Abstract
Amyloid fibrils are associated with multiple neurodegenerative disorders, such as Alzheimer’s disease. Although biological membranes are involved in fibril plaque formation, the role of lipid membrane composition in fibril formation and toxicity is not well understood. We investigated the effect of cholesterol on the interaction of model lipid membranes with amyloid-β peptide (Aβ). With atomic force microscopy we demonstrated that binding of Aβ (1–42) to DOPC bilayer, enriched with 20% cholesterol, resulted in an intriguing formation of small nonuniform islands loaded with Aβ. We attribute this effect to the presence of nanoscale electrostatic domains induced by cholesterol in DOPC bilayers. Using frequency-modulated Kelvin probe force microscopy we were able to resolve these nanoscale electrostatic domains in DOPC monolayers. These findings directly affect our understanding of how the presence of cholesterol may induce targeted binding of amyloid deposits to biomembranes. We postulate that this nonhomogeneous electrostatic effect of cholesterol has a fundamental nature and may be present in other lipid membranes and monolayers.
Although amyloid fibril plaque accumulations have been observed on the surface neuronal cells in vivo in test subjects with Alzheimer’s disease (1,2) the role of cell membrane composition and the presence of cholesterol on fibril plaque formation and toxicity are still not well understood. Studies have shown that amyloid interacts with the membrane and that it is vital in amyloid fibril formation and toxicity (3,4). Although cholesterol is an important constituent of lipid rafts and is thought to regulate various important functions of the membrane (5), the role of cholesterol in the molecular mechanism of amyloid toxicity is not clear. Cholesterol has been shown to influence the fluidity of total brain extract, cell death, and the extent to which Aβ fibrillogenesis occurs (6). The effect of this sterol on the membrane is very complex and is still debated to this day (7,8).
We recently discovered an interesting electrostatic effect of cholesterol on pulmonary surfactant BLES and showed that cholesterol inhibits surfactant function (9). These intriguing electrostatic properties of cholesterol may be important for understanding the interaction of the plasma membrane with amyloid-forming peptides. Lipid bilayers and monolayers are widely used to mimic biological membranes (7) to study their structure and interaction with biomolecules (6,10,11).
In this work we used atomic force microscopy (AFM) and frequency-modulated Kelvin probe force microscopy (FM-KPFM) (see the Supporting Material) to investigate the effect of cholesterol on the structure of dioleoyl-sn-glycero-3-phosphocholine (DOPC) bilayers and monolayers and to study how this affects Aβ (1–42) binding and fibril formation. DOPC bilayers with and without cholesterol were prepared as described in the Supporting Material; deposited on mica; incubated with Aβ (1–42) solution in buffer; and then rinsed with water and imaged in water. As shown in Fig. 1 a, Aβ deposits on the pure DOPC bilayers were small, spherical, and uniformly distributed across the lipid membrane surface with no preferential binding sites or clustering. Amyloid fibril formation on DOPC membranes with 20% cholesterol showed quite different and striking results (Fig. 1 b)—amyloid deposits were binding to the lipid membrane in a nonuniform, selective manner, which resulted in the formation of nanoscale islands or domains (from tens to hundreds nm in size) enriched with amyloid deposits (Fig. 1, and see the Supporting Material).
Figure 1.
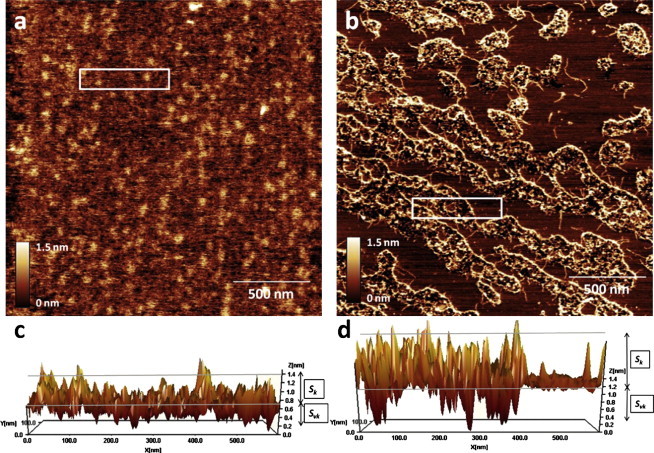
AFM topography images of Aβ binding to the lipid membrane. (a) Pure DOPC membrane after 1 h incubation with Aβ (1–42). (b) DOPC membrane with 20% cholesterol after 1 h incubation with Aβ (1–42). (c) Surface roughness (see the Supporting Material) of the pure DOPC membrane with Aβ deposits corresponding to the rectangular area marked in panel a. (d) Surface roughness of the cholesterol-enriched DOPC membrane with Aβ deposits corresponding to the rectangular area marked in panel b.
