Abstract
Rationale: Diabetic peripheral neuropathy is common and causes significant morbidity. Obstructive sleep apnea (OSA) is also common in patients with type 2 diabetes. Because OSA is associated with inflammation and oxidative stress, we hypothesized that OSA is associated with peripheral neuropathy in type 2 diabetes.
Objectives: To assess the relationship between OSA and peripheral neuropathy in patients with type 2 diabetes.
Methods: A cross-sectional study of adults with type 2 diabetes recruited randomly from the diabetes clinic of two UK hospitals.
Measurements and Main Results: Peripheral neuropathy was diagnosed using the Michigan Neuropathy Screening Instrument. OSA (apnea-hypopnea index ≥ 5 events/h) was assessed using home-based, multichannel respiratory monitoring. Serum nitrotyrosine was measured by ELISA, lipid peroxide by spectrophotometer, and microvascular function by laser speckle contrast imaging. Two hundred thirty-four patients (mean [SD] age, 57 [12] yr) were analyzed. OSA prevalence was 65% (median apnea-hypopnea index, 7.2; range, 0–93), 40% of which were moderate to severe. Neuropathy prevalence was higher in patients with OSA than those without (60% vs. 27%, P < 0.001). After adjustment for possible confounders, OSA remained independently associated with diabetic neuropathy (odds ratio, 2.82; 95% confidence interval, 1.44–5.52; P = 0.0034). Nitrotyrosine and lipid peroxide levels (n = 102, 74 with OSA) were higher in OSA and correlated with hypoxemia severity. Cutaneous microvascular function (n = 71, 47 with OSA) was impaired in OSA.
Conclusions: We describe a novel independent association between diabetic peripheral neuropathy and OSA. We identified increased nitrosative/oxidative stress and impaired microvascular regulation as potential mechanisms. Prospective and interventional studies are needed to assess the impact of OSA and its treatment on peripheral neuropathy development and progression in patients with type 2 diabetes.
Keywords: obstructive sleep apnea, diabetic neuropathy, nitrosative stress, microvascular function, laser speckle contrast imaging
At a Glance Commentary
Scientific Knowledge on the Subject
Obstructive sleep apnea (OSA) is known to be common in patients with type 2 diabetes (T2DM). However, the consequences of OSA complicating T2DM are unclear, particularly in regard to diabetes-related micro and macrovascular complications.
What This Study Adds to the Field
We provide evidence that OSA is independently associated with diabetic peripheral neuropathy (DPN). We also found that the severity of DPN correlated with the degree of OSA and the severity of nocturnal hypoxemia. In addition, we identified potential mechanisms linking OSA and DPN, including increased nitrosative stress and impaired microvascular blood flow regulation. Our results therefore indicate that OSA complicating T2DM may aggravate and amplify glucose toxicity, which has significant implications for tissues susceptible to diabetes complications.
Diabetic peripheral neuropathy (DPN) is common and results in great morbidity, mortality, and significant economic burden (1). Known DPN risk factors include increasing age and the duration and degree of the antecedent hyperglycemia (2, 3), as well as hypertension, dyslipidemia, and obesity (4, 5). Putative mechanisms for DPN include increased oxidative/nitrosative stress, advanced glycation end-product formation, activation of the hexosamine and polyol pathways, and perturbations of protein kinase C, resulting in direct cellular damage and functional and/or structural defects involving the extracellular matrix and/or microvasculature (6, 7). Despite our improved understanding of the pathogenesis of DPN, disease-modifying treatments are still lacking (with the exception of improved glycemia) (4, 6). Hence, improved understanding of DPN pathogenesis is important to identify new treatments (6).
Obstructive sleep apnea (OSA) is a common disorder that is highly prevalent in patients with type 2 diabetes (T2DM) (8, 9). It is characterized by upper airway instability during sleep, resulting in markedly reduced (hypopnea) or absent (apnea) airflow (10). These apnea/hypopnea episodes are usually accompanied by cyclical oxygen desaturations and cyclical changes in blood pressure and heart rate (10). Because OSA is associated with many of the pathophysiological deficits that are found in diabetes (11–13), it seems reasonable to speculate that OSA could play an important role in the development or progression of DPN. The primary aim of this study was therefore to explore the interrelationships of OSA and DPN in subjects with T2DM. A secondary aim was to explore the potential pathophysiological mechanisms.
Methods
We conducted an observational cross-sectional study in adults with T2DM. Patients with respiratory disease (including prediagnosed OSA), end-stage renal disease, or nondiabetic neuropathy (< 1%) were excluded. Patients were recruited casually from the outpatient diabetes departments of two UK hospitals. Patients were approached in the waiting area before they had seen the clinicians and without any prior knowledge of their medical condition. We avoided any reference to snoring during the recruitment process. Consent was obtained, and ethnicity was determined in accordance with the UK decennial census by the study participants. The project was approved by the Warwickshire Research Ethics Committee (REC number 08/H1211/145).
DPN was assessed using the Michigan Neuropathy Screening Instrument (MNSI) (see Figure E1 in the online supplement) (14–18). DPN was diagnosed if the MNSI examination score was greater than 2 and/or MNSI questionnaire score was greater than or equal to 7 (17, 19). Foot insensitivity to a 10-g monofilament (applied to 10 foot locations) was defined as fewer than eight correct responses (19).
OSA was assessed by a single overnight home-based cardiorespiratory sleep study using a portable multichannel device (Alice PDX; Philips Respironics, Best, The Netherlands) and scored in accordance with the American Academy of Sleep Medicine guidelines (20). An apnea-hypopnea index (AHI) greater than or equal to 5 events/h was consistent with the diagnosis of OSA (21).
All patients were approached, and serum nitrotyrosine and plasma lipid peroxide were assessed in duplicate in all subjects who agreed. 3-Nitrotyrosine was measured by ELISA (Oxiselect; Cell Biolabs Inc., San Diego, CA) and lipid peroxide by spectrophotometry.
