, Jr., Hull JT, Hughes RJ, Ronda JM, Czeisler CA. Sleep and wakefulness out of phase with internal biological time impairs learning in humans. J Cogn Neurosci. 2006;18:508–21. doi: 10.1162/jocn.2006.18.4.508. [DOI] [PubMed] [Google Scholar]
- 13.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol (Lond) 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockley SW, Evans EE, Scheer FA, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 15.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 18.Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49:331–9. doi: 10.1016/j.neuron.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Brainard GC, Hanifin JP. Photons, clocks, and consciousness. J Biol Rhythms. 2005;20:314–25. doi: 10.1177/0748730405278951. [DOI] [PubMed] [Google Scholar]
- 20.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra3. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 22.Brainard GC, Hanifin JP, Rollag MD, et al. Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86:433–6. doi: 10.1210/jcem.86.1.7277. [DOI] [PubMed] [Google Scholar]
- 23.Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns' weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351:1829–37. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 24.SleepWatch and Basic Motionlogger User's Guide. Ardsley, NY: Ambulatory Monitoring, Inc.; 2010. [Google Scholar]
- 25.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–5. [Google Scholar]
- 26.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 27.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 28.Loh S, Lamond N, Dorrian J, Roach G, Dawson D. The validity of psychomotor vigilance tasks of less than 10-minute duration. Behav Res Methods Instrum Comput. 2004;36:339–46. doi: 10.3758/bf03195580. [DOI] [PubMed] [Google Scholar]
- 29.Lamond N, Dawson D, Roach GD. Fatigue assessment in the field: validation of a hand-held electronic psychomotor vigilance task. Aviat Space Environ Med. 2005;76:486–9. [PubMed] [Google Scholar]
- 30.Roach GD, Dawson D, Lamond N. Can a shorter psychomotor vigilance task be used as a reasonable substitute for the ten-minute psychomotor vigilance task? Chronobiol Int. 2006;23:1379–87. doi: 10.1080/07420520601067931. [DOI] [PubMed] [Google Scholar]
- 31.Lamond N, Jay SM, Dorrian J, Ferguson SA, Roach GD, Dawson D. The sensitivity of a palm-based psychomotor vigilance task to severe sleep loss. Behav Res Methods. 2008;40:347–52. doi: 10.3758/brm.40.1.347. [DOI] [PubMed] [Google Scholar]
- 32.Reeves D, Kane R, Winter K, Raynsford K, Pancella T. Automated Neuropsychological Assessment Metrics (ANAM): Test Administrator's Guide, Version 1. St Louis, MO: Missouri Institute of Mental Health; 1993. [Google Scholar]
- 33.Bleiberg J, Kane R, Reeves D, Garmoe W, Halpern E. Factor analysis of computerized and traditional tests used in mild brain injury research. Clin Neuropsychol. 2000;14:287–94. doi: 10.1076/1385-4046(200008)14:3;1-P;FT287. [DOI] [PubMed] [Google Scholar]
- 34.Kabat M, Kane R, Jefferson A, DiPino R. Construct validity of selected Automated Neuropsychological Assessment Metrics (ANAM) Battery Measures. Clin Neuropsychol. 2001;15:498–507. doi: 10.1076/clin.15.4.498.1882. [DOI] [PubMed] [Google Scholar]
- 35.Short P, Cernich A, Kabat M, Wilken J, Kane R. Initial construct validation of frequently employed ANAM measures through structural equation modeling. Arch Clin Neuropsychol. 2007;22(Suppl 1):63–77. doi: 10.1016/j.acn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Reeves D, Winter K, Bielberg J, Kane R. ANAM genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol. 2007;22:S15–37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Schiflett S, Eddy DR, Schlegel RE, French J, Shebab R. National Aeronautics and Space Administration, Life Sciences Project Division. Houston, TX: Johnson Space Center; 1995. Performance Assessment Workstation (PAWS), Final Report, IML-2 Mission. [Google Scholar]
