Abstract
Purpose
To determine the efficacy of intravitreal ranibizumab 2.0 mg in patients with recalcitrant neovascular age-related macular degeneration (AMD).
Methods
This single-masked, randomized, prospective, pilot study enrolled patients with subfoveal neovascular AMD. All study eyes had persistent subretinal (SRF) or intraretinal fluid (IRF) on spectral-domain optical coherence tomography (SD-OCT) <30 days following at least 6 monthly intravitreal injections of ranibizumab or bevacizumab. Patients were randomized 2 : 1 to receive either ranibizumab 2.0 or 0.5 mg. Following three-loading treatments 4-weeks apart, both groups were treated using a ‘treat and extend' regimen guided by eye-tracked SD-OCT through month 12. The primary end point was the mean change in best-corrected visual acuity (BCVA) at month 6.
Results
Nine eyes of 9 patients (mean age±SD, 82.0±5.8 years) were enrolled. Seven eyes received ranibizumab 2.0 mg and two eyes received 0.5 mg. Owing to the small number of patients enrolled, no statistical comparison could be made between the two dosages. At month 6, the mean improvement in BCVA was +6.1±3.7 (W=0, P<0.001) ETDRS letters and +2.0 ETDRS letters in the 2.0 and 0.5 mg groups, respectively. In the 2.0 mg group, there was a statistically significant decline in central foveal thickness, SRF and maximum pigment epithelial detachment height at 6 months compared with baseline. No adverse events were reported in either group.
Conclusion
Ranibizumab 2.0 mg has the potential to maintain or improve BCVA in some patients with persistent or recurrent SRF or IRF secondary to neovascular AMD despite prior monthly intravitreal anti-vascular endothelial growth factor therapy with the standard dose.
Keywords: age-related macular degeneration, choroidal neovascularisation, vascular endothelial growth factor, ranibizumab
Introduction
Age-related macular degeneration (AMD) is the most common cause of severe vision loss in the developed world in people over the age of 50 years.1 Neovascular AMD is characterized by the development of abnormal neovascular tissue beneath the retinal pigment epithelium (RPE) (type 1), beneath the retina (type 2) or within the retina (type 3).2 This neovascular tissue can lead to haemorrhage and exudation resulting in sub-RPE fluid, subretinal fluid (SRF), or intraretinal fluid (IRF) with devastating visual consequences.
The success of intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy in the improvement or stabilization of vision in patients with neovascular AMD has led to its rapid acceptance as the standard of care. In particular, ranibizumab (Lucentis, Genentech Inc., South San Francisco, CA, USA) and bevacizumab (Avastin, Genentech Inc.) have enjoyed widespread use. Currently of the two, only ranibizumab is FDA-approved for the treatment of neovascular AMD.
Ranibizumab is a monoclonal antibody fragment that binds to all isoforms of VEGF-A. Unlike bevacizumab, ranibizumab is affinity matured to ensure that there is increased binding to VEGF-A. The drug is administered locally by injecting 0.5 mg in a 0.05 ml solution intravitreally. Randomized clinical trials established the efficacy of monthly ranibizumab to improve best-corrected visual acuity (BCVA) by at least three ETDRS lines in 25–40% of patients, and stabilize the vision in 95% of patients.1, 3
Despite its general success, monthly anti-VEGF therapy for neovascular AMD is not without disadvantages. First, a monthly injection regimen requires frequent patient visits, increases the risk of injection-related adverse events, and places a significant resource burden on ophthalmologists and the healthcare system. Attempts to minimize the frequency of injections have led to other treatment regimens, including ‘as needed' (optical coherence tomography (OCT)-guided or ‘pro re nata' (PRN)) and ‘treat and extend'. In the ‘as needed' regimen, patient visits are mandated monthly, but treatment only prescribed if fluid is present on OCT.4 In the ‘treat and extend' regimen, treatments are given at each visit, but the duration of follow-up is extended so long as the OCT remains fluid-free.5 Recurrence of fluid indicates treatment with a shortened duration of follow-up until the OCT becomes fluid-free. Eyes in which the dosing interval cannot be extended beyond 4 weeks continue receiving injections at this interval. This study is the first prospective randomized trial to employ a ‘treat and extend' regimen in its design.
