Abstract
Purpose
To better understand the effects of severe glaucoma on the thickness of the retinal ganglion cell (RGC) and inner plexiform (IP) layers measured with frequency-domain optical coherence tomography.
Methods
In experiment 1, macular cube scans were obtained in 11 patients with glaucoma and the thickness of both the RGC and IP layers were measured at locations corresponding to 3, 5, and 7° eccentricity. For patients, only locations with total deviation losses of −15 dB or worse on perimetry were included. In experiment 2, higher resolution, horizontal midline scans were obtained from 30 controls in order to obtain a precise measure of the thickness of the RGC and IP layers of the healthy retina.
Results
In regions of severe field loss (experiment 1), glaucoma decreased the thickness of both layers, leaving a residual layer. The residual thickness of the IP layer was larger than the residual thickness of the RGC layer. In healthy controls (experiment 2), the RGC layer was about 57% of the RGC+IP layer thickness at 3° as compared with only 36% at 10°, in agreement with a recent histological study.
Conclusion
Glaucomatous optic neuropathy, with severe losses in visual field sensitivity, decreases the thickness of both the RGC and IP layers, but leaves a residual thickness of both. The IP layer contributes slightly more than the RGC to this residual, even just outside the center of the fovea where the RGC layer thickness exceeds the IP layer thickness in controls.
Keywords: glaucoma, OCT, ganglion cell layer, inner plexiform layer
Introduction
Glaucomatous optic neuropathy has been traditionally diagnosed based upon structural (optic disc appearance) and functional (visual field (VF)) abnormalities. In the last 10 years, imaging techniques have allowed for structural measurements of the nerve fiber rim and retinal nerve fiber layer (RNFL) thicknesses and for quantitative comparisons of structural thickness to VF loss.1, 2, 3, 4, 5, 6, 7, 8 However, because these techniques measure RNFL thickness, they provide an indirect measure of local retinal ganglion cell (RGC) damage.
With the advent of the higher resolution frequency-domain optical coherence tomography (fdOCT), it became possible to measure local RGC thickness in all the important macular region.9, 10 In a recent study, Raza et al11 found a good correlation between local RGC plus inner plexiform (IP) layer thickness and local loss of VF sensitivity in patients with glaucoma, within about central 8° of fixation. Interestingly, the RGC+IP layer thickness reached an asymptotic thickness of about 45 μm, no matter how extreme the field loss was. The authors suggested that this relatively large residual, which was about one-half of the RGC+IP layer at its thickest point, might be due to a relative preservation of the IP layer.
Raza et al,11 however, could not test this hypothesis, as it was not possible to reliably discern separate RGC and IP layers in their images. In the present study, we will make use of higher resolution scans. In experiment 1, we analyzed local changes in both RGC and IP layer thicknesses in patients with severe VF losses secondary to glaucoma, scanned under conditions allowing for better visualization of both the RGC and IP layers.
To better understand how the relative thickness of the RGC and IP layers varies with eccentricity in healthy eyes, we also examined the thickness of these layers in higher resolution horizontal OCT line scans. The results of experiment 2 will be compared with a recent histological study of the postmortem human retina.12
Materials and methods
Experiment 1
Retinal layers from patients with glaucoma were studied with cube scans and an eye-tracking system, which improved the definition between RGC and IP layers. To directly measure the relative loss in RGC and IP layers due to glaucoma, regions with severe losses in field sensitivity and clearly discernable separate RGC and IP layers were examined.
Participants
We tested 11 patients with glaucoma (mean age 65±8.21 years) (Table 1) who were diagnosed based on ophthalmological examination and VF. Each patient was invited to take part in the study only if the inclusion criteria was satisfied, which included the presence of severe VF loss (−15 dB) inside the central 10°, reliable VFs and absence of systemic diseases or any other ophthalmological pathology besides glaucoma. Results were compared with 20 age-matched, healthy controls with no ophthalmological or systemic diseases.
