Abstract
Rationale: Although commonly used as the primary outcome measure of clinical trials in pulmonary arterial hypertension (PAH), the minimal important difference (MID) of the 6-minute walk test (6MWT) has not been well defined for this population of patients.
Objectives: To estimate the MID in the 6MWT in patients with PAH.
Methods: Study subjects from the clinical trial of tadalafil in PAH, a 16-week, parallel-group, randomized clinical trial of patients who were treatment naive or on background therapy with an endothelin receptor antagonist, were eligible. 6MWT was performed using a standardized protocol. Distributional and anchor-based methods were used to estimate the MID; the latter method used the Physical Component Summary Score (PCS) of the Medical Outcomes Study 36-item short form (SF-36).
Measurements and Main Results: Four hundred five subjects were analyzed. Domains of the SF-36 were weakly to modestly associated with 6MWT. Change in the PCS of the SF-36 was most strongly associated with change in 6MWT (r = 0.40, P < 0.001) and thus was selected as the anchor for subsequent anchor-based analyses. Distributional analyses yielded estimates of the MID ranging from 25.1 to 38.5 m, whereas anchor-based analyses yielded an estimate of 38.6 m.
Conclusions: Using both distributional and anchor-based methods, the estimated consensus MID in the 6MWT for PAH is approximately 33 m. These results have important implications for (1) assessing treatment responses from clinical trials and metaanalyses of specific PAH therapy, and (2) sample size calculations for future study design.
Keywords: pulmonary hypertension, outcome measures, 6-minute walk test, minimal important difference
At a Glance Commentary
Scientific Knowledge on the Subject
Although commonly used as the primary outcome measure for clinical trials in pulmonary arterial hypertension (PAH), little is known about clinically relevant changes in the 6-minute walk test (6MWT).
What This Study Adds to the Field
This study reports the minimal important difference for the 6MWT in PAH, thereby providing a framework for assessing clinical response to therapy and for planning future clinical trials.
Pulmonary arterial hypertension (PAH) comprises a group of clinical disorders characterized by progressive increase in pulmonary artery pressure that often leads to right ventricular failure and death (1). Despite recent advances in therapies for PAH, mortality remains high (2). Although improvements in survival remain the ultimate goal of therapy for PAH, alternate outcome measures have been used to assess efficacy of medications. The 6-minute walk test (6MWT) has been used as a primary outcome measure in many studies of various forms of PAH (3–9). Based on the cumulative data regarding 6MWT in PAH, regulatory agencies currently accept exercise capacity as a primary outcome measure for clinical trials in PAH. Furthermore, these regulatory bodies have approved pharmacologic agents for PAH therapy based on small but statistically significant differences in 6-minute walk distances (6MWD) between treatment and placebo arms. A recent metaanalysis of randomized controlled trials of PAH therapy in patients with pulmonary hypertension reported a weighted mean improvement in 6MWT in subjects in the treatment arm of 42.8 m (range, 10–93 m; 95% confidence interval [CI], 27.8–57.8 m) (10). However, despite its widespread use in clinical trials of PAH, the minimal important difference (MID) of the 6MWT in patients with PAH has not been thoroughly evaluated (11, 12).
The MID is the smallest change or difference in an outcome measure, perceived as beneficial, that would justify a change in the patient’s medical management (13). Although the MID has been determined for the 6MWT in the chronic obstructive pulmonary disease (COPD) population (14, 15), the congestive heart failure population (16), and idiopathic pulmonary fibrosis population (17), this parameter has been reported in only one group of patients with PAH (18). In that study, only distributional-based methods were used to determine the MID; these methods rely solely on statistical methodology and do not include the relationship between patient-important outcome measures, such as health-related quality of life (19). Furthermore, MID estimates derived from distributional-based methods differ sometimes substantially across different methods (20).