- 38.Shehab RL, Schlegel RE, Schiflett SG, Eddy DR. The NASA Performance Assessment Workstation: cognitive performance during head-down bed rest. Acta Astronaut. 1998;43:223–33. doi: 10.1016/s0094-5765(98)00156-8. [DOI] [PubMed] [Google Scholar]
- 39.Eddy DR, Schiflett SG, Schlegel RE, Shehab RL. Cognitive performance aboard the life and microgravity spacelab. Acta Astronaut. 1998;43:193–210. doi: 10.1016/s0094-5765(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 40.Barnes RG, Forbes MJ, Arendt J. Shift type and season affect adaptation of the 6-sulphatoxymelatonin rhythm in offshore oil rig workers. Neurosci Lett. 1998;252:179–82. doi: 10.1016/s0304-3940(98)00585-0. [DOI] [PubMed] [Google Scholar]
- 41.Gibbs M, Hampton S, Morgan L, Arendt J. Predicting circadian response to abrupt phase shift: 6-sulphatoxymelatonin rhythms in rotating shift workers offshore. J Biol Rhythms. 2007;22:368–370. doi: 10.1177/0748730407302843. [DOI] [PubMed] [Google Scholar]
- 42.Thorne H, Hampton S, Morgan L, Skene DJ, Arendt J. Differences in sleep, light, and circadian phase in offshore 18:00-06:00 h and 19:00-07:00 h shift workers. Chronobiol Int. 2008;25:225–35. doi: 10.1080/07420520802106850. [DOI] [PubMed] [Google Scholar]
- 43.Dumont M, Benhaberou-Brun D, Paquet J. Profile of 24-h light exposure and circadian phase of melatonin secretion in night workers. J Biol Rhythms. 2001;16:502–11. doi: 10.1177/074873001129002178. [DOI] [PubMed] [Google Scholar]
- 44.Hansen M, Garde H, Hansen J. Diurnal urinary 6-sulfatoxymelatonin levels among healthy Danish nurses during work and leisure time. Chronobiol Int. 2006;23:1203–15. doi: 10.1080/07420520601100955. [DOI] [PubMed] [Google Scholar]
- 45.Midwinter MJ, Arendt J. Adaptation of the melatonin rhythm in human subjects following night-shift work in Antarctica. Neurosci Lett. 1991;122:195–198. doi: 10.1016/0304-3940(91)90856-o. [DOI] [PubMed] [Google Scholar]
- 46.Ross JK, Arendt J, Horne J, Haston W. Night-shift work in Antarctica: Sleep characteristics and bright light treatment. Physiol Behav. 1995;57:1169–74. doi: 10.1016/0031-9384(95)00018-e. [DOI] [PubMed] [Google Scholar]
- 47.Bearn J, Franey C, Arendt J, Checkley SA. A study of the effects of desipramine treatment alone and in combination with L-triiodothyronine on 6-sulphatoxymelatonin excretion in depressed patients. Br J Psychiatry. 1989;155:341–7. doi: 10.1192/bjp.155.3.341. [DOI] [PubMed] [Google Scholar]
- 48.Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82:3763–70. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- 49.Skene DJ, Bojkowski CJ, Currie JE, Wright J, Boulter PS, Arendt J. 6-Sulphatoxymelatonin production in breast cancer patients. J Pineal Res. 1990;8:269–76. doi: 10.1111/j.1600-079x.1990.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 50.Aldhous ME, Arendt J. Radioimmunoassay for 6-sulphatoxymelatonin in urine using an iodinated tracer. Ann Clin Biochem. 1988;25:298–303. doi: 10.1177/000456328802500319. [DOI] [PubMed] [Google Scholar]
- 51.Nelson W, Tong YL, Lee J, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–23. [PubMed] [Google Scholar]
- 52.Kronauer RE, Forger DB, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 1999;14:500–15. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- 53.Jewett ME, Forger DB, Kronauer RE. Revised limit cycle oscillator model of human circadian pacemaker. J Biol Rhythms. 1999;14:493–9. doi: 10.1177/074873049901400608. [DOI] [PubMed] [Google Scholar]
- 54.May CD, Dean DA, II, Jewett ME. A revised definition of core body temperature phase that incorporates both state variables of a limit-cycle human circadian pacemaker model improves model stability at low circadian amplitudes. Society for Research on Biological Rhythms meeting. 2002;8:133. [Google Scholar]
- 55.Light model, cognitive throughput model, and subjective alertness model. Model equation summary Brigham and Women's Hospital, Harvard Medical School. CPSS version 1.2 Software Release Notes. [Google Scholar]