Many patients prescribed monthly anti-VEGF manifest recalcitrant fluid. The Comparison of Age-Related Macular Degeneration Treatment Trials (CATT) research group recently reported that despite monthly treatment with anti-VEGF for 1 year, 53.2% of patients prescribed ranibizumab and 70.9% of patients prescribed bevacizumab showed evidence of persistent fluid on OCT.4 Persistent fluid may cause structural changes in the retina with resultant decreased visual acuity. It is possible that reducing or completely eliminating fluid in patients with neovascular AMD may improve visual outcomes. As increasing the frequency of injections further increases the treatment burden, this study was specifically designed to target those patients with recalcitrant fluid, and evaluate whether increasing the monthly dose of ranibizumab by fourfold (from 0.5 to 2.0 mg) would lead to improved functional and anatomical outcomes.
Materials and methods
Study design
The evaluation of high-dose ranibizumab (2.0 mg) in the management of AMD in patients with perSistent/recurrenT macular fluid (LAST) study was a 12-month, single-centre, single-masked randomized controlled trial. The Western Institutional Review Board approved the study and all patients provided written informed consent for study participation. The study was conducted in accordance with the Declaration of Helsinki. All applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Study treatment
Patients were randomized in a 2 : 1 ratio to receive either ranibizumab 2.0 mg (40 mg/ml, high-dose) or ranibizumab 0.5 mg (10 mg/ml, standard dose) ranibizumab (Lucentis, Genentech Inc.) in a 0.05 ml intravitreal dose. Patients were masked to their study assignment. After randomization, patients in each group received an intravitreal injection of their assigned dose every 4 weeks for three-loading doses. Following this, patients were managed using a modified ‘treat and extend' protocol.5 In brief, patients were reviewed 4 weeks following their third and subsequent injections; if there was SRF or IRF noted on spectral-domain OCT (SD-OCT), patients received an injection and were reviewed again in 4 weeks. If there was no SRF or IRF, patients were not treated, but were re-evaluated again at 2-week intervals, receiving treatment at 6 weeks if SRF or IRF was present, or at 8 weeks regardless of the presence or absence of fluid. With this protocol the maximum interval between treatments was 8 weeks. Recurrence of fluid required a shortened review back to 4 weeks following injection. Presence of a vascularized pigment epithelial detachment (PED) alone was not sufficient for inclusion in this study, although recurrence or increase in PED size during the study was an indication for the treatment.
Patient Selection
Eligibility criteria included patients 50 years of age or older; BCVA in the study eye between 20/30 and 20/400 (inclusive) using an ETDRS chart at 4 m; subfoveal choroidal neovascularization (CNV) secondary to AMD; and documented SRF or IRF on SD-OCT <30 days following at least 6 monthly injections of intravitreal anti-VEGF therapy (either ranibizumab 0.5 mg/0.05 ml or bevacizumab 1.25 mg/0.05 ml), the most recent being ranibizumab. Patient exclusions included: contraindication to intravitreal injection; prior treatment with thermal laser or photodynamic therapy in the study eye; prior treatment with an intravitreal anti-VEGF agent (other than ranibizumab) or dexamethasone within 30 days of baseline; intravitreal triamcinolone within 6 months of baseline; history of retinal vein occlusion or diabetic retinopathy, intra-ocular surgery within 2 months of baseline; any previous vitreo-retinal surgery; presence of fibrosis or haemorrhage >50% of the subfoveal CNV based on fluorescein angiography; current vitreous haemorrhage or intra-ocular inflammation; history of rhegmatogenous retinal detachment or macular hole (stage 3 or 4) in the study eye; uncontrolled glaucoma (defined as an intra-ocular pressure of ≥30 mm Hg despite anti-glaucomatous medications); history of cerebrovascular accident, transient ischaemic attack or myocardial infarction within 3 months of study enrolment; and current participation in another medical investigation or trial.
