Abstract
Objective(s)
Average two-year survival following cardiac transplantation is approximately 80%. The evolution and subsequent approval of larger pulsatile and, more recently, continuous flow mechanical circulatory support (MCS) technology for destination therapy (DT) offers the potential for triage of some patients awaiting cardiac transplantation to DT.
Methods
The National Heart, Lung and Blood Institute Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) is a national multi-institutional study of chronic mechanical circulatory support. Between June 2006 and December 2011, 127 pulsatile and 1160 continuous flow pumps (24% of total primary LVADs) carried an initial strategy of DT therapy.
Results
By multivariable analysis, risk factors (p<0.05) for mortality following DT included older age, larger body mass index, history of cancer, history of cardiac surgery, INTERMACS level I (cardiogenic shock), dialysis, increased BUN, use of a pulsatile flow device and use of a RVAD. Among continuous flow LVAD patients who were not in cardiogenic shock, a particularly favorable survival was associated with no cancer, patients not in cardiogenic shock, and BUN < 50, resulting in one and two year survival of 88 and 80%.
Conclusions
1) Evolution from pulsatile to continuous flow technology has dramatically improved one and two year survival; 2) Destination Therapy is not appropriate for patients with rapid hemodynamic deterioration; or severe right ventricular failure 4) Important subsets of continuous flow DT patients now enjoy survival which is competitive with heart transplantation out to about two years.
INTRODUCTION
Durable mechanical circulatory support (MCS) systems have evolved into therapies suitable for multi-year support. In the United States (U.S.), the historical development of such support devices was linked to cardiac transplantation, addressing the universal shortage of suitable donors for cardiac transplantation. The vast majority of durable devices have been implanted as bridge-to-transplant (BTT) therapy, with a small subset implanted as a bridge to ventricular recovery. When MCS therapy in the United States was expanded to include the intent of long-term “destination” therapy (DT) in 2003,1 Medicare and most other providers considered DT appropriate only for patients not considered eligible for cardiac transplantation, based on inferior demonstrated survival with MCS compared to transplantation.
However, the landscape of devices, their expected durability, and patient outcomes have rapidly evolved over the past four years. This study was undertaken to examine, through a national MCS database, the hypothesis that “mechanical circulatory support as Destination Therapy has evolved to a level that justifies consideration of selected patients for DT who are transplant eligible.”
MATERIALS AND METHODS
INTERMACS Database
INTERMACS is a National Heart, Lung, and Blood Institute (NHLBI) - sponsored registry for durable (suitable for patient discharge) U.S. Food and Drug Administration (FDA) - approved mechanical circulatory support devices implanted in the United States (US) The term “Interagency” emphasizes the unique collaboration between the NHLBI as the funding and scientific support agency, the FDA as the regulatory agency, and the Center for Medicaid and Medicare Services (CMS) as the federal reimbursement agency.2 Information collected in the INTERMACS database includes patient profile data, implant and device data, scheduled follow-up information, and event-driven data. The occurrence of infection, device failure, neurologic events, and death trigger the acquisition of additional relevant data elements. Participation in INTERMACS is a requirement for hospitals to be reimbursed by CMS for the implantation of MCS devices intended for permanent or “Destination” therapy. Patient enrollment in INTERMACS was commenced on June 23, 2006. Between June 23, 2006 and December 31, 2011, 5,614 patients who received a durable ventricular assist device or total artificial heart were entered into the INTERMACS database.
Study Group
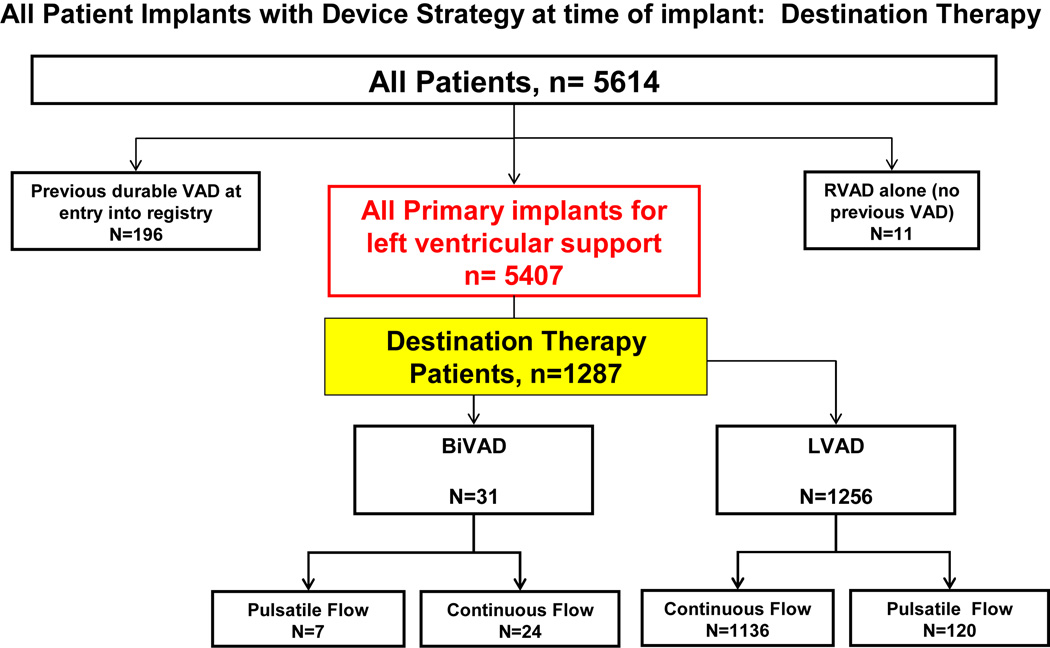
Of these 5,614 registry patients, 1,287 patients received a ventricular assist device (VAD) as DT. (Figure 1) These 1,287 patients are the subject of this analysis. The study inclusion criteria are listed in Table 1. At the time of implant, 1,256 patients received an LVAD only, and 31 received biventricular support. In the overall experience, 127 DT implants were pulsatile devices and 1,160 were continuous flow pumps. (see again Figure 1).
Figure 1.
Categorization of all 5,614 patients entered into INTERMACS between June 23, 2006 and December 31, 2011. The group Destination Therapy (n=1,287) constitutes the study group.
Table 1.
Intermacs June 2006 – December 2011: Destination Therapy
Inclusions/Exclusion Criteria
| ||
| Study Group description | ||
| Institutions contributing data: 104 | ||
| Patients: | 1287 | |
| Total deaths with a device in place | 314 | |
| Total heart transplants | 52 | |
| Total device removal due to recovery | 3 | |
| Total Device Exchanges | 52 | |
| Total Transfers* | 11 | |
| Follow-up: Through December 31, 2011 | ||
11 patients had their care transferred to a non-INTERMACS center and were censored at the time of care transfer LVAD, left ventricular assist device; BIVAD, biventricular-assist device; FDA, United States Food and Drug Administratior.
Missing DT Patients from INTERMACS
Patients receiving MCS implants in the United States who are entered into INTERMACS must fulfill two criteria: 1) the device implanted must be FDA approved; and 2) the patient must provide informed consent for entry of his/her data into INTRMACS. For FDA-approved devices, INTERMACS receives data on device implant and survival/mortality at 48 hours for all patients, even if consent is not obtained. Further follow-up is available only if patient consent is obtained. INTERMACS audits and screening logs indicate that 9.6% of patients suitable for INTERMACS were not entered with full data collection due to failure to obtain informed consent. INTERMACS receives no information for patients who receive an investigational device as part of a clinical trial.
Follow-up
All patients are followed as part of the requirements of INTERMACS until one of 3 end points are reached: death, transplant, or device explant for recovery. Data collection at routine follow-up intervals (see Appendix 1) occurs for a variety of routine clinical variables in addition to data forms which are “triggered” by specific adverse events. Among the 1,287 DT patients, follow-up was available in greater than 99% of patients at the follow-up date of 12/31/11.
Adverse Event Definitions
Standardized definitions for adverse events were established during the initial phase of INTERMACS, developed with the participation and agreement of experts in the field, FDA, and industry. The adverse event definitions are included in Appendix 2.
INTERMACS Profiles
The INTERMACS profiles represent a reclassification of New York Heart Assocition (NYHA) Class IV heart failure.3 The profiles are listed in Appendix 3.