Microvascular assessment was performed on a casually chosen representative patient subset using laser speckle contrast imaging (Moor Instruments Ltd, Devon, UK) (22, 23).
All assessments in the study were blinded.
Data analysis was performed using SPSS 15.0 software (SPSS Inc, Chicago, IL). Data are presented as mean (SD) or median (interquartile range). Independent continuous variables were compared using the Student t test or the Mann-Whitney test. Categorical variables were compared using the Chi-square test. Correlations between continuous variables were performed using the Pearson or Spearman tests. Differences between independent groups were assessed by analysis of variance. Analysis of covariance was used to assess the impact of covariates on the differences between several independent groups. To assess whether OSA status, OSA severity, or hypoxemia measures are independent predictors of DPN, multiple logistic regression (forced entry method) was used. Multiple linear regression (forced entry method) was used to assess independent predictors of continuous variables. Variables included in the regression models were based on known outcome-related risk factors and/or variables that differed between patients with and without OSA. To further explore the impact of baseline differences on the associations observed, a subgroup of 70 patients with and 70 without OSA were group matched for a variety of risk factors. A P value less than 0.05 was considered significant unless stated otherwise.
For detailed methodology and details on model building, please see the online supplement.
Results
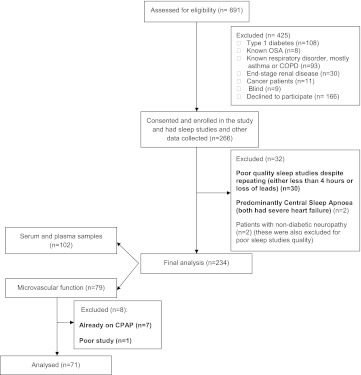
We recruited 266 patients; 32 were excluded, leaving 234 patients for analysis (Figure 1). Of these 234 patients, 58% were men, 55% were white, and 45% were South Asian.
Figure 1.
The consort diagram for our study. COPD = chronic obstructive pulmonary disease; CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea.
OSA and DPN Prevalence
The overall prevalence of DPN was 48%. The overall prevalence of OSA was 65%. Of the 151 patients with OSA, 60% had mild (AHI, 5 to < 15), 23% had moderate (AHI, 15 to < 30), and 17% had severe (AHI ≥ 30) OSA.
OSA and Clinical Characteristics in T2DM
Patients with OSA (OSA+) were older, had longer diabetes duration, higher systolic blood pressure and obesity measures, and were sleepier compared with those without OSA (OSA−) (Table 1).
TABLE 1.
PARTICIPANT CHARACTERISTICS IN RELATION TO OBSTRUCTIVE SLEEP APNEA STATUS
| OSA− (n = 83) | OSA+ (n = 151) | P Value | |
| Male | 34 (41%) | 101 (67%) | <0.001 |
| White | 32 (39%) | 97 (64%) | <0.001 |
| Age, yr | 54.7 (11.9) | 58.5 (11.3) | 0.02 |
| Diabetes duration, yr | 9 (5–15) | 11 (7–17) | 0.02 |
| Body mass index, kg/m2 | 30.2 (27.3–35.0) | 34.4 (30.9–39.5) | <0.001 |
| Waist circumference, cm | 105.5 (96.0–115.0) | 116.0 (107.5–125.5) | <0.001 |
| Hip, cm | 106.0 (98.0–117.0) | 114.0 (105.0–125.0) | <0.001 |
| Waist/hip ratio | 0.97 (0.93–1.02) | 1.01 (0.96–1.05) | 0.002 |
| Neck circumference, cm | 38.0 (36.5–41.3) | 43.0 (39.0–46.0) | <0.001 |
| Height, cm | 163.5 (8.3) | 167.8 (10.0) | 0.001 |
| Systolic blood pressure, mm Hg | 125.5 (115.0–135.5.0) | 130.0 (123.5–140.0) | 0.002 |
| Diastolic blood pressure, mm Hg | 78.50 (71.0–85.00) | 78.00 (71.00–84.50) | 0.88 |
| HbA1c, % | 7.7 (7.0–8.7) | 8.3 (7.3–9.3) | 0.05 |
| Total cholesterol, mmol/L | 3.7 (3.4–4.5) | 3.7 (3.3–4.3) | 0.57 |
| Triglycerides, mmol/L | 1.5 (1.0–2.1) | 1.8 (1.3–2.5) | 0.03 |
| HDL, mmol/L | 1.2 (0.9–1.4) | 1.1 (0.9–1.2) | 0.02 |
| Estimated GFR, ml/min/1.73 m2 | 92.92 (25.16) | 82.41 (26.41) | 0.003 |
| TSH | 1.6 (1.0–2.2) | 1.7 (1.2–2.4) | 0.32 |
| Epworth sleepiness score | 5.0 (2.0–12.0) | 8.0 (4.0–13.0) | 0.003 |
| Smoking (current or ex-smoker) | 32 (39%) | 62 (41%) | 0.71 |
| Alcohol (drinks alcohol) | 12 (15%) | 12 (35%) | 0.001 |
| Oral antidiabetes treatment | 81 (98%) | 137 (91%) | 0.05 |
| Insulin | 34 (41%) | 91 (60%) | 0.005 |
| Insulin dose, units | 61 (35–88) | 80 (56–118) | 0.007 |
| ACE inhibitors | 40 (48%) | 69 (46%) | 0.71 |
| Antihypertensive agents | 61 (74%) | 129 (85%) | 0.03 |
| Lipid-lowering treatment | 71 (86%) | 125 (83%) | 0.58 |
| Stroke or TIA | 60 (7%) | 18 (12%) | 0.28 |
| Ischemic heart disease | 14 (17%) | 33 (22%) | 0.40 |
| PVD | 1 (1%) | 10 (7%) | 0.06 |
| Albuminuria | 20 (24%) | 65 (43%) | 0.007 |
| Sight-threatening retinopathy | 17 (21%) | 72 (48%) | <0.001 |
Definition of abbreviations: ACE = angiotensin-converting enzyme; GFR = glomerular filtration rate; HDL = high-density lipoprotein; IQR = interquartile range; OSA = obstructive sleep apnea; PVD = peripheral vascular disease; TIA = transient ischemic attack; TSH = thyroid-stimulating hormone.