Outcome measures
The primary end point was the mean change in BCVA measured using ETDRS letters between baseline and month 6. Secondary end points included: quantitative and qualitative measurements relating to retinal structure analysed from SD-OCT images (Heidelberg Spectralis HRA+OCT, Heidelberg Engineering Inc., Vista, CA, USA) every 3 months and fundus fluorescein angiograms (Topcon 50IA, Topcon Corporation, Paramus, NJ, USA) every 6 months; mean number of injections in each group and adverse events. The active eye-tracking (TruTrack) and automatic follow-up scan (AutoRescan) features of the Heidelberg Spectralis HRA+OCT were employed to enable accurate comparisons between follow-up scans. Quantitative data analysed from the SD-OCT included: ‘PED' (vertical height from Bruch's membrane to the top of the RPE); ‘SRF' (vertical height from the top of the RPE to the inner segment/outer segment (IS/OS) junction) and ‘IRF' (vertical height from the IS/OS junction to the inner-limiting membrane). All of these measurements were taken at the fovea with the use of digital calipers. Central foveal thickness (CFT) was measured using the Heidelberg retinal volume central 1 mm circle. Presence of sub-RPE, SRF, or IRF and maximum PED height and diameter anywhere on the foveal scan were also documented. In addition, qualitative assessment was performed to determine any change in the total volume of any PED, SRF, or IRF within the 20 × 20 macular grid from baseline and from 3 months prior. Fluorescein angiography was analysed using Topcon IMAGEnet software (Topcon Medical Systems, Inc., Oakland, NJ, USA) and the mean area and maximum linear diameter of leakage measured. All measurements were taken by two independent observers (AF and NK) and averaged. When there was discordance between the two observers of greater >20%, the scans were reviewed and a decision was reached by open arbitration.
Analysis
The Wilcoxon signed rank test was performed to compare outcome measures in the ranibizumab 2.0 mg group between baseline and 6 and 12 months.
Results
Patient demographics and baseline characteristics
Nine eyes of 9 patients of Vitreous Retina Macula Consultants of New York, NY participated in this study. Seven eyes were randomized to the ranibizumab 2.0 mg group and 2 eyes to the ranibizumab 0.5 mg group. Patient demographics and characteristics are summarized in Table 1. Patients were not matched by baseline characteristics before assignment to the high-dose and standard-dose groups. No patients were lost to follow-up. One patient in the 0.5 mg ranibizumab group missed a single treatment during the loading phase due to unrelated illness.
Table 1. Patient demographics and baseline characteristics.
| High-dose group (2.0 mg; n=7) | Standard-dose group (0.5 mg; n=2) | |
|---|---|---|
| Gender (male/female) | 2/5 | 1/1 |
| Mean age (years±SD, (range)) | 80.1±5.4 (72–87) | 88.0 |
| Mean VA score in study eye (ETDRS letters±SD) | 63.7±8.5 | 61.5 |
| Mean number of intravitreal anti-VEGF injections before enrolment (total, (bevacizumab/ranibizumab)) | 24.0 (7.6/16.4) | 23.5 (4.0/19.5) |
Abbreviations: VA, visual acuity, VEGF, vascular endothelial growth factor.
End points
At month 6, the ranibizumab 2.0 mg group gained a mean±SD of 6.1±3.7 (W=0, P<0.001) ETDRS letters, while the ranibizumab 0.5 mg group gained a mean of 2.0 ETDRS letters. At month 12, the ranibizumab 2.0 mg group gained a mean of 4.1±4.5 (W=4.5, 0.10<P<0.20) ETDRS letters, while the 0.5 mg ranibizumab group gained a mean of 3.0 ETDRS letters. In the ranibizumab 2.0 mg group, there was a statistically significant decline in CFT (−40±38 μm, W=0, P<0.001), ‘SRF' (−50±75 μm, W=1.5, 0.02<P<0.05) and maximum PED height (−52±82 μm, W=1, 0.02<P<0.05) at 6 months and in the area of leakage on fluorescein angiogram at 6 (−0.92±0.95 μm, W=0, P<0.001) and 12 months (−1.87±0.89 μm, W=0, P<0.001). The results are summarized in Table 2 and Figure 1. Individual case reports are presented in Figures 2 and 3. No adverse events were reported in either group.
