Abstract
Rationale: HIV–tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS) is an immunopathological reaction to mycobacterial antigens induced by antiretroviral therapy. Prednisone reduces morbidity in TB-IRIS, but the mechanisms are unclear.
Objectives: To determine the effect of prednisone on the inflammatory response in TB-IRIS (antigen-specific effector T cells, cytokines, and chemokines).
Methods: Blood was taken from participants in a randomized placebo-controlled trial of prednisone for TB-IRIS, at 0, 2, and 4 weeks. Participants received prednisone at a dosage of 1.5 mg/kg/day for 2 weeks followed by 0.75 mg/kg/day for 2 weeks, or placebo at identical dosages.
Measurements and Main Results: Analyses included IFN-γ enzyme-linked immunospot (ELISPOT), reverse transcription-polymerase chain reaction on peripheral blood mononuclear cells after restimulation with heat-killed Mycobacterium tuberculosis, Luminex multiplex cytokine analysis of corresponding tissue culture supernatants, and Luminex multiplex cytokine analysis of serum. Fifty-eight participants with TB-IRIS (31 receiving prednisone, 27 receiving placebo) were included. In serum, significant decreases in IL-6, IL-10, IL-12 p40, tumor necrosis factor-α, IFN-γ, and IFN-γ–induced protein-10 concentrations during prednisone, but not placebo, treatment were observed. No differences in ELISPOT responses comparing prednisone and placebo groups were shown in response to ESAT-6 (early secreted antigen target-6), Acr1, Acr2, 38-kD antigen, or heat-killed H37Rv M. tuberculosis. Purified protein derivative ELISPOT responses increased over 4 weeks in the prednisone group and decreased in the placebo group (P = 0.007).
Conclusions: The beneficial effects of prednisone in TB-IRIS appear to be mediated via suppression of predominantly proinflammatory cytokine responses of innate immune origin, not via a reduction of the numbers of antigen-specific T cells in peripheral blood.
Keywords: human immunodeficiency virus, tuberculosis, immune reconstitution inflammatory syndrome, glucocorticoids
At a Glance Commentary
Scientific Knowledge on the Subject
Corticosteroid therapy has been shown to reduce morbidity and improve symptoms in paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome (TB-IRIS), a syndrome that may complicate antiretroviral therapy in TB-coinfected patients.
What This Study Adds to the Field
This study, nested within a clinical trial of prednisone for TB-IRIS, suggests that prednisone (but not placebo) reduces serum concentrations of IL-6, IL-10, IL-12 p40, TNF-α, IFN-γ, and IP-10 over 4 weeks of treatment. These findings also suggest that corticosteroids may act in TB-IRIS via suppression of proinflammatory cytokine concentrations.
Inflammatory pathology driven by the immune response to mycobacteria is a characteristic feature of treatment in tuberculosis (TB), leprosy, and other nontuberculous mycobacterial infections. Paradoxical reactions after commencement of TB treatment (1, 2), immune reconstitution inflammatory syndrome (IRIS) after initiation of antiretroviral therapy (ART) (3–5), and type 1 and 2 reactions in patients being treated for leprosy (6, 7) are all examples of treatment-induced immunopathology. Corticosteroids are used to modulate these immunopathological reactions and in certain forms of TB, such as meningitis and pericarditis, adjunctive treatment with corticosteroids has been shown to improve clinical outcomes (8–10). The benefit of corticosteroids is likely related to reducing pathological inflammation caused by the immune response, but mechanisms are not well characterized.
Paradoxical TB-IRIS occurs in 8–43% of HIV-infected patients who start ART while receiving treatment for TB (3). Within a few weeks of starting ART patients develop recurrent symptoms of TB and worsening of clinical and radiographic features of TB, such as enlargement of TB lymph nodes (3, 4). We have reported a randomized placebo-controlled clinical trial of prednisone for the treatment of paradoxical TB-IRIS (11). A 4-week course of prednisone reduced the duration of hospitalization and the number of outpatient therapeutic procedures. In addition, participants receiving prednisone experienced significant improvement in TB-IRIS symptoms and chest radiographs, and more rapid reduction in C-reactive protein (CRP). There was no mortality difference and no excess of severe infections in the prednisone-treated participants in this trial.
The immunological mechanisms underlying paradoxical TB-IRIS are only partially elucidated. Expansion of mycobacteria-specific effector T cells that produce IFN-γ after the start of ART has been associated with TB-IRIS in several studies (12–14). However, we have questioned the causal role of such expansions because we observed similar expansions in control patients with TB starting ART who did not develop TB-IRIS, and some patients with TB-IRIS did not demonstrate expansions at the time of IRIS onset (14). Proinflammatory cytokines, in particular tumor necrosis factor (TNF)-α, IFN-γ, and IL-6, likely play an important role in pathogenesis as their concentrations are significantly raised in patients with TB-IRIS (15).
In this study we analyzed samples collected during the randomized controlled trial of prednisone for paradoxical TB-IRIS (11) to assess the effect of prednisone on T-cell expansions, cytokine and chemokine gene expression, and protein concentrations after in vitro stimulation and cytokine/chemokine concentrations in vivo. The aim was to determine the effect of corticosteroids on the aberrant immune response in TB-IRIS. Some of the results of these studies have been previously reported in the form of an abstract (16).
Methods
Setting
The study was conducted at GF Jooste Hospital, a community-based referral hospital in Cape Town, South Africa. Most patients are commenced on TB treatment and ART in community primary care clinics, but are referred to GF Jooste Hospital when complications occur for investigations and/or admission. Patients with a first episode of TB are treated with rifampicin, isoniazid, ethambutol, and pyrazinamide for 2 months followed by rifampicin and isoniazid for 4 months. For subsequent episodes the treatment duration is 8 months, including streptomycin for the first 2 months. At the time of the study the preferred ART regimen for patients on TB treatment was stavudine, lamivudine, and efavirenz.
Clinical Trial
Between June 2, 2005 and December 20, 2007 we enrolled participants into a randomized placebo-controlled clinical trial of prednisone for the treatment of paradoxical TB-IRIS. The methods have been described in detail (11), but are summarized here. Consecutive patients with suspected paradoxical TB-IRIS referred to the hospital were screened using standardized case definitions for paradoxical TB-IRIS (3). We limited enrollment to four TB-IRIS manifestations. Only patients with new or recurrent tuberculosis symptoms and one or more of the following manifestations were enrolled: (1) infiltrate on chest radiograph, (2) enlarging lymph node(s), (3) serous effusion(s), or (4) cold abscess(es). Patients with immediately life-threatening TB-IRIS were excluded. TB diagnosis was made on the basis of culture, smear microscopy, or clinicoradiological diagnosis (17, 18).