Statistical Methods
Standard Kaplan-Meier actuarial methods as well as parametric depictions were used to examine survival and freedom from other specific events. Standard methods were used to examine whether differences among variables were likely due to chance. A p-value ≤ 0.05 was considered significant. Competing outcomes depictions employed standard methodology as described by McGiffin, Naftel, Kirklin and colleagues.4 Risk factors for mortality were examined via multivariable analysis in the Hazard Function Domain. The variables entered into the multivariable model are listed in Appendix 4. The risk factor equations are listed in Appendix 5.
Transplant Survival
Expected survival following cardiac transplantation was drawn from the Registry of the International Society for Heart and Lung Transplantation.5
Quality of Life Studies
All institutions were encouraged to apply quality of life instruments to patients receiving MCS, beginning pre-implantation and repeated at specified intervals thereafter. The selected quality of life instruments included the EQ5D, and the EQVAS score. The EQ5D is a standardized instrument designed as a measure of health outcome and applicable to a wide range of health conditions and treatments. This test is primarily designed for self-completion by the respondents, examining the dimensions of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. The EQVAS instrument includes a standard vertical 20 cm visual analogue scale (similar to a thermometer) for recording an individual’s self-rating regarding their personal general health-related quality of life state.
RESULTS
Evolution of Destination Therapy
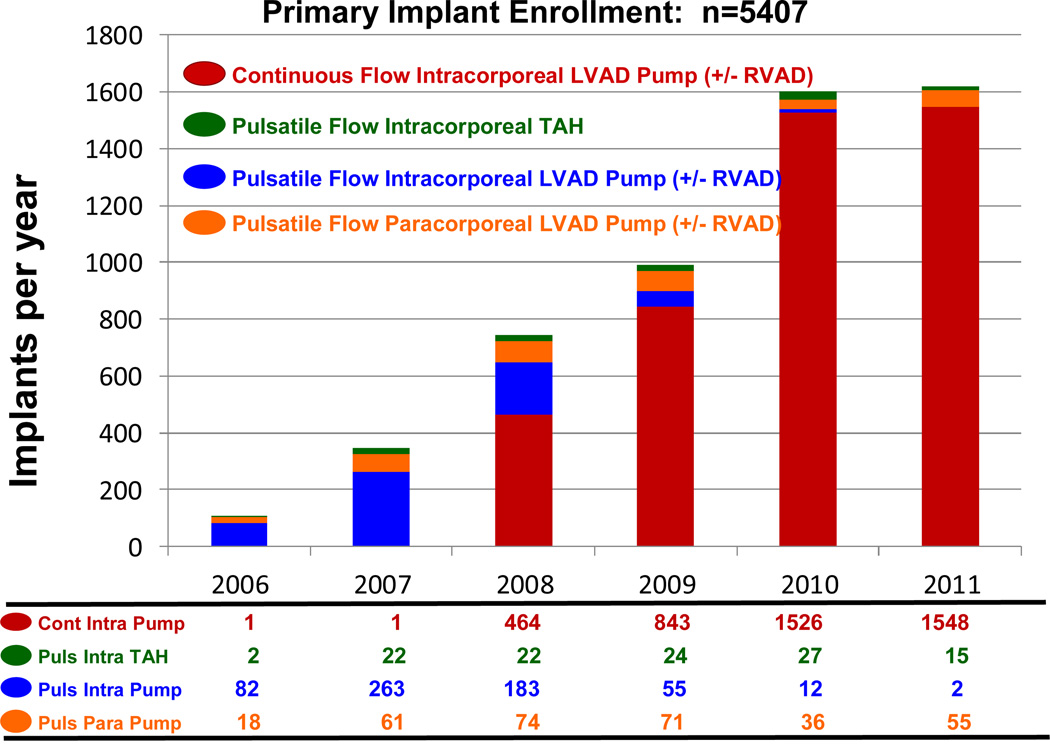
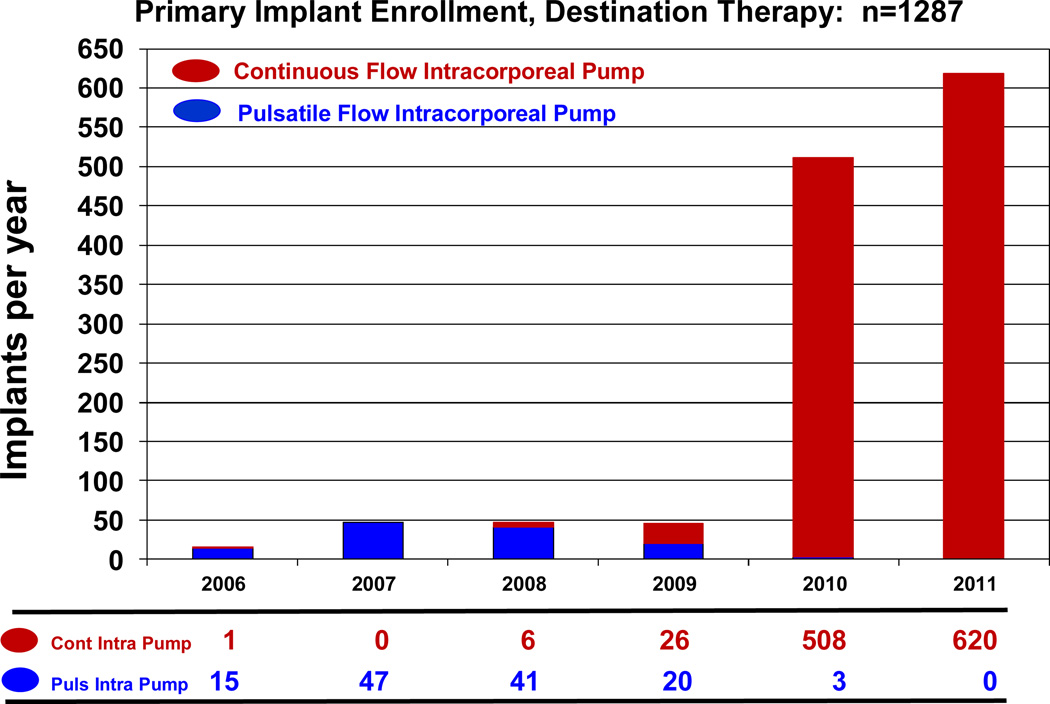
During the early phase of INTERMACS, continuous flow technology was not yet FDA approved for adult patients. During the era in which essentially only pulsatile pumps were entered into INTERMACS (June, 2006 – December, 2007), only 13% of pumps were implanted with a strategy of DT. With the approval of the HeartMate II continuous flow device for DT in January, 2010, the number and proportion of pumps implanted as DT progressively increased (Table 2). During the year 2011, 96% of primary device implants (for any strategy) were continuous flow pumps (Figure 2). Destination Therapy accounted for 38% of implants during 2011, and all DT patients received continuous flow devices (Figure 3).
Table 2.
Primary Device Strategy by Year of Implant: June 2006 – December 2011
| Implant Year | |||||||
|---|---|---|---|---|---|---|---|
| Device strategy | 2006 No. (%) |
2007 No. (% |
2008 No. (%) |
2009 No. (%) |
2010 No. (%) |
2011 No. (%) |
Total |
| Bridge to recovery | 4(3.9) | 14(4.0) | 15(2.0) | 12(1.2) | 12(0.8) | 15(0.9) | 72 |
| Bridge to transplanta | 45(43.7) | 148(42.6) | 367(49.4) | 491(49.4) | 463(28.9) | 370(22.8) | 1884 |
| Bridge to transplant candidacy | 36(35.0) | 132(38.0) | 302(40.6) | 433(43.6) | 598(37.4) | 600(37.0) | 2101 |
| Destination therapyb | 16(15.6) | 47(13.5) | 47(6.3) | 47(4.7) | 511(31.9) | 620(38.3) | 1288c |
| Rescue therapy | 2(1.9) | 6(1.7) | 12(1.6) | 5(0.5) | 8(0.5) | 5(0.3) | 38 |
| Other | 0(0.00) | 0(0.00) | 0(0.00) | 5(0.5) | 9(0.6) | 10(0.6) | 24 |
| Total | 103 | 347 | 743 | 993 | 16014 | 1620 | 5407 |
Patient currently listed for transplant
Patient definitely not eligible for transplant
One patient, age 18 years, was considered a pediatric patient and was not included in the study group
Figure 2.
Primary device implant by year, stratified by device type, for the entire INTERMACS experience. LVAD, left ventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart; Cont, continuous flow; Intra, intracorporeal; Puls pulsatile; Para, paracorporeal.
Figure 3.
Destination Therapy device implants by year, stratified by device type.