Table 2. Outcome measures at baseline, 6 and 12 months.
| Baseline±SD | 6 months (change from baseline±SD, P-value) | 12 months (change from baseline) | ||||
|---|---|---|---|---|---|---|
| |
High-dose group (2.0 mg) |
Standard-dose group (0.5 mg) |
High-dose group (2.0 mg) |
Standard-dose group (0.5 mg) |
High-dose group (2.0 mg) |
Standard-dose group (0.5 mg) |
| Mean VA (ETDRS letters) | 63.7±8.5 | 61.5±14.8 | 69.9 (+6.1±3.7, W=0, P<0.001) | 63.5 (+2.0) | 67.9 (+4.1±4.5, W=4.5, 0.10<P<0.20) | 64.5 (+3.0) |
| Central foveal thickness (μm) | 325±108 | 397±192 | 285 (−40±38, W=0, P<0.001) | 289 (−108) | 297 (−28±46, W=5, 0.10<P<0.20) | 325 (−73) |
| ‘PED' (μm) | 100±68 | 156±101 | 102 (+2±35, W=13, P>0.20) | 73 (−83) | 108 (+8±48, W=13, P>0.20) | 85 (−71) |
| ‘SRF' (μm) | 122±113 | 94±49 | 73 (−50±75, W=1.5, 0.02<P<0.05) | 110 (+16) | 70 (−53±115, W=8, P>0.20) | 108 (+14) |
| ‘IRF' (μm) | 114±45 | 276±245 | 112 (−2±21, W=14, P>0.20) | 96 (−180) | 106 (−8±47, W=12, P>0.20) | 107 (−169) |
| Maximum PED height on the foveal scan (μm) | 222±126 | 237±53 | 169 (−52±82, W=1, 0.02<P<0.05) | 149 (−88) | 166 (−56±106, W=3, 0.05<P<0.10) | 143 (−94) |
| Maximum PED diameter on the foveal scan (μm) | 2399±818 | 3336±496 | 2310 (−89±192 W=7, P>0.20) | 3259 (−77) | 2145 (−254±260, W=3, 0.05<P<0.10) | 3033 (−304) |
| Presence of any sub-RPE fluid (number, (%)) | 7/7 (100%) | 2/2 (100%) | 7/7 (100%) | 2/2 (100%) | 7/7 (100%) | 2/2 (100%) |
| Presence of any SRF (number, (%)) | 7/7 (100%) | 2/2 (100%) | 4/7 (57%) | 1/2 (50%) | 5/7 (71%) | 1/2 (50%) |
| Presence of any IRF (number, (%)) | 1/7 (14%) | 1/2 (50%) | 2/7 (29%) | 1/2 (50%) | 4/7 (57%) | 1/2 (50%) |
| Area of leakage on fluorescein angiography (mm2) | 10.56±3.85 | 11.19±1.42 | 9.64 (−0.92±0.95, W=0, P<0.001) | 9.89 (−1.30) | 8.95 (−1.87±0.89, W=0, P<0.001) | 7.84 (−3.35) |
| Greatest linear diameter of leakage on fluorescein angiography (mm) | 4.40±0.73 | 4.22±0.31 | 4.14 (−0.26±0.39, W=3, 0.05<P<0.10) | 3.97 (−0.36) | 4.12 (−0.35±0.38, W=3, 0.10<P<0.20) | 3.89 (−0.44) |
| Mean number of injections (±SD) | N/A | N/A | 6.0 (±0.0) | 5.0 | 6.0 (±0.0) | 5.0 |
Abbreviations: IRF, intraretinal fluid; PED, pigment epithelial detachment; SRF, subretinal fluid; VA, visual acuity; ‘IRF', vertical height from inner segment/outer segment junction to internal limiting membrane at the fovea; ‘PED', vertical height from Bruch's membrane to top of retinal pigment epithelium at the fovea; ‘SRF', vertical height from top of retinal pigment epithelium to inner segment/outer segment junction at the fovea.
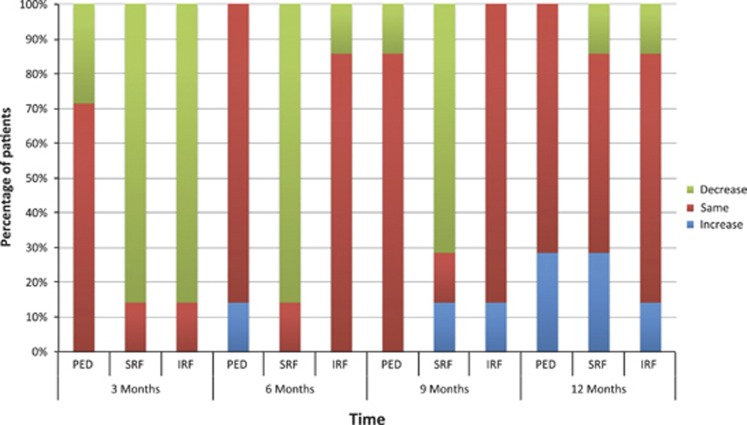
Figure 1.