Table 1. Demographic data of the patients with glaucoma.
| Patient no | Code | Age | Gender | Eye | VA | IOP | TD (10°) | MD | PSD | TG |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7343 | 62 | M | OS | 20/30 | 8 | −13.18 | −16.90 | 13.62 | POAG (NTG) |
| 2 | 7467 | 72 | M | OD | 20/25 | 13 | −3.87 | −2.95 | 7.35 | POAG |
| 3 | 7481 | 59 | M | OD | 20/15 | 21 | −13.18 | −12.07 | 14.16 | POAG |
| 4 | 7494 | 64 | F | OD | 20/20 | 14 | −7.62 | −7.68 | 8.37 | POAG |
| 5 | 7673 | 61 | F | OS | 20/20 | 12 | −15.81 | −18.39 | 13.89 | POAG |
| 6 | 7755 | 61 | F | OD | 20/15 | 13 | −17.06 | −13.03 | 16.68 | POAG (NTG) |
| 7 | 7811 | 81 | F | OS | 20/25 | 10 | −9.25 | −6.51 | 8.99 | POAG |
| 8 | 7831 | 76 | F | OS | 20/30 | 15 | −28.00 | −26.68 | 10.00 | XFG |
| 9 | 7269 | 52 | M | OD | 20/30 | 14 | −19.31 | −14.71 | 13.79 | POAG |
| 10 | 7245 | 64 | F | OS | 20/20 | 12 | −29.68 | −29.10 | 6.46 | POAG |
| 11 | 7322 | 66 | M | OD | 20/25 | 10 | −10.93 | −10.68 | 10.45 | POAG |
| Mean | 65.27 | 12.91 | −15.26 | −14.43 | 11.25 | |||||
| SD | 8.21 | 3.39 | −8.01 | −8.05 | 3.33 |
Abbreviations: F, female; IOP, intraocular pressure (at the time of the visual field evaluation and OCT measurements); M, male; MD, mean deviation; NTG, normal tension glaucoma; POAG, primary open angle glaucoma; PSD, pattern standard deviation; TG, type of glaucoma; TD, total deviation (inside 10 degrees); VA, visual acuity; XFG, exfoliative glaucoma.
Equipment and procedures
Standard automated perimetry
VFs were performed using the Humphrey Field Analyzer program 24-2 SITA Standard (Carl Zeiss Meditec, Dublin, CA, USA). The total deviation (TD) score, which is the difference in dB in the patient's sensitivity when compared with an age-matched control group, was used for the analysis. The 16 field points of the 24-2 pattern within the central 10° were examined to identify the regions with a TD value equal to −15 dB or worse.
OCT
All the individuals were scanned with an fdOCT (Spectralis HRA-OCT; Heidelberg Engineering, Vista, CA, USA) using the eye-tracking feature and a three-dimensional/volume macular scan protocol, which consisted of 128 horizontal lines (B-scans) across a 6 × 6 mm2 area, a protocol routinely employed in our clinic. These scans, centered on the fovea, were about 20° in height and width and had images of the necessary quality, that is, with no blinking or movement artifacts, for the segmentation procedure described below
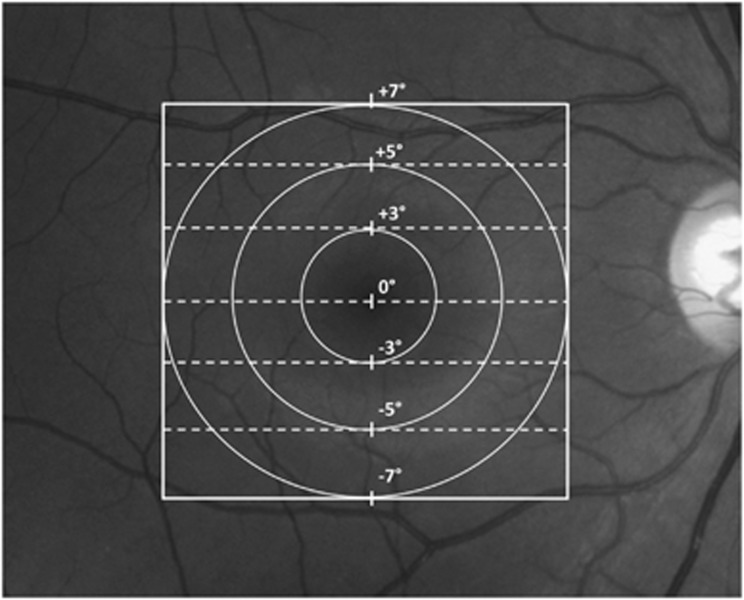
The OCT images were exported to a program written in Matlab 711 (MathWorks, Natick, MA, USA). The retinal layers were segmented with a computer-aided, manual segmentation procedure,9, 13 previously shown to produce reliable and reproducible results.14 The images corresponding to the seven scan lines were analyzed (Figure 1). In particular, for each scan the following borders were determined: (1) RNFL/RGC; (2) RGC/IP layer and (3). IP layer/ inner nuclear layer (INL). The central line scan passes through the foveal center and is the 0° line (Figure 1).