Reasons for TX Ineligibility
A variety of contraindications to transplants were cited, some of which were absolute while others were potentially reversible during VAD support (Table 3). The most frequently cited contraindication was advanced age (38% of patients), followed by renal dysfunction (20%), high body mass index (14%), and pulmonary hypertension (12%). Approximately 35% were considered potentially modifiable, indicating the possibility of later suitability for transplantation.
TABLE 3.
Transplant Contraindications – Adult Primary Implants: INTERMACS June 2006 – December 2011
| Contraindications | No. (%) (N = 1287) |
|---|---|
| Modifiable | |
| Renal dysfunction | 256 (20%) |
| High body mass index | 182 (14%) |
| Pulmonary hypertension | 157 (12%) |
| Still smoking | 90 (7%) |
| Severe diabetes | 87 (7%) |
| Alcohol abuse | 41 (3%) |
| Illicit drug use | 40 (3%) |
| Repeated non-compliance | 40 (3%) |
| Limited social support | 38 (3%) |
| Limited cognition/understanding | 21 (1.6%) |
| Malnutrition/cachexia | 20 (1.6%) |
| Severe depression | 10 (0.8%) |
| Musculoskeletal limitation | 10 (0.8%) |
| Risk of recurrent infection | 9 (0.7%) |
| Current infection | 9 (0.7%) |
| Heparin-induced thrombocytopenia | 9 (0.7%) |
| Recent pulmonary embolus | 3 (0.2%) |
| Non-modifiable | |
| Advanced age | 487 (38%) |
| Peripheral vascular disease | 89 (7%) |
| Pulmonary disease | 80 (6%) |
| History of solid-organ cancer | 64 (5%) |
| Patient refuses transplant | 54 (4%) |
| Frailty | 48 (4%) |
| Fixed pulmonary hypertension | 41 (3%) |
| Multiple sternotomies | 32 (2%) |
| History of lymphoma leukemia | 29 (2%) |
| Major stroke | 18 (1.4%) |
| Contraindication to immunotherapy | 15 (1.2%) |
| Other major psychiatric diagnosis | 8 (0.6%) |
| Allosensitization | 6 (0.5%) |
| Mediastinal radiation | 4 (0.3%) |
| Thoracic aortic disease | 4 (0.3%) |
| Other comorbidity | 118 (9%) |
Outcome of Patients with Initial DT Strategy
As noted in the previous section, many of the contraindications to transplantation at the time of device implant were considered potentially reversible. As their clinical situation evolved over time, some patients were reconsidered for cardiac transplantation, or, potentially, for device explant. These events are tracked by the competing outcomes depictions in Figure 4 for pulsatile devices and Figure 5 for continuous flow devices. In the current era of continuous flow DT devices, less than 5% of patients undergo transplantation or explant within 2 years.
Figure 4.
Competing outcomes depiction for pulsatile flow left ventricular assist devices (LVAD) implanted with a strategy of destination therapy. All outcome events are mutually exclusive, such that the some of all probabilities at any point in time equals 100%.
*Includes LVAD only as well as LVAD plus an RVAD implanted for RV failure at original LVAD implant or anytime thereafter.
Figure 5.
Competing Outcomes Depiction for continuous flow left ventricular assist devices implanted with a strategy of Destination Therapy. The depiction is as in Figure 4.
Adverse Events
Infection and Bleeding were the most common adverse events, followed by respiratory failure, neurologic events, and renal dysfunction (Table 4). Of note, the adverse event profile differed between pulsatile and continuous flow pumps. (Table 4).
Table 4.
Adverse Event Rates (Events/100 Patient Months) in the First 12 Months for Destination Therapy Patients
| Pulsatile (n=127) | Continuous (n=1160) | Hazard | ||||
|---|---|---|---|---|---|---|
| Adverse Event | Events | Rate | Events | Rate | Ratio2 | p-value |
| Device malfunction | 38 | 3.69 | 100 | 1.15 | 3.21 | < 0.0001 |
| Bleeding | 150 | 14.56 | 1040 | 11.94 | 1.22 | 0.008 |
| Cardiac/vascular | ||||||
| Right heart failure | 14 | 1.36 | 151 | 1.73 | 0.78 | 0.75 |
| Myocardial infarction | 0 | 0.00 | 3 | 0.03 | --- | --- |
| Cardiac arrhythmia | 55 | 5.34 | 339 | 3.89 | 1.37 | 0.009 |
| Pericardial drainage | 10 | 0.97 | 54 | 0.62 | 1.57 | 0.06 |
| Hypertension* | 27 | 2.62 | 73 | 0.84 | 3.13 | < 0.0001 |
| Arterial non-CNS thrombosis | 5 | 0.49 | 17 | 0.20 | 2.49 | 0.01 |
| Venous thrombotic event | 11 | 1.07 | 56 | 0.64 | 1.66 | 0.03 |
| Hemolysis | 0 | 0.00 | 55 | 0.63 | --- | --- |
| Infection | 236 | 22.91 | 705 | 8.09 | 2.83 | < 0.0001 |
| Neurologic dysfunction | 30 | 2.91 | 162 | 1.86 | 1.57 | 0.006 |
| Renal dysfunction | 30 | 2.91 | 141 | 1.62 | 1.80 | < 0.0001 |
| Hepatic dysfunction | 7 | 0.68 | 50 | 0.57 | 1.18 | 0.24 |
| Respiratory failure | 41 | 3.98 | 230 | 2.64 | 1.51 | 0.004 |
| Wound dehiscence | 10 | 0.97 | 19 | 0.22 | 4.45 | < 0.0001 |
| Psychiatric episode | 21 | 2.04 | 78 | 0.90 | 2.28 | < 0.0001 |
| Total burden | 685 | 66.50 | 3273 | 37.56 | 1.77 | < 0.0001 |
With current reporting, identification of hypertension with continuous flow pumps is unreliable
The hazard ratio is the rate for pulsatile pump divided by the rate for continuous flow pump.
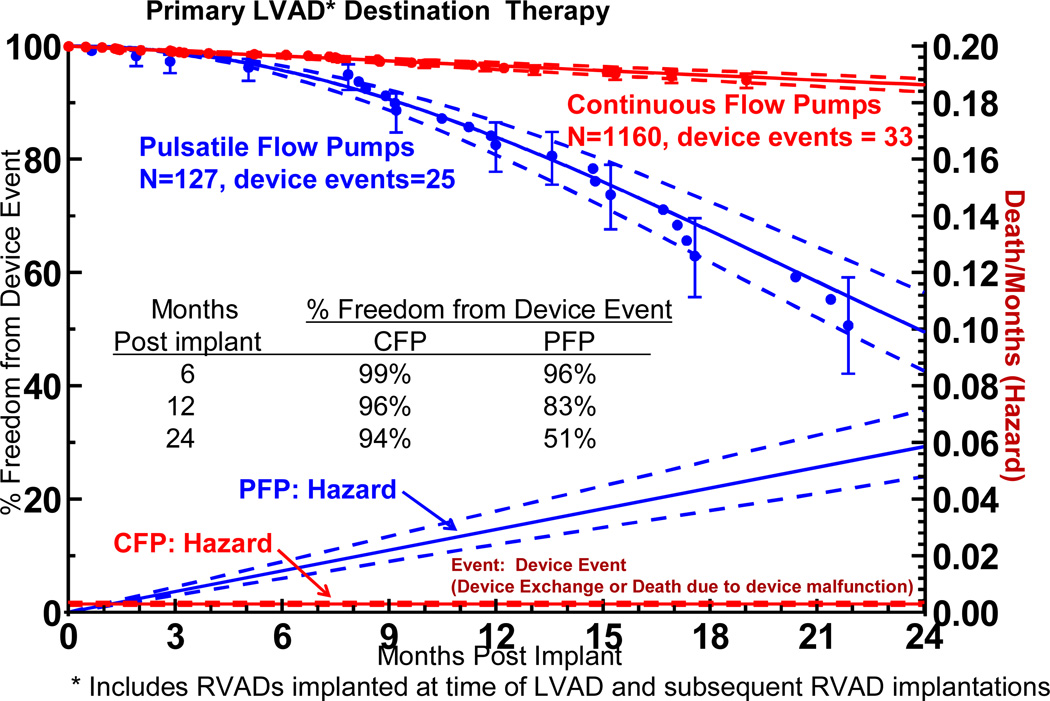
Device malfunction, bleeding, infection, stroke, renal dysfunction, and respiratory failure all occurred with a significantly higher event rate with pulsatile pumps. Device malfunction severe enough to require pump exchange or cause death was strikingly different between pump types (Figure 6). The hazard function (instantaneous risk) for device failure progressively increased over the 2 years of follow-up for pulsatile pumps, whereas the hazard function for continuous flow devices remained low and essentially constant. For pulsatile pumps, the freedom from device exchanged was only 51% at 24 months, compared to 94% for continuous flow pumps.