Percentage of patients in the ranibizumab 2.0 mg arm who demonstrated a total volume decrease, no change or increase in retinal PED, SRF or IRF throughout the entire 20 × 20 macular SD-OCT scan at each time-point compared with 3 months prior.
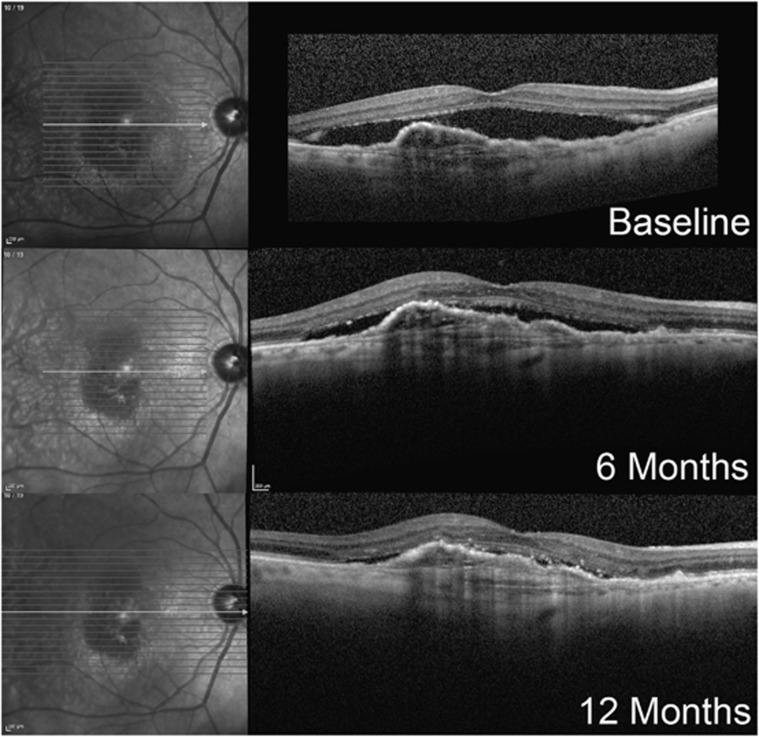
Figure 2.
Case 1 (2.0 mg Ranibizumab group). An 85-year-old male developed type 1 neovascular AMD in his right eye 3 years prior. Over this period he received 28 injections of intravitreal bevacizumab and 19 injections of intravitreal ranibizumab. The patient had been maintained on a 3-weekly alternating bevacizumab and ranibizumab dosing regimen, after SRF had been noted to recur within a standard 4-week dosing regimen. Despite this, at no time did the SRF or sub-RPE fluid completely resolve. In the year before enrolment, the BCVA had slowly declined from 20/50 to 20/80 OD. The patient received 12 intravitreal injections of ranibizumab 2.0 mg at 4-weekly intervals over the first 12 months of the trial. During this period his BCVA improved from 56 ETDRS letters at baseline to 60 letters at 6 months to 61 letters at 12 months. SRF fluid (vertical height from the top of the RPE to the IS/OS junction) at the fovea decreased from 311 μm at baseline to 109 μm at 6 months and 49 μm at 12 months. The retinal pigment epithelial detachment remained stable in size.
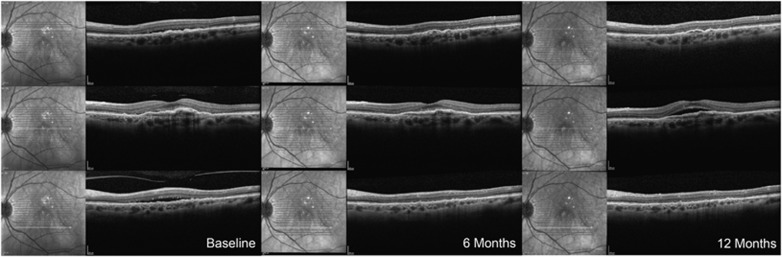
Figure 3.
