Abstract
Rationale: Bronchopulmonary dysplasia (BPD) is a major complication of premature birth. Risk factors for BPD are complex and include prenatal infection and O2 toxicity. BPD pathology is equally complex and characterized by inflammation and dysmorphic airspaces and vasculature. Due to the limited availability of clinical samples, an understanding of the molecular pathogenesis of this disease and its causal mechanisms and associated biomarkers is limited.
Objectives: Apply genome-wide expression profiling to define pathways affected in BPD lungs.
Methods: Lung tissue was obtained at autopsy from 11 BPD cases and 17 age-matched control subjects without BPD. RNA isolated from these tissue samples was interrogated using microarrays. Standard gene selection and pathway analysis methods were applied to the data set. Abnormal expression patterns were validated by quantitative reverse transcriptase–polymerase chain reaction and immunohistochemistry.
Measurements and Main Results: We identified 159 genes differentially expressed in BPD tissues. Pathway analysis indicated previously appreciated (e.g., DNA damage regulation of cell cycle) as well as novel (e.g., B-cell development) biological functions were affected. Three of the five most highly induced genes were mast cell (MC)-specific markers. We confirmed an increased accumulation of connective tissue MCTC (chymase expressing) mast cells in BPD tissues. Increased expression of MCTC markers was also demonstrated in an animal model of BPD-like pathology.
Conclusions: We present a unique genome-wide expression data set from human BPD lung tissue. Our data provide information on gene expression patterns associated with BPD and facilitated the discovery that MCTC accumulation is a prominent feature of this disease. These observations have significant clinical and mechanistic implications.
Keywords: microarray, tryptase, chymase, carboxypeptidase A3, bronchopulmonary dysplasia
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Bronchopulmonary dysplasia (BPD) is a major complication of premature birth, particularly for infants born at less than 32 weeks of gestation or weighing less than 1500 g. The overall cost of treating infants with BPD in the United States is estimated to be $2.4 billion, second only for pediatric lung disease to the cost for treating asthma. Due to the relatively limited availability of clinical samples, an understanding of the molecular pathogenesis of this disease and its causal mechanisms and disease-associated biomarkers is limited.
What This Study Adds to the Field
We present a comprehensive data set describing gene expression changes in the lung tissue of subjects with BPD. These studies improve our understanding of the molecular pathogenesis of BPD, and reveal that accumulation of connective tissue mast cells is a significant feature of disease pathology.
Bronchopulmonary dysplasia (BPD) is a lung disease of the premature infant defined by dependence on supplemental oxygen for more than 28 days post-partum and/or at 36 weeks corrected gestational age. In the United States, more than 500,000 babies are born prematurely each year, with approximately 60,000 at high risk for BPD (< 1,500 g) and 10,000 diagnosed with this disease (1). The overall cost of treating infants with BPD in the United States were estimated to be $2.4 billion annually, second only for pediatric lung disease to the costs for treating asthma, and far exceeding the cost of treating cystic fibrosis (2). The incidence of BPD has increased over the last 30 years, largely due to higher survival rates of premature babies. Unfortunately, no effective therapies, other than those providing symptomatic relief, are currently available.
Despite major advances in neonatal medicine, including surfactant therapy and the application of gentle ventilator strategies, premature infants remain at risk for morbidity and mortality due to the complications of BPD. In addition to gestational age at birth, there are a number of contributing risk factors for BPD including infant birth weight and growth rate, infection associated with lung inflammation, barotrauma, and oxygen toxicity, and environmental tobacco smoke exposure. Similarly, disease is associated with complex and heterogeneous pathology. Clinical interventions, which have significantly increased survival of very premature infants, have resulted in a change in BPD-associated lung pathology. The “new” BPD is commonly described as arrested lung development, primarily associated with failure of alveogenesis and peripheral vascular dysmorphia (3).
Pathological heterogeneity and the relative lack of clinical samples available for detailed molecular studies have significantly hampered progress in the development of disease-associated biomarkers and therapeutic interventions. Efforts have been made over the past few years to identify putative biomarkers relevant to the disease processes occurring in BPD (4–8), (for review, see Reference 9). However, correlation with severity of respiratory disease and risk of chronic lung disease has overall been poor (10). Various animal models of BPD, such as in nonhuman primates and rodents, have helped to understand the relationship between risk factors and disease pathogenesis (11–15). Although animal models are valuable tools, due to their marked differences in immune systems and lung structure, there remain significant limitations regarding the clinical relevance of observations made using these animal models. Additional information on disease-associated biomarkers derived from human specimens is highly warranted.
Our group has developed a unique biorepository of lung tissues obtained from premature infants, including subjects diagnosed with and without BPD. This collection has been previously described (3, 16–19). In the current study, we characterized genome-wide expression patterns in BPD and non-BPD tissues in an effort to gain a better understanding of disease-related processes.
Methods
Human Tissue Samples and RNA Isolation
Tissues were obtained under protocols approved by the Institutional Review Board and Privacy Board of the University of Rochester. Consent for autopsy, including a release of tissue for research, was obtained before collection of tissues. The study meets the requirements of the Health Insurance Portability and Accountability Act (privacy) compliance. All tissues and data were deidentified before release to investigators. Lung samples were harvested within 6 hours of death and snap frozen in liquid nitrogen. Estimated gestational age (EGA) was based on obstetrical dating of the last menstrual period and early trimester ultrasound fetal measurements, confirmed by physical examination assessment at birth. Data from this sample collection have been previously published (16–20). Twenty-eight of these samples were selected for genome-wide expression profiling, including 11 BPD and 9 non-BPD control cases, matched for gestational age at birth and at death, as well as 4 cases with culture-positive, acute overwhelming sepsis and 4 cases of early postnatal acute cardiovascular collapse secondary to immaturity or necrotizing enterocolitis (Table 1). Histopathological sections from four subjects with a diagnosis of BPD, obtained at the Cincinnati Children’s Hospital Medical Center, were used for replication.
TABLE 1.