Figure 6.
Actuarial freedom from device exchange or death secondary to device malfunction or device complication, stratified by device type. The lower curves represent the hazard function for this event for each device type. The dashed lines enclose the 70% confidence limits. *See notation in Figure 4.
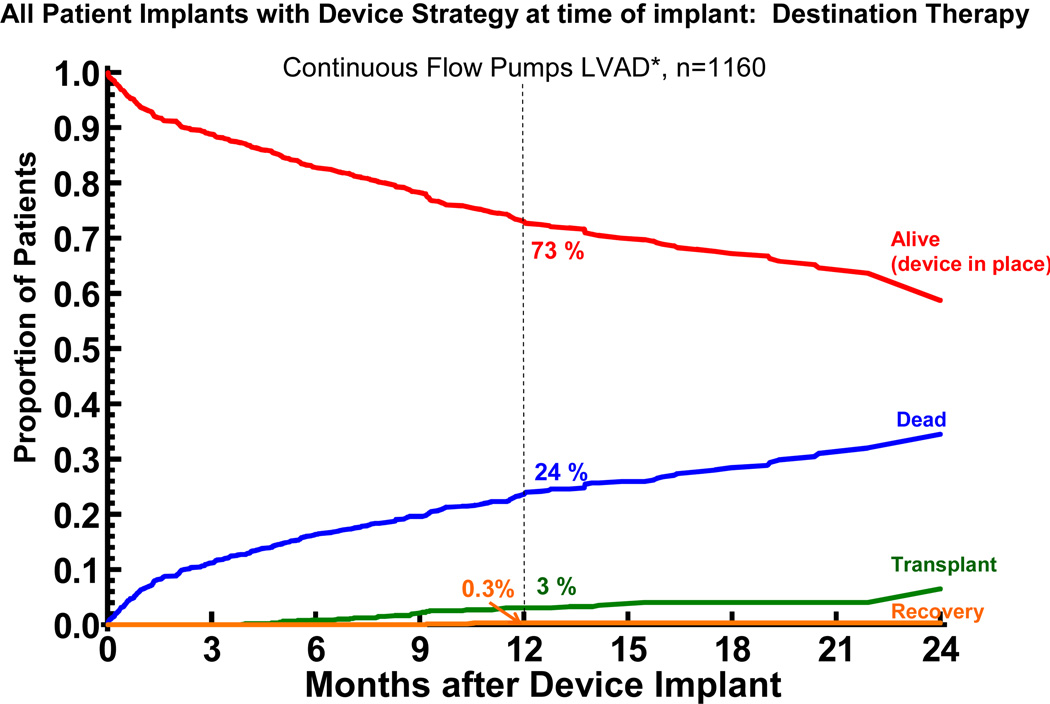
Survival
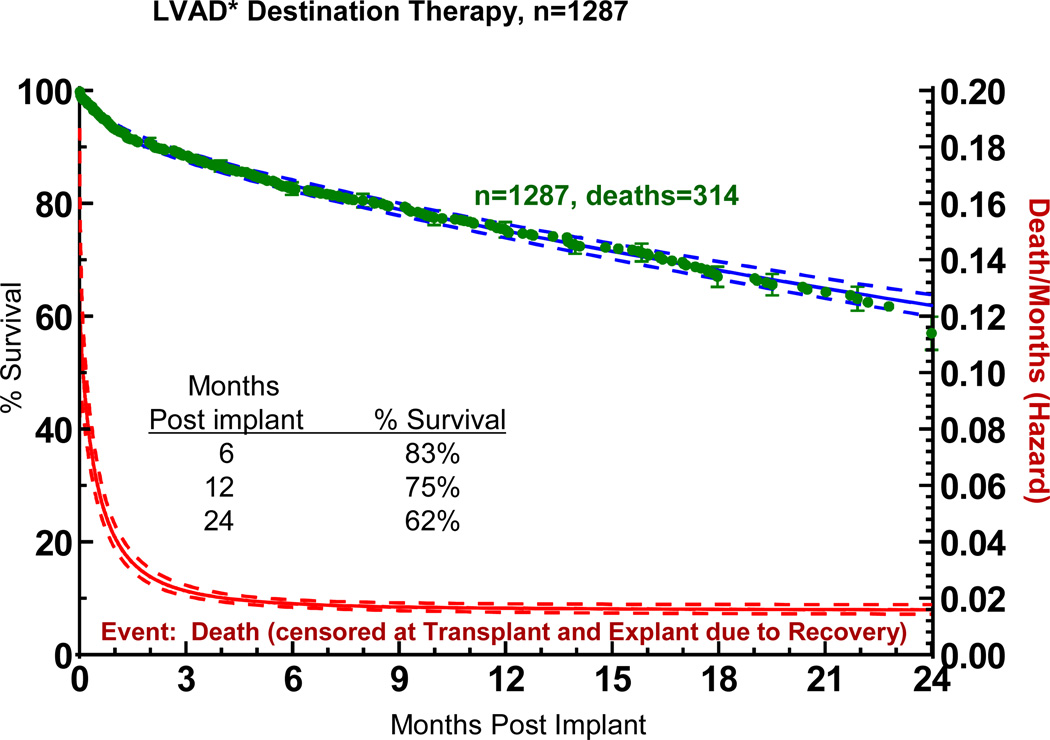
The overall actuarial survival among all DT patients was 75% at 1 year and 62% at 2 years (Figure 7). The hazard function shows a rapidly falling early phase which merges with a constant phase at about 3 months.
Figure 7.
Actuarial survival among 1,287 patients receiving destination therapy LVAD support. Patients are censored at time of transplant or explant due to recovery. The lower curve represents the hazard function. The dashed lines enclose the 70% confidence limits. *See notation in Figure 4.
Cause of Death
The primary causes of death differed according to time interval post implant as well as device type (Tables 5 and 6). Cardiac failure was the major cause of early mortality, and 32% of those patients received their device while in cardiogenic shock. No other specific cause of death was predominately related to either device type or interval from implant to death
TABLE 5.
Causes of Death in 1287 LVAD* Destination Therapy Patients INTERMACS June 2006 – December 2011
| Pulsatile Flow Pumps | ||||||
|---|---|---|---|---|---|---|
| Early (≤ mon) | Later (>1 mon) | Total | ||||
| (n = 13) | (n = 53) | (n = 66) | ||||
| Primary cause of death | No | % | No | % | No | % |
| Cardiac failure | ||||||
| RV failure | 2 | 15% | 4 | 8% | 6 | 9% |
| Arrhythmia/Other | 1 | 8% | 6 | 11% | 7 | 11% |
| Infection | 1 | 8% | 8 | 15% | 9 | 14% |
| CNS event | 5 | 38% | 7 | 13% | 12 | 18% |
| Multiorgan failure | 0 | 0% | 3 | 6% | 3 | 5% |
| Respiratory failure | 2 | 15% | 1 | 2% | 3 | 5% |
| Bleeding | ||||||
| Gastrointestinal | 0 | 0% | 0 | 0% | 0 | 0% |
| Surgical | 1 | 8% | 0 | 0% | 1 | 1% |
| Other Bleeding | 1 | 8% | 2 | 4% | 3 | 5% |
| Device failure | 0 | 0% | 3 | 6% | 3 | 5% |
| Renal failure | 0 | 0% | 1 | 2% | 1 | 1% |
| Hepatic failure | 0 | 0% | 1 | 2% | 1 | 1% |
| Malignancy | 0 | 0% | 1 | 2% | 1 | 1% |
| Arterial embolism | 0 | 0% | 0 | 0% | 0 | 0% |
| Cardiac tamponade | 0 | 0% | 0 | 0% | 0 | 0% |
| Withdrawal of support | 0 | 0% | 0 | 0% | 0 | 0% |
| Other | 0 | 0% | 16 | 30% | 16 | 24% |
| Total | 13 | 100% | 53 | 100% | 66 | 100% |
CNS, central nervous system; INTERMACS, Interagency Registry for Mechanical Circulatory Support; LVAD, left ventricular assist device.
includes RVADs implanted at the time of LVAD or subsequent to LVAD implant.
TABLE 6.