Data presented as median (IQR) or mean (SD). Categorical variables presented as number (% of OSA status). Analysis performed using the Chi-square test for categorical variables, the independent t test for normally distributed variables, and the Mann-Whitney U test for nonnormally distributed variables.
The Relationship between OSA and DPN
The overall DPN prevalence was higher in OSA+ compared with OSA− patients (60 vs. 27%, P < 0.001). This relationship between OSA and DPN was present irrespective of ethnicity (Figure E2).
The Relationship between OSA and Clinical Signs and Symptoms of DPN
The overall foot insensitivity prevalence was 37%. Foot insensitivity was higher in OSA+ compared with OSA− patients (50 vs. 15%, P < 0.001, respectively). OSA+ patients had more abnormalities on all aspects of the neurological examination (Table 2).
TABLE 2.
THE RELATIONSHIP BETWEEN OBSTRUCTIVE SLEEP APNEA STATUS AND ASPECTS OF FOOT EXAMINATION USING THE MICHIGAN NEUROPATHY SCREENING INSTRUMENT
| OSA− (n = 83) | OSA+ (n = 151) | P Values | |
| Inspection | 34 (41) | 100 (67) | <0.001 |
| Ulcers | 0 (0) | 8 (5) | 0.03 |
| Ankle reflexes | 25 (30) | 87 (58) | <0.001 |
| Vibration | 19 (23) | 90 (60) | <0.001 |
| 10-g Monofilament | 12 (15) | 75 (50) | <0.001 |
Data presented as No. (% of abnormal test in the particular OSA group). This was a univariate analysis performed using the Chi-square test. The Bonferroni correction was applied, and P < 0.01 was considered significant.
Based on the MNSI questionnaire, OSA+ patients had a higher prevalence of skin hypersensitivity (33 vs. 13%, P = 0.001). A previous history of “open sore on the foot” was also more common in OSA+ patients (27 vs. 7%, P < 0.001), consistent with findings using the monofilament. The rest of the questionnaire components were not significantly different between OSA+ and OSA− patients (data not shown).
A Multivariate Analysis of the Relationship between OSA, Its Severity, and DPN
To assess whether the relationship between OSA and DPN is secondary to or independent of the differences observed in baseline characteristics, logistic regression (forced entry method) was used (Table 3). Despite some attenuation by adiposity measures, OSA remained independently associated with DPN (OR, 2.82; 95% CI, 1.44–5.52; P = 0.003) after adjustment for main possible confounders (model 1, Table 3). Further adjustment by inserting other possible confounders into the model did not affect the relationship between OSA and DPN and did not improve the model R2 (models 4 and 5, Table 3). Replacing body mass index (BMI) with waist circumference or waist/hip ratio in model 1 did not change the significant relationship between OSA and DPN (models 2 and 3, Table 3). Other independent associations with DPN included waist circumference (OR, 1.03; 95% CI, 1.004–1.05; P = 0.02), insulin use (OR, 2.59; 95% CI, 1.34–5.01; P = 0.005), and diabetes duration (OR, 1.06; 95% CI, 1.01–1.11; P = 0.01). Inserting OSA as a three-category (no OSA, mild OSA, and moderate to severe OSA) rather than a dichotomous (no OSA and OSA) variable into model 1 demonstrated that both mild (OR, 3.04; 95% CI, 1.48–6.25; P = 0.002) and moderate to severe OSA (OR, 2.43; 95% CI, 1.06–5.57; P = 0.04) were independently associated with DPN. Furthermore, models that included AHI quartiles and nadir nocturnal oxygen saturation instead of OSA found that these variables were also independently associated with DPN (see online supplement).
TABLE 3.
ASSESSING THE IMPACT OF POSSIBLE CONFOUNDERS ON THE ASSOCIATION BETWEEN OBSTRUCTIVE SLEEP APNEA AND DIABETIC PERIPHERAL NEUROPATHY (BASED ON THE MICHIGAN NEUROPATHY SCREENING INSTRUMENT) USING DIFFERENT LOGISTIC REGRESSION MODELS (FORCED ENTRY METHOD)
| Model | Nagelkerke R2 | OR | 95% CI | P Value |
| Unadjusted: OSA | 0.13 | 4.09 | 2.28–7.35 | <0.001 |
| Model 1 | 0.25 | 2.82 | 1.44–5.52 | 0.003 |
| Model 2 | 0.26 | 2.76 | 1.41–5.40 | 0.003 |
| Model 3 | 0.25 | 3.33 | 1.72–6.47 | <0.001 |
| Model 4 | 0.28 | 2.77 | 1.36–5.62 | 0.005 |
| Model 5 | 0.29 | 2.72 | 1.34–5.55 | 0.006 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; DPN = diabetic peripheral neuropathy; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; OR = odds ratio; OSA = obstructive sleep apnea.
The ORs reported are the odds for having DPN in OSA+ compared with OSA− patients. All patients (n = 234) were included in all models. Models 1, 2, and 3 include only the main possible confounders for the relationship between OSA and DPN, whereas models 4 and 5 are adjusted for all variables in our database.
Model 1: OSA + ethnicity + sex + age at diabetes diagnosis + diabetes duration + eGFR + insulin use + BMI + mean arterial pressure + HbA1c + alcohol intake (units/wk).
Model 2: as model 1 but replacing BMI with waist circumference.
Model 3: as model 1 but replacing BMI with waist hip ratio.