Figure 1.
Macular locations of the scans at 0, 3, 5, and 7°.
Analysis of RGC and IP layer thicknesses at fixed eccentricities
For all patients and controls, the thickness of the RGC+IP, RGC, and IP layers were determined at three retinal eccentricities, 3, 5, and 7° (circles in Figure 1), by averaging the thicknesses at the points where the seven scan lines (dashed lines in Figure 1) crossed the circles. Thus, there were four points at 3° eccentricity, 8 points at 5°, and 12 points at 7°. For each individual, the average thickness at each eccentricity was determined. To simplify the presentation of the data, the thicknesses for upper and lower scans of corresponding eccentricities were averaged, as the values were not statistically different.
For the patients, the same analysis was performed, but only the locations associated with losses of −15 dB or more were included. Based on the relationship between RGC and VF location,15 we estimated that the RGCs at 3, 5, and 7° on the retina corresponded to the light falling on the receptors at about 5.1, 6.5, and 8.1°, respectively. When the field point did not correspond exactly to the ganglion cell location according to the relationship suggested,11, 15 we used a linear interpolation.
Experiment 2
To obtain a more precise measure of the relative thickness of the RGC and IP layers in healthy eyes, we obtained high-resolution line scans averaged across the horizontal meridian.
Participants
For this experiment, 30 eyes from 30 healthy controls were studied (35.7±14.0 years); 20 of these eyes were part of a control group previously published.13 All the participants satisfied the inclusion criteria for controls, which were absence of ophthalmological or systemic diseases. Participants included in experiment 2 were not the same as those included in experiment 1.
Equipment and procedures
OCT
All the individuals were scanned with a fdOCT (Spectralis HRA-OCT; Heidelberg Engineering) using the eye-tracking feature. To obtain high-resolution scans, 100 horizontal line scans taken through the fovea were averaged. The exported scans were segmented as described in experiment 1, and measurements of the thickness of the RGC+IP, RGC, and IP layers were obtained as well
In both experiments, informed consent was obtained from all subjects before their participation. Procedures followed the tenets of the Declaration of Helsinki, and the committee of the Institutional Board of Columbia University approved the protocol.