Causes of Death in 1287 LVAD* Destination Therapy Patients INTERMACS June 2006 – December 2011
| Continuous Flow Pumps | ||||||
|---|---|---|---|---|---|---|
| Early (≤ mon) | Later (>1 mon) | Total | ||||
| (n = 72) | (n = 176) | (n = 248) | ||||
| Primary cause of death | No | % | No | % | No | % |
| Cardiac failure | ||||||
| RV failure | 6 | 8% | 11 | 6% | 17 | 7% |
| Arrhythmia/Other | 12 | 17% | 34 | 19% | 46 | 19% |
| Infection | 5 | 7% | 17 | 10% | 22 | 9% |
| CNS event | 8 | 11% | 15 | 9% | 23 | 9% |
| Multiorgan failure | 12 | 17% | 11 | 6% | 23 | 9% |
| Respiratory failure | 3 | 4% | 9 | 5% | 12 | 5% |
| Bleeding | ||||||
| Gastrointestinal | 2 | 3% | 2 | 1% | 4 | 2% |
| Surgical | 4 | 6% | 1 | 1% | 5 | 2% |
| Other Bleeding | 5 | 7% | 13 | 7% | 18 | 7% |
| Device failure | 0 | 0% | 6 | 3% | 6 | 2% |
| Renal failure | 1 | 1% | 4 | 2% | 5 | 2% |
| Hepatic failure | 2 | 3% | 3 | 2% | 5 | 2% |
| Malignancy | 0 | 0% | 4 | 2% | 4 | 2% |
| Arterial embolism | 0 | 0% | 5 | 3% | 5 | 2% |
| Cardiac tamponade | 0 | 0% | 0 | 0% | 0 | 0% |
| Withdrawal of support | 1 | 1% | 8 | 5% | 9 | 4% |
| Other | 11 | 15% | 33 | 19% | 44 | 18% |
| Total | 72 | 100% | 176 | 100% | 248 | 100% |
CNS, central nervous system; INTERMACS, Interagency Registry for Mechanical Circulatory Support; LVAD, left ventricular assist device.
includes RVADs implanted at the time of LVAD or subsequent to LVAD implant.
Risk Factors for Mortality
Risk factors for mortality in the early and constant phases identified by multivariable analysis are listed in Table 7.
Table 7.
Risk Factors for Death in Destination Therapy Patients – Adult Primary Implants: INTERMACS, June 2006 – December 2011
| Early hazard | Constant hazard | |||
|---|---|---|---|---|
| Risk factors | HR | p-value | HR | p-value |
| Age (older) | 1.241 | .01 | ||
| BMI (higher) | 1.042 | .03 | ||
| History of cancer | 1.89 | .04 | ||
| History of cardiac surgery | 1.69 | .001 | ||
| Dialysis | 3.14 | .004 | ||
| BUN | 1.083 | .009 | ||
| INTERMACS Level 1 | 4.58 | <.0001 | ||
| INTERMACS Level 2 | 2.35 | .02 | ||
| Use of pulsatile LVAD | 2.63 | <.0001 | ||
| RVAD in same operation | 3.22 | .002 | ||
The hazard ratio denotes the increased risk from 60 to 70 years
The hazard ratio denotes the increased risk of a 5 unit increase in BMI
The hazard ratio denotes the increased risk of a 10 unit increase in BUN
Older Age
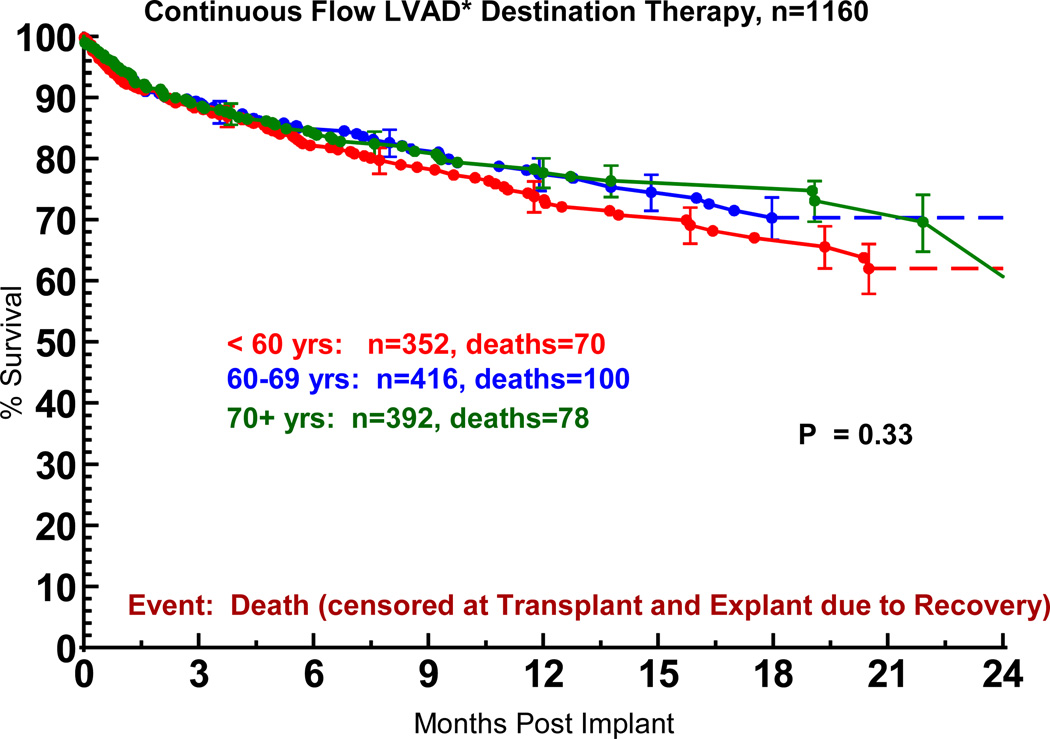
The hazard ratio of 1.24 reflects the increase in risk from age 60 to 70 years. Perhaps not surprising is the finding that elderly patients receiving DT had a lower general risk profile compared to younger patients. Without risk-adjustment, the actuarial survival for older and younger patients was similar (Figure 8).
Figure 8.
Actuarial survival following Destination Therapy LVAD, stratified by age at implant. Patients are censored at transplant or explant for recovery. LVAD, left ventricular assist device; BIVAD, biventricular assist device. *See notation in Figure 4.
INTERMACS Level 1
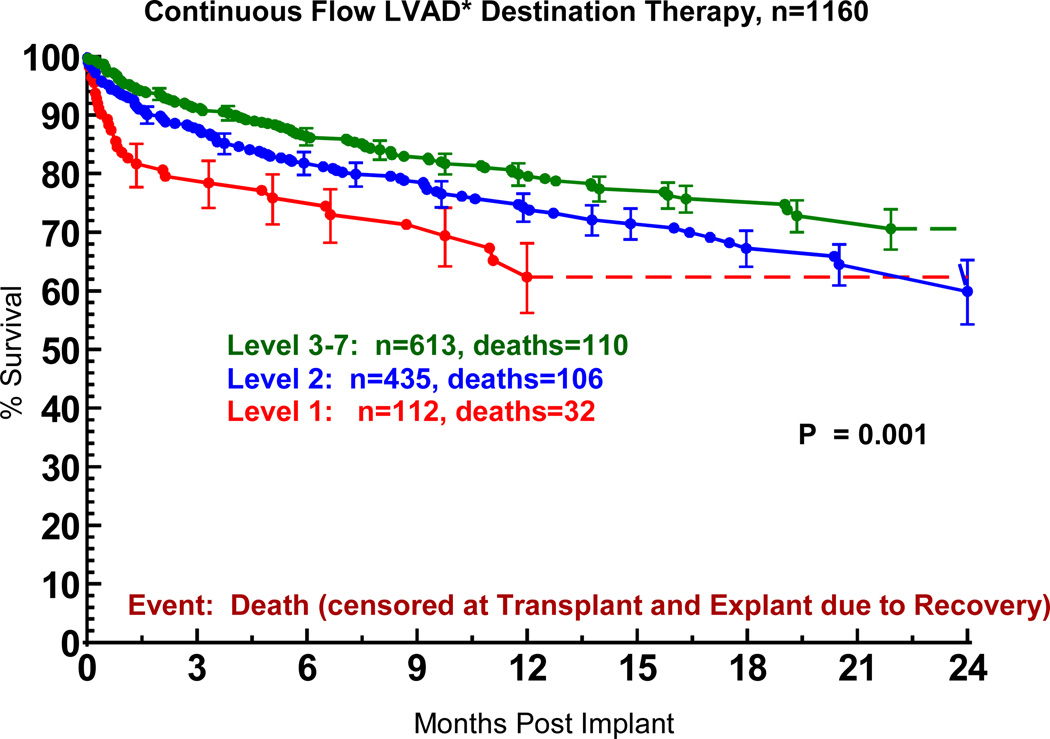
INTERMACS Level 1 identifies those patients in cardiogenic shock at the time of VAD implant (see Appendix 3). The progressive decrease in the proportion of patients in Level 1 at implant over the course of the study is documented in Table 8. The inferior survival when DT was undertaken in the face of cardiogenic shock is apparent in Figure 9.