Model 4: OSA + age at diabetes diagnosis + ethnicity + sex + diabetes duration + BMI + alcohol intake + HbA1c+ insulin use + mean arterial pressure + eGFR + PVD + smoking + total cholesterol + triglycerides + HDL + oral antidiabetes treatment + antihypertensive agents + lipid-lowering therapy + antiplatelets + recruitment site.
Model 5: As for model 4 but both BMI and waist circumference included in the model.
A Multivariate Analysis of the Relationship between OSA, Its Severity, and the Clinical Signs of DPN
Using the monofilament test to detect the “at-risk foot” as an outcome, OSA remained independently associated with foot insensitivity (OR, 3.97; 95% CI, 1.80–8.74; P = 0.001, Nagelkerke R2, 0.34) after adjustment as in model 1 in Table 3. Similar to DPN, both mild (OR, 4.93; 95% CI, 2.10–11.57; P < 0.001) and moderate to severe (OR, 2.83; 95% CI, 1.13–7.13; P = 0.03) OSA and AHI quartiles were independently associated with the at-risk foot after adjustment (see online supplement).
In addition, OSA and AHI quartiles were independently associated with reduced/absent ankle reflexes and vibration perception (see online supplement).
The Relationship of OSA Severity and DPN Severity
DPN severity (MNSI examination score categories: < 2, 2 to < 4, and ≥ 4) correlated significantly with OSA severity and nocturnal hypoxemia severity, independently of age, obesity, diabetes duration, sex, and estimated glomerular filtration rate in the case of AHI (Table 4). There was also a significant trend of higher DPN prevalence in patients with lower nocturnal nadir oxygen saturation (61 vs. 52 vs. 41 vs. 38% for nadir oxygen saturation quartiles < 77% vs. 77 to < 83% vs. 83 to < 87% and ≥ 87%, respectively; P = 0.02 for the trend). There was, however, no significant increase in DPN prevalence (as binary variable) between patients with mild (60%), moderate (57%), and severe (62%) OSA.
TABLE 4.
THE RELATIONSHIP BETWEEN DIABETIC PERIPHERAL NEUROPATHY SEVERITY BASED ON THE MICHIGAN NEUROPATHY SCREENING INSTRUMENT EXAMINATION SCORE AND OBSTRUCTIVE SLEEP APNEA AND NOCTURNAL HYPOXEMIA SEVERITY USING THE KRUSKAL-WALLIS H TEST
| MNSIe | AHI* | ODI* | Time Spent with Oxygen Saturations < 90%* | Nadir Nocturnal Oxygen Saturations* |
| Univariate analysis | ||||
| Group 1: < 2 (n = 90) | 4.7 (1.6–12.2) | 4.7 (1.7–13.6) | 0.9 (0.1–4.9) | 83.5 (79.0–89.0) |
| Group 2: 2 to <4 (n = 100) | 7.2 (2.4–16.0) | 6.5 (2.7–13.1) | 1.2 (0.1–5.6) | 83.0 (78.0–88.0) |
| Group 3: ≥ 4 (n = 44) | 8.9 (6.8–27.0) | 9.8 (6.0–26.8) | 2.2 (0.2–9.6) | 80.0 (71.5–84.8) |
| P value for the trend | < 0.001 | < 0.001 | 0.174 | 0.004 |
| Adjusted analysis | ||||
| Group 1 | 5.5 (4.4–6.9) | 5.6 (4.4–6.9) | 2.5 (1.7–3.4) | 84.0 (82.2–85.4) |
| Group 2 | 6.3 (5.1–7.8) | 5.9 (4.7–7.2) | 2.3 (1.7–3.2) | 83.8 (82.3–85.3) |
| Group 3 | 11.0 (7.4–16.2) | 9.5 (6.4–14.0) | 2.7 (1.4–4.9) | 80.9 (77.0–84.1) |
| P value after adjustment | 0.02 | 0.08 | 0.89 | 0.26 |
Definition of abbreviations: AHI = apnea-hypopnea index; IQR = interquartile range; MNSIe = Michigan Neuropathy Screening Instrument examination; ODI = oxygen desaturation index.
Data presented as median (IQR). Adjusted P values are adjusted for sex, age, body mass index, diabetes duration, and estimated glomerular filtration rate. Adjusted P values were calculated using analysis of covariance. Interaction between sex and MNSIe categories was not significant in any of the analyses performed. Data in the adjusted analysis presented as mean (95% confidence interval). For patients' characteristics by MNSIe group, please refer to Table E1 in the online supplement.
These parameters are used as scale variables.
OSA and DPN: A Matched-Group Analysis
The above findings indicate that OSA is independently associated with DPN after adjusting for the differences observed between patients with and without OSA. However, we believed that minimizing these differences by matching for as many DPN risk factors as possible would be advantageous to further test this relationship. We were able to group match 140 (70 with and 70 without OSA) patients for BMI and diabetes duration among others (for detailed characterization, see Table E2). DPN prevalence remained higher in the OSA+ group (53 vs. 24%, P = 0.001; OSA+ vs. OSA−, respectively). The prevalence of the at-risk foot based on the monofilament examination was also higher in the OSA+ group (43 vs. 13%, P < 0.001). After adjustment as in model 4 in Table 3, OSA remained independently associated with DPN (OR, 3.92; 95% CI, 1.54–9.96; P = 0.004; Nagelkerke R2, 0.31) and the at-risk foot (based on monofilament perception) (OR, 5.56; 95% CI, 1.82–16.97; P = 0.003, Nagelkerke R2, 0.37).