Results
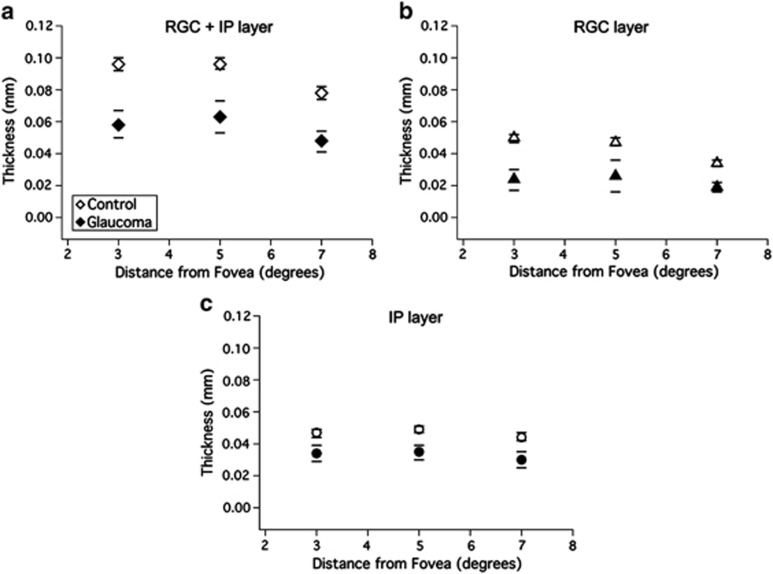
Figure 2 shows the mean (±2SE) thicknesses for the control group (open symbols) and patients (filled symbols) with severe glaucomatous loss, described for experiment 1. At all eccentricities, there was a marked difference between the combined RGC+IP layer thicknesses (Figure 2a) of the two groups (P<0.001, t-test). This difference was due to changes in thickness of both the RGC and IP layers, meaning that glaucoma decreases the thickness of both layers. In particular, the mean thicknesses for the RGC layer (Figure 2b) were smaller for the patient group (24, 26, and 19 μm) compared with those for the control group (50, 47, and 34 μm) (P<0.001, t-test) at all the three eccentricities. The IP layer thicknesses (Figure 2c) were also smaller for the patient group (34, 35, and 30 μm) compared with those of the control group (46, 49, and 44 μm; P<0.01, t-test), and approximately constant with eccentricity for both groups. The residual RGC+IP layer thickness remaining after severe glaucomatous loss is a combination of the residual RGC and IP layers, with the IP making a slightly larger contribution.
Figure 2.
The average RGC+IP (a), RGC (b), and IP (c) layer thickness at 3, 5, and 7° from fovea for the control group (open circles) and glaucoma patients (filled circles). Bars represent ±2SE from each group.
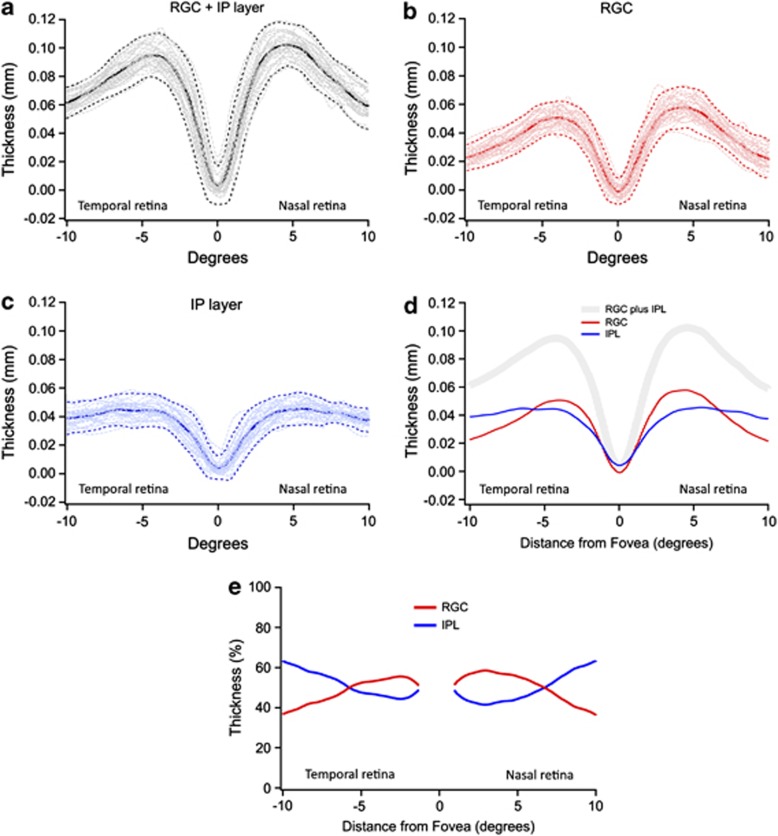
Figure 3 represents the results of experiment 2 and shows the thickness profiles for the RGC+IP (panel a), RGC (b), and IP (c) layers measured along the horizontal meridian of the 30 controls (thin gray lines). Solid lines represent the mean and dashed lines represent the ±2 SD (95% CI) for this group.