Participants were randomized to receive either prednisone at 1.5 mg/kg/day for 2 weeks followed by 0.75 mg/kg/day for 2 weeks or placebo administered identically. If significant clinical deterioration occurred after 2 weeks of follow-up, the study protocol allowed participants to be switched to open-label prednisone or earlier if life-threatening deterioration occurred. Follow-up was for 12 weeks. The primary end point was the cumulative number of days hospitalized, and number of outpatient therapeutic procedures performed counted as one additional hospital day (11).
Timing of Blood Samples
Blood samples were taken from participants enrolled in the trial before starting study medication (Week 0) and then at Weeks 2 and 4. Placebo-treated participants who were switched to open-label prednisone were not excluded from this immunology study, but any sample taken after the switch to prednisone was excluded.
Participants
One hundred and ten patients were enrolled in the clinical trial (55 received placebo and 55 prednisone). Blood samples were taken from the first 73 participants in the clinical trial for this immunology study provided that laboratory staff were available to process specimens during working hours. If at least one repeat specimen (at 2 or 4 wk) was available then the participant was included in these analyses. The inclusion and exclusion of participants were not subject to systematic bias because the treatment allocation was randomized and unknown to clinical or laboratory staff at the time of specimen processing. To analyze results by treatment response, participants were classified as improvers (symptoms improving at Week 2 and no new symptoms) or nonimprovers (symptoms unchanged or worsened at Week 2 by comparison with Week 0 symptoms) irrespective of treatment allocation.
Ethical Permission and Trial Registration
The University of Cape Town Human Research Ethics Committee approved the study (337/2004). Written informed consent was obtained from all participants. The trial was registered with the International Standard Randomized Controlled Trial Number register (ISRCTN 21322548).
Peripheral Blood Mononuclear Cell Isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from 30 ml of blood collected in sodium-heparin BD Vacutainers (Preanalytical Solutions, BD Diagnostics, Franklin Lakes, NJ), using the Ficoll separation method (Ficoll-Paque PLUS, GE Healthcare Bio-Sciences AB, Uppsala, Sweden) on the day of collection.
ELISPOT Assay
ELISPOT analysis was performed as previously described (14, 19–21) on the same day. Antigenic stimuli were endotoxin free and were assayed in duplicate wells. Concentrations used were as follows: early secreted antigen target (ESAT)-6, 5 μg/ml; α-crystallin 1, 10 μg/ml; α-crystallin 2, 20 μg/ml; and 38-kD antigen, 2.5 μg/ml. Purified protein derivative (PPD) was used at 200 IU/ml (4.6 μg/ml) and heat-killed H37Rv Mycobacterium tuberculosis (MTB) was used at a multiplicity of infection (MOI) of 1:1 (200,000 H37Rv:200,000 PBMCs). Control wells included phytohemagglutinin (5 μg/ml) and no antigenic stimulus. The number of IFN-γ spot-forming cells/106 PBMCs on ELISPOT plates was counted on an ImmunoSpot series 3B analyzer (Cellular Technology Ltd, Cleveland, OH). Forty-one participants were included in the ELISPOT analysis (16 placebo-treated and 25 prednisone-treated).
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction after Mycobacterium tuberculosis Stimulation of PBMCs
After PBMC isolation, cells at 2.5 × 106/ml in RPMI–10% fetal calf serum were rested overnight in an incubator at 37°C in 5% CO2. Thereafter the PBMCs were restimulated with heat-killed H37Rv Mycobacterium tuberculosis for 24 hours at an MOI of 1:1 and unstimulated cultures were also incubated. After restimulation, PBMCs were harvested and lysed in 350 μl of buffer RLT for lysis (Qiagen, Valencia, CA). Lysates were collected for RNA analysis and tissue culture supernatants were preserved at –80°C until used in the Luminex multiplex experiments.
RNA was extracted from PBMC lysates according to the RNeasy mini kit spin protocol for isolation of total RNA from animal cells (Qiagen) as per the manufacturer’s instructions and stored at –80°C until further use. Primers and probes for reverse transcription-polymerase chain reaction (RT-PCR) were purchased from Applied Biosystems (Foster City, CA) as predesigned inventoried assay reagents. We used the following TaqMan gene expression assays: IL-1β, Hs00174097_m1 (catalogue number); IL-2, Hs00174114_m1; IL-4, Hs00174122_m1; IL-6, Hs00985639_m1; IL-8, Hs01038788_m1; IL-10, Hs00174086_m1; IL-12 p40, Hs01011518_m1; IL-13, Hs00174379_m1; IL-15, Hs00542562_m1; IL-17A, Hs00174383_m1; IL-22, Hs00220924_m1; IL-27, Hs00377399_m1; TNF-α, Hs00174128_m1; IFN-γ, Hs00174143_m1; granulocyte-macrophage colony-stimulating factor (GM-CSF), Hs00171266_m1; CCL3, Hs00234142_m1; CCL4, Hs99999148_m1; CCL5, Hs00174575_m1. RNA concentration was determined by NanoDrop ND 1000 (Thermo Scientific, Wilmington, DE) and samples were diluted to give an RNA working solution concentration of approximately 10 ng/μl. RT-PCR was performed according to the TaqMan RNA-to-CT 1-Step kit protocol (Applied Biosystems, Foster City, CA). The reaction mixture was prepared using the following outlined procedure: 1 μl of TaqMan gene expression assay, 10 μl of 2× buffer, 0.5 μl of RT enzyme, and 8.5 μl of diluted mRNA for each reaction. β-Actin was used as an endogenous control throughout. RT-PCR was performed on an ABI PRISM 7000 platform under the following universal thermal cycling conditions: reverse transcription at 48°C for 15 minutes, enzyme activation at 95°C for 15 seconds (40 cycles), annealing/primer extension at 60°C for 1 minute (40 cycles). Transcript abundance was calculated by subtracting the cycle threshold (CT) of β-actin from the CT of the gene of interest to derive a ΔCT value. Fold induction of genes in response to heat-killed H37Rv stimulation was calculated by the ΔΔCT method: the ΔCT of the unstimulated sample was subtracted from the ΔCT of the stimulated sample and 2 was then raised to the power of –ΔΔCT. Values obtained were normalized by log10 transformation and these values are reported. RT-PCR was performed in 25 participants (9 placebo-treated and 16 prednisone-treated), who had cells available for RNA extraction at Week 0 and at least one additional time point.