With the many new therapeutic agents available for potential treatment of pulmonary hypertension, it is important to establish an MID in the most widely used outcome measure, the 6MWT. The MID for the 6MWT in PAH could be used to assess response to therapy in clinical trials (21). In addition to reporting the mean difference in 6MWT, investigators could report the proportion of subjects who demonstrated clinically relevant responses to therapy. This would provide more pertinent information regarding the efficacy of the intervention and allow analysis of clinical characteristics of responders. Importantly, the MID of the 6MWT is also intended to be used to determine sample size in the design of future interventional trials, thereby ensuring the most adequately powered study design. Therefore, we sought to determine the MID for the 6MWT in patients with PAH, using data from the large clinical trial of tadalafil in PAH (5), using both anchor-based and distributional methods.
Methods
The Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) trial was a double-masked, placebo-controlled study of 405 patients with PAH who were either treatment naive or on background therapy with the endothelin receptor antagonist bosentan. Patients were randomized to receive either tadalafil 2.5, 10, 20, or 40 mg orally once daily or placebo for 16 weeks (5). The primary study outcome was change from baseline to week 16 in 6MWD. Secondary outcome measures included quality of life assessed by the Medical Outcomes Study 36-item short form version 2 (SF-36); this was collected at baseline and at Week 16 (22). The 6MWT was performed according to consensus guidelines (23). A practice test was required as part of the study protocol.
The SF-36 is a generic instrument used to assess health-related quality of life (24). The SF-36 has been validated and demonstrated responsiveness to change in a variety of patient groups and is commonly used in PAH clinical trials (25, 26). Because the Physical Component Summary Score (PCS) has been shown to be the most responsive parameter of the SF-36 to change in patients with chronic lung disease, the PCS was selected as the anchor for anchor-based analyses, as discussed below and in the online supplement (27).
Estimation of the Minimally Important Difference
Both anchor-based and distributional methods for determining the MID were used. The anchor-based methods for determination of the MID use measures for which an MID has been established (the anchor) to estimate the MID for another metric. Furthermore, the anchor must have a relatively strong linear relationship with the metric of interest; therefore, PCS was chosen as the anchor (28). Prior studies of the PCS in chronic diseases have defined the MID as 5 units (22). The numerical value of the MID for the 6MWD was then determined for PCS MID of 5 units using the linear regression of change in PCS against change in 6MWD.
The distributional methods used are: (1) effect size (ES), (2) standardized response mean (SRM), (3) standard error of the measurement (SEMeas), and (4) 0.5 times the SD of the baseline measure (0.5 SD). ES is defined as the average of the difference between end-of-treatment and baseline scores divided by the SD of the baseline scores (29). The SRM uses the SD of the change of the measure and therefore accounts for the covariance between the baseline and end-of-study measures (30). The SEMeas was calculated by multiplying the SD of the baseline measurement by the square root of the difference of 1 minus the intraclass coefficient of the measure (31). Additionally, we used another distributional method, 0.5 SD, which simply involves multiplying the SD of the baseline measurement by 0.5 (32).
These estimates of MID were then triangulated to generate a clinically and statistically relevant measure of change in 6MWD. This methodology, as described by Leidy and Wyrwich, involves the synthesis of qualitative and quantitative data from both a statistical and clinical perspective and is the logical extension of using multiple methods of estimation of the MID in this study (33).
Results
As shown in Table 1, 405 subjects who completed the PHIRST trial were included in this analysis. The majority of subjects were white women who were, on average, 53 years of age. Most had idiopathic PAH (IPAH, 60.8%), but approximately one-quarter had PAH related to connective tissue disease (CTD-PAH). Nearly two-thirds of the subjects were World Health Organization (WHO) functional class III, and one-third were WHO functional class II; a minority were in functional classes I and IV. Hemodynamics revealed moderate to severe PAH, with a mean pulmonary artery pressure of 54 ± 13 mm Hg, a mean cardiac index of 2.6 ± 0.7 L/min/m2, and a mean pulmonary vascular resistance (PVR) of 11.1 ± 5.2 Wood units. The mean 6MWD was 343.2 ± 76.8 m, suggesting moderate functional impairment. The change in 6MWD between baseline and end-of-study was 33 m (95% CI, 15–50 m).
TABLE 1.