Case 3 (2.0 mg Ranibizumab group). A 72-year-old female with a 26-month history of neovascular AMD in her left eye had undergone 21 injections of intravitreal ranibizumab. Despite this, there was persistent SRF fluid and vascularized pigment epithelial detachment. BCVA in that eye was 20/63. The patient received 12 monthly injections of intravitreal ranibizumab 2.0 mg over the first 12 months of the study. At month 3, there was significant reduction in SRF throughout the posterior pole. This improvement was maintained at month 6, but the SRF recurred gradually between months 9 and 12. The BCVA in that eye was 60, 71, 71, 70, and 67 ETDRS letters at baseline and months 3, 6, 9, and 12, respectively.
Discussion
There is biological evidence to support studies of high-dose intravitreal ranibizumab for the treatment of neovascular AMD. In pharmacokinetic studies on cynomalous monkeys, the peak retinal and vitreous concentrations are ∼2.9 × and 3.6 × higher for ranibizumab 2.0 mg compared with ranibizumab 0.5 mg.6 No dose–response curves for intravitreal ranibizumab in humans have ever been established. However, in findings from the anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) and Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the treatment of Neovascular Age-Related Macular Degeneration (MARINA) study groups, there was a marginal trend towards better vision in patients receiving ranibizumab 0.5 mg vs ranibizumab 0.3 mg.1, 3
To our knowledge, the LAST trial is the first prospective clinical trial of neovascular AMD to publish results of high-dose ranibizumab (2.0 mg), the first to utilize a ‘treat and extend' protocol, and the first trial to exclusively use the active eye-tracking (TruTrack) and automatic follow-up scan (AutoRescan) features of the Heidelberg Spectralis HRA-OCT to allow for accurate comparisons between study visits. In our study there was a statistically significant improvement in the ranibizumab 2.0 mg group in BCVA, CFT, ‘SRF', and maximum PED height at 6 months, and the area of leakage on fluorescein angiogram at 6 and 12 months. Owing to the small number of patients recruited, it was not appropriate to perform meaningful statistical comparative analysis between the 2.0 and 0.5 mg ranibizumab groups, or for the ranibizumab 0.5 mg group alone. The results of the ranibizumab 0.5 mg group were heavily influenced by one patient, who demonstrated marked flattening of a subfoveal PED, and the resolution of cystoid IRF despite previously demonstrating recalcitrant fluid following eight injections of intravitreal bevacizumab and five injections of intravitreal ranibizumab. There is no clear explanation as to why this occurred. No adverse events were reported in either group. This is consistent with an early clinical dose-escalation study (Study FVF2425g), in which 15 patients tolerated doses up to 2.0 mg lyophilized ranibizumab (RhuFab V2) without any serious ocular adverse events.7
Despite the inability to compare the two study arms, the trial has several strengths. The study only included patients who had recalcitrant fluid. Patients with recalcitrant fluid may be at risk of progressive retinal degeneration, limiting their functional potential. In addition, they may have higher levels of intravitreal VEGF, warranting a higher dose of ranibizumab. Benefit of the ranibizumab 2.0 mg was demonstrated in some of the study patients. However, determining which patients might respond to the higher dose is not currently possible. Although three other unpublished studies have assessed the role of ranibizumab 2.0 mg for neovascular AMD,8, 9, 10 only one of these has investigated patients with recalcitrant fluid despite treatment with a monthly anti-VEGF agent. The SAVE study was a phase I–II, multicenter, open-label, controlled clinical trial assessing ranibizumab 2.0 mg injections for recalcitrant neovascular AMD (defined as having sub-RPE, SRF, or IRF on SD-OCT despite monthly ranibizumab 0.5 mg injections).9 BCVA improved from baseline at month 8 by 4.8 letters and 3.8 letters in the 4-week and 6-week follow-up arms, respectively. There was a corresponding improvement in SD-OCT central subfield thickness in both arms. The authors concluded that some patients may benefit from ranibizumab 2.0 mg compared with the commercially available 0.5 mg dose. This finding is consistent with our study. The largest study to date on ranibizumab 2.0 mg for subfoveal neovascular AMD is the HARBOR study, which enrolled 1098 patients.8 This 24-month study compared the efficacy and safety of ranibizumab 2.0 mg vs ranibizumab 0.5 mg administered monthly and on a PRN basis for treatment naive patients. The study's primary end point at 12 months failed to demonstrate superiority of monthly ranibizumab 2.0 mg over monthly ranibizumab 0.5 mg. However, given that our study only included patients with recalcitrant fluid and HARBOR did not, their findings are not directly transferable to our study.