SUBJECT DEMOGRAPHICS INCLUDING AGE AND PATHOLOGICAL DIAGNOSIS
| Sample ID | Phenotype | Diagnosis | GA at Birth (wk) | GA at Death (wk) |
| Group 1: Control subjects | ||||
| Early gestation | ||||
| 35 | Control | Extreme prematurity, RDS | 23 | 23.9 |
| 52 | Control | HIE, NLD to very mild RDS | 24.5 | 26.1 |
| 53 | Control | RDS, Pulmonary hemorrhage | 24.4 | 25.0 |
| 56 | Control | NLD to very mild RDS, central line event | 26.6 | 28.5 |
| Late gestation | ||||
| 8 | Control | Neuromuscular abnormality, NLD | 40 | 40.6 |
| 30 | Control | HIE, NLD | 38.5 | 38.6 |
| 46 | Control | HIE, acute meconium aspiration | 40.9 | 41.0 |
| 50 | Control | HIE, NLD | 41 | 41.4 |
| 51 | Control | NLD to very mild RDS, central line event; chorioamnionitis | 32.3 | 32.7 |
| Group 2: BPD | ||||
| Early BPD | ||||
| 10 | BPD | BPD | 26 | 27.7 |
| 11 | BPD | BPD; necrotizing enterocolitis | 26 | 34.1 |
| 14 | BPD | BPD; necrotizing enterocolitis | 26 | 28.4 |
| 17 | BPD | BPD; cytomegalovirus + | 25 | 34.1 |
| 49 | BPD | BPD; rhinovirus + | 24.7 | 31.8 |
| 58 | BPD | BPD, Acute pneumonia | 25.4 | 34.0 |
| Late BPD | ||||
| 18 | BPD | BPD | 27 | 40.7 |
| 19 | BPD | Healed BPD; recurrent necrotizing enterocolitis | 28 | 50.6 |
| 33 | BPD | BPD; cytomegalovirus + | 29.2 | 43.1 |
| 44 | BPD | BPD | 28 | 45.0 |
| 47 | BPD | BPD; cor pulmonale | 29.1 | 46.5 |
| Group 3: Non-BPD lung disease | ||||
| Acute inflammation, minimal prior lung disease | ||||
| 13 | Others | Chorioamnionitis; probable sepsis; RDS | 27 | 27.1 |
| 29 | Others | Chorioamnionitis; RDS; pulmonary hemorrhage | 28 | 28.1 |
| 39 | Others | Resolved mild RDS; necrotizing enterocolitis | 32 | 33.1 |
| 40 | Others | Resolved mild RDS; necrotizing enterocolitis | 31.2 | 32.3 |
| Severe sepsis/ pneumonia | ||||
| 3 | Others | HIE; Necrotizing pneumonia; probable aspiration | 42 | 43.3 |
| 5 | Others | Necrotizing enterocolitis; Escherichia coli sepsis | 28 | 33.0 |
| 20 | Others | E. coli sepsis | 31 | 31.6 |
| 24 | Others | Herpes simplex viral sepsis and pneumonia | 27.7 | 28.4 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; GA = gestational age; HIE = hypoxic ischemic encephalopathy; NLD = no lung disease; RDS = respiratory distress syndrome.
Frozen tissue was homogenized in Trizol regent (Invitrogen, Carlsbad, CA), and total RNA was purified using a protocol including an on-column DNase I treatment (MiniPrep kit; Agilent Technologies, Santa Clara, CA). The quality of purified RNA was assessed using a Bioanalyzer (Agilent Technologies). Only RNA samples with an RNA concentration greater than 100 ng/μl and an RNA integrity number greater than 6 were used for microarray analysis.
Microarray Profiling
RNA samples were analyzed using the Affymetrix GeneChip U133 Plus 2.0 microarray (Santa Clara, CA). RNA from individual samples was analyzed according to manufacturer’s recommendations. Expression values were extracted from. CEL files using Robust Multiarray Average (RMA) as implemented in BioConductor (http://www.bioconductor.org). Raw and RMA normalized data files are accessible at the NCBI Gene Expression Omnibus repository (http://www.ncbi.nlm.nih.gov/geo/).
Data Analysis
Significance in gene expression difference between 11 BPD cases and 9 non-BPD control cases was defined using t test P less than 0.05 and fold change greater than or equal to 2. Genes meeting these criteria were used for pathway analysis. Ingenuity Pathway Analysis software was used.
Quantitative Reverse Transcriptase–Polymerase Chain Reaction
Quantitative reverse transcriptase–polymerase chain reaction (qPCR) was performed as previously described (26) using predeveloped commercial (Applied Biosystems, Carlsbad, CA) or noncommercial (http://pga.mgh.harvard.edu/primerbank) assays (see Table E1 in the online supplement). Gene expression levels were calculated relative to PPIA (cyclophilin A) as an internal, endogenous control, according to the ddCT method.
Immunohistochemistry
Immunostaining was performed on formalin-fixed, paraffin-embedded lung tissue sections as previously described (19). For human samples, total mast cell numbers were identified by tryptase expression (M7052, 1:1000; Dako, Carpinteria, CA), and the connective tissue mast cell subpopulation (MCTC) was identified by chymase expression (MCA1930, 1:500; ABD Serotec, Raleigh, NC). The number of mast cells per field (200×) was defined in 10 random fields and was summarized as the total number of cells/field for each subject. At least eight non-BPD and 11 BPD samples were studied. Control slides were stained with either secondary antibody alone, purified IgG, or preimmune serum. For some analyses, the anatomical location of the cell (parenchyma, mucosal, perivascular, peribronchiolar) was further defined. In this case, data were normalized for the number of specific fields containing that anatomical feature. For mouse samples, mast cell numbers were identified by chymase (Cma1; Mcp5) expression (MCA1930, 1:200; ABD Serotec), using the Mouse-on-Mouse kit (Vector Labs, Burlingame, CA).
Animal Model
Generation and analysis of mice deficient in expression of both FGFR3 and FGFR4 (FGFR3/4) was performed as we recently described (21). Whole lung tissue RNA was isolated from FGFR3/4 mutant and wild-type control mice at 1 month of age and subjected to qPCR analysis for CPA3, TPSAB1, and TPSB2 using gene-specific primers.
Statistical Analysis
Statistical analysis of microarray data was performed as described above. For qPCR and immunohistochemistry (IHC), group means and group variation were used to calculate significance according to the nonparametric Mann-Whitney U test.
Results
Subject Demographics
From our biorepository collection, we studied lung tissue samples from a total of 28 subjects (Table 1). Eleven of these subjects died with a clinical and pathological diagnosis of BPD. Nine control subjects without BPD, matched for age at birth and death, displayed evidence of mild lung pathology (pneumonia, respiratory distress syndrome, or alveolar hemorrhage). Another eight subjects with significant, non-BPD pulmonary pathology, primarily consisting of inflammation associated with sepsis, were used as an additional comparison group. Our primary analysis was to distinguish all the subjects with BPD (n = 11) from all control subjects with mild lung pathology (n = 9). Subanalyses examined gene expression patterns stratified by age, as defined by “early” (< 27 wk EGA at birth; < 35 wk EGA at death) or “late” (> 27 wk EGA at birth; > 35 wk EGA at death) gestation. When matched for age at birth or death, only one sample was reclassified; sample 51 was defined as late at birth, but early at death.
Pathways Affected in BPD
RNA was isolated from grossly dissected distal lung tissue and processed as described in Methods. All RNA samples studied passed quality control assessments and were interrogated for genome-wide expression using the Affymetrix Hu133 Plus 2.0 array, essentially as previously described (22, 23). Normalized and background corrected data were extracted using RMA. The entire preprocessed data set has been made publicly available (NCBI Gene Expression Omnibus, http://www.ncbi.nlm.nih.gov/geo/).
Prior studies have indicated a significant diminution of vessel formation in BPD lungs. Therefore, we examined the microarray-based expression patterns of vascular molecules, including cell surface proteins (PECAM, TIE2, FLT1, ENG), growth factors (VEGF, ANG1), and signaling molecules (HIF1A, HIF3A) in our data set. Seven of nine genes examined (and 19 of 26 probe sets examined) demonstrated evidence for reduced expression (fold change < 2) in BPD when compared with controls (Table E2). Among the seven vascular marker genes demonstrating reduced expression, HIF3A and TIE2 were significantly reduced (P < 0.05) in BPD tissue. Additionally, we examined expression of nitric oxide synthase (NOS) genes, because nitric oxide has been implicated in BPD pathogenesis (24–26). All three NOS genes (NOS1, NOS2, NOS3) showed some evidence for decreased expression, and NOS2 was significantly reduced (P < 0.02) in BPD. These data validate that our approach is able to reliably capture known alterations associated with BPD pathology and provide novel molecular insight.