CHARACTERISTICS OF STUDY POPULATION
| Placebo | Tadalafil 2.5 mg | Tadalafil 10 mg | Tadalafil 20 mg | Tadalafil 40 mg | Overall | |
| Age, yr | 55 (15) | 54 (16) | 53 (15) | 52 (15) | 53 (15) | 54 (15) |
| Women, n (%) | 65 (79) | 64 (78) | 68 (84) | 62 (76) | 59 (75) | 318 (78) |
| White, n (%) | 73 (88) | 65 (80) | 65 (80) | 61 (76) | 64 (82) | 327 (81) |
| PAH etiology, n (,%) | ||||||
| Idiopathic | 54 (66) | 45 (55) | 52 (64) | 50 (61) | 46 (58) | 247 (61) |
| Collagen vascular | 16 (20) | 16 (20) | 24 (30) | 21 (26) | 19 (24) | 96 (24) |
| Anorexigen | 2 (2) | 5 (6) | 1 (1) | 4 (5) | 4 (5) | 16 (4) |
| ASD/surgical | 10 (12) | 16 (20) | 4 (5) | 7 (8) | 10 (13) | 47 (11) |
| WHO FC baseline, n (%) | ||||||
| I | 1 (1) | 1 (1) | 0 (0) | 0 (0) | 2 (3) | 4 (1) |
| II | 23 (28) | 29 (35) | 24 (30) | 28 (34) | 26 (33) | 130 (32) |
| III | 56 (68) | 49 (60) | 55 (68) | 54 (66) | 51 (65) | 265 (65) |
| IV | 2 (2) | 3 (4) | 2 (2) | 0 (0) | 0 (0) | 7 (2) |
| 6MWT baseline, m | 343 (84) | 347 (71) | 336 (77) | 337 (74) | 352 (78) | 343 (77) |
| Mean Δ 6MWT, m | 8.7 (60.2) | 24.6 (61.6) | 29.9 (63.0) | 37.1 (47.8) | 41.9 (49.3) | 28.5 (57.5) |
| RAP, mm Hg | 7 (4) | 8 (4) | 9 (6) | 7 (4) | 8 (4) | 8 (4) |
| Mean PAP, mm Hg | 49 (12) | 55 (14) | 51 (16) | 58 (12) | 54 (8) | 54 (13) |
| Cardiac index, L/min/m2 | 2.3 (0.6) | 2.4 (0.7) | 2.5 (0.8) | 2.7 (0.6) | 2.7 (1.0) | 2.5 (0.7) |
| PCWP, mm Hg | 9 (3) | 11 (7) | 10 (5) | 9 (4) | 9 (4) | 10 (5) |
| PVR, Wood units | 11 (5) | 11 (5) | 11 (6) | 12 (5) | 11 (6) | 11 (5) |
| Concomitant bosentan use, n (%) | 45 (55) | 43 (52) | 41 (51) | 45 (55) | 42 (53) | 216 (53) |
Definition of abbreviations: 6MWT = 6-min walk test; ASD = atrial septal defect; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; surgical = PAH after surgical repair of congenital systemic to pulmonary shunts; WHO FC = World Health Organization functional class.
All values presented are mean (SD) unless otherwise specified.