Luminex Multiplex Assay for Cytokine/Chemokine Concentrations in Supernatants and Serum
Supernatants from stimulated and unstimulated 24-hour cultures (see above) were later assayed for the following cytokines and chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p40, IL-13, IL-15, TNF-α, IFN-γ, IP-10, GM-CSF, CCL3, CCL4, and CCL5. These assays were performed in 96-well filter plates on the Bio-Plex platform (Bio-Rad Laboratories, Hercules, CA) using customized Milliplex kits (Millipore, St. Charles, MO) according to the manufacturer’s instructions. Assays were performed in 29 participants (12 placebo-treated and 17 prednisone-treated). The change in concentrations, subtracting background from stimulated, was used for comparisons.
Serum was stored at –80°C and, using the same protocols, the following cytokines/chemokines were assayed in serum samples at a later time point: IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12 p40, TNF-α, IFN-γ, IP-10, GM-CSF, CCL3, and CCL4. Assays were performed in 58 participants (27 placebo-treated and 31 prednisone-treated). In all Luminex multiplex experiments samples from the same patient were included on the same 96-well plate.
IL-8 Sandwich ELISA
A human IL-8 ELISA core kit (Koma Biotechnology, Seoul, South Korea) was used and the assay was performed according to the manufacturer’s instructions.
Statistical Analysis
Medians are presented with the interquartile range (IQR) and means are presented with the standard deviation. Categorical variables were compared by chi-square or Fisher exact test and continuous variables by Wilcoxon signed-rank test. Unpaired and paired normally distributed variables were compared by Student unpaired and paired t tests. Paired nonparametric variables were analyzed by paired Wilcoxon signed-rank test. To factor multiple comparisons, P values were multiplied by n – 1 (Bonferroni correction) where n = number of comparisons. STATA 10.1 (StataCorp, College Station, TX) and GraphPad Prism version 5.0a software (GraphPad Software, San Diego, CA) were used.
Results
Serial samples were available from 58 participants (27 placebo-treated and 31 prednisone-treated). Among these 58 participants, 33 were female (57%) and the median age was 31 years (IQR, 26–35). The median CD4+ lymphocyte count before ART was 56 cells/μl (IQR, 29–94) and at study enrollment the median CD4+ cell count was 116 cells/μl (IQR, 60–234). There were no significant differences between the placebo- and prednisone-treated participants in terms of demographics or TB and HIV disease characteristics (Table 1). ELISPOT was performed in 41 participants, RT-PCR in 25 participants, Luminex multiplex on supernatants of cultures stimulated with heat-killed H37Rv MTB of 29 participants, and Luminex multiplex on serum of 58 participants.
TABLE 1.
CLINICAL AND DEMOGRAPHIC CHARACTERISTICS OF PARTICIPANTS INCLUDED IN IMMUNOLOGICAL ANALYSES
| Variable | Placebo Treated (n = 27) | Prednisone Treated (n = 31) | P Value |
| Sex | |||
| Male | 13 (48%) | 12 (39%) | |
| Female | 14 (52%) | 19 (61%) | 0.8 |
| Age | 31 (27–36) | 31 (26–35) | 0.6 |
| Previous TB | 6 (22%) | 10 (32%) | 0.6 |
| Basis of TB diagnosis | |||
| Cultured Mycobacterium tuberculosis | 20 (74%) | 17 (55%) | |
| Acid-fast bacilli smear positive | 5 (19%) | 4 (13%) | |
| Clinicoradiological diagnosis | 2 (7%) | 10 (32%) | 0.07 |
| Extrapulmonary TB at TB diagnosis | 15 (56%) | 16 (52%) | 0.8 |
| CD4 count before ART, cells/μl | 58 (20–95) | 55 (31–94) | 0.6 |
| CD4 count at enrollment, cells/μl* | 107 (49–202) | 117 (73–278) | 0.2 |
| C-reactive protein at enrollment, mg/L | 100 (80–143) | 94 (42–150) | 0.6 |
| Duration TB treatment to ART, d | 43 (26–79) | 69 (35–96) | 0.09 |
| Duration ART to TB-IRIS symptom onset, d | 10 (7–17) | 14 (7–22) | 0.1 |
| TB-IRIS clinical manifestations† | |||
| New or enlarging lymph node(s) | 11 (41%) | 9 (29%) | 0.4 |
| New or expanding pulmonary infiltrate(s) | 6 (22%) | 9 (29%) | 0.8 |
| New or enlarging serous effusion(s) | 3 (11%) | 2 (6%) | 0.7 |
| Recurrent symptoms and infiltrate(s) on CXR but without baseline CXR available for comparison | 10 (37%) | 14 (45%) | 0.6 |
Definition of abbreviations: ART = antiretroviral therapy; CXR = chest radiograph; TB = tuberculosis; TB-IRIS = tuberculosis-associated immune reconstitution inflammatory syndrome.
Values shown represent numbers (percentage) or median (interquartile range). P values compare placebo- and prednisone-treated participants.
CD4 count at enrollment available for 24 placebo-treated and 27 prednisone-treated participants.
Participants may have had more than one TB-IRIS manifestation, and thus manifestations sum to more than 100%
ELISPOT
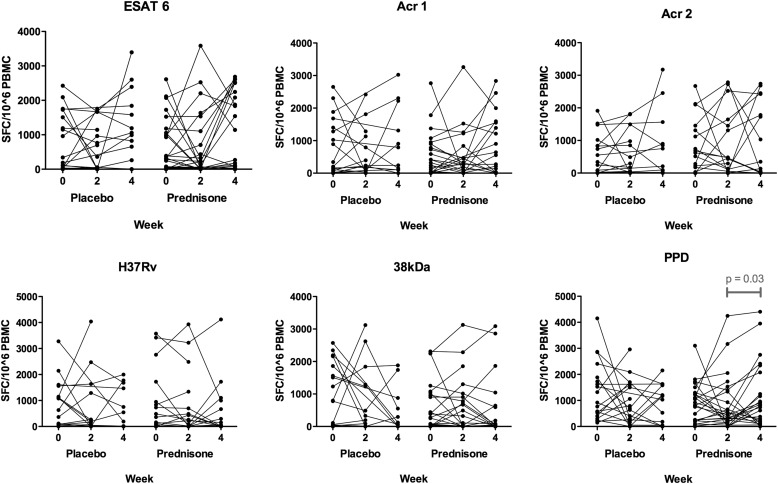
There were no significant differences in median number of spot-forming cells on comparing placebo- and prednisone-treated participants at each of the three time points (Weeks 0, 2, and 4) for any of the six antigenic stimuli (Figure 1). Longitudinal within-group analysis by paired Wilcoxon signed-rank test to compare Week 0 with Week 2, Week 0 with Week 4, and Week 2 with Week 4 revealed a difference only in the PPD-stimulated wells: there was an increase in the median number of spot-forming cells (SFCs) from 449 SFCs/106 PBMCs (IQR, 197–1,368) at Week 2 to 800 (IQR, 188–2,078) at Week 4 (P = 0.03) in prednisone-treated participants.