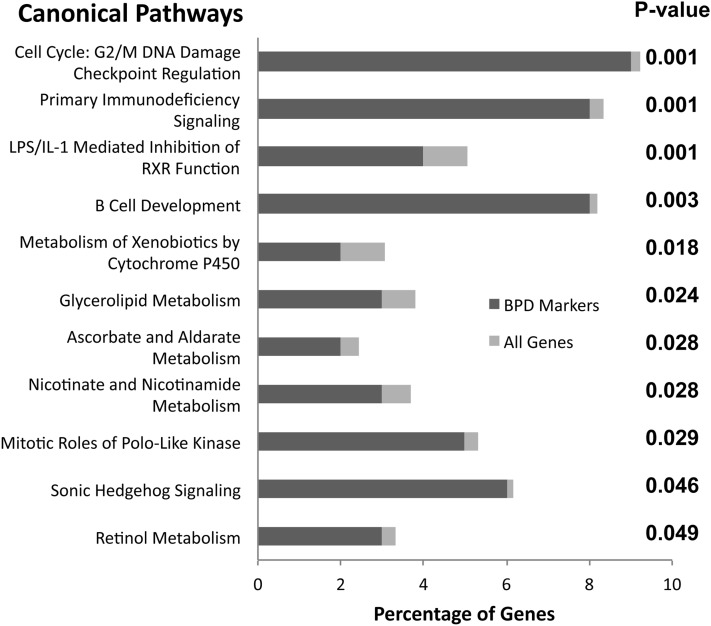
We focused further studies on a set of 159 genes with significant differences in expression between BPD and controls (P < 0.05; greater than twofold change) as defined by our microarray data set. A complete list of these genes is presented in Table E3. We tested for canonical pathways overrepresented in these 159 genes using Ingenuity Pathways Analysis software (Figure 1). Discovered biological processes significantly associated with BPD included cell-cycle regulation (DNA damage-related, Polo-like kinase) and immune-cell regulation (immunodeficiency signaling, B-cell development). Additionally, gene expression in BPD was associated with specific developmental and biochemical pathways, including some previously shown to be important specifically in the lung (sonic hedgehog signaling, retinol metabolism). These results largely confirmed prior knowledge that BPD has a complex disease pathology associated with changes in cell proliferation, oxidant stress–related DNA damage repair, inflammatory cell infiltration, and ongoing developmental processes.
Figure 1.
Canonical pathways affected in bronchopulmonary dysplasia (BPD) lungs. To estimate general biological mechanisms that are altered during BPD pathogenesis, we performed pathways analysis using Ingenuity software, as described in Methods. We identified 159 genes whose expression was significantly altered in BPD lung tissue and assessed overrepresentation by this gene set in known pathways. Shown are “canonical” pathways, ranked by Fisher-exact t test P value, listing the percentage of the BPD-associated gene set in each pathway (dark gray bar), versus the percentage of all genes in that pathway (light gray bar).
BPD-associated Gene Expression
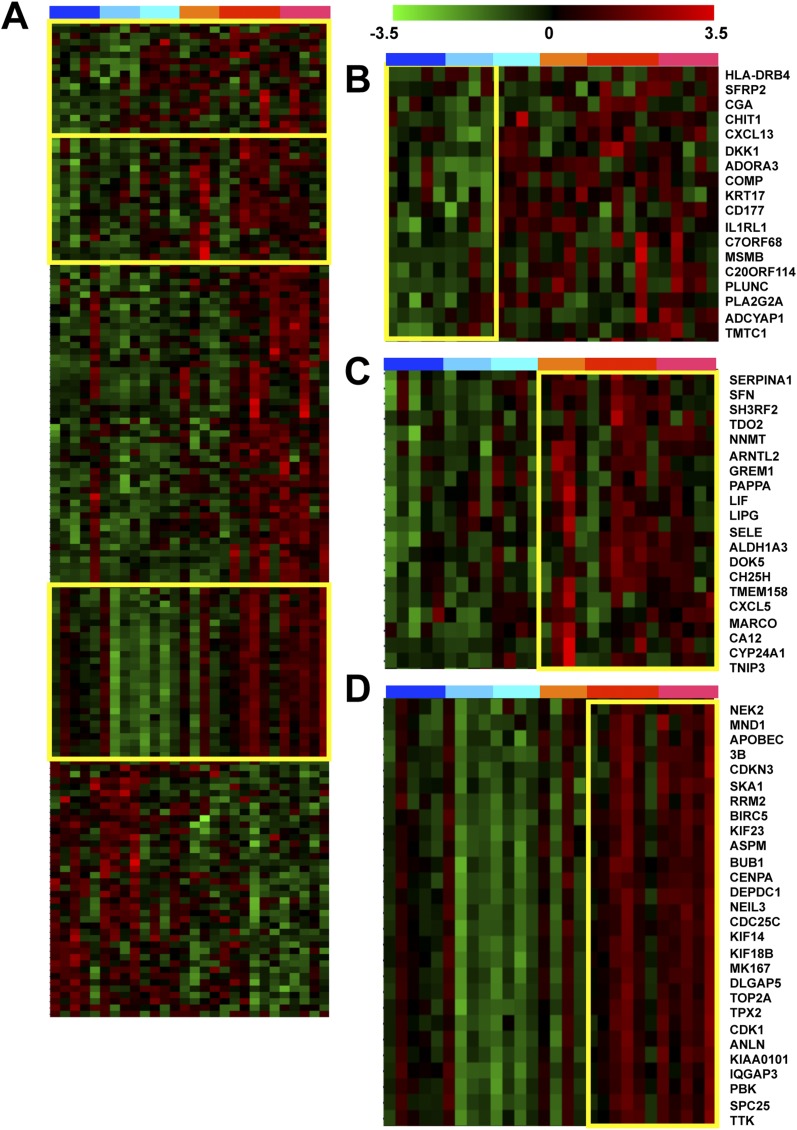
Next, we analyzed global expression patterns for the 159 genes in all samples (Figure 2), to assess age-related changes in expression and disease specificity. Significant variability in expression of individual genes within subject groups was apparent, as expected given the complex and diverse pathology associated with this disease. Even so, genes displaying consistent increases or decreases in expression in BPD, as compared with controls, were apparent. Although most gene expression changes identified in BPD lungs were specific, a subset of genes induced in BPD tissues showed similar changes in non-BPD lung disease tissues (Figure 2B). Another subset of genes displayed similar expression patterns in BPD and sepsis only, not in non–sepsis-related lung injury (Figure 2C). Interestingly, a majority of the genes significantly affected in BPD did not display evidence for age-dependent changes in expression in controls. However, a subset of genes showed a trend for reduced expression over time in controls but persistent expression over time in BPD tissues (Figure 2D).
Figure 2.
Gene expression patterns in bronchopulmonary dysplasia (BPD) lungs. (A) Shown are gene expression patterns for a set of 159 genes significantly affected (P value < 0.05, fold change > 2) in BPD lung tissue as compared with controls. Columns represent individual subjects including (defined at bar on top); early gestation age control lungs (dark blue), late gestation control lungs (light blue), non-BPD lung injury (aqua), non-BPD injury with sepsis (orange), early gestation age BPD (red), and late gestation BPD (pink). Expression patterns for individual genes are in rows, with high expression levels in red and low expression levels in green, defined as indicated by the scale bar. Yellow boxes indicate areas represented in B–D. (B) As in A, highlighting a set of genes affected in both BPD and in general injury samples (including sepsis). (C) As in A, highlighting a set of genes affected in both BPD and sepsis-associated samples only. (D) As in A, highlighting a set of genes whose expression is associated with gestational age in control subjects but not in subjects with BPD.