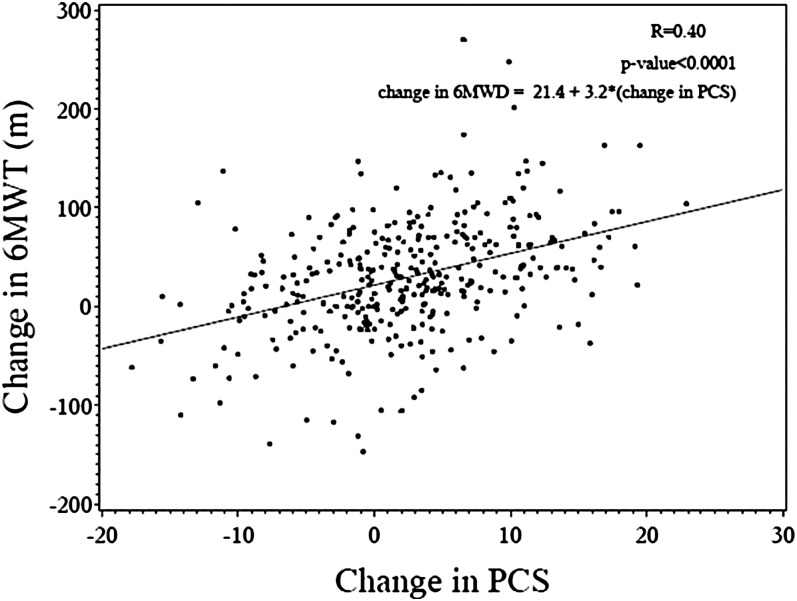
SF-36 data for each domain and the PCS and mental component summary score (MCS) are presented in the PHIRST study (5). On average, there were improvements in each of the eight domains and summary scores comparing baseline to end-of-study assessments. As shown in Figure 1, the relationship between change in PCS and change in 6MWD was moderate, with an r = 0.40 (P < 0.001). Other relationships between SF-36 domains and 6MWD were examined (data not shown); PCS demonstrated the strongest association and therefore was selected as the anchor for subsequent analyses (34).
Figure 1.
Change in 6-minute walk distance (6MWD) versus change in Physical Component Summary (PCS) score. Linear regression of change in 6MWD on change in PCS of the Medical Outcomes Study 36-item short form for subjects in the Pulmonary Arterial Hypertension and Response to Tadalafil trial, including regression equation.
Data, including demographic, functional class, 6MWD, and SF-36 at baseline, were available on 405 subjects who were included in the distributional analyses. Baseline and end-of-study 6MWT and SF-36 were available on 348 subjects who were ultimately included in the anchor-based analyses. There were no significant differences in the demographic, functional, or hemodynamic characteristics between these groups.
MID Calculations
Table 2 shows the point estimates for the MID for the 6MWT using the anchor-based, ES, SRM, SEMeas, and 0.5 SD methods. The anchor-based analysis using a change in PCS of 5 units yielded an estimate of 38.6 m. ES analyses corresponding to moderate (0.5) effect sizes yielded an estimate of 38.4 m; with SRM-based methods, the estimate was 28.8 m. SEMeas estimate was 25.1 m, and the estimate calculated using 0.5 SD resulted in a value of 38.5 m. The consensus of these values led to an estimated MID of the 6MWT in PAH of 33 m.
TABLE 2.
ANCHOR- AND DISTRIBUTIONAL-BASED ESTIMATES OF THE MINIMAL IMPORTANT DIFFERENCE FOR THE 6-MINUTE WALK TEST
| Method | MID Estimate (m) |
| Anchor method (MID PCS = 5) | 38.6 |
| ES [Eos − baseline/SD(bl)] | 38.4 |
| SRM [Eos − baseline/SD(Eos − baseline)] | 28.8 |
| SEMeas [SD(baseline) × ] | 25.1 |
| 0.5 SD [0.5 × SD of baseline 6MWT] | 38.5 |
Definition of abbreviations: 6MWT = 6-minute walk test; ICC = intraclass correlation coefficient; Eos = end of study; ES = effect size; MID = minimal important difference; PCS = physical component summary score; SEMeas = standard error of the measurement; SRM = standardized response mean.
Table 3 shows the point estimates for the MID for the 6MWT for certain subgroups in the study. Overall, the estimates for individual subgroups were similar to the estimates for the overall cohort (range, from 24.4–40.6 m); for instance, no significant differences were noted between estimates for the treatment-naive group and the background-therapy group. Although the anchor-based estimate for the CTD group was smaller than other subgroup estimates (24.4 m), this estimate remained in the range of estimates from the overall cohort (Table 1).
TABLE 3.