The Relationship of OSA and Nitrosative and Oxidative Stress
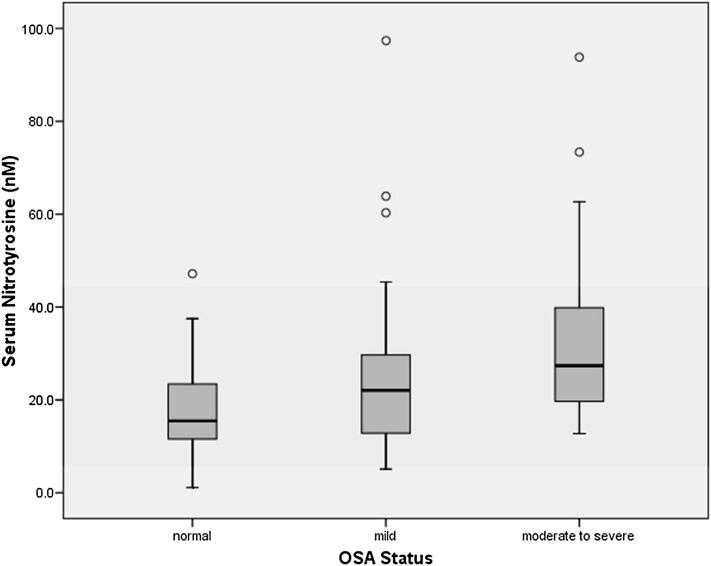
To explore possible mechanisms that underlie the relationship between OSA and DPN, serum nitrotyrosine and plasma lipid peroxide levels were measured in a cohort of 102 patients (29 without and 73 with OSA; for patient characteristics, see Tables E3 and E4). Nitrotyrosine levels were higher in patients with DPN compared with those without (25.6 [17.7–35.8] nM vs. 19.5 [11.5–29.6] nM, P = 0.01) and in OSA+ patients compared with OSA− patients (23.5 [16.7–36.1] nM vs. 15.5 [11.5–24.3] nM, P = 0.007). There was a stepwise increase in nitrotyrosine abundance between patients without OSA (n = 29) and patients with mild (n = 45) and moderate to severe OSA (n = 28) (P < 0.001 for the trend using analysis of variance) (Figure 2), with significant differences between moderate to severe OSA and mild OSA (P = 0.04) and patients without OSA (P < 0.001). The difference between moderate to severe OSA and no OSA remained significant after adjusting for age, BMI, and diabetes duration (P = 0.011).
Figure 2.
The relationship between obstructive sleep apnea (OSA) and serum nitrotyrosine levels in patients with type 2 diabetes without OSA (n = 29) and with mild (n = 45) and moderate to severe OSA (n = 28). P value for the trend, P < 0.001. P = 0.04 for mild versus moderate to severe OSA. P < 0.001 for normal versus moderate to severe OSA. Normal: patients with type 2 diabetes but without OSA.
Serum nitrotyrosine levels correlated with OSA severity and nocturnal hypoxemia measures (AHI [r = 0.38, P < 0.001], time spent with oxygen saturations < 80% [r = 0.23, P = 0.02], oxygen desaturation index [ODI] [r = 0.35, P < 0.001], and nadir nocturnal oxygen saturations [r = −0.21, P = 0.03]).
Using multiple linear regression, and after adjusting for age at diabetes diagnosis, sex, ethnicity, diabetes duration, BMI, HbA1c, and mean arterial pressure, OSA (AHI ≥ 10) (B = 0.19, P = 0.005), OSA (AHI ≥ 15) (B = 0.17, P = 0.01), AHI (B = 0.24, P = 0.001), ODI (B = 0.24, P = 0.002), and nadir nocturnal oxygen saturations (B = −0.30, P = 0.003) were all independently associated with nitrotyrosine levels. OSA (AHI ≥ 5) (B = 0.12, P = 0.11) was not associated with nitrotyrosine levels after adjustment.
Lipid peroxide levels were higher in patients with DPN (21.1 [3.9–42.5] μM/ml) compared with those free from DPN (12.2 [2.9–24.6] μM/ml, P = 0.01) and in those with OSA (18.4 [8.3–37.4] μM/ml) compared with those without OSA (7.9 [0.8–22.8] μM/ml, P = 0.01), which remained significant after adjusting for age, BMI, and diabetes duration (P = 0.02) (see online supplement).
The Relationship of OSA and Microvascular Blood Flow Regulation
Microvascular assessment was performed in 71 patients (47 with OSA; 28 mild, 11 moderate, 8 severe). For patients characteristics see Tables E5 and E6. Patients with OSA had lower basal microvascular flux and lower acetylcholine and sodium nitroprusside–induced flux, whereas heating-induced flux was not different between the groups. After adjustment for mean arterial pressure and maximal vasodilatation, basal, acetylcholine and sodium nitroprusside–induced flux remained significantly lower in OSA+ patients (Table 5). After adjustment for ethnicity, sex, age at diabetes diagnosis, diabetes duration, BMI, OSA, AHI, and nocturnal hypoxemia, measures remained independently associated with basal and sodium nitroprusside–induced microvascular function (Tables 5 and E7).
TABLE 5.
ASSESSMENT OF MICROVASCULAR BLOOD FLOW AND ENDOTHELIAL FUNCTION IN TYPE 2 DIABETES WITH AND WITHOUT OBSTRUCTIVE SLEEP APNEA
| OSA− (n = 24) | OSA+ (n = 47) | P Value, Unadjusted | P Value, after Adjustment | |
| Conductance | ||||
| Baseline | 0.40 (0.28–0.48) | 0.20 (0.16–0.31) | <0.001 | <0.001 |
| Heating | 1.82 (1.43–2.03) | 1.66 (1.28–2.07) | 0.37 | 0.42 |
| Ach | 1.43 (1.09–1.83) | 1.07 (0.75–1.29) | 0.002 | 0.1 |
| SNP | 1.61 (1.15–2.14) | 1.16 (0.62–1.41) | 0.001 | <0.001 |
| Flux in relation to maximum vasodilatation | ||||
| Baseline | 0.22 (0.16–0.29) | 0.14 (0.10–0.17) | <0.001 | 0.002 |
| Ach | 0.81 (0.670.902) | 0.63 (0.430.77) | 0.005 | 0.26 |
| SNP | 0.93 (0.77–1.13) | 0.57 (0.41–0.89) | <0.001 | 0.001 |
Definition of abbreviations: Ach = acetylcholine; IQR = interquartile range; OSA = obstructive sleep apnea; SNP = sodium nitroprusside.