The amyloid deposits observed on the DOPC/cholesterol membrane were clusters of spherical oligomers and short fibrils. To estimate the disruptive effect of Aβ deposits on the lipid membranes, we evaluated the roughness parameters of the membrane surfaces for both samples. The surface of the pure DOPC membrane with Aβ deposits was relatively smooth with Aβ deposits slightly sinking into the membrane (Fig. 1, a and c); this correlates with our previous data (11). The domains of clustered Aβ deposits on the DOPC/cholesterol bilayer showed rough surfaces corresponding to the domains saturated with Aβ deposits and smooth areas of pure membrane (Fig. 1, b and d). The core roughness Sk (12), (see Fig. S1 in the Supporting Material) is slightly lower for pure DOPC membrane (0.7 ± 0.1 nm) than for cholesterol-enriched DOPC membrane (0.8 ± 0.1 nm). The reduced valley depth Svk is significantly higher for the cholesterol-enriched DOPC membrane (0.5 ± 0.1 nm) than for the pure DOPC membrane (0.24 ± 0.03 nm), which corresponds to increased damage (deeper holes produced) in the membrane by Aβ deposits.
It was reported that cholesterol induces a small thickening of the DOPC membrane (13), which may result in the formation of topographical domains that we observe; this indicates the coexistence of liquid-ordered and liquid-disordered phases (5). In addition, using FM-KPFM (14,15), we show that these domains are also electrostatic in nature (Fig. 2). Fig. 2 shows AFM topography (Fig. 2, a and b) and corresponding FM-KPFM surface potential images (Fig. 2, c and d) of pure DOPC monolayer (Fig. 2, a and c) and monolayer with 20% cholesterol (Fig. 2, b and d). The pure DOPC lipid monolayer is smooth and (Fig. 2 a) has uniform featureless surface potential (Fig. 2 c), whereas the DOPC lipid monolayer with 20% of cholesterol shows domains in topography (Fig. 2 b) and in surface potential (Fig. 2 d). These domains have a surface potential difference of 61 ± 8 mV measured from potential image cross sections (see the Supporting Material).
Figure 2.
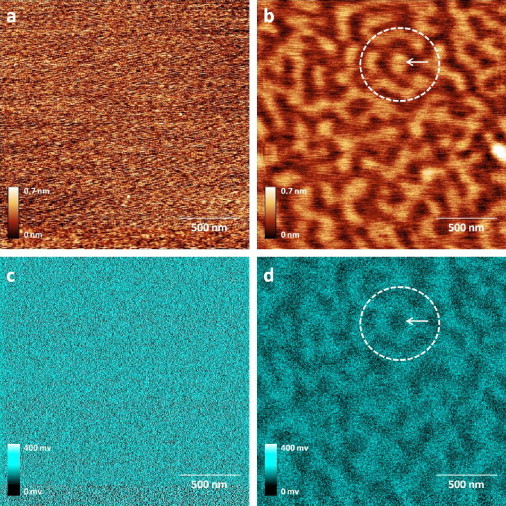
AFM and corresponding FM-KPFM images of lipid monolayers with and without cholesterol: AFM topography images of pure DOPC monolayer (a) and DOPC monolayer with 20% cholesterol (b); corresponding FM-KPFM images of surface potential distribution of pure DOPC monolayer (c) and DOPC monolayer with 20% cholesterol (d). AFM and FM-KPFM images were collected with SmartSPM (AIST-NT). The arrow in the circular region shows that the areas enriched with cholesterol are higher in topography (b) but lower in potential (d).