Figure 1.
Enzyme-linked immunospot results in response to overnight restimulation of peripheral blood mononuclear cells (PBMCs) with six different antigen stimuli at 0, 2, and 4 weeks, comparing placebo- and prednisone-treated participants. Antigen stimuli used were as follows: early secreted antigen target-6 (ESAT-6, 5 μg/ml), α-crystallin 1 (Acr1; 10 μg/ml), α-crystallin 2 (Acr2; 20 μg/ml), and 38-kD antigen (2.5 μg/ml); purified protein derivative (PPD) was used at 200 IU/ml (4.6 μg/ml) and heat-killed H37Rv Mycobacterium tuberculosis was used at a multiplicity of infection of 1:1 (200,000 H37Rv:200,000 PBMCs). Four patients in the placebo-treated arm switched to open-label prednisone at Week 2 and thus their Week 4 samples were excluded from analysis. Statistical comparisons of median values for each time point comparing placebo- and prednisone-treated participants showed no significant differences. Within-group paired comparisons between time points (0 vs. 2, 0 vs. 4, and 2 vs. 4 wk) revealed only one significant difference (P < 0.05): a rise in the number of spot-forming cells (SFCs) to PPD in the prednisone-treated participants from 2 to 4 weeks (from 449 SFCs/106 PBMCs [IQR, 197–1,368] at Week 2 to 800 [IQR, 188–2,078] at Week 4 [P = 0.03]).
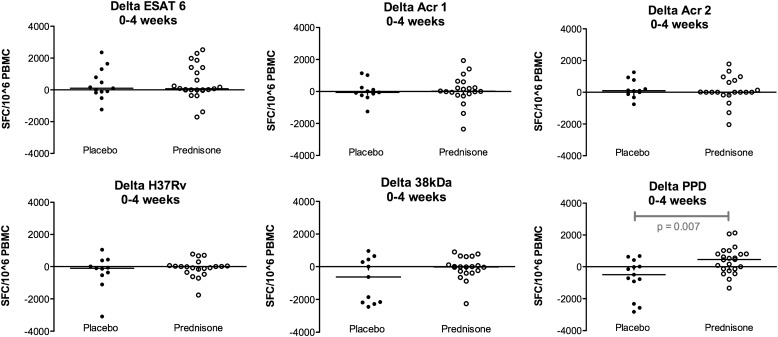
Next, delta values (Δ = Week 4 ELISPOT value – Week 0 ELISPOT value) were calculated and delta values for each antigenic stimulus were compared for placebo- and prednisone-treated participants. The only significant difference was for the PPD-stimulated samples: between Weeks 0 and 4 in placebo-treated participants the median ELISPOT value decreased by 496 SFCs/106 PBMCs (IQR, –1,623 to +217) and in prednisone-treated participants the median ELISPOT value increased by 455 SFCs/106 PBMCs (IQR, –140 to +807) (P = 0.007) (Figure 2).
Figure 2.
Changes in enzyme-linked immunospot (ELISPOT) responses between Week 0 and Week 4, comparing placebo- and prednisone-treated patients. These results relate to the same experiments as Figure 1, but show the change (delta) in responses over 4 weeks in placebo- and prednisone-treated participants. The only significant difference (P < 0.05) was with purified protein derivative (PPD): between Weeks 0 and 4 in placebo-treated participants the median ELISPOT value decreased by 496 spot-forming cells (SFCs)/106 peripheral blood mononuclear cells (PBMCs) (IQR, –1,623 to +217) and in prednisone-treated participants the median ELISPOT value increased by 455 SFCs/106 PBMCs (IQR, –140 to +807) (P = 0.007). 38kDa = 38-kD antigen; Acr1 = α-crystallin 1; Acr2 = α-crystallin 2; ESAT-6 = early secreted antigen target-6.
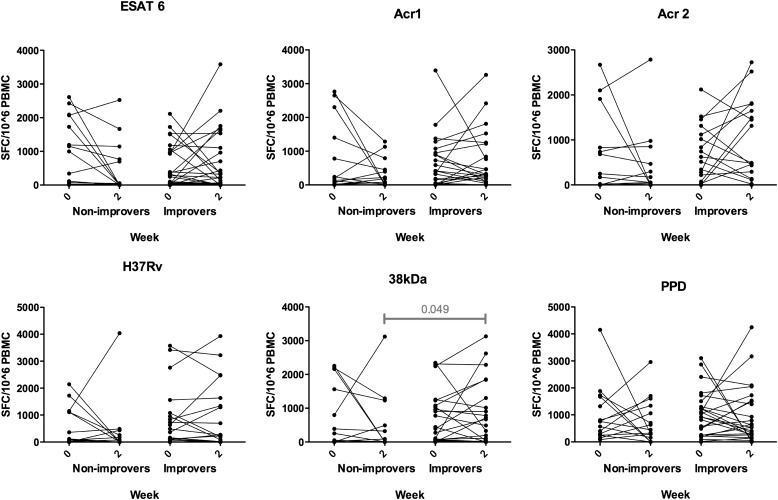
To assess the relationship between ELISPOT responses and change in symptoms over time, the median ELISPOT value at Week 0 and at Week 2 were compared for those who did, or did not, experience improvement by Week 2 irrespective of whether they were randomized to prednisone or placebo. Thereafter, within-group analyses comparing 0- and 2-week values were performed. No significant differences were shown, apart from borderline significant difference for 38-kD antigen, on comparing the values of those who improved with the values of those who did not improve at Week 2. In improvers the median was 580 SFCs/106 PBMCs (IQR, 30–1,435) and in nonimprovers it was 10 SFCs/106 PBMCs (IQR, 0–678) (P = 0.049) (Figure 3).
Figure 3.