Figure 3.
RGC+IP (a), RGC (b), and IP (c) layer thicknesses as a function of eccentricity for 30 controls (gray lines). Solid lines represent the average and dashed lines, ±2 SD. (d) Comparison of the mean thickness of the RGC+IP (gray), RGC (red), and IP (blue) layers. (e) The RGC (red) and IPL (blue) thicknesses are expressed as a percentage of the total RGC+IPL thickness.
Notice that outside the fovea, the IP layer stays approximately constant (Figure 3c), whereas the RGC layer shows the expected parafoveal thickening (Figure 3b), reaching a maximum between 4 and 5° from the center of the fovea. The RGC layer contribution to the RGC+IP layer thickness is larger than that of the IP layer, close to the fovea, whereas the reverse is true beyond around 6° (Figure 3d). Figure 3e plots the RGC (red) and IPL (blue) thickness as percent of the total RGC+IPL thickness. For example, the RGC thickness is about 57% of the RGC+IP layer thickness at 3°. However, there is an impressive change in the relative contributions from around 6–10°. At 10° (2.8 mm), the RGC layer is responsible for only 36% of the RGC+IP layer thickness.
Discussion
With severe glaucomatous loss, both the RGC (Figure 2b) and IP (Figure 2c) layers show a marked decrease in thickness, although there is a larger absolute change in RGC thickness near the fovea (eg, 3°). In any case, in agreement with previous work,11 there is a sizable residual thickness of the RGC+IP layer that remains in regions where the sensitivity of the VF is markedly depressed. This residual thickness seen in patients with severe glaucoma has contributions from both the RGC and IP layers, with the IP layer contribution being slightly larger than that of the RGC layer.
Experiment 2, with higher resolution scans, provided a measurement of the RGC and IP layers across the horizontal meridian of healthy controls. The RGC thickness increases outside the center of the fovea, reaches a peak around 4.5°, and then decreases again (Figure 3b). On the other hand, from about 3° outward, the IP layer is approximately constant, on average 41 μm, where it exceeds the thickness of the RGC layer beyond about 6° (Figure 3c).
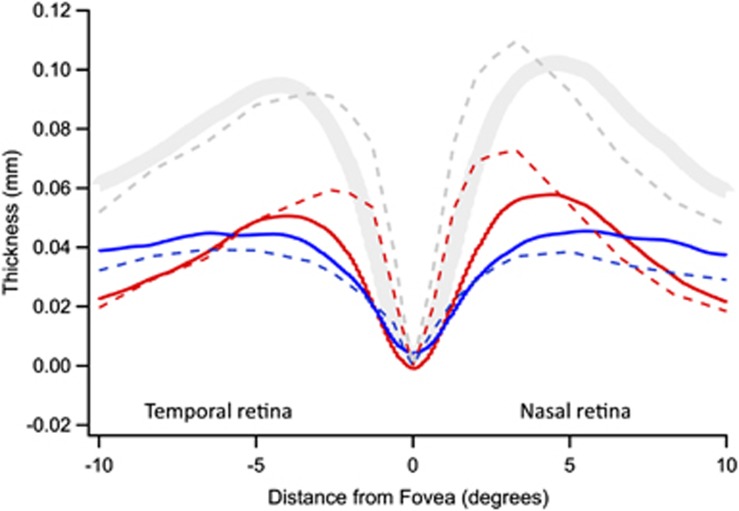
Figure 4 shows a comparison of our OCT data (solid curves from Figure 3) with Curcio et al's12 histological analysis of postmortem human tissue (dashed curves). A significant finding of this study is that the two sets of data show good qualitative agreement, that is, the RGC layer is thicker than the IP layer within about 7° (2 mm), whereas the reverse is true beyond this eccentricity. Quantitatively, absolute thickness of the RGC layer is greater in the histological study. Given the various differences in the methodologies used, it is hard to know what to attribute this difference to.
Figure 4.