ANCHOR- AND DISTRIBUTIONAL-BASED ESTIMATES OF THE MINIMAL IMPORTANT DIFFERENCE FOR PULMONARY ARTERIAL HYPERTENSION SUBGROUPS
| R value* (Δ6MWD vs. ΔPCS) | Distributional (m) |
||||||
| Subgroup | Baseline 6MWD | Change 6MWD | Anchor (m) | ES | SRM | 0.5 SD | |
| IPAH (n = 246) | 346 (76.6) | 30.2 (59.3) | 0.32 | 39.0 | 38.3 | 29.6 | 38.3 |
| CVD-PAH (n = 96) | 326 (81.2) | 12.4 (51.5) | 0.51 | 24.4 | 40.6 | 25.7 | 40.6 |
| Treatment naive (n = 177) | 339 (78.1) | 25.5 (60.8) | 0.47 | 38.7 | 39.0 | 30.4 | 39.1 |
| Background therapy (n = 197) | 354 (74.9) | 31.2 (54.4) | 0.33 | 36.4 | 37.4 | 27.2 | 37.4 |
Definition of abbreviations: 6MWT = 6-minute walk test; ES = effect size; MID = minimal important difference; PCS = physical component summary score; SRM = standardized response mean.
All results presented as mean (SD) unless otherwise specified.
All P values < 0.0001.
Discussion
In this study, we report several estimates of the MID for the 6MWT for PAH. To our knowledge, this is the first report of the MID for the 6MWT in PAH using both anchor-based and distributional methods. Our data show that estimates of MID for the 6MWT are remarkably similar between these methods. The consistency of the estimates derived from different methods supports the validity and robustness of this estimate of the MID. Based on these findings, we estimate the MID of the 6MWT in PAH to be approximately 33 m.
Determination of an MID for an outcome measure is an integral component in the validation of that measure in a particular disease state and in the elucidation of the distinction between statistical and clinical significance. Because sample size heavily influences the power to detect a statistically significant change in the outcome of interest in any study (i.e., the larger the sample size, the smaller the statistically significant change that can be detected), assessing the clinical relevance of the minimum detectable difference is of paramount importance when designing and reporting the results of clinical trials. However, despite its importance, few parallel-group randomized clinical trials across the spectrum of human disease report the MID for the respective outcome measures used (35). To our knowledge, only two prior studies in PAH have provided estimates of MID for clinically relevant outcome measures: stroke volume by cardiac magnetic resonance imaging (36), SF-36 (18), and 6MWT (18).
The estimate of the MID for the 6MWT in PAH reported in the current study differs slightly from the prior study of patients with PAH (18), which reported an MID of 41 m (range, 18.7–74.15 m). Similar to the current study, Gilbert and colleagues (18) used a large, randomized controlled study (the SUPER trial [4]) of sildenafil therapy in PAH. The patient populations included in the SUPER and PHIRST trials had similar proportions of IPAH and APAH subjects, similar distribution of WHO functional class, and similar disease severity as assessed by baseline 6MWD and hemodynamics. Thus, perhaps it is to be expected that the estimates were similar between studies. For instance, when compared with other randomized, double-masked, parallel group studies in PAH, distributional estimates of the MID, calculated by 0.5 times SD of the baseline 6MWD, fall within a range of 35.7 to 45 m (data not shown) (4–9, 37).
The estimate of MID for the 6MWT in PAH is remarkably similar to estimates of the MID for the 6MWT in other disease states (Table 4). Using the National Emphysema Treatment Trial data, Puhan and colleagues described an MID of around 26 m, derived by both anchor-based and distributional methods (14). Holland and colleagues found an MID estimate of 25 m using similar methodology in a smaller cohort of patients with COPD who participated in pulmonary rehabilitation (38). Recently, du Bois and colleagues reported an MID ranging from 24 to 45 m in the 6MWT for patients with idiopathic pulmonary fibrosis, again comparable to the current estimates for MID in patients with PAH (17). Thus, even the anchor-based estimate of MID for the CTD group in the current study (24.4 m), despite differing quantitatively from the other subgroup estimates in the stratified analyses, falls within a range of expected variability. Whether the consistency of these MID estimates across pulmonary disease states reflects an intrinsic characteristic of response to any disease or similarities in the functional limitations independent of disease pathogenesis is not known.
TABLE 4.