Enzyme-linked immunospot responses at Weeks 0 and 2, comparing participants who had no improvement in symptoms during this time with patients who reported symptom improvement, regardless of treatment allocation. Statistical comparisons were made between nonimprovers and improvers at each time point and within-group paired analyses comparing 0- and 2-week time points. There was a significant difference (P < 0.05) for 38-kD antigen (38kDa), comparing values of responders with those of nonresponders at Week 2. In improvers the median was 580 spot-forming cells (SFCs)/106 peripheral blood mononuclear cells (PBMCs) (IQR, 30–1,435) and in nonimprovers it was 10 SFCs/106 PBMCs (IQR, 0–678) (P = 0.049). Acr1 = α-crystallin 1; Acr2 = α-crystallin 2; ESAT 6 = early secreted antigen target-6; PPD = purified protein derivative.
RT-PCR for Cytokine/Chemokine Gene Up-regulation in Response to MTB Stimulation
There were significant reductions in mean log fold induction in placebo-treated participants at Week 4 (compared with Week 0) for IL-1β, IL-6, IL-13, GM-CSF, and CCL3 (see Table E1 in the online supplement). There was a significant increase in mean log fold induction for IL-22 at Week 4 in placebo-treated participants and a decrease in prednisone-treated participants at Week 4 for IL-4 and IL-10 genes (all compared with Week 0). After Bonferroni correction the only change that remained significant was the decrease in mean log fold induction from 0.32 (± 0.42) at Week 0 to –0.26 (± 0.29) for IL-4 at Week 4 in prednisone-treated participants (Pcorr = 0.03).
To address the hypothesis that gene expression in unstimulated samples could reflect transcription by non-antigen–specific cells and that this would be specifically modified by prednisone, we analyzed ΔCT values in unstimulated samples for four cytokines known to be of predominantly myeloid cell origin (TNF-α, IL-6, IL-10, IL-12 p40) (Table E3). For TNF-α, IL-10, and IL-12 p40, these values did not change significantly over 4 weeks in either treatment arm. For IL-6 in prednisone (but not placebo)–treated participants there was a significant reduction in gene expression measured at Week 2 compared with Week 0 (ΔCT rose from 12.79 [IQR, 11.81–14.55] to 16.47 [IQR, 13.30–17.37]; P = 0.04), but not at Week 4 compared with Week 0.
Luminex Multiplex Analysis of Tissue Culture Supernatants
For placebo-treated participants in relation to Week 0 there were increases in concentrations of IFN-γ at Week 2, decreased IL-13 at Week 2, and decreased IL-1β, IL-6, IL-10, TNF-α, GM-CSF, CCL3, and CCL4 at Week 4 (Table E2). In prednisone-treated participants GM-CSF and CCL3 decreased at Week 2 and IL-1β and IL-6 decreased at both Week 2 and Week 4 in relation to the Week 0 value. After Bonferroni correction, none of these differences remained significant.
Luminex Multiplex Analysis of Serum
Luminex multiplex analysis of serum from prednisone-treated participants showed significant declines from Week 0 to Week 2 and from Week 0 to Week 4, after correcting for multiple comparisons, for IL-6, IL-10, IL-12 p40, IP-10, and TNF-α concentrations (Table 2 and Figure 4). For IFN-γ there was a significant decrease from Week 0 to Week 4, but not at Week 2. There was no significant decrease in IL-8, CCL3, or CCL4 in prednisone-treated participants after correction for multiple comparisons. In placebo-treated participants none of these cytokines or chemokines declined significantly at the Week 2 or Week 4 time point compared with Week 0 after correction for multiple comparisons. Concentrations of IL-1β, IL-2, and GM-CSF were undetectable in most samples and there was no significant change in values over 4 weeks in either treatment group.
TABLE 2.
LUMINEX MULTIPLEX ASSAY OF CYTOKINE AND CHEMOKINE CONCENTRATIONS IN SERUM, COMPARING PARTICIPANTS TREATED WITH PLACEBO VERSUS PATIENTS TREATED WITH PREDNISONE AT WEEKS 0, 2, AND 4 ON TRIAL
| Placebo Treated |
Prednisone Treated |
|||||||||||||
| Week 0 | Week 2 | Week 4 | P | Pcorr | P | Pcorr | Week 0 | Week 2 | Week 4 | P | Pcorr | P | Pcorr | |
| (n = 27) | (n = 24) | (n = 18) | (0–2) | (0–2) | (0–4) | (0–4) | (n = 31) | (n = 31) | (n = 29) | (0–2) | (0–2) | (0–4) | (0–4) | |
| IL-1β | 0 | 0 | 0 | 1.0 | 1.0 | 0 | 0 | 0 | 1.0 | 0.5 | ||||
| 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | |||||||||
| IL-2 | 0 | 0 | 0 | 1.0 | 1.0 | 0 | 0 | 0 | 0.55 | 0.53 | ||||
| 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | |||||||||
| IL-6 | 35.5 | 17.2 | 17.2 | 0.005* | 0.05 | 0.03* | 0.36 | 21.7 | 9.0 | 7.7 | <0.0001* | <0.001* | 0.0002* | 0.002* |
| 20.4–57.1 | 6.8–30.2 | 5.0–37.0 | 11.8–42.7 | 0–11.5 | 0–16.3 | |||||||||