The small sample size of our study allowed for detailed anatomical analysis of all patients. Case 3 (Figure 3) demonstrates an initial response to ranibizumab 2.0 mg followed by recurrence of fluid at 9 months. This suggests that tachyphylaxis, reported with standard-dose intravitreal bevacizumab11 and ranibizumab12, 13 use, may also occur with high-dose ranibizumab. Although improvement in BCVA, CFT, ‘SRF', maximum PED height and the area of leakage on fluorescein angiogram were significant for the ranibizumab 2.0 mg group at month 6, only the area of leakage on fluorescein angiogram remained significant at month 12. A similar trend is demonstrated in Figure 1, in which the percentage of patients in the ranibizumab 2.0 mg arm with an increase in fluid (compared with 3 months prior) appears to increase between months 9 and 12. This effect may be expected, as patients with persistent fluid have the most to gain initially from an alternative to the standard ranibizumab 0.5 mg; thereafter any gains demonstrate diminishing returns and recurrence of fluid is documented as an increase.
In summary, this pilot study suggests that ranibizumab 2.0 mg has the potential to maintain or improve BCVA and anatomical outcomes in some patients with persistent or recurrent SRF or IRF secondary to neovascular AMD despite prior standard anti-VEGF therapy.
Acknowledgments
Peggy Guerrero for assistance co-ordinating the trial.
This work was supported by the Macula Foundation, Inc. and Genentech, Inc. K Bailey Freund is an advisor to Genentech and Regeneron. Jason S Slakter receives support from Genentech, Regeneron and Novartis; James M Klancnik (Jnr) by Genentech and Regeneron; Richard S Spaide by Genentech.
References
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration. Retina. 2010;30:1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Group CR, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbert M, Zweifel SA, Freund KB. Long-term follow-up for type 1 (subretinal pigment epithelium) neovascularization using a modified “treat and extend” dosing regimen of intravitreal antivascular endothelial growth factor therapy. Retina. 2010;30:1368–1375. doi: 10.1097/IAE.0b013e3181d50cbf. [DOI] [PubMed] [Google Scholar]
- Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P, Villate N, Feuer Wea.RhuFab V2 (anti-VEGF antibody fragment) in neovascular AMD: safety, tolerability and efficacy of multiple, escalating dose intravitreal injections Invest Ophthalmol Vis Sci 2003. ARVO44E-Abstract 970. [Google Scholar]
- Ho AC. Efficacy and Safety of 2.0 mg and 0.5 mg Ranibizumab in Patients with Subfoveal Age-related Macular Degeneration: The HARBOR Study. American Academy of Ophthalmology: Orlando, USA; 2011. [Google Scholar]
- Chen E, Mariani A, Brown DM. SAVE (Superdose AntiVEgf) Trial - 2.0-mg Intravitreal Ranibizumab for Recalcitrant Neovascular Age-Related Macular Degeneration (Poster) Association for Research in Vision and Ophthalmology: Fort Lauderdale, USA; 2011. [Google Scholar]
- Chan C, Abraham P, Sarraf D, Nuthi ASD, Lin SG, McCannel CA. High-dose (2.0 mg) vs. 0.5 mg Ranibizumab for Treating Vascularized Pigment Epithelial Detachment in Age-Related Macular Degeneration. American Academy of Ophthalmology: Orlando, USA; 2011. [Google Scholar]
- Schaal S, Kaplan HJ, Tezel TH. Is there tachyphylaxis to intravitreal anti-vascular endothelial growth factor pharmacotherapy in age-related macular degeneration. Ophthalmology. 2008;115:2199–2205. doi: 10.1016/j.ophtha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Eghøj MS, Sørensen TL. Tachyphylaxis during treatment of exudative age-related macular degeneration with ranibizumab. Br J Ophthalmol. 2012;96:21–23. doi: 10.1136/bjo.2011.203893. [DOI] [PubMed] [Google Scholar]
- Gasperini JL, Fawzi AA, Khondkaryan A, Lam L, Chong LP, Eliott D, et al. Bevacizumab and ranibizumab tachyphylaxis in the treatment of choroidal neovascularisation. Br J Ophthalmol. 2012;96:14–20. doi: 10.1136/bjo.2011.204685. [DOI] [PubMed] [Google Scholar]