MINIMAL IMPORTANT DIFFERENCE ESTIMATES FOR THE 6-MINUTE WALK TEST IN OTHER CHRONIC CARDIOPULMONARY DISEASES
| Method and Estimate (m) |
||||
| Study Population | Reference Number | Distributional | Anchor | Other |
| COPD | 14* | 25.7 to 30.6 | 18.9 to 26.4 | |
| COPD | 38† | 25.5 to 26.5 | 24.5 | |
| COPD | 34‡ | 29 to 42 | ||
| COPD | 15§ | 54 (37 to 71) | ||
| IPF | 17║ | 45 (42 to 47) | 24 | |
| DPLD | 42** | 33 | 30.5 (19 to 45) | |
| CHF | 16†† | −43 (−48.6 to −47) | ||
| Elderly | 43‡‡ | |||
| Small meaningful change | 19 to 22 | −21 to −54 | ||
| Substantial change | 47 to 49 | Unable to estimate | ||
Definition of abbreviations: CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; DPLD = diffuse parenchymal lung disease; IPF = idiopathic pulmonary fibrosis; NETT = National Emphysema Treatment Trial.
From NETT study; severe COPD only.
Small cohort (n = 44); anchor was patient-reported change.
Poor correlations between 6MWT and patient-reported outcomes precluded anchor-based analyses; study cohort included patients with both moderate and severe COPD.
Patient-reported global rating of change; comparisons to other patients.
Large cohort; criterion reference used for anchor-based analysis.
Small cohort (n = 48); anchor was patient-reported change in symptoms.
Small cohort (n = 45); patient-reported global rating of change; effect sizes larger for deterioration than improvement.
Cohort of elderly in strength training trial, subacute stroke survivors, or prospective community-based study; determined both smallest meaningful change and substantial change based on standard error of the measurement, ES = 0.2, and ES = 0.5.
The current study used several distributional-based methods and an anchor-based method to estimate the MID in an effort to generate a robust and reliable estimate; however, there is no consensus about which method or quantity of change in the construct of a particular method truly represents the MID. There remain strengths and weaknesses of each of the methods used (19, 28). In the current study, the estimates derived from the distributional methods varied based on the SD of the parameter used in the calculations. The ES method uses the SD of the baseline 6MWT, whereas the SRM method uses the SD of the change in 6MWT (end-of-study minus baseline). Because the variation of the baseline 6MWT is larger than the variation of the change in 6MWT over time, the ES method will yield a larger MID estimate, as it does in the current study. However, because an intervention study using an exercise measure such as 6MWT is intended to interpret changes over time, the SRM method is likely more appropriate (34).
Anchor-based estimates of MID use an external clinical or patient-based measure to group patients by magnitude of response: no change, small, moderate, or large positive (or negative) changes (19). The anchor chosen should be relevant to the disease and have clinical usefulness in the disease state. In the current study, we investigated the relationship between change in each of the eight domains of the SF-36 along with the summary scores (PCS and MCS). The strongest correlation between parameters of the SF-36 and 6MWT was found with PCS; this parameter has particular relevance in PAH as the primary symptom in the disease is dyspnea on exertion. Furthermore, preliminary data suggest an independent relationship between PCS and survival in PAH (39). Therefore, PCS appears to have both face validity and clinical usefulness in PAH and, thus, was an appropriate anchor for this analysis.