To further assess the reliability of our microarray data set, and our methods for identifying disease gene expression biomarkers, we validated expression of a set of genes by qPCR (Figures 3 and 4). Genes were chosen for validation studies based on their inclusion in the set of 159 differentially expressed genes, their magnitude of change in expression, and our interest in their potential biological function. In addition to comparing all BPD and all controls, we stratified our analysis by EGA to compare disease-related patterns in subjects earlier in age (< 27 wk EGA at birth; < 35 wk EGA at death) or later in age (> 27 wk EGA at birth; > 35 wk EGA at death).
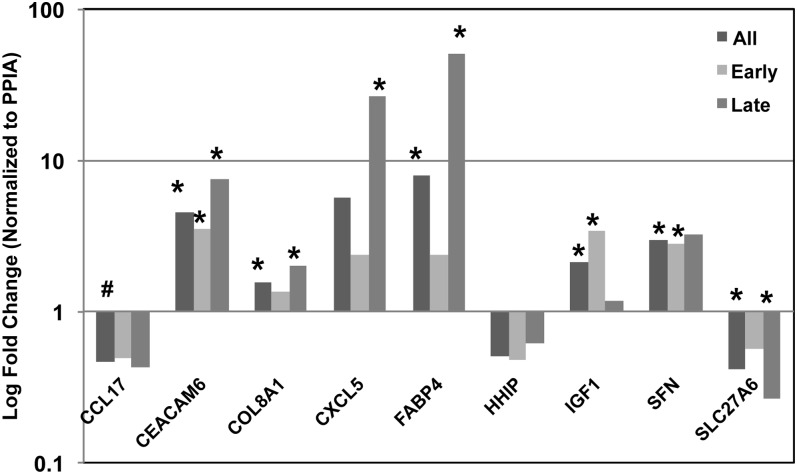
Figure 3.
Gene expression validation. We used quantitative reverse transcriptase polymerase chain reaction (qPCR) to confirm expression patterns predicted by microarray analysis. We selected nine genes, based on their magnitude of change and biological interest. Shown are fold differences in expression (log scale) for each gene, comparing all samples, or samples stratified by gestational age. All genes demonstrated evidence for replication, with seven of nine displaying significant differences between bronchopulmonary dysplasia and controls. (*P value < 0.05; #P value < 0.10).
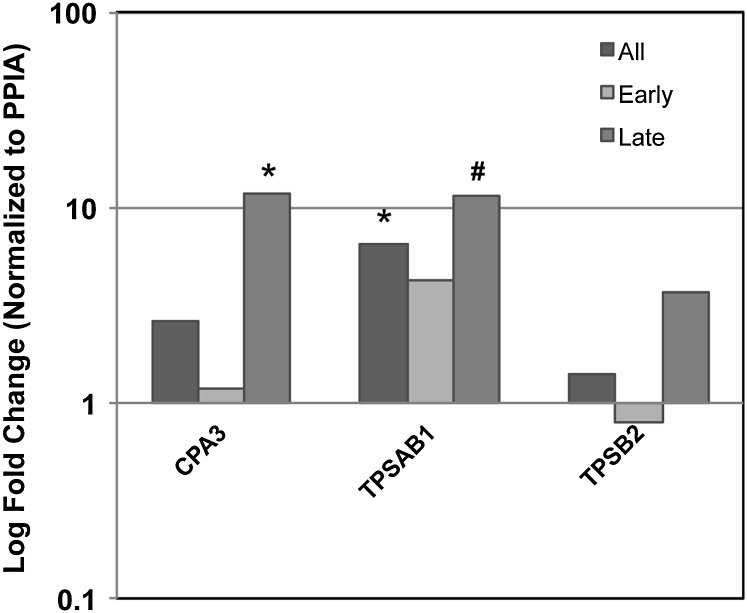
Figure 4.
Mast cell–specific gene expression validation. We used quantitative reverse transcriptase polymerase chain reaction (qPCR) to confirm expression patterns for mast cell–specific genes in bronchopulmonary dysplasia (BPD) lung tissue. We selected 3 mast cell–specific genes that were among the 10 most differentially expressed of the 159 significantly affected gene set. Shown are fold differences in expression (log scale) for each gene, comparing all samples, or samples stratified by gestational age. All genes demonstrated evidence for significant increases in expression, with two of three displaying significant differences between BPD and controls. (*P value < 0.05; #P value < 0.10).
We confirmed a significant difference (P < 0.05) in expression between BPD and controls for seven out of a total of nine genes tested, including CCL17, CEACAM6, COL8A1, CXCL5, FABP4, HHIP, IGF1, SFN, and SLC27A6 (Figure 3). The additional two genes showed some evidence for differential expression by qPCR, consistent with our microarray results. CCL17 was decreased twofold and demonstrated a trend for significance in all samples (P = 0.06). HHIP showed a twofold reduction in all samples. In addition, we also examined the expression of KITLG, BLP, and IL4, which have previously been reported to be altered in BPD (Table E4). We observed no evidence for difference in the expression of these genes in the microarray data set. IL4 expression was virtually undetectable in lung tissue samples by qPCR (CT > 35). KITLG and BLP showed appreciable expression levels but did not show any significant changes in their expression by qPCR.
Identification of Mast Cell Gene Signature
Among the list of 159 BPD-associated genes, we examined those with the greatest magnitude of change (Table 2). Three of the top five most highly induced genes were mast cell–specific markers, suggesting an increase in mast cells in BPD tissues. Furthermore, nine probe sets, independently measuring these three genes, were among the 25 most highly increased in BPD. Interestingly, the single most highly induced gene encodes CPA3, a marker specific for connective tissue–type mast cells (MCTC), a subpopulation of mast cells that are not frequently found within the lung but have been associated with chronic obstructive pulmonary disease (COPD) and severe asthma (27).
TABLE 2.
RANKED LIST (BASED ON FOLD CHANGE BETWEEN BRONCHOPULMONARY DYSPLASIA AND CONTROL) OF 25 MOST AFFECTED GENES AMONG 159 SIGNIFICANTLY DYSREGULATED IN BRONCHOPULMONARY DYSPLASIA
| Gene Symbol | Gene Title | Probeset ID | P Value | Fold Change | Gene Class |
| CPA3 | Carboxypeptidase A3 (mast cell) | 205624_at | <0.01 | 7.14 | Mast cells |
| CCL18 | Chemokine (C-C motif) ligand 18 (pulmonary and activation-regulated) | 32128_at | <0.01 | 6.74 | Chemokine |
| IGHA1 | Immunoglobulin heavy constant alpha 1 | 217022_s_at | 0.02 | 6.90 | Immunoglobulin |
| CCL18 | Chemokine (C-C motif) ligand 18 (pulmonary and activation-regulated) | 209924_at | <0.01 | 6.27 | Chemokine |
| TPSAB1 | Tryptase alpha/beta 1 | 216474_x_at | 0.01 | 6.16 | Mast cell |
| TPSAB1 | Tryptase alpha/beta 1 | 207134_x_at | 0.01 | 5.15 | Mast cell |
| TPSAB1 | Tryptase alpha/beta 1 | 205683_x_at | <0.01 | 5.03 | Mast cell |
| ALDH1A3 | Aldehyde dehydrogenase 1 family, member A3 | 203180_at | <0.01 | 4.60 | |
| TPSAB1 | Tryptase alpha/beta 1 | 217023_x_at | 0.01 | 4.38 | Mast cell |
| TPSAB1 | Tryptase alpha/beta 1 | 210084_x_at | 0.01 | 4.34 | Mast cell |
| FABP4 | Fatty acid binding protein 4, adipocyte | 235978_at | 0.01 | 3.87 | |
| PLUNC | Palate, lung and nasal epithelium carcinoma associated | 220542_s_at | 0.03 | 4.28 | |
| FABP4 | Fatty acid binding protein 4, adipocyte | 203980_at | 0.01 | 4.28 | |
| DNAJC5B | DnaJ (Hsp40) homolog, subfamily C, member 5 beta | 232798_at | 0.01 | 4.14 | |
| TPSB2 | Tryptase alpha/beta 1 /// tryptase beta 2 | 207741_x_at | 0.01 | 4.10 | Mast cell |
| ATP6V0D2 | ATPase, H+ transporting, lysosomal 38kDa, V0 subunit d2 | 1553153_at | 0.01 | 3.82 | ATPase |
| ATP6V0D2 | ATPase, H+ transporting, lysosomal 38kDa, V0 subunit d2 | 1553155_x_at | 0.01 | 3.78 | ATPase |
| CXCL13 | Chemokine (C-X-C motif) ligand 13 (B-cell chemoattractant) | 205242_at | 0.04 | 3.66 | Chemokine |
| C1orf186 | Chromosome 1 open reading frame 186 | 230381_at | <0.01 | 3.82 | ORFs |
| MEG8 | Maternally expressed (in Callipyge) 8 | 240083_at | <0.01 | 3.45 | |
| TPSAB1 | Tryptase alpha/beta 1 | 215382_x_at | 0.01 | 3.64 | Mast cell |
| VSIG1 | V-set and immunoglobulin domain containing 1 | 243764_at | 0.02 | 3.45 | Immunoglobulin |
| DNAJC5B | DnaJ (Hsp40) homolog, subfamily C, member 5 beta | 1554258_a_at | 0.01 | 3.36 | |
| EREG | Epiregulin | 205767_at | 0.01 | 3.30 | |
| TPSAB1 | Tryptase alpha/beta 1 | 216485_s_at | 0.01 | 3.29 | Mast cell |
Mast cells have previously been suggested as playing a role in BPD and have been observed in some animal models of this disease. qPCR for mast cell–specific marker expression, for both mucosal-type mast cells (MCT) (TPSAB1, TPSB2) and MCTC (CPA3) populations, was consistent with increases in these cells, particularly in late-stage BPD samples (Figure 4). CPA3 showed an approximately threefold increase and was significantly increased in late-stage BPD samples. TPSB2 was increased 3.5-fold in late-stage BPD samples.
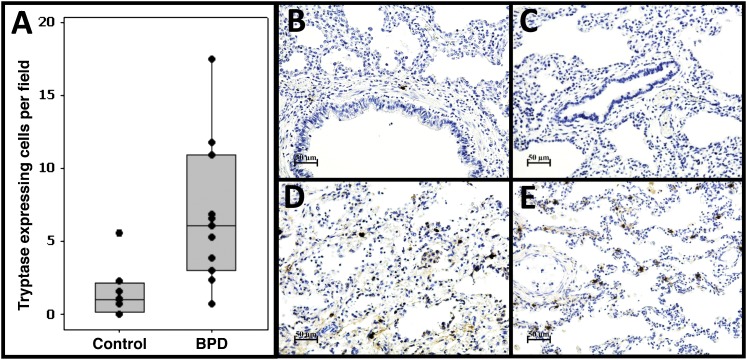
To confirm our gene expression data and more thoroughly assess the microarray-based prediction of increased mast cells in BPD lungs, we performed IHC for mast cell markers. Immunostaining for tryptase (Figure 5 and Figure E1), a marker for all mast cell subtypes, confirmed a significant increase in total mast cell number in our subjects with BPD (6.8 vs. 1.5 cells/field, P = 0.005). No staining was observed in negative control experiments (Figure E1).
Figure 5.
Increased mast cell accumulation in bronchopulmonary dysplasia (BPD) lungs. We used immunohistochemistry to identify tryptase-expressing cells, a general mast cell–specific marker. (A) Quantitative analysis of the number of tryptase-expressing cells in BPD and non-BPD control lungs. Shown are the average numbers of stained cells/field for each individual subject (dots), the group means (bar), and interquartile range (box). (B, C) Examples of staining patterns in age-matched control subjects. (D, E) Examples of staining patterns in BPD lungs. Magnification is as indicated on scale bars. A significant approximately fivefold increase in tryptase-positive cells is observed in BPD lungs.
Increased Connective Tissue Mast Cells Are Associated with BPD
As mentioned above, mast cell subtypes in the lung have been appreciated, each with different anatomical distributions and characteristic secretory products. Therefore, we assessed the distribution of tryptase-expressing cells in (1) the parenchymal region of the lung, (2) adjacent to the airways, and (3) adjacent to the large/intermediate vasculature (Figure E1). We tested for region-specific differences between BPD and control tissues. We observed a significant increase in tryptase-staining cells in BPD in the parenchymal region (6.5 vs. 1.3 cells/field, P = 0.004) only, with no differences in the airway or vascular regions (Figure E1). Interestingly, at this early developmental stage, a majority of tryptase-staining cells appeared to be in the parenchymal region, and not the airway, for both groups. This observation is limited by the proportion of tissue occupied by each region studied, especially for large airways and vessels. In these predominantly peripheral lung samples, each field has a larger proportion of parenchyma than airways or vessel.
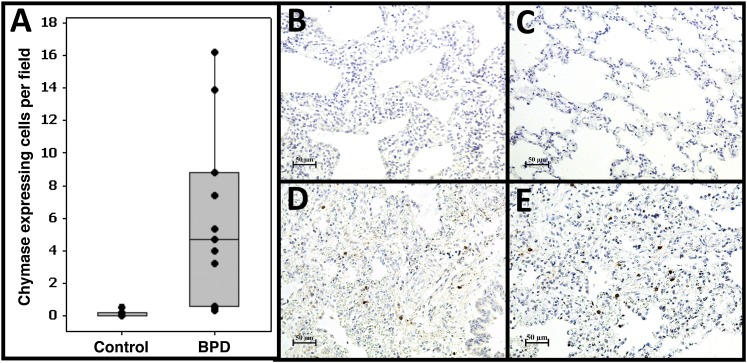
Gene expression profiling identified both general mast cell (tryptase) and MCTC-specific (CPA3) markers increased in BPD tissue. Furthermore, excessive tryptase-expressing cell accumulation was observed predominantly in the parenchymal region of the lungs in BPD, a location where connective tissue mast cells are typically found. Therefore, we performed immunostaining for chymase, an MCTC-specific marker, in our tissues (Figure 6). We observed a greater than 50-fold increase in chymase-expressing cells in BPD (0.1 vs. 5.9 cells/field, P = 0.0003). No staining was observed in negative control slides (Figure E2). As expected, the chymase-expressing MCTC mast cells were highly restricted to the parenchymal region (97% of all positive cells in BPD, markedly fewer but 100% of all positive cells in non-BPD).
Figure 6.
Increased connective tissue–type mast cell accumulation in bronchopulmonary dysplasia (BPD) lungs. We used immunohistochemistry to identify chymase-expressing cells, a specific marker for connective tissue–type mast cells. (A) Quantitative analysis of the number of chymase-expressing cells in BPD and non-BPD control lungs. Shown are the average numbers of stained cells/field for each individual subject (dots), the group means (bar), and interquartile range (box). (B, C) Examples of staining patterns in age-matched control subjects. (D, E) Examples of staining patterns in BPD lungs. Magnification is as indicated on scale bars. A significant approximately 50-fold increase in chymase-positive cells is observed in BPD lungs.
Using an independent cohort of subjects obtained from Cincinnati Children’s Hospital Medical Center, we attempted to validate that increases in MCTC accumulation were not restricted to our original study cohort. We investigated histopathological specimens from four subjects with a diagnosis of BPD, each of which showed a frequency of MCTC (1.3, 1.9, 2.1, 2.4 chymase-expressing cells/field) substantially above controls (0.0–0.5 chymase-expressing cells/field). These data demonstrate that the specific accumulation of MCTC represents a general, and previously unappreciated, aspect of BPD pathology.
Increased Expression of MCTC Markers in a Mouse Model of BPD-like Pathology
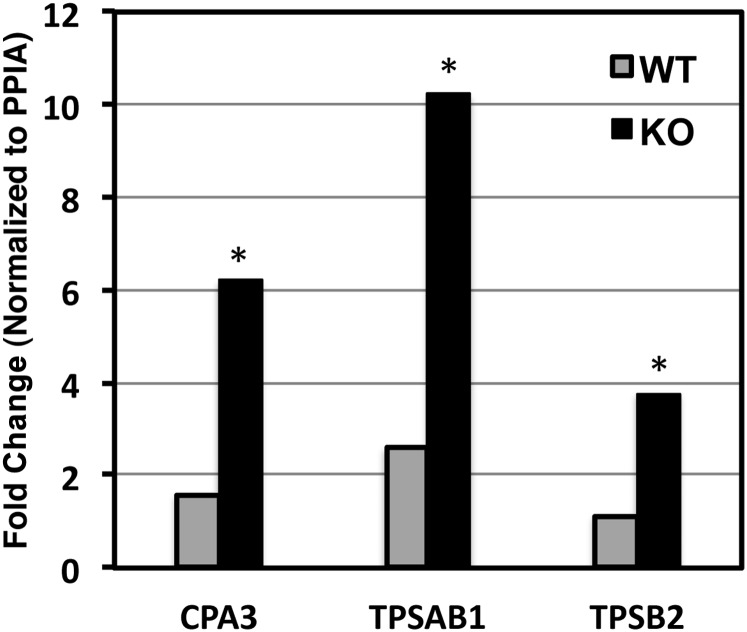
We previously reported lung development abnormalities in mice lacking expression of both fibroblast growth factor receptor (FGF)-3 and -4 (21, 28). The FGFR3/4 mutant mouse phenocopies certain aspects of human BPD pathology, including chronic lung disease characterized by structural changes in distal lung architecture consistent with developmental arrest, dysplastic elastin fiber formation, and alveolar enlargement. Interestingly, we also noted previously underappreciated proximal airway phenotypes in this model that are reminiscent of pathology observed in individuals with BPD (squamous changes to conducting airway epithelium, bronchiolar exudates). Critically, we noted mild monocytic infiltrates in both the proximal and distal airways. Given our observations demonstrating robust accumulation of MCTC in human BPD lung samples, and the presence of BPD-like pathologies in the FGFR3/4 mutant mouse lungs, we tested them for the presence of mast cells. Similar to our observations in human lung tissue from subjects with BPD, we noted significant increases in the expression of both general mast cell (tryptase) markers TPSAB1 (4-fold, P < 0.05) and TPSB2 (3.5-fold, P = 0.05) and the connective tissue-type mast cell marker CPA3 (4-fold, P < 0.05), at 1 month of age in the FGFR3/4 mice (Figure 7). Immunostaining for chymase supported these data (Figure E3), showing immunoreactive cells in airway walls and in distal airspaces of mutant mice. Dramatically fewer total inflammatory cells, and chymase-staining cells, were observed in control mice.
Figure 7.
Increased mast cell marker expression in animal model of bronchopulmonary dysplasia (BPD). We used quantitative real-time polymerase chain reaction to assess gene expression for the mast cell markers CPA3, TPSAB1, and TPSB2 in whole lung tissue obtained from wild-type (WT, n ≥ 3) or FGFR3/4 compound deficient (KO, n ≥ 3) mice at 1 month of age. Shown are mean relative gene expression values for each group and for each gene. (*P value < 0.05).
Discussion
Using a unique biorepository of autopsy tissues, we sought to comprehensively define gene expression changes from the lung tissue of premature babies who died with a diagnosis of BPD. As our population was relatively small, we presumed that we would only be able to detect changes in expression that were both consistent between subjects and large in magnitude. Initially, we assessed the expression of vasculature-associated genes. We detected a reduction in expression for many of these genes, consistent with the well-described abnormalities in vascular morphogenesis associated with BPD. Animal models of BPD have been associated with reduced mRNA and protein for vascular endothelial growth factor and its receptors (17, 29, 30). We also identified significant changes in the expression of additional nonvascular genes previously identified to be dysregulated in this disease, including increases in IGF1 (31) and FABP4 (32). Validation of these expression patterns by qPCR demonstrated a high degree of reliability for microarray-based predictions. Overall, we validated significant changes in expression for 9 of 12 genes tested by qPCR, indicating an observed false discovery rate of 25%. Collectively, validated detection of genes previously identified as differentially expressed in BPD strongly supports the methods we used to identify gene expression biomarkers for BPD.
Our approach also facilitated the identification of “pathways” associated with BPD, using analytical methods that are robust to false discovery for individual genes. These analyses identified pathways associated with known disease features, including developmental, inflammatory, and cell proliferation processes. Interestingly, we specifically identified a signature of DNA damage-related cell cycle regulation, which has been suggested as a specific mechanism contributing to disease processes in humans and in animal models of disease (33, 34). We also observed dysregulation of genes associated with Hedgehog signaling, a pathway previously shown to be critical for lung development in the pseudoglandular/canalicular stages (35, 36) but whose role in BPD pathogenesis is unclear. Furthermore, we identified evidence of B-lymphocyte development alterations in BPD. Although inflammation in general (and more recently, lymphocytes in particular) has been widely appreciated as a component of disease pathology (37, 38), a role for B cells has not been previously reported. We suggest that further exploration of these pathways may provide novel information regarding disease mechanisms.
In BPD tissues, we discovered a robust gene expression signature predictive of increased mast cell accumulation. The normal human lung contains a small number of tryptase-containing mucosal mast cells, typically associated with larger airways, which are involved in allergic responses. We validated the mast cell–specific gene expression patterns by qPCR and by demonstrating an approximately fivefold increase in the total number of mast cells in BPD tissues, as defined by IHC for tryptase. Increased mast cell accumulation has been previously identified as a feature of BPD (39) and has been observed in some animal models of disease (8, 40), but the significance of these prior observations is not clear. Subramaniam and colleagues identified a role for increased expression of bombesin-like peptides (BLP) in a baboon model of BPD (8). In this model, they observed BLP-dependent increases in “parenchymal” mast cells (8). In the baboon model, BLP showed both chemotactic and pro-proliferative effects on mast cells. We did not observe any significant differences in the expression of BLP, KITL, IL4, or other factors known to promote mast cell accumulation or differentiation. Ongoing studies are aimed at identifying mechanisms responsible for regulating mast cell recruitment, differentiation, and function in human BPD.
Functional and molecular heterogeneity of mast cells was first described more than 20 years ago (41). Recently, there has been a growing appreciation of the role of subpopulations of mast cells, which differ in terms of their anatomical distribution and molecular function, in human lung diseases. The phenotype of mast cells previously associated with BPD in humans or in animal models of similar disease, whether mucosal or connective tissue type, was not determined. Our gene expression signature included mast cell markers specific for MCTC. Furthermore, a large proportion of the mast cells observed in BPD (and non-BPD) tissues, identified by tryptase expression, were found in the parenchymal region, where MCTC are typically observed. Using IHC, we found an approximately 50-fold increase in chymase-expressing cells, almost exclusively in the parenchyma region, in our subjects with BPD. Furthermore, we validated the presence of chymase-expressing MCTC in an independent cohort of patients. These data strongly suggest increased MCTC accumulation is a feature of BPD. Excessive accumulation of connective tissue mast cells (MCTC), expressing both tryptase and chymase, has recently been identified in COPD (42) and severe asthma (27). Future studies are warranted to define how this observation contributes to clinical and/or pathophysiological commonalities among these diseases.
Infants with BPD remain at risk for reduced exercise tolerance, reactive airway disease, and progressive emphysema in the first years of life and at least into young adulthood (43, 44). They continue to have increased sensitivity to environmental stimuli, such as cigarette smoke, and to viral infections (45, 46), with enhanced inflammation and bronchoconstriction. Persistent, resident mast cells have been associated with similar symptoms in patients with asthma. In fact, mast cell proteases were recently identified as mediators of airway hypersensitivity in animal model of asthma (47). Their ability to release histamine, leukotrienes, bronchoconstrictive prostaglandins, and potentially tissue-destructive peptidases make mast cells likely candidates to exacerbate inflammatory responses to lung irritants.
If mast cells participate in BPD pathogenesis or symptomatology, inhibition of mast cell secretion might be expected to be therapeutic in the disease. Interestingly, cromolyn sodium, a commonly used inhibitor of mast cell degranulation, has previously been tested as a therapeutic for chronic lung disease in prematurity. Two small randomized clinical trials were performed to assess the early use of this therapy, in the first month of life, in preventing BPD (48–50). Neither trial demonstrated a difference in mortality or use of oxygen at 28 days or 36 weeks postmenstrual age. Use of cromolyn in treatment of established chronic lung disease of the newborn has not been studied in a randomized trial. However, mast cells in the skin, which are predominantly connective tissue, dual chymase-tryptase expressing mast cells like those demonstrated to predominate in BPD lung tissue, are poorly inhibited by cromolyn, and so it may not be expected to have efficacy for BPD (51). Alternative methods of targeting mast cells, and mast cell–derived mediators, for intervention in BPD may be warranted.
To determine if mast cell recruitment occurs in animal models of neonatal chronic lung disease, we studied our recently described model of FGFR3/4 deficiency (21). This line of mice displays many BPD-like lung pathologies including airspace enlargement and dysregulated elastogenesis, as well as an underappreciated monocytic cell accumulation, but lacks many BPD features. This model was particularly relevant, as we previously reported excessive IGF1 expression in these mice, as previously reported in human BPD lungs (31), and as we confirmed in our current human specimens. In these mice, we found significant increases in the expression of the mast cell markers identified in our human BPD subjects, specifically including the MCTC marker CPA3. These data suggest that accumulation of MCTC is a prominent feature of human BPD, and at least one animal model of the disease. Interestingly, Wagenaar and colleagues (52) have reported increased expression of mast cell protease in newborn rats exposed to hyperoxia. Furthermore, these data suggest the FGFR3/4 mutant mouse may provide a useful model system for assessing the mechanism of mast cell accumulation in BPD-like lung injury and the physiological role of these cells in disease pathogenesis.
In summary, to the best of our knowledge, this study provides the first ever genome-wide analysis of expression changes at the mRNA level in BPD lungs. Data analysis implicates previously identified as well as novel genes and pathways that are involved in disease pathogenesis. The data provide confirmation that mast cell accumulation is a feature of human BPD. Furthermore, they facilitated the novel observation that the disease-relevant mast cells are multi-protease producing, connective tissue type, as recently reported for COPD and severe asthma. These observations have significant clinical and mechanistic implications.
Supplementary Material
Acknowledgments
The authors thank Jody Gascon for technical assistance and Dr. Steve Welle and the URMC Functional Genomics Center for support with microarray analysis.
Footnotes
Supported by University of Rochester Clinical and Translational Science Institute grant NIH UL1 RR024160-03 and University of Rochester Strong Children’s Research Center grant NIH T32HD057821.
Author Contributions: S.B. assisted with experimental design, led primary data collection and analysis, and contributed to writing the manuscript. D.G. assisted with primary data collection and analysis and contributed to writing the manuscript. D.L.K. contributed to sample processing, immunohistochemistry, and to primary data collection. H.L.H. contributed to sample collection and processing and to primary data collection. S.K.S. contributed to sample collection and processing and to primary data collection. V.A.L. contributed to sample collection and processing and to primary data collection. L.M. led human sample collection. S.S. contributed to sample collection and processing and to primary data collection. S.E.W. provided tissue samples for replication. T.J.M. directed experimental design, primary data collection and analysis, provided animal specimens for analysis, and was a main contributor to writing the manuscript. G.S.P. directed human specimen collection and processing, assisted with experimental design, primary data collection and analysis, and was a main contributor to writing the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201203-0406OC on June 21, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955 [DOI] [PubMed] [Google Scholar]
- 2.Zimmerman JJ. Bronchoalveolar inflammatory pathophysiology of bronchopulmonary dysplasia. Clin Perinatol 1995;22:429–456 [PubMed] [Google Scholar]
- 3.Chess PR, D'Angio CT, Pryhuber GS, Maniscalco WM. Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 2006;30:171–178 [DOI] [PubMed] [Google Scholar]
- 4.Schrama AJ, Bernard A, Poorthuis BJ, Zwinderman AH, Berger HM, Walther FJ. Cord blood Clara cell protein CC16 predicts the development of bronchopulmonary dysplasia. Eur J Pediatr 2008;167:1305–1312 [DOI] [PubMed] [Google Scholar]
- 5.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed 2008;93:F455–F461 [DOI] [PubMed] [Google Scholar]
- 6.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 2009;123:1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ. Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res 1996;40:250–256 [DOI] [PubMed] [Google Scholar]
- 8.Subramaniam M, Sugiyama K, Coy DH, Kong Y, Miller YE, Weller PF, Wada K, Wada E, Sunday ME. Bombesin-like peptides and mast cell responses: relevance to bronchopulmonary dysplasia? Am J Respir Crit Care Med 2003;168:601–611 [DOI] [PubMed] [Google Scholar]