Data presented as median (IQR) or ratios. Blood flux was measured in arbitrary perfusion units. Conductance is calculated by dividing flux by the mean arterial pressure. Analysis was performed using the Mann-Whitney U test. Adjusted P values were calculated using linear regression. For patient characteristics please refer to Tables E5 and E6 in the online supplement. For the results of the adjusted analysis using forced entry method please refer to Table E7 in the online supplement.
Discussion
To our knowledge, this is the first report identifying a novel independent association between OSA and DPN in patients with T2DM. Different markers of OSA severity correlated with the DPN severity, and DPN prevalence increased with worsening hypoxemia. The OSA prevalence in our sample is consistent with other studies in subjects with T2DM (8, 9). The DPN prevalence in our cohort is also similar to previous studies (14, 24).
As expected, demographic and metabolic factors differed between patients with and without OSA. Nevertheless, although these differences contributed to the observed relationship between OSA and DPN, OSA remained independently associated with DPN even after adjustment for these possible confounders. Furthermore, OSA remained independently associated with DPN when the groups were matched for obesity and several other DPN risk factors.
Our data also show that OSA is independently associated with the at-risk foot (based on the 10-g monofilament test). Interestingly, the association between OSA and the monofilament test seemed stronger than that with the MNSI. This difference may reflect the different modalities assessed by the 10-g monofilament test (which tests for advanced foot insensitivity sufficient to result in ulceration) and the MNSI (a test for DPN) (see online supplement for further information). It is worth noting that all patients with foot ulceration in our sample also experienced OSA and that a previous “sore on the foot” is more common in patients with OSA. This provides clinical confirmation of an independent association between OSA and the inability to feel a 10-g monofilament.
Potential Mechanisms That May Link OSA and DPN
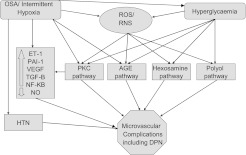
There are several possible explanations for a relationship between OSA and DPN (Figure 3). OSA has been shown to increase advanced glycation end-products production (12) and has been associated with altered protein kinase C signaling (25), which plays an important role in cellular response to hypoxia (26). OSA is associated with decreased endothelial nitric oxide synthase and increased endothelin-1 levels (27). OSA is also associated with hypercoagulability (increased plasminogen activator inhibitor-1) (28) and inflammation (11). The repetitive episodes of reoxygenation after hypoxemia in patients with OSA simulate ischemia–reperfusion injury, which results in the generation of reactive oxygen species (11, 29). Furthermore, OSA has been recently identified as a “missed” cause in patients with idiopathic peripheral neuropathy (30, 31). The role and importance of hypoxemia is supported by our finding of a correlation between DPN severity and measures of nocturnal hypoxemia as well as the increasing prevalence of DPN with worsening intermittent hypoxemia.
Figure 3.
The postulated mechanisms linking obstructive sleep apnea (OSA) to diabetic peripheral neuropathy (DPN) (and microvascular complications). AGE = advance glycation end-products; ET-1 = endothelin-1; HTN = hypertension; NF-KB = nuclear factor kappa-light-chain-enhancer of activated B cells; NO = nitric oxide; PAI-1 = plasminogen activator inhibitor-1; RNS = reactive nitrogen species; ROS = reactive oxygen species; PKC = protein kinase C; TGF = tissue growth factor; VEGF = vascular endothelial growth factor. For more details, please refer to the text.
In addition, patients with chronic obstructive pulmonary disease (who have sustained hypoxemia) are also known to be at increased risk of peripheral neuropathy (32).
Mild OSA Is Associated with Increased Prevalence of DPN
Mild and moderate to severe OSA, AHI, and nadir nocturnal oxygen saturations were all found to be independently associated with the presence of DPN. In addition, the severity of DPN was found to be associated with OSA severity (as judged by AHI and nocturnal hypoxemia measures). However, there was no increase in DPN (as a binary variable) prevalence between patients with mild and those with moderate and severe OSA. The significant increase of DPN in patients with mild OSA could reflect the relatively long diabetes duration of these subjects, which could amplify the impact of mild OSA/intermittent hypoxemia in vulnerable tissues. Thus, assessing patients with shorter diabetes duration might yield different results. The lack of a further increase in DPN prevalence in patients with AHI greater than or equal to 15 could reflect the small number of patients in that category, the relative insensitivity of the MNSI (compared with nerve electrophysiology) to stage DPN severity, or perhaps a threshold effect of hypoxemia. These issues will need to be explored in larger numbers of patients using a spectrum of quantitative measurements to stage DPN severity.
Sleepiness in Patients with T2DM and OSA
An intriguing finding is that our population was not excessively sleepy as assessed by the Epworth Sleepiness Score; even in patients with OSA, the median score was less than what is considered suggestive of hypersomnolence. This suggests that sleepiness per se cannot be used to case identify OSA in patients with T2DM.