Limitations
Despite the use of multiple methods to estimate the MID for the 6MWT in this study, several limitations exist. First, although PCS has face validity and clinical usefulness in PAH, as a generic measure of health-related quality of life, it is less responsive to change than disease-specific measures (27). However, among the parameters of the SF-36 and other metrics, such as preference-based instruments, the PCS demonstrates the most responsiveness to change in chronic lung disease (27). To account for this diminished responsiveness, we chose to examine effect sizes of a moderate magnitude (change in PCS = 5 units). Thus, we expect the MID estimate derived from the anchor-based method adequately represents the MID, despite the limitations of the PCS as an anchor. Use of additional patient-reported outcomes to use as anchors would be ideal, but were not available in this dataset or did not have an established MID to use as an anchor (20). The US Food and Drug Administration has presented results of a pooled analysis of 13 randomized, double-masked, parallel group studies in PAH that correlates change in 6MWD with change in PVR index (PVRI), showing that change in PVRI is moderately associated with change in 6MWD (40). However, the clinical significance of change in PVR (or PVRI), specifically related to a patient-important outcome, has recently been drawn into question. van de Veerdonk and colleagues reported that although baseline PVR predicted outcome in a large cohort of patients with IPAH, change in PVR over time was not related to survival (41). Furthermore, there are no data from randomized trials relating changes in hemodynamic parameters to changes in quality of life. Therefore, the usefulness of determining an MID for a surrogate parameter such as PVR may be limited, in particular if the surrogate correlates poorly with the outcome that it is supposed to represent. Third, the study cohort was predominantly composed of patients with New York Heart Association functional class II or III disease. This limited sample may impact the estimate derived from distributional analyses, as the SD for the 6MWT may be smaller than that of a cohort that includes patients from functional classes I through IV and thus limit the generalizability of this estimate. However, because the SD of the change in 6MWT (for the SRM method) was used, this particular limitation may be mitigated.
Conclusions
Using both anchor-based and distributional-based methods, the estimated MID for the 6MWT in patients with PAH is around 33 m. The anchor-based method and distributional-based estimates were very similar and comparable to estimates of MID for other chronic lung diseases. This MID estimate provides a strong basis from which clinical trials of PAH-specific therapy can be interpreted and future interventional studies can be planned.
Supplementary Material
Footnotes
Supported by National Heart, Lung, and Blood Institute grant K23 HL093387 (S.C.M.).
Author Contributions: S.C.M.: conception/design, analysis and interpretation of data, drafting and revising manuscript, final approval of manuscript; M.A.P.: conception/design, analysis and interpretation of data, revision and final approval of manuscript; D.L.: analysis and interpretation of data, revision and final approval of manuscript; R.A.W.: conception/design, analysis and interpretation of data, revision and final approval of manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201203-0480OC on June 21, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chin KM, Kim NH, Rubin LJ. The right ventricle in pulmonary hypertension. Coron Artery Dis 2005;16:13–18 [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573–1619 [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Oudiz RJ, Beardsworth A, Brundage BH, Simonneau G, Ghofrani HA, Sundin DP, Galie N. Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2011;30:632–643 [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157 [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009;119:2894–2903 [DOI] [PubMed] [Google Scholar]
- 6.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903 [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008;149:521–530 [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165:800–804 [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008;117:3010–3019 [DOI] [PubMed] [Google Scholar]
- 10.Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J 2007;153:1037–1047 [DOI] [PubMed] [Google Scholar]
- 11.Macchia A, Mariani J, Comignani PD, Tognoni G. Clinical trials using vasodilators in pulmonary arterial hypertension: where do we go from here? Rev Recent Clin Trials 2011;6:228–234 [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Badesch DB, Delcroix M, Fleming TR, Gaine SP, Galie N, Gibbs JS, Kim NH, Oudiz RJ, Peacock A, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54:S97–S107 [DOI] [PubMed] [Google Scholar]
- 13.Beaton DE, Boers M, Wells GA. Many faces of the minimal clinically important difference (MCID): a literature review and directions for future research. Curr Opin Rheumatol 2002;14:109–114 [DOI] [PubMed] [Google Scholar]
- 14.Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN, Wise RA, Sciurba F. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011;37:784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997;155:1278–1282 [DOI] [PubMed] [Google Scholar]
- 16.O’Keeffe ST, Lye M, Donnellan C, Carmichael DN. Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart 1998;80:377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med 2011;183:1231–1237 [DOI] [PubMed] [Google Scholar]
- 18.Gilbert C, Brown MC, Cappelleri JC, Carlsson M, McKenna SP. Estimating a minimally important difference in pulmonary arterial hypertension following treatment with sildenafil. Chest 2009;135:137–142 [DOI] [PubMed] [Google Scholar]
- 19.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 2008;61:102–109 [DOI] [PubMed] [Google Scholar]
- 20.Turner D, Schunemann HJ, Griffith LE, Beaton DE, Griffiths AM, Critch JN, Guyatt GH. The minimal detectable change cannot reliably replace the minimal important difference. J Clin Epidemiol 2010;63:28–36 [DOI] [PubMed] [Google Scholar]
- 21.Man-Son-Hing M, Laupacis A, O'Rourke K, Molnar FJ, Mahon J, Chan KB, Wells G. Determination of the clinical importance of study results. J Gen Intern Med 2002;17:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinksi MA, Dewey JE. How to score version 2 of the SF-36 Health Survey. Lincoln, RI: QualityMetric, Inc. 2000.