Table 8.
Patient Profile Level –Adult Primary Implants: INTERMACS, June 2006 – December 2011, Destination Therapy
| Level | June 2006– Dec 2009 (N=156) No. (%) |
Jan 2010– Dec 2011 (N=1131) No. (%) |
|---|---|---|
| 1. Critical cardiogenic shock | 29 (18.6) | 110 (9.7) |
| 2. Progressive Decline | 64 (41.0 | 422 (37.3) |
| 3. Stable but inotrope-dependent | 36 (23.1) | 346 (30.6) |
| 4. Recurrent advanced HF | 20 (12.8) | 177 (15.6) |
| 5. Exertion intolerant | 2 (1.3) | 43 (3.8) |
| 6. Exertion limited | 3 (1.9) | 21 (1.9) |
| 7. Advanced NYHA class III | 2 (1.3)8 | 12 (1.1) |
HF, heart failure; INTERMACS; Interagency Registry for Mechanical Assisted Circulatory Support; NYHA, New York Heart Association.
Figure 9.
Actuarial survival following Destination Therapy LVAD, stratified by INTERMACS level at time of implant. The INTERMACS levels are defined in Appendix 3. LVAD, left ventricular assist device; BIVAD, biventricular assist device. *See notation in Figure 4.
Severe Right Ventricular Failure
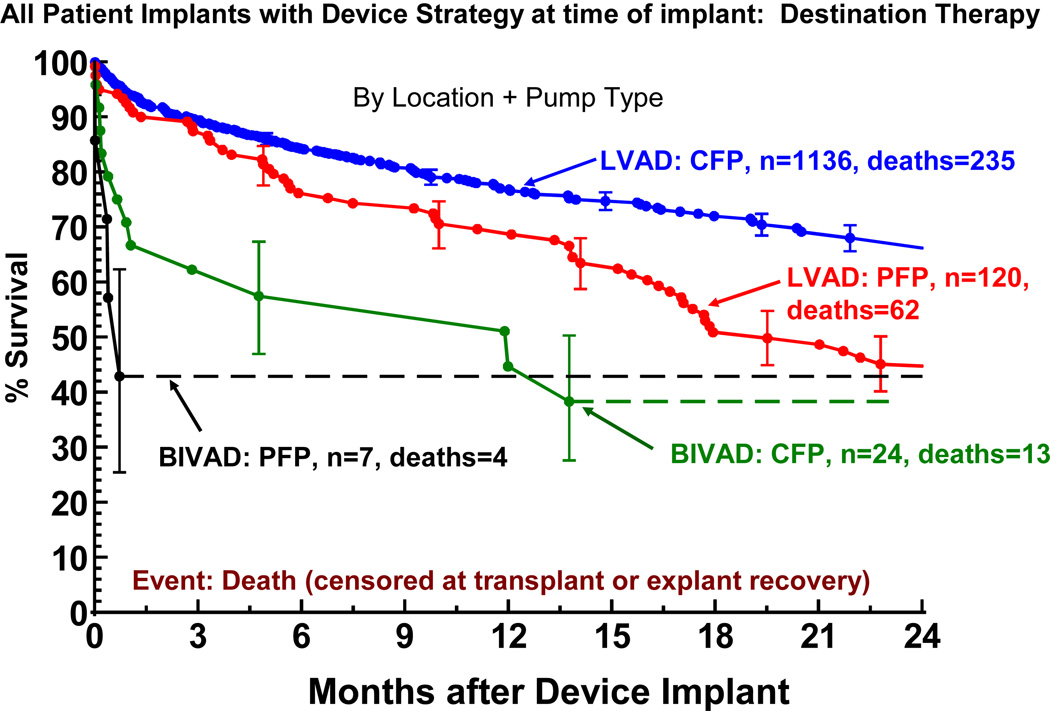
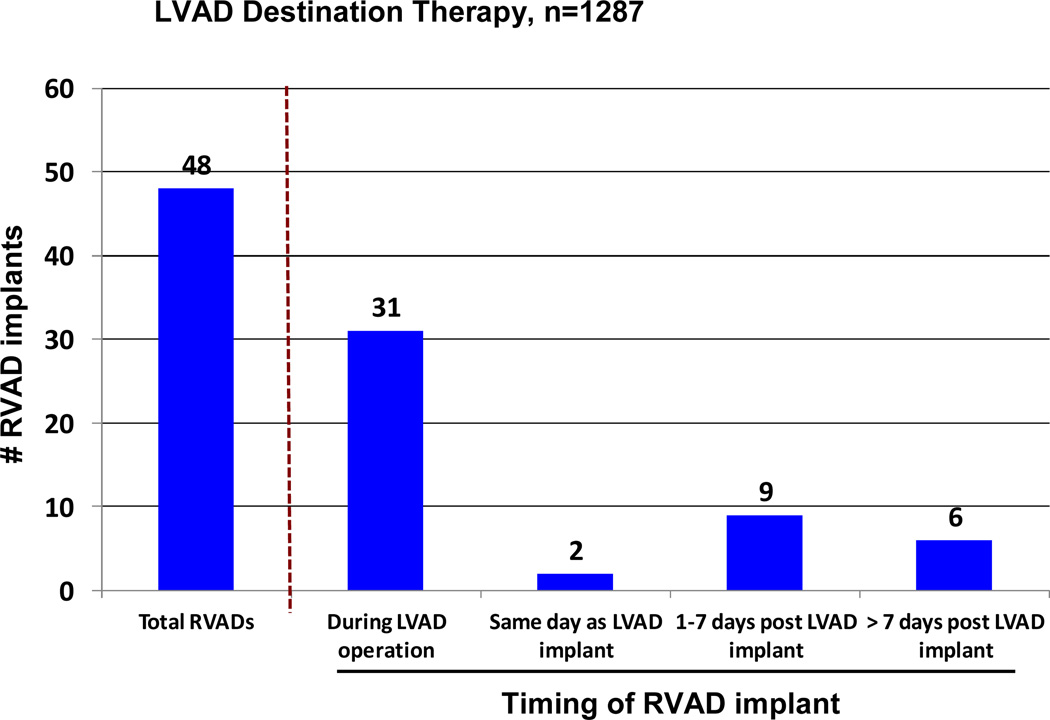
Right ventricular failure sufficient to require BIVAD support at the time of implant was the strongest predictor of mortality in the constant phase (see again Table 7). The need for biventricular support resulted in a marked reduction in survival for both pulsatile and continuous flow LVADs (Figure 10). The likelihood of RVAD implant at time of DT was 5.5% for pulsatile LVADS, but fell to 2.1% with continuous flow technology. Among the 48 RVAD implants, 31 (65%) occurred at the time of LVAD implant, 2 (4%) later the same day, 9 (19%) between 0 and 7 days, and 6 (12%) more than 7 days post LVAD. (Figure 11). RVAD explant occurred in 9 of 48 (19%) patients receiving an RVAD. The duration of RVAD support ranged from 2 to 37 days (mean 18). The likelihood of needing biventricular support was 3 times as high for patients in Level 1 compared to Levels 3–7.
Figure 10.
Actuarial survival following Destination Therapy LVAD, stratified by device location and pump type. Patients are censored at transplant or device explant for recovery. LVAD, left ventricular assist device; CFP, continuous flow pump PFP; pulsatile flow pump; BiVAD, biventricular assist device (implanted at time of LVAD implant).
Figure 11.
Bar chart indicating the timing of RVAD implant. LVAD, left ventricular assist device; RVAD, right ventricular assist device.