- 56.Gronfier C, Wright KP, Jr., Kronauer RE, Jewett ME, Czeisler CA. Efficacy of a single sequence of intermittent bright light pulses for delaying circadian phase in humans. Am J Physiol Endocrinol Metab. 2004;287:E174–E81. doi: 10.1152/ajpendo.00385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol (Lond) 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single pulses of bright light in humans. Sleep. 2000;23:A22–3. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 60.Gronfier C, Kronauer RE, Wright KP, Jr., Czeisler CA. Phase-shifting effectiveness of intermittent light pulses: relationship to melatonin suppression. Society for Research on Biological Rhythms. 2000;7:134. [Google Scholar]
- 61.Lockley SW, Dijk DJ, Kosti O, Skene DJ, Arendt J. Alertness, mood and performance rhythm disturbances associated with circadian sleep disorders in the blind. J Sleep Res. 2008;17:207–16. doi: 10.1111/j.1365-2869.2008.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 63.Dean DA, II, Fletcher A, Hursh SR, Klerman EB. Developing mathematical models of neurobehavioral performance for the “Real World”. J Biol Rhythms. 2007;22:246–58. doi: 10.1177/0748730407301376. [DOI] [PubMed] [Google Scholar]
- 64.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 65.National Sleep Foundation. 2009 Sleep In America Poll: Summary of Findings. Washington, DC: 2009. [Google Scholar]
- 66.Schoenborn CA, Adams PF. Centers for Disease C, Prevention, eds. 2004-2006. Hyattsville, MD: NCHS Health E-stats; 2008. Sleep duration as a correlate of smoking, alcohol use, leisure-time physical inactivity, and obesity among adults: United States. [Google Scholar]
- 67.Wright KP, Jr., Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–77. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lockley SW, Skene DJ, Butler LJ, Arendt J. Sleep and activity rhythms are related to circadian phase in the blind. Sleep. 1999;22:616–23. doi: 10.1093/sleep/22.5.616. [DOI] [PubMed] [Google Scholar]
- 69.Groemer G, Gruber V, Bishop S, Peham D, Wolf L, Hogl B. Human performance data in a high workload environment during the simulated Mars expedition “AustroMars”. Acta Astronaut. 2009;66:780–7. [Google Scholar]
- 70.Hull JT, Wright KP, Jr., Czeisler CA. The influence of subjective alertness and motivation on human performance independent of circadian and homeostatic regulation. J Biol Rhythms. 2003;18:329–38. doi: 10.1177/0748730403253584. [DOI] [PubMed] [Google Scholar]
- 71.O'Griofa M, Blue R, Cohen K, O'Keefe D. Sleep stability and cognitive function in an Arctic Martian Analogue. Aviat Space Environ Med. 2011;82:434–40. doi: 10.3357/asem.2570.2011. [DOI] [PubMed] [Google Scholar]
- 72.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 73.Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–7. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 74.Rusak B, Zucker I. Biological rhythms and animal behavior. Ann Rev Psychol. 1975;26:137–71. doi: 10.1146/annurev.ps.26.020175.001033. [DOI] [PubMed] [Google Scholar]
- 75.Johnson MP, Duffy JF, Dijk DJ, Ronda JM, Dyal CM, Czeisler CA. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J Sleep Res. 1992;1:24–9. doi: 10.1111/j.1365-2869.1992.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 76.Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra3. doi: 10.1126/scitranslmed.3000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright KP, Jr., Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–R7. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 78.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 79.Johnson LC. Sleep deprivation and performance. In: Webb WB, editor. Biological rhythms, sleep, and performance. New York: John Wiley & Sons Ltd.; 1982. pp. 111–41. [Google Scholar]
- 80.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–7. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 81.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6:407–20. [PubMed] [Google Scholar]
- 82.Human Factors as a Field. Expert Pages. [cited 2010 August 20]. Available from: http://expertpages.com/news/humanfac.htm.