Nitrosative and Oxidative Stress as a Potential Link between OSA and DPN
The higher serum nitrotyrosine and lipid peroxide levels in our patients with DPN is consistent with reports in experimental DPN implicating nitrosative and oxidative stress in DPN pathogenesis (33) by mechanisms including reducing nerve perfusion and impairing vascular reactivity of epineurial arterioles (34, 35). Nitrosative stress also affects all cell types in the peripheral nervous system, including endothelial and Schwann cells of the peripheral nerve, neurons, astrocytes, and oligodendrocytes of the spinal cord, and neurons and glial cells of dorsal root ganglia (36). Nitrosative stress is associated with the development of thermal hyper- and hypoalgesia, mechanical hypoalgesia, tactile allodynia, and small sensory nerve fiber degeneration (35). More recently, the inhibition of nitrosative stress has been shown to result in improvement of experimental neuropathy in diabetic rodent models (37). To our knowledge, this is the first report of an association of OSA with oxidative/nitrosative stress in patients with T2DM. The significant correlation between serum nitrotyrosine and nocturnal/during sleep hypoxemia measures suggests that nitrosative stress is a potential mechanistic link between OSA and DPN. Another report in patients without diabetes showed that endothelial expression of nitrotyrosine correlated with AHI despite adjustment for age and adiposity (27).
OSA and Its Association with Microvascular Function
In parallel, microvascular/endothelial dysfunction have been implicated in the pathogenesis of diabetes-related microvascular complications, including DPN (38, 39). Our data are therefore consistent with a role for impaired microvascular blood flow regulation as a link between OSA with DPN. Patients with OSA had lower blood flow at baseline and after stimulation with acetylcholine and sodium nitroprusside. These differences (with exception of acetylcholine) persisted even when adjusted for maximal vasodilatation, mean arterial pressure, ethnicity, sex, age at diabetes diagnosis, diabetes duration, and BMI. Our data also show that AHI, ODI, and nadir oxygen saturations are also independently associated with microvascular blood flow regulation abnormalities in patients with T2DM (Table E7). The impaired response to sodium nitroprusside might in part be due to impaired response to nitric oxide secondary to oxidative stress (40).
The impact of OSA on microvascular blood flow regulation in patients with T2DM has not previously been reported. Previously, impaired brachial artery flow–mediated dilatation was identified in obese nondiabetic patients with OSA (41). The same report assessed forearm skin microcirculation using laser Doppler flowmetry and also found that OSA was associated with lower baseline blood flow compared with subjects without OSA. However, their findings differed from those reported herein in that the response to acetylcholine and sodium nitroprusside was not impaired by OSA (41). These findings suggest that when OSA is complicated by diabetes there are additional deficits of vasoactive agent metabolism or action, which is believed to mediate the pathogenetic effects of oxidative/nitrosative stress in the development of DPN (42).
Potential Clinical Implications
The data reported herein provide a rationale for further prospective and interventional studies to assess the impact of OSA and its treatment on DPN development and progression in patients with T2DM. To date, trials examining the impact of continuous positive airway pressure (CPAP) in patients with T2DM have mainly focused on metabolic indices. However, the impact of CPAP on diabetes complications is unknown. Although CPAP had a beneficial impact on glycemic indices in some studies (43), others did not show a benefit (44). The association between mild OSA and DPN in this report, if confirmed, may also have implications for the threshold for OSA treatment, because some authorities only offer CPAP treatment in moderate to severe OSA.
Study Limitations
The main limitation of our study is its cross-sectional nature and the lack of an interventional arm; hence, causation cannot be proven. The findings of our observational study, however, should provide the basis for conducting prospective observational and interventional studies in patients with T2DM. We have used home-based portable multichannel respiratory devices rather than inpatient overnight polysomnography. However, this approach is well established and validated (45). The MNSI is not the gold standard for diagnosing or staging DPN, but it has been validated against nerve conduction studies (15, 46) and has been used widely in landmark studies (14, 16, 17, 19). We chose to use the MNSI (in concert with the 10-g monofilament) because it offers the advantage of consisting of robust, meaningful, clinically detectable endpoints.
Conclusions
We have identified a novel association between DPN and OSA in patients with T2DM. In addition, we identified some novel potential mechanistic links, including elevated nitrosative stress in patients with OSA and T2DM that correlated with OSA severity and also abnormal microvascular blood flow regulation in these patients. Prospective studies are required to determine the role of OSA and intermittent hypoxemia in the development and progression of DPN in patients with “early” and advanced diabetes as well as the impact of CPAP treatment on DPN.
Supplementary Material
Acknowledgments
Dr. Abd Tahrani is a research training fellow supported by the National Institute for Health Research. The authors thank Dr. Fahmy Hanna, Dr. Thang Han, Mrs. Helen Hodgson, and Mrs. Rebecca Barakam for their help in recruitment. They also thank the National Institute for Health Research in the UK, the UK Novo Nordisk Research Foundation, and Sanofi Aventis for supporting and funding this project.
Footnotes
Funded by the National Institute for Health Research (UK), the UK Novo Nordisk Research Foundation, and Sanofi Aventis.
The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research, or the Department of Health.