Pulsatile Pump Technology
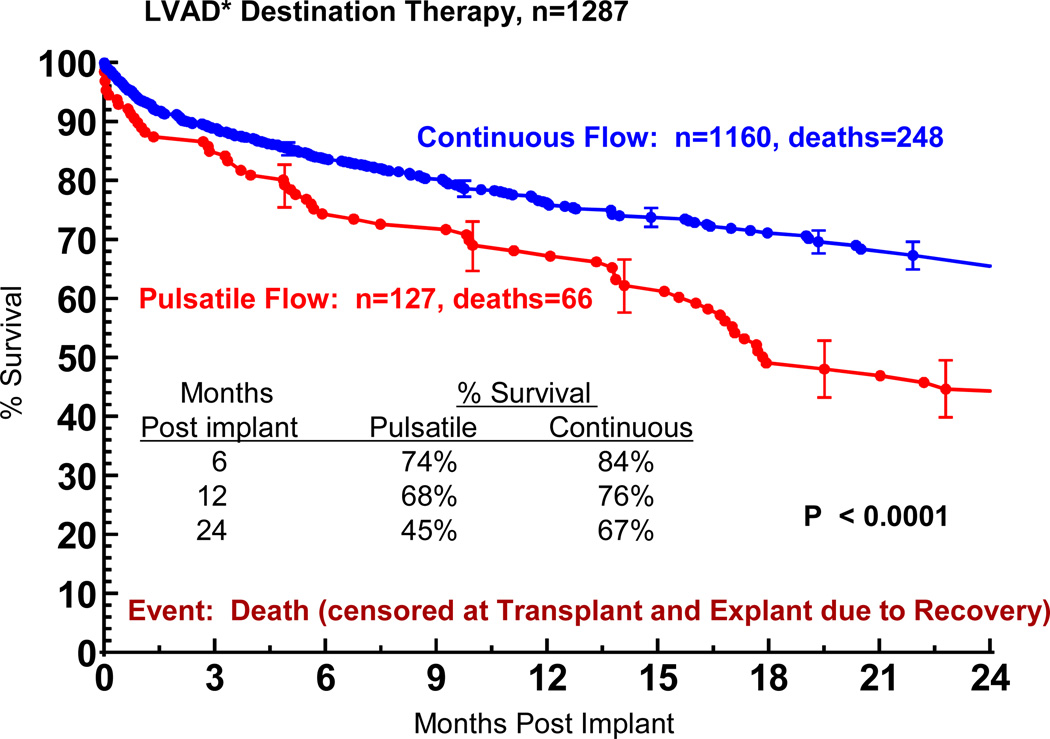
In this experience, the use of a pulsatile pump yielded inferior survival compared to a current continuous flow device (Figure 12), in which the 1 and 2 year actuarial survival were 76% and 67%. In general, pulsatile pump patients were slightly older and had somewhat worse renal and hepatic function compared to continuous flow patients (Table 9).
Figure 12.
Actuarial survival among Destination Therapy patients, stratified by device type. Patients are censored at time of transplant or explant due to recovery. LVAD, left ventricular assist device; BIVAD, biventricular assist device. *See notation in Figure 4.
TABLE 9.
INTERMACS: Primary Implants – DT Analysis – AATS Implant dates: June 23, 2006 – December 31, 2011 Pre-implant Baseline Characteristics
| Pre-implant Characteristics | PFP N=127 |
CFP N=1160 |
p-value |
|---|---|---|---|
| Age (yrs) | 54.70 | 63.56 | <0.0001 |
| Albumin (g/dL) | 3.19 | 3.38 | 0.02 |
| Total bilirubin (mg/dL) | 1.59 | 1.37 | 0.09 |
| BMI (kg per meter2) | 32.40 | 28.14 | <0.0001 |
| BNP (pg/ml) | 1286.82 | 1255.79 | 0.84 |
| BSA (m2) | 2.27 | 2.04 | <0.0001 |
| BUN (MG/DL) | 38.83 | 33.91 | 0.01 |
| Cholesterol (mg/dL) | 117.93 | 126.80 | 0.15 |
| Cardiac index (L/min per sq meter) | 2.09 | 2.15 | 0.64 |
| Creatinine (mg/dL) | 1.81 | 1.54 | 0.02 |
| CRP (mg/L) | 23.47 | 14.43 | 0.39 |
| Diastolic blood pressure (mmHg) | 61.46 | 62.89 | 0.18 |
| Hemoglobin (mg/dL) | 11.20 | 11.33 | 0.48 |
| Heart Rate | 9185 | 84.91 | <0.0001 |
| INR (international units) | 1.38 | 1.33 | 0.27 |
| LVEDD | 6.81 | 6.74 | 0.58 |
| Platelet (K/uL) | 199.29 | 187.06 | 0.09 |
| Pre-albumin (mg/dL) | 15.30 | 18.85 | 0.0024 |
| Protein C (%) | 77.17 | 84.70 | 0.59 |
| Protein S (%) | 70.29 | 77.47 | 0.54 |
| Pulmonary diastolic pressure (mmHg) | 29.35 | 24.61 | <0.0001 |
| Pulmonary systolic pressure (mmHg) | 54.49 | 50.10 | 0.01 |
| Pulmonary wedge pressure (mmHg) | 26.75 | 23.21 | 0.0035 |
| Pulmonary vascular resistance (PVR) (wood units) | 3.08 | 2.75 | 0.31 |
| RA pressure (mmHg) | 15.75 | 11.35 | <0.0001 |
| SGOT/AST (u/L) | 122.94 | 54.23 | 0.06 |
| SGPT/ALT(u/L) | 133.19 | 52.42 | 0.01 |
| Sodium (mmol/L) | 134.23 | 134.99 | 0.13 |
| Systolic blood pressure (mmHg) | 99.40 | 104.90 | 0.0004 |
| WBC (K/uL) | 9.86 | 8.39 | 0.0008 |
BMI, Body mass index; BNP, brain natriuretic peptide; BSA, body surface area; BUN, blood urea nitrogen; CRP, C-reactive protein; LVEDD, left ventricular end diastolic dimension; RA, right atrial; WBC, white blood cell count; PFP, pulsatile flow pump; CFP, continuous flow pump.
Quality of Life
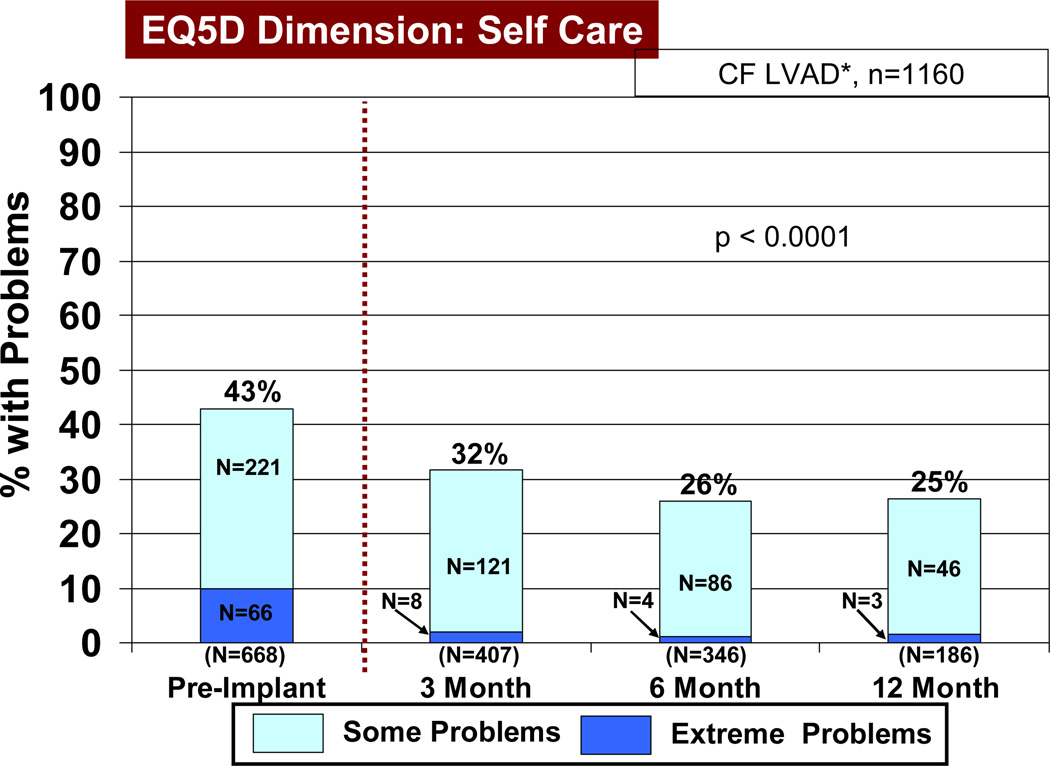
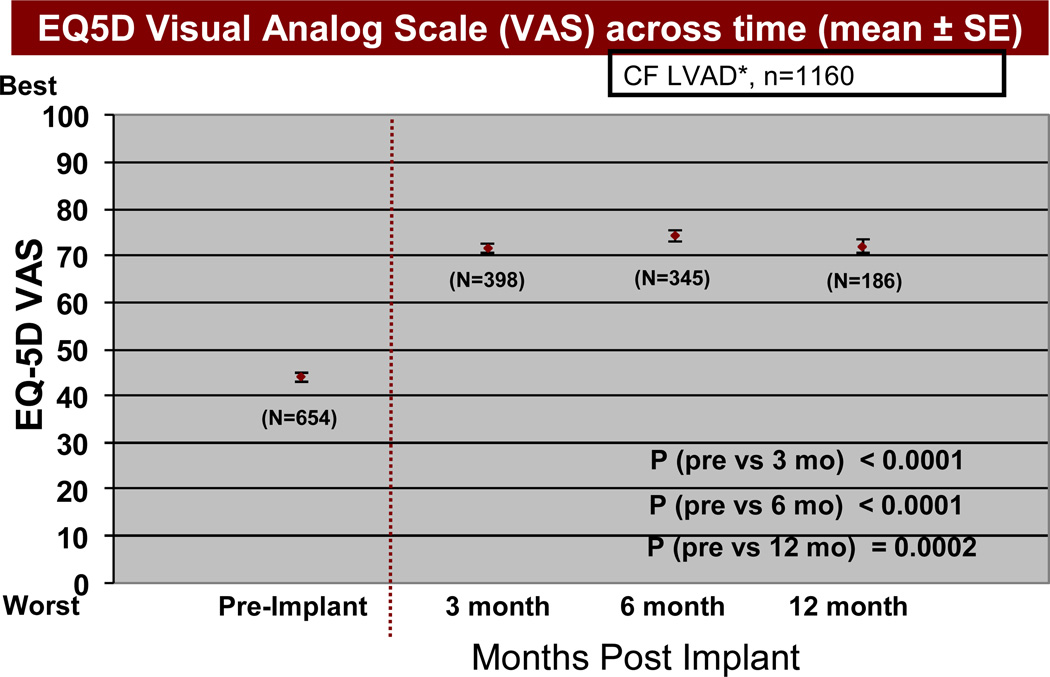
Based on the EQ5D quality of life instruments, patients receiving a continuous flow pump as destination therapy noted a significant reduction (p<0.05) in both some and extreme problems with mobility, self-care (Figure 13), usual activities of living (Figure 14), and anxiety/depression, comparing pre-implant with 3, 6, and 12 months post-implant. In the area of pain and discomfort, significantly fewer patients noted this symptom complex post implant, but 6% of patients interviewed continued to have extreme symptoms of pain and discomfort up to one year after LVAD implant. The EQ5D visual analog scale (see details under Material and Methods) demonstrated prompt improvement within 3 months following VAD implant, which was maintained during the first year following implant (Figure 15).