- 83.Barger LK, Lockley SW, Rajaratnam SM, Landrigan CP. Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9:155–64. doi: 10.1007/s11910-009-0024-7. [DOI] [PubMed] [Google Scholar]
- 84.HRP. Houston, TX: Lyndon B. Johnson Space Center; 2009. Human Research Program Integrated Research Plan. HRP-47065. National Aeronautics and Space Administration. Revision A. [Google Scholar]
- 85.Kelly TL, Neri DF, Grill JT, et al. Nonentrained circadian rhythms of melatonin in submariners scheduled to an 18-hour day. J Biol Rhythms. 1999;14:190–6. doi: 10.1177/074873099129000597. [DOI] [PubMed] [Google Scholar]
- 86.Palinkas LA, Suedfeld P. Psychological effects of polar expeditions. Lancet. 2008;371:153–63. doi: 10.1016/S0140-6736(07)61056-3. [DOI] [PubMed] [Google Scholar]
- 87.Viola A, James L, Schlangen L, Dikj D. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scan J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 88.Schubert EF, Kim JK. Solid-state light sources getting smart. Science. 2005;308:1274–8. doi: 10.1126/science.1108712. [DOI] [PubMed] [Google Scholar]
- 89.Mistlberger RE, Skene DJ. Nonphotic entrainment in humans? J Biol Rhythms. 2005;20:339–52. doi: 10.1177/0748730405277982. [DOI] [PubMed] [Google Scholar]
- 90.Eastman CI. What the placebo literature can tell us about light therapy for SAD. Psychopharmacol Bull. 1990;26:495–504. [PubMed] [Google Scholar]
- 91.Byrne B, Rollag MD, Hanifin JP, Reed C, Brainard GC. Bright light imagery does not suppress melatonin. J Pineal Res. 2000;29:62–64. doi: 10.1034/j.1600-079x.2000.290109.x. [DOI] [PubMed] [Google Scholar]
- 92.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 93.Mars Science Laboratory. Pasadena, CA: Jet Propulsion Laboratory; [cited 2010 July 9]. Available from: http://mars.jpl.nasa.gov/msl/ [Google Scholar]
- 94.NASA wants to bring Martian rocks to Earth. MSNBC.com. [cited 2010 Jul 9]. Available from: http://www.msnbc.msn.com/id/37213273/ns/technology_and_science-space/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score) plotted across duration of study. Each symbol represents a different subject (symbols consistent with Figures S2 and S4)

aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score) plotted across time of day of fitted acrophase. Each symbol represents a different subject (symbols consistent with Figures S1 and S4).

aMT6s amplitudes at fitted acrophase for all subjects (n = 18, aMT6s μg/hr z-score, 8 acrophases excluded due to missing actigraphy to categorize sleep/wake). Closed circles indicate acrophases that occurred during sleep and open circles indicate acrophases that occurred during wake. There was no difference in amplitude when the peak occurred during wake or sleep (unpaired Student's t, P = 0.25).

aMT6s acrophase times vs. cortisol acrophase times for 7 subjects. Each symbol represents a different subject (symbols consistent with Figures S1 and S2).