| IL-8 | 70.6 | 72.7 | 66.9 | 0.25 | 0.26 | 50.6 | 49.9 | 50.8 | 0.008* | 0.09 | 0.12 | |||
| 40.7–146.0 | 31.0–132.9 | 26.2–150.9 | 35.2–117.1 | 20.7–72.5 | 26.6–85.6 | |||||||||
| IL-10 | 8.8 | 10.1 | 8.5 | 0.16 | 0.56 | 15.0 | 8.8 | 9.5 | 0.001* | 0.01* | 0.0001* | 0.001* | ||
| 4.6–25.7 | 4.1–22.4 | 3.6–17.9 | 7.0–22.5 | 4.6–17.2 | 2.5–14.4 | |||||||||
| IL-12 p40 | 25.0 | 13.0 | 25.5 | 0.13 | 0.94 | 11.9 | 0 | 0 | 0.0001* | 0.001* | 0.0002* | 0.002* | ||
| 11.9–55.8 | 0–32.8 | 4.4–219.9 | 0–56.1 | 0–10.2 | 0–16.5 | |||||||||
| TNF-α | 51.1 | 47.3 | 41.3 | 0.08 | 0.24 | 52.4 | 26.9 | 25.9 | <0.0001* | <0.001* | <0.0001* | <0.001* | ||
| 34.4–105.1 | 22.0–95.6 | 24.4–102.9 | 33.1–106.2 | 21.1–44.3 | 17.7–50.2 | |||||||||
| IFN-γ | 27.4 | 24.6 | 29.9 | 0.10 | 0.54 | 27.2 | 15.9 | 12.4 | 0.10 | 0.0007* | 0.008* | |||
| 12.8–68.9 | 8.2–71.9 | 15.7–52.9 | 18.2–57.8 | 4.1–45.9 | 2.5–49.6 | |||||||||
| IP-10 | 3,801 | 3,330 | 2,736 | 0.07 | 0.008* | 0.09 | 5,487 | 3,728 | 3,126 | 0.0001* | 0.001* | 0.0005* | 0.006* | |
| 2,299–9,840 | 1,972–4,229 | 1,943–3,648 | 2,798–9,998 | 2,309–5,162 | 2,068–4,896 | |||||||||
| GM-CSF | 0 | 0 | 0 | 0.50 | 1.0 | 0 | 0 | 0 | 0.94 | 0.38 | ||||
| 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | |||||||||
| CCL3 | 29.8 | 23.3 | 35.2 | 0.79 | 0.04* | 0.45 | 26.6 | 15.5 | 24.3 | 0.66 | 0.63 | |||
| 0–78.6 | 0–66.8 | 3.3–169.6 | 0–50.4 | 0–47.2 | 0–85.1 | |||||||||
| CCL4 | 71.6 | 53.0 | 69.1 | 0.03* | 0.37 | 0.19 | 78.3 | 81.9 | 81.8 | 0.02* | 0.26 | 0.73 | ||
| 40.8–100.4 | 37.4–78.8 | 56.1–132.1 | 55.0–110.7 | 44.6–100.1 | 53.4–115.5 | |||||||||
Definition of abbreviations: GM-CSF = granulocyte-macrophage colony-stimulating factor; IP-10 = IFN-γ–induced protein-10; Pcorr = P value after Bonferroni correction; TNF-α = tumor necrosis factor-α.
Results shown are cytokine/chemokine concentrations assayed with Luminex multiplex in serum. Median and interquartile range values are presented and units are picograms per milliliter. P values were calculated for comparisons between 0- and 2-week values and between 0- and 4-week values for each treatment group separately, using the Wilcoxon matched-pairs test. Four patients in the placebo-treated arm switched to open-label prednisone at Week 2 and thus their Week 4 samples were excluded from analysis.
Significant differences. Uncorrected P values are shown, and where the uncorrected P value was less than 0.05 a corrected P value is also shown. The Bonferroni correction for multiple comparisons (Pcorr = P × 12 – 1 = P × 11) was used.
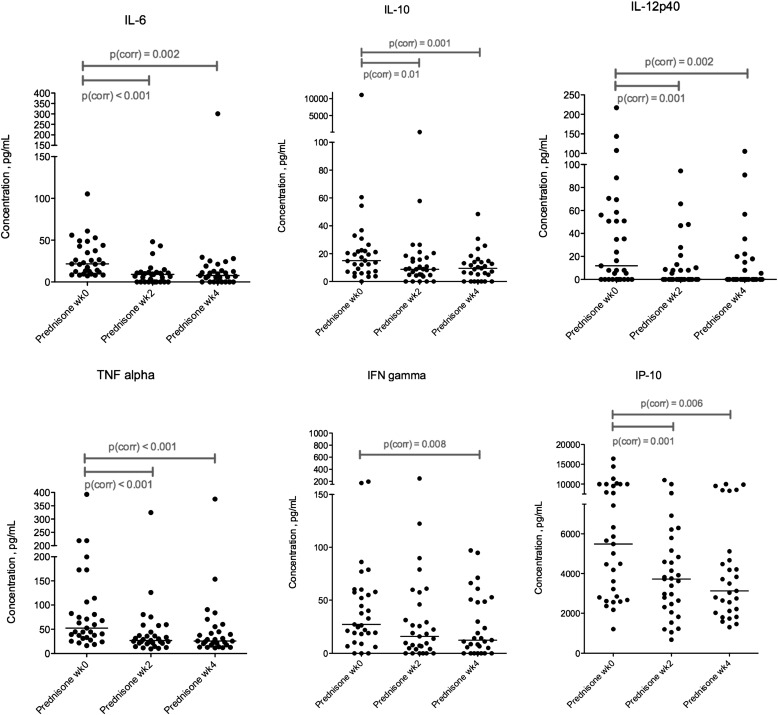
Figure 4.
Serum Luminex multiplex assay showing results for IL-6, IL-10, IL-12 p40, tumor necrosis factor (TNF)-α, IFN-γ, and IFN-γ–induced protein-10 (IP-10) concentrations in prednisone-treated participants at Weeks 0, 2, and 4. Statistical comparisons were performed using the matched-pairs Wilcoxon test and corrected for multiple comparisons with the Bonferroni correction. There were significant reductions (Pcorr < 0.05) between Weeks 0 and 2 for all these cytokines/chemokine apart from IFN-γ. There were significant reductions for all between Weeks 0 and 4. Full results of these experiments are shown in Table 2.
In addition, we compared the change (delta) in serum cytokine concentrations between Week 0 and Week 2 for those participants who experienced symptom improvement versus those who experienced no improvement or deteriorated during this time irrespective of treatment allocation (Table E4). No significant differences between these two groups were demonstrated for any of the 12 cytokines/chemokines.
Discussion
We have previously demonstrated in a randomized clinical trial that corticosteroid therapy results in clinical benefit in paradoxical TB-IRIS (11). We have also previously demonstrated that TB-IRIS is associated with hypercytokinemia (15), the most consistently elevated cytokines being IL-6, TNF-α, and IFN-γ. In the current study we sought to identify the immune mechanism of action of prednisone in TB-IRIS and thus potentially gain insight into the pathogenesis of TB-IRIS.
Corticosteroids are known to exert antiinflammatory effects on immune cells through direct effects on transcription of inflammatory mediators via the glucocorticoid-responsive element, indirect genomic effects via interference with other transcriptional factors such as NF-κB and AP-1, and nongenomic effects on antiinflammatory proteins (22). These effects result in increased transcription of a number of antiinflammatory mediators and decreased transcription of proinflammatory cytokines, chemokines, enzymes, receptors, and adhesion molecules. In addition, corticosteroids have been shown to reduce T-cell survival by enhancing apoptosis (23).