Figure 13.
Percent of patients with some and extreme problems with self care before and at intervals after Destination Therapy with a continuous flow (CF) left ventricular assist device (LVAD). See Materials and Methods for definition of EQ5D. RVAD, right ventricular assist device.
Figure 14.
Percent of patients with some or extreme problems with usual activities before and at intervals after Destination Therapy with a continuous flow pump. The depiction and abbreviations are as in Figure 14.
Figure 15.
EQ5D visual analog scale (see Materials and Methods for definition) before and at intervals after Destination Therapy with a continuous flow pump. The abbreviations are as in Figure 13.
Current Expected Survival Following Cardiac Transplantation
The current average survival after cardiac transplantation, as a benchmark for comparing post-transplant survival against DT, is derived from the most recent International Society for Heart and Lung Transplant Registry.5 The average 2-year risk-unadjusted survival in the most recent cohort is approximately 80% (Figure 16)
Figure 16.
Survival after cardiac transplantation, stratified by era. Reproduced with permission, International Society for Heart and Lung Transplantation Registry.
Survival Justification for Potential Triage from Transplant to DT Therapy
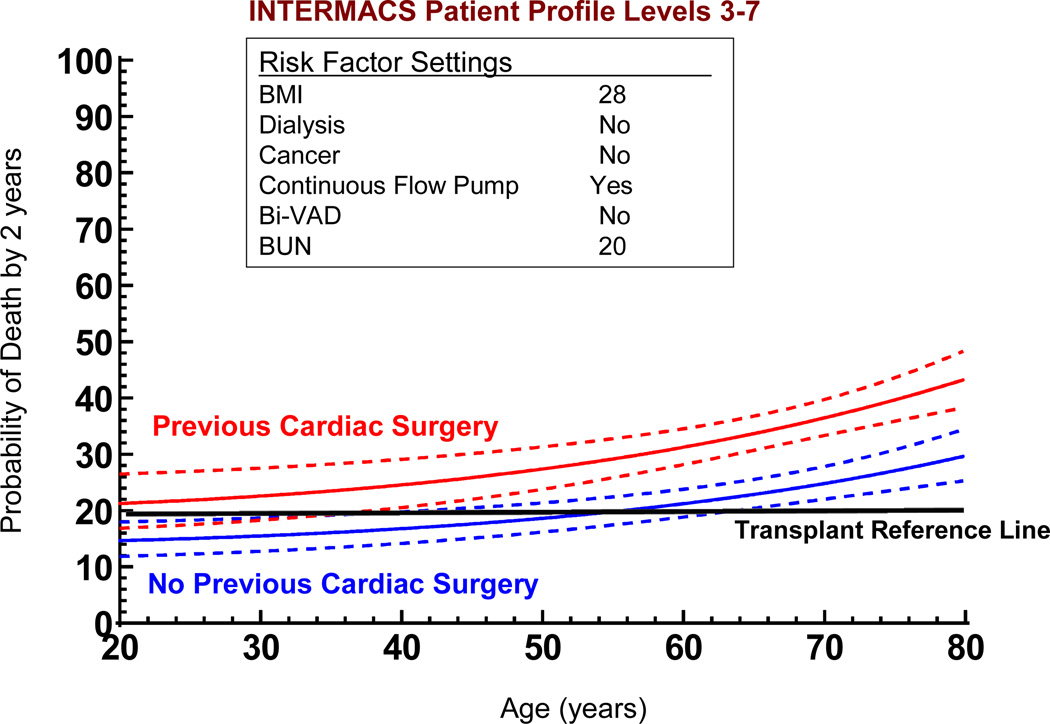
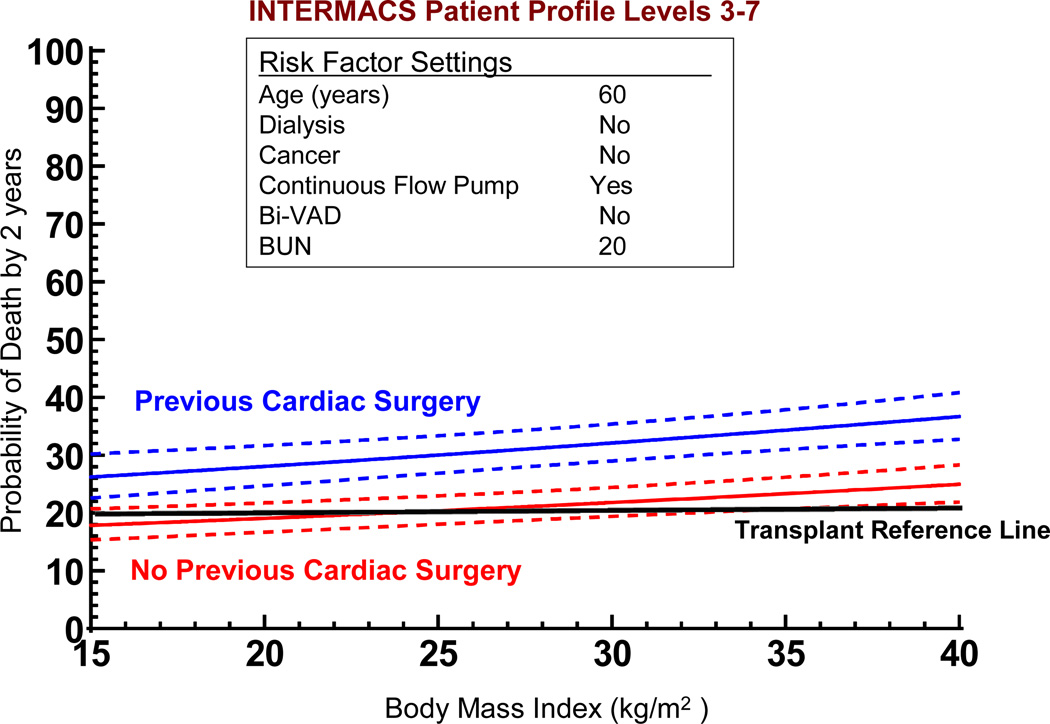