Comparison between OCT (solid) and histological measurements (dashed) of human retina. The histological results are from Figure 7 of Curcio et al,12 assuming that 0.289 mm equals 1°.
Our results have implications for the clinical interpretation of the thinning of the inner retinal layers seen in patients with glaucoma. First, severe vision loss due to glaucoma leads to a thinning, but not a complete disappearance of RGC and IP layers. Thus, the mere presence of these layers does not necessarily imply a residual visual function. Second, the relative thicknesses of the three inner retinal layers (RNF, RGC, and IP) change with eccentricity in the healthy retina. Thus, commercially available software should ideally provide separate measures of each layer, not combined measures as is often the case in current systems. Separate measures should soon be possible, as the quality of both the scans and the segmentation algorithms are improving. These improvements will also allow us to overcome a major limitation of the present study. Because of the time needed to hand segment scans, we do not have sufficient data to examine either the effect of disease progression or the type of glaucoma on the residual layers remaining.
In summary, the results support the following hypothesis. Glaucoma decreases the thickness of both the RGC and IP layers in areas with severe VF losses, but leaves a residual thickness. The IP layer contributes slightly more than the RGC layer to this residual, even in the central retina.
Acknowledgments
We thank Dr Robert Ritch, whose patients' data were used in this analysis, and Dr Curcio for supplying the data from Figure 7 of Curcio et al.12 We also thank the Edith C Blum Foundation, New York Glaucoma Research Institute (Dr De Moraes). This work was supported by NIH/NEI RO1-EY02115 and CAPES BEX 4181/08-5.
The authors declare no conflict of interest.
References
- Zangwill LM, Williams J, Berry CC, Knauer S, Weinreb RN. A comparison of optical coherence tomography and retinal nerve fiber layer photography for detection of nerve fiber layer damage in glaucoma. Ophthalmol. 2000;107:1309–1315. doi: 10.1016/s0161-6420(00)00168-8. [DOI] [PubMed] [Google Scholar]
- Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43 (7:2213–2220. [PubMed] [Google Scholar]
- Bowd C, Zangwill LM, Medeiros FA, Tavares IM, Hoffmann EM, Bourne RR, et al. Structure–function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2006;47 (7:2889–2895. doi: 10.1167/iovs.05-1489. [DOI] [PubMed] [Google Scholar]
- Sihota R, Sony P, Gupta V, Dada T, Singh R. Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci. 2006;47 (5:2006–2010. doi: 10.1167/iovs.05-1102. [DOI] [PubMed] [Google Scholar]
- Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26 (6:688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Anderson SC, Wall M, Raza AS, Kardon RH. A test of a linear model of glaucomatous structure-function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Invest Ophthalmol Vis Sci. 2009;50 (9:4254–4266. doi: 10.1167/iovs.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Progress Ret Eye Res. 2010;29 (4:249–271. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MT, Zangwill LM, Weinreb RN, Rao HL, Alencar LM, Medeiros FA. Structure–function relationships using the cirrus spectral domain optical coherence tomography and standard automated perimetry. J Glaucoma. 2012;21 (1:49–54. doi: 10.1097/IJG.0b013e31822af27a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Hood DC, Cho JS, Ghadiali Q, De Moraes CG, Zhang X, et al. Measurement of local retinal ganglion cell layer thickness in patients with glaucoma using frequency-domain optical coherence tomography. Arch Ophthalmol. 2009;127 (7:875–881. doi: 10.1001/archophthalmol.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan O, Chopra V, Lu AT. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmol. 2009;116 (12:2305–2314. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza AS, Cho J, de Moraes CG, Wang M, Zhang X, Kardon RH, et al. Macular retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011;129 (12:1529–1536. doi: 10.1001/archophthalmol.2011.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Messinger JD, Sloan KR, Mitra A, McGwin G, Spaide RF. Human chorioretinal layer thickness measured in macula-wide, high-resolution histologic sections. Invest Ophthalmol Vis Sci. 2011;52:3943–3954. doi: 10.1167/iovs.10-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50 (5:2328–2336. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88 (1:113–123. doi: 10.1097/OPX.0b013e3181fc3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47 (22:2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]