Our most important finding was that serum concentrations of IL-6, IL-10, IL-12 p40, IP-10, and TNF-α were reduced in prednisone-treated participants at 2 and 4 weeks on trial compared with baseline after correcting for multiple comparisons. The decrease in IFN-γ occurred later, at the Week 4 time point. Similar decreases were not seen in placebo-treated participants. Many of these cytokines are of myeloid origin (IL-10, IL-12 p40, IP-10) or of combined myeloid and lymphoid origin (TNF-α and IL-6). These findings concur with reports that suggest myeloid cells may play an important role in TB-IRIS pathogenesis (24, 25).
We have previously reported that among patients treated with prednisone in this trial, CRP fell from a median 104 mg/L at Week 0 to 35 mg/L at Week 2. In placebo-treated patients the decrease was more gradual (11). This corresponds with our finding here that there is a significant fall in IL-6 in prednisone-treated but not placebo-treated patients, as IL-6 is the major stimulus of CRP production (26). The reduction in the regulatory cytokine IL-10 may have been a direct corticosteroid effect or as a consequence of decreased inflammatory activity.
We previously reported dynamic and heterogeneous expansions and contractions of tuberculosis-specific IFN-γ–secreting T cells in both TB-IRIS case subjects and control subjects who do not develop IRIS (14). Similar ELISPOT patterns were noted during the 4-week course of treatment among patients in this trial. These were largely unaffected by prednisone therapy. The only significant finding was the difference in PPD-specific cells with an increase in prednisone-treated participants and a decrease in placebo-treated participants from Week 0 to 4. A plausible explanation is that the antiinflammatory effect of prednisone at tissue sites of IRIS resulted in recirculation of these effector cells into peripheral blood and reduced recruitment. We thus found no evidence that the effect of prednisone in TB-IRIS was mediated via a reduction in numbers of antigen-specific effector T cells in peripheral blood, although it did reduce IFN-γ production at Week 4. It is possible that this effect was mediated at a transcriptional level although we did not demonstrate this in ex vivo restimulation assays.
Although prednisone did not reduce numbers of mycobacteria-specific T cells in peripheral blood it may reduce their trafficking to sites of inflammation, a hypothesis that we were unable to explore in our experiments which were focused on studies of peripheral blood.
The findings of the 24-hour stimulation experiments, which assessed changes in cytokine/chemokine gene expression by RT-PCR and protein concentration by Luminex multiplex in response to MTB stimulation, showed few differences, and only a reduction in gene expression of IL-4 at Week 4 in prednisone-treated participants remained significant after correcting for multiple comparisons. We interpret this to indicate that prednisone administered to patients does not have a significant effect that persists in tissue culture experiments during 24-hour ex vivo restimulation. An alternative explanation for the discrepant findings between the tissue culture and serum Luminex multiplex experiments is that the tissue culture experiments reflect production by cells in peripheral blood whereas measurement of serum cytokine and chemokine protein concentrations using Luminex may reflect production by cells in peripheral blood as well as “spillover” of cytokines and chemokines produced by activated cells at sites of tissue inflammation.
A clinical trial conducted in Vietnam demonstrated that adjunctive dexamethasone for TB meningitis reduced mortality (10). In this trial, as was the case in our study, corticosteroids had no significant effect on ELISPOT responses to ESAT-6 and there were no effects on monocyte-derived cytokine concentrations after ex vivo restimulation of whole blood with mycobacterial antigens (27). In contrast to our findings, dexamethasone did not significantly alter concentrations of a number of cytokines and chemokines assayed. However, in the Vietnamese study concentrations were assayed in cerebrospinal fluid not serum. Similarly, in children with TB meningitis, prednisone did not significantly alter TNF-α, IL-1β, and IFN-γ concentrations in cerebrospinal fluid (28). The difference in our findings could be that we analyzed blood. TB-IRIS frequently causes a systemic inflammatory syndrome characterized by fever, tachycardia, and weight loss and this is reflected in high proinflammatory cytokine concentrations in blood (15), which are beneficially modulated by corticosteroids. This may also reflect that corticosteroids have different immunological effects in TB-IRIS compared with their use as adjunctive treatment at the time of TB diagnosis. A study of high-dose prednisolone in patients with HIV-associated smear-positive pulmonary TB and CD4+ cell counts equal to or exceeding 200 cells/μl did, however, demonstrate a reduction in serum TNF-α in patients taking prednisolone (29).
Strengths of our study were that treatment allocation was randomized, reducing potential biases; there were no significant differences in demographic and disease characteristics between treatment groups; and participants were extensively investigated to exclude alternative causes for deterioration. An important limitation was the small sample size in the 24-hour stimulation assays because insufficient serial samples were available. This may have limited power to detect small differences. There was a trend toward a longer duration between TB treatment and ART initiation and a trend toward more empiric diagnoses of TB among prednisone-treated participants, but the differences were not statistically significant.
Our findings suggest that the mechanism of corticosteroid action in TB-IRIS is at least partially mediated by a reduction in concentrations of proinflammatory cytokines. It raises the question as to whether more targeted antiinflammatory therapy with TNF-α blockers or an IL-6 blocker (tocilizumab) (30) may be effective for severe cases such as enlarging intracerebral tuberculomas that are potentially life-threatening and may respond poorly to corticosteroids (31).
Supplementary Material
Acknowledgments
The authors are indebted to Priscilla Mouton, the study nurse, for her hard work. The authors also acknowledge the assistance of Tolu Oni with patient recruitment.
Footnotes
Supported by the Medical Research Council of South Africa, the Wellcome Trust (081667, 084323, 088316, 084670), and EDCTP (060613). G. Meintjes and D.J.P. received SATBAT research training that was funded by the Fogarty International Center and the NIH (NIH/FIC 1 U2RTW007373-01A1 and U2RTW007370). G. Maartens was supported in part by grant U2RTW007370 from the Fogarty International Center. D.J.P. was supported by funding from the U.S. Agency for International Development and PEPFAR via the Perinatal HIV Research Unit. R.J.W. received funding from the United Kingdom (MRC) (u.1175.02.002.00014.01). The Gulf Drug Co. (Durban, South Africa) donated the prednisone and placebo tablets.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center, the National Institutes of Health, USAID, or the United States Government. The sponsors had no role in study design; data collection, analysis, and interpretation; writing of the manuscript; or the decision to submit for publication.