- 23.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117 [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care 1994;32:40–66 [DOI] [PubMed] [Google Scholar]
- 25.Pepke-Zaba J, Beardsworth A, Chan M, Angalakuditi M. Tadalafil therapy and health-related quality of life in pulmonary arterial hypertension. Curr Med Res Opin 2009;25:2479–2485 [DOI] [PubMed] [Google Scholar]
- 26.Pepke-Zaba J, Gilbert C, Collings L, Brown MC. Sildenafil improves health-related quality of life in patients with pulmonary arterial hypertension. Chest 2008;133:183–189 [DOI] [PubMed] [Google Scholar]
- 27.Puhan MA, Guyatt GH, Goldstein R, Mador J, McKim D, Stahl E, Griffith L, Schunemann HJ. Relative responsiveness of the Chronic Respiratory Questionnaire, St. Georges Respiratory Questionnaire and four other health-related quality of life instruments for patients with chronic lung disease. Respir Med 2007;101:308–316 [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002;77:371–383 [DOI] [PubMed] [Google Scholar]
- 29.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care 1989;27:S178–S189 [DOI] [PubMed] [Google Scholar]
- 30.Zou GY. Quantifying responsiveness of quality of life measures without an external criterion. Qual Life Res 2005;14:1545–1552 [DOI] [PubMed] [Google Scholar]
- 31.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861–873 [DOI] [PubMed] [Google Scholar]
- 32.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–592 [DOI] [PubMed] [Google Scholar]
- 33.Leidy NK, Wyrwich KW. Bridging the gap: using triangulation methodology to estimate minimal clinically important differences (MCIDs). COPD 2005;2:157–165 [DOI] [PubMed] [Google Scholar]
- 34.Puhan MA, Mador MJ, Held U, Goldstein R, Guyatt GH, Schunemann HJ. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur Respir J 2008;32:637–643 [DOI] [PubMed] [Google Scholar]
- 35.Chan KB, Man-Son-Hing M, Molnar FJ, Laupacis A. How well is the clinical importance of study results reported? An assessment of randomized controlled trials. CMAJ 2001;165:1197–1202 [PMC free article] [PubMed] [Google Scholar]
- 36.van Wolferen SA, van de Veerdonk MC, Mauritz GJ, Jacobs W, Marcus JT, Marques KM, Bronzwaer JG, Heymans MW, Boonstra A, Postmus PE, et al. Clinically significant change in stroke volume in pulmonary hypertension. Chest 2011;139:1003–1009 [DOI] [PubMed] [Google Scholar]
- 37.Humbert M, Barst RJ, Robbins IM, Channick RN, Galie N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004;24:353–359 [DOI] [PubMed] [Google Scholar]
- 38.Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010;91:221–225 [DOI] [PubMed] [Google Scholar]
- 39.Mathai SC, Bueso M, Lechtzin N, Boyce D, Singh S, Zaiman A, Girgis R, Hassoun P. The impact of health-related quality of life on outcomes in pulmonary arterial hypertension [abstract]. Am J Respir Crit Care Med 2010;181:A4829 [Google Scholar]
- 40.2010 Meeting Materials, Cardiovascular and Renal Drugs Advisory Committee [accessed 2012 April 16]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm192863.htm
- 41.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511–2519 [DOI] [PubMed] [Google Scholar]
- 42.Holland AE, Hill CJ, Conron M, Munro P, Mc Donald CF. Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir Med 2009;103:1430–1435 [DOI] [PubMed] [Google Scholar]
- 43.Perera S, Mody AH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.