Author Contributions: A.A.T.: conception, design, analysis, interpretation, writing first draft, and final approval. A.A.: design, reviewing draft, and final approval. N.T.R.: statistical analysis and interpretation, reviewing draft, and final approval. S.B.: design, reviewing draft, and final approval. K.D.: design, reviewing draft, and final approval. S.M.: design, reviewing draft, and final approval. B.J.: reviewing draft and final approval. M.K.P.: reviewing draft and final approval. A.H.B.: design, reviewing draft, and final approval. M.J.S.: conception, design, analysis, interpretation, and final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201112-2135OC on June 21, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia 2000;43:957–973 [DOI] [PubMed] [Google Scholar]
- 2.Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, Drash AL, Becker DJ, Kuller LH, Greene DA. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989;38:1456–1461 [DOI] [PubMed] [Google Scholar]
- 3.Partanen J, Niskanen L, Lehtinen J, Mervaala E, Siitonen O, Uusitupa M. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med 1995;333:89–94 [DOI] [PubMed] [Google Scholar]
- 4.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 5.Tesfaye S, Chaturvedi N, Eaton SEM, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH. the EURODIAB Prospective Complications Study Group: Vascular Risk Factors and Diabetic Neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 6.Tahrani AA, Askwith T, Stevens MJ. Emerging drugs for diabetic neuropathy. Expert Opin Emerg Drugs 2010;15:661–683 [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 8.Einhorn D, Stewart DA, Erman MK, Gordon N, Philis-Tsimikas A, Casal E. Prevalence of sleep apnea in a population of adults with type 2 diabetes mellitus. Endocr Pract 2007;13:355–362 [DOI] [PubMed] [Google Scholar]
- 9.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Pi Sunyer FX, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care 2009;32:1017–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc 2008;5:154–160 [DOI] [PubMed] [Google Scholar]
- 11.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 2009;32:447–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan KC, Chow WS, Lam JC, Lam B, Bucala R, Betteridge J, Ip MS. Advanced glycation endproducts in nondiabetic patients with obstructive sleep apnea. Sleep 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 13.Ip MSM, Tse HF, Lam B, Tsang KWT, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med 2004;169:348–353 [DOI] [PubMed] [Google Scholar]
- 14.Factors in development of diabetic neuropathy Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes 1988;37:476–481 [PubMed] [Google Scholar]
- 15.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 16.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care 2006;29:340–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyraz O, Saracoglu M. The effect of obesity on the assessment of diabetic peripheral neuropathy: a comparison of Michigan patient version test and Michigan physical assessment. Diabetes Res Clin Pract 2010;90:256–260 [DOI] [PubMed] [Google Scholar]
- 19.Pambianco G, Costacou T, Strotmeyer E, Orchard TJ. The assessment of clinical distal symmetric polyneuropathy in type 1 diabetes: A comparison of methodologies from the Pittsburgh Epidemiology of Diabetes Complications Cohort. Diabetes Res Clin Pract 2011;92:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007; Westchester, IL: American Academy of Sleep Medicine [Google Scholar]
- 21.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–276 [PMC free article] [PubMed] [Google Scholar]
- 22.Roustit M, Millet C, Blaise S, Dufournet B, Cracowski JL. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res 2010;80:505–511 [DOI] [PubMed] [Google Scholar]
- 23.Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci 2006;27:503–508 [DOI] [PubMed] [Google Scholar]
- 24.de Wytt CN, Jackson RV, Hockings GI, Joyner JM, Strakosch CR. Polyneuropathy in Australian outpatients with type II diabetes mellitus. J Diabetes Complications 1999;13:74–78 [DOI] [PubMed] [Google Scholar]
- 25.Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKC{delta}. Am J Physiol Heart Circ Physiol 2008;294:H920–H927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen JL, Lin HH, Kim KJ, Lin A, Ou JH, Ann DK. PKC delta signaling: a dual role in regulating hypoxic stress-induced autophagy and apoptosis. Autophagy 2009;5:244–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH. Vascular inflammation in obesity and sleep apnea. Circulation 2010;121:1014–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet-function and fibrinolytic-activity in hypertensive and normotensive sleep-apnea. Sleep 1995;18:188–194 [DOI] [PubMed] [Google Scholar]
- 29.Lavie L. Oxidative stress: a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis 2009;51:303–312 [DOI] [PubMed] [Google Scholar]
- 30.de Seze J. Obstructive sleep apnoea: an underestimated cause of peripheral neuropathy. J Neurol Neurosurg Psychiatry 2007;78:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lüdemann P, Dziewas R, Sörös P, Happe S, Frese A. Axonal polyneuropathy in obstructive sleep apnoea. J Neurol Neurosurg Psychiatry 2001;70:685–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oncel C, Sevin B, Cam M, Akdag B, Taspinar B, Evyapan F. Peripheral neuropathy in chronic obstructive pulmonary disease. COPD 2010;7:11–16 [DOI] [PubMed] [Google Scholar]
- 33.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy. Diabetes 2005;54:3435–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obrosova IG, Mabley JG, Zsengellér Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J 2005;19:401–403 [DOI] [PubMed] [Google Scholar]
- 35.Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG. Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int J Mol Med 2009;23:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drel VR, Lupachyk S, Shevalye H, Vareniuk I, Xu W, Zhang J, Delamere NA, Shahidullah M, Slusher B, Obrosova IG. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-{alpha}. Endocrinology 2010;151:2547–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stavniichuk R, Drel VR, Shevalye H, Maksimchyk Y, Kuchmerovska TM, Nadler JL, Obrosova IG. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative/nitrosative stress and p38 MAPK activation. Exp Neurol 2011;230:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Y, Suo L, Yu H, Wang C, Tang H. Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Diabetes Res Clin Pract 2004;65:95–104 [DOI] [PubMed] [Google Scholar]
- 39.Quattrini C, Harris ND, Malik RA, Tesfaye S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care 2007;30:655–659 [DOI] [PubMed] [Google Scholar]
- 40.Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med 2008;18:109–116 [DOI] [PubMed] [Google Scholar]
- 41.Yim-Yeh S, Rahangdale S, Nguyen ATD, Stevenson E, Novack V, Veves A, Malhotra A. vascular dysfunction in obstructive sleep apnea and type 2 diabetes mellitus. Obesity (Silver Spring) 2011;19:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pop-Busui R, Marinescu V, Van Huysen C, Li F, Sullivan K, Greene DA, Larkin D, Stevens MJ. Dissection of metabolic, vascular, and nerve conduction interrelationships in experimental diabetic neuropathy by cyclooxygenase inhibition and acetyl-l-carnitine administration. Diabetes 2002;51:2619–2628 [DOI] [PubMed] [Google Scholar]
- 43.Dawson A, Abel SL, Loving RT, Dailey G, Shadan FF, Cronin JW, Kripke DF, Kline LE. CPAP therapy of obstructive sleep apnea in type 2 diabetics improves glycemic control during sleep. J Clin Sleep Med 2008;4:538–542 [PMC free article] [PubMed] [Google Scholar]
- 44.West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007;62:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep 1997;20:1077–1085 [PubMed] [Google Scholar]
- 46.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg 2006;108:477–481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.