- 9.Thompson A, Bhandari V. Pulmonary biomarkers of bronchopulmonary dysplasia. Biomark Insights 2008;3:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truog WE, Ballard PL, Norberg M, Golombek S, Savani RC, Merrill JD, Parton LA, Cnaan A, Luan X, Ballard RA. Inflammatory markers and mediators in tracheal fluid of premature infants treated with inhaled nitric oxide. Pediatrics 2007;119:670–678 [DOI] [PubMed] [Google Scholar]
- 11.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, et al. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 2004;170:1188–1196 [DOI] [PubMed] [Google Scholar]
- 12.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol 2010;108:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Reilly MA, Marr SH, Yee M, McGrath-Morrow SA, Lawrence BP. Neonatal hyperoxia enhances the inflammatory response in adult mice infected with influenza A virus. Am J Respir Crit Care Med 2008;177:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunday ME, Shan L, Subramaniam M. Immunomodulatory functions of the diffuse neuroendocrine system: implications for bronchopulmonary dysplasia. Endocr Pathol 2004;15:91–106 [DOI] [PubMed] [Google Scholar]
- 15.Maniscalco WM, Watkins RH, O’Reilly MA, Shea CP. Increased epithelial cell proliferation in very premature baboons with chronic lung disease. Am J Physiol Lung Cell Mol Physiol 2002;283:L991–L1001 [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, Stripp BR. Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am J Respir Crit Care Med 2002;166:1498–1509 [DOI] [PubMed] [Google Scholar]
- 17.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–L823 [DOI] [PubMed] [Google Scholar]
- 18.Lee MK, Pryhuber GS, Schwarz MA, Smith SM, Pavlova Z, Sunday ME. Developmental regulation of p66Shc is altered by bronchopulmonary dysplasia in baboons and humans. Am J Respir Crit Care Med 2005;171:1384–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980 [DOI] [PubMed] [Google Scholar]
- 20.Pryhuber GS, Huyck HL, Staversky RJ, Finkelstein JN, O'Reilly MA. Tumor necrosis factor-alpha-induced lung cell expression of antiapoptotic genes TRAF1 and cIAP2. Am J Respir Cell Mol Biol 2000;22:150–156 [DOI] [PubMed] [Google Scholar]
- 21.Srisuma S, Bhattacharya S, Simon DM, Solleti SK, Tyagi S, Starcher B, Mariani TJ. Fibroblast growth factor receptors control epithelial-mesenchymal interactions necessary for alveolar elastogenesis. Am J Respir Crit Care Med 2010;181:838–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharya S, Srisuma S, Demeo DL, Shapiro SD, Bueno R, Silverman EK, Reilly JJ, Mariani TJ. Molecular biomarkers for quantitative and discrete COPD phenotypes. Am J Respir Cell Mol Biol 2009;40:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kho AT, Bhattacharya S, Tantisira KG, Carey VJ, Gaedigk R, Leeder JS, Kohane IS, Weiss ST, Mariani TJ. Transcriptomic analysis of human lung development. Am J Respir Crit Care Med 2010;181:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCurnin DC, Pierce RA, Willis BC, Chang LY, Yoder BA, Yuhanna IS, Ballard PL, Clyman RI, Waleh N, Maniscalco W, et al. Postnatal estradiol up-regulates lung nitric oxide synthases and improves lung function in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2009;179:492–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis CW, Gonzales LW, Ballard RA, Ballard PL, Guo C, Gow AJ. Expression of nitric oxide synthases and endogenous NO metabolism in bronchopulmonary dysplasia. Pediatr Pulmonol 2008;43:703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rieger-Fackeldey E, Hentschel R. Bronchopulmonary dysplasia and early prophylactic inhaled nitric oxide in preterm infants: current concepts and future research strategies in animal models. J Perinat Med 2008;36:442–447 [DOI] [PubMed] [Google Scholar]
- 27.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, Castro M, Gaston B, Israel E, Schwartz LB, et al. Mast cell phenotype, location, and activation in severe asthma: data from the severe asthma research program. Am J Respir Crit Care Med 2011;183:299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 1998;125:3615–3623 [DOI] [PubMed] [Google Scholar]
- 29.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001;164:1981–1987 [DOI] [PubMed] [Google Scholar]
- 30.Tambunting F, Beharry KD, Waltzman J, Modanlou HD. Impaired lung vascular endothelial growth factor in extremely premature baboons developing bronchopulmonary dysplasia/chronic lung disease. J Investig Med 2005;53:253–262 [DOI] [PubMed] [Google Scholar]
- 31.Chetty A, Andersson S, Lassus P, Nielsen HC. Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr Pulmonol 2004;37:128–136 [DOI] [PubMed] [Google Scholar]
- 32.Ghelfi E, Karaaslan C, Berkelhamer S, Akar S, Kozakewich H, Cataltepe S. Fatty acid binding proteins and peribronchial angiogenesis in bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2011;45:550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maniscalco WM, Watkins RH, Roper JM, Staversky R, O'Reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased VEGF expression. Pediatr Res 2005;58:549–556 [DOI] [PubMed] [Google Scholar]
- 34.O’Reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: braking to facilitate repair. Am J Physiol Lung Cell Mol Physiol 2001;281:L291–L305 [DOI] [PubMed] [Google Scholar]
- 35.Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev 2003;17:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet 1998;20:58–61 [DOI] [PubMed] [Google Scholar]
- 37.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94 [DOI] [PubMed] [Google Scholar]
- 38.Speer CP. Inflammation and bronchopulmonary dysplasia. Semin Neonatol 2003;8:29–38 [DOI] [PubMed] [Google Scholar]
- 39.Lyle RE, Tryka AF, Griffin WS, Taylor BJ. Tryptase immunoreactive mast cell hyperplasia in bronchopulmonary dysplasia. Pediatr Pulmonol 1995;19:336–343 [DOI] [PubMed] [Google Scholar]
- 40.Schultz ED, Potts EN, Mason SN, Foster WM, Auten RL. Mast cells mediate hyperoxia-induced airway hyper-reactivity in newborn rats. Pediatr Res 2010;68:70–74 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA 1986;83:4464–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson CK, Mori M, Bjermer L, Lofdahl CG, Erjefalt JS. Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:206–217 [DOI] [PubMed] [Google Scholar]
- 43.Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA. Reduced exercise capacity in children born very preterm. Pediatrics 2008;122:e287–e293 [DOI] [PubMed] [Google Scholar]
- 44.Aukland SM, Rosendahl K, Owens CM, Fosse K, Eide GE, Halvorsen T. Neonatal bronchopulmonary dysplasia predicts abnormal pulmonary HRCT in long term survivors of extreme preterm birth. Thorax 2009;64:405–410 [DOI] [PubMed] [Google Scholar]
- 45.Kinney JS, Robertsen CM, Johnson KM, Gaddis M, Wheeler R, Jackson MA, Daily DK. Seasonal respiratory viral infections. Impact on infants with chronic lung disease following discharge from the neonatal intensive care unit. Arch Pediatr Adolesc Med 1995;149:81–85 [DOI] [PubMed] [Google Scholar]
- 46.Hennessy EM, Bracewell MA, Wood N, Wolke D, Costeloe K, Gibson A, Marlow N. Respiratory health in pre-school and school age children following extremely preterm birth. Arch Dis Child 2008;93:1037–1043 [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW, Erle DJ, Abrink M, et al. The alphavbeta6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest 2012;122:748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watterberg KL, Murphy S. Failure of cromolyn sodium to reduce the incidence of bronchopulmonary dysplasia: a pilot study. The Neonatal Cromolyn Study Group. Pediatrics 1993;91:803–806 [PubMed] [Google Scholar]
- 49.Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbell AB, Palmer TW. Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 1997;156:1523–1529 [DOI] [PubMed] [Google Scholar]
- 50.Ng GY, Ohlsson A. Cromolyn sodium for the prevention of chronic lung disease in preterm infants. Cochrane Database Syst Rev 2001; (2):CD003059. [DOI] [PubMed] [Google Scholar]
- 51.Irani AM. Ocular mast cells and mediators. Immunol Allergy Clin North Am 2008;28:25–42 [DOI] [PubMed] [Google Scholar]
- 52.Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 2004;36:782–801 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.