Considering the charged nature of Aβ (16), we expect that electrostatic domains created in the DOPC lipid membranes by cholesterol attract the Aβ peptide, thus inducing nonhomogeneous islands or domains that are densely packed with amyloid deposits (shown on Fig. 1 b). Earlier, we discovered that cholesterol-induced nanoscale electrostatic domains are crucial for the function of pulmonary surfactant and its interaction with charged nanoparticles (9). We postulate that this previously unknown electrostatic effect of cholesterol is not specific to pulmonary surfactant films and extends to other self-assembled amphiphilic structures such as lipid monolayers and lipid membranes, and, therefore, can alter the interaction of charged or polar biomolecules with the surface of lipid membrane. In this work, we demonstrated that this electrostatic effect of cholesterol may serve as a driving force for amyloid binding to lipid membranes and thus supports the hypothesis that cholesterol is involved in the mechanism of amyloid toxicity.
Acknowledgments
We thank Dr. J. Sanderson and Dr. S. Attwood for critical reading of the manuscript and useful discussion. We also greatly acknowledge AIST-NT (Novato, CA) for lending us the SmartSPM instrument to collect the frequency-modulation Kelvin probe force microscopy images and technical support.
This work was supported by Canadian Foundation for Innovation and Ontario Research Fund grants to Z.L., Natural Sciences and Engineering Research Council of Canada (NSERC) operating grant to Z.L., and NSERC Canada Graduate Scholarship to E.D.
Supporting Material
References and Footnotes
- 1.Chiti F., Dobson C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 2.Roberson E.D., Mucke L. 100 years and counting: prospects for defeating Alzheimer’s disease. Science. 2006;314:781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman R., Pellarin R., Caflisch A. Amyloid aggregation on lipid bilayers and its impact on membrane permeability. J. Mol. Biol. 2009;387:407–415. doi: 10.1016/j.jmb.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Jang H., Arce F.T., Lal R. Truncated β-amyloid peptide channels provide an alternative mechanism for Alzheimer’s disease and Down syndrome. Proc. Natl. Acad. Sci. USA. 2010;107:6538–6543. doi: 10.1073/pnas.0914251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 6.Yip C.M., Elton E.A., McLaurin J. Cholesterol, a modulator of membrane-associated Aβ-fibrillogenesis and neurotoxicity. J. Mol. Biol. 2001;311:723–734. doi: 10.1006/jmbi.2001.4881. [DOI] [PubMed] [Google Scholar]
- 7.Ohvo-Rekilä H., Ramstedt B., Slotte J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002;41:66–97. doi: 10.1016/s0163-7827(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 8.Bonn M., Roke S., Müller M. A molecular view of cholesterol-induced condensation in a lipid monolayer. J. Phys. Chem. B. 2004;108:19083–19085. [Google Scholar]
- 9.Finot E., Leonenko Y., Leonenko Z. Effect of cholesterol on electrostatics in lipid-protein films of a pulmonary surfactant. Langmuir. 2010;26:1929–1935. doi: 10.1021/la904335m. [DOI] [PubMed] [Google Scholar]
- 10.Choucair A., Chakrapani M., Johnston L.J. Preferential accumulation of Aβ (1–42) on gel phase domains of lipid bilayers: an AFM and fluorescence study. Biochim. Biophys. Acta. 2007;1768:146–154. doi: 10.1016/j.bbamem.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Hane F., Drolle E., Leonenko Z. Amyloid-β aggregation on model lipid membranes: an atomic force microscopy study. J. Alzheimers Dis. 2011;26:485–494. doi: 10.3233/JAD-2011-102112. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q., Fan X., Chen J. Characterization of bioscoured cotton fabrics using FT-IR ATR spectroscopy and microscopy techniques. Carbohydr. Res. 2006;341:2170–2175. doi: 10.1016/j.carres.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Kucerka N., Pencer J., Katsaras J. Influence of cholesterol on the bilayer properties of monounsaturated phosphatidylcholine unilamellar vesicles. Eur. Phys. J E Soft Matter. 2007;23:247–254. doi: 10.1140/epje/i2007-10202-8. [DOI] [PubMed] [Google Scholar]
- 14.Zerweck U., Loppacher C., Eng L. Accuracy and resolution limits of Kelvin probe force microscopy. Phys. Rev. B. 2005;71 125424:1–9. [Google Scholar]
- 15.Moores B., Hane F., Leonenko Z. Kelvin probe force microscopy in application to biomolecular films: frequency modulation, amplitude modulation, and lift mode. Ultramicroscopy. 2010;110:708–711. doi: 10.1016/j.ultramic.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 16.Moores B., Drolle E., Leonenko Z. Effect of surfaces on amyloid fibril formation. PLoS ONE. 2011;6:e25954. doi: 10.1371/journal.pone.0025954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