Author Contributions: The clinical trial was designed by G. Maartens, R.J.W., and G. Meintjes. G. Meintjes, K.R., D.J.P., and M.X.R. assessed and managed participants in the clinical trial and recorded clinical data. C.M. and G. Meintjes analyzed clinical data. The immunology study was designed by R.J.W., G. Meintjes, K.A.W., and R.C. The laboratory experiments were conducted by K.H.S., K.A.W., G. Meintjes, K.M., R.T., R.S., and A.C.-B. Laboratory data analysis was performed by G. Meintjes, K.H.S., and A.C.-B. G. Meintjes wrote the first draft of the manuscript, which was critically reviewed by all authors.
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201201-0094OC on June 14, 2012
Author disclosures are available with the text of this article at www.atsjournals.org
References
- 1.Hawkey CR, Yap T, Pereira J, Moore DA, Davidson RN, Pasvol G, Kon OM, Wall RA, Wilkinson RJ. Characterization and management of paradoxical upgrading reactions in HIV-uninfected patients with lymph node tuberculosis. Clin Infect Dis 2005;40:1368–1371 [DOI] [PubMed] [Google Scholar]
- 2.Cheng VC, Yam WC, Woo PC, Lau SK, Hung IF, Wong SP, Cheung WC, Yuen KY. Risk factors for development of paradoxical response during antituberculosis therapy in HIV-negative patients. Eur J Clin Microbiol Infect Dis 2003;22:597–602 [DOI] [PubMed] [Google Scholar]
- 3.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008;8:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med 2009;30:797–810 (x). [DOI] [PubMed] [Google Scholar]
- 5.Phillips P, Bonner S, Gataric N, Bai T, Wilcox P, Hogg R, O'Shaughnessy M, Montaner J. Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis 2005;41:1483–1497 [DOI] [PubMed] [Google Scholar]
- 6.Walker SL, Lockwood DN. Leprosy type 1 (reversal) reactions and their management. Lepr Rev 2008;79:372–386 [PubMed] [Google Scholar]
- 7.Girdhar BK, Girdhar A, Chakma JK. Advances in the treatment of reactions in leprosy. Indian J Lepr 2007;79:121–134 [PubMed] [Google Scholar]
- 8.Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 2000;84:183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strang JI, Kakaza HH, Gibson DG, Allen BW, Mitchison DA, Evans DJ, Girling DJ, Nunn AJ, Fox W. Controlled clinical trial of complete open surgical drainage and of prednisolone in treatment of tuberculous pericardial effusion in Transkei. Lancet 1988;2:759–764 [DOI] [PubMed] [Google Scholar]
- 10.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 2004;351:1741–1751 [DOI] [PubMed] [Google Scholar]
- 11.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 2010;24:2381–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS 2006;20:F1–F7 [DOI] [PubMed] [Google Scholar]
- 13.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, Huffam S, Oelrichs R, Sophea P, Saphonn V, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis 2009;200:1736–1745 [DOI] [PubMed] [Google Scholar]
- 14.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, Seldon R, Pepper DJ, Rebe K, Mouton P, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis–associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med 2008;178:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tadokera R, Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Seldon R, Chegou NN, Maartens G, Rangaka MX, Rebe K, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J 2011;37:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meintjes G, Skolimowska K, Wilkinson K, Conesa-Botella A, Matthews K, Tadokera R, Seldon R, Pepper D, Maartens G, Wilkinson R. Corticosteroid modulated myeloid immune activation in HIV/TB-IRIS. Abstract 146 presented at the 19th Conference on Retroviruses and Opportunistic Infections, Seattle, WA, March 5–8, 2012.
- 17.Wilson D, Nachega J, Morroni C, Chaisson R, Maartens G. Diagnosing smear-negative tuberculosis using case definitions and treatment response in HIV-infected adults. Int J Tuberc Lung Dis 2006;10:31–38 [PubMed] [Google Scholar]
- 18.Saranchuk P, Boulle A, Hilderbrand K, Coetzee D, Bedelu M, van Cutsem G, Meintjes G. Evaluation of a diagnostic algorithm for smear-negative pulmonary tuberculosis in HIV-infected adults. S Afr Med J 2007;97:517–523 [PubMed] [Google Scholar]
- 19.Wilkinson KA, Kon OM, Newton SM, Meintjes G, Davidson RN, Pasvol G, Wilkinson RJ. Effect of treatment of latent tuberculosis infection on the T cell response to Mycobacterium tuberculosis antigens. J Infect Dis 2006;193:354–359 [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson KA, Simsova M, Scholvinck E, Sebo P, Leclerc C, Vordermeier HM, Dickson SJ, Brown JR, Davidson RN, Pasvol G, et al. Efficient ex vivo stimulation of Mycobacterium tuberculosis–specific T cells by genetically detoxified Bordetella pertussis adenylate cyclase antigen toxoids. Infect Immun 2005;73:2991–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, Maskell N, Davies R, Pasvol G, Lalvani A. Ex vivo characterization of early secretory antigenic target 6–specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis 2005;40:184–187 [DOI] [PubMed] [Google Scholar]
- 22.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005;353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 23.Payne DN, Adcock IM. Molecular mechanisms of corticosteroid actions. Paediatr Respir Rev 2001;2:145–150 [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS 2009;23:143–145 [DOI] [PubMed] [Google Scholar]
- 25.Oliver BG, Elliott JH, Price P, Phillips M, Saphonn V, Vun MC, Kaldor JM, Cooper DA, French MA. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis 2010;202:1728–1737 [DOI] [PubMed] [Google Scholar]
- 26.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons CP, Thwaites GE, Quyen NT, Chau TT, Mai PP, Dung NT, Stepniewska K, White NJ, Hien TT, Farrar J. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J Immunol 2005;175:579–590 [DOI] [PubMed] [Google Scholar]
- 28.Donald PR, Schoeman JF, Beyers N, Nel ED, Carlini SM, Olsen KD, McCracken GH. Concentrations of interferon γ, tumor necrosis factor α, and interleukin-1β in the cerebrospinal fluid of children treated for tuberculous meningitis. Clin Infect Dis 1995;21:924–929 [DOI] [PubMed] [Google Scholar]
- 29.Mayanja-Kizza H, Jones-Lopez E, Okwera A, Wallis RS, Ellner JJ, Mugerwa RD, Whalen CC. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J Infect Dis 2005;191:856–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 2008;4:767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackmore TK, Manning L, Taylor WJ, Wallis RS. Therapeutic use of infliximab in tuberculosis to control severe paradoxical reaction of the brain and lymph nodes. Clin Infect Dis 2008;47:e83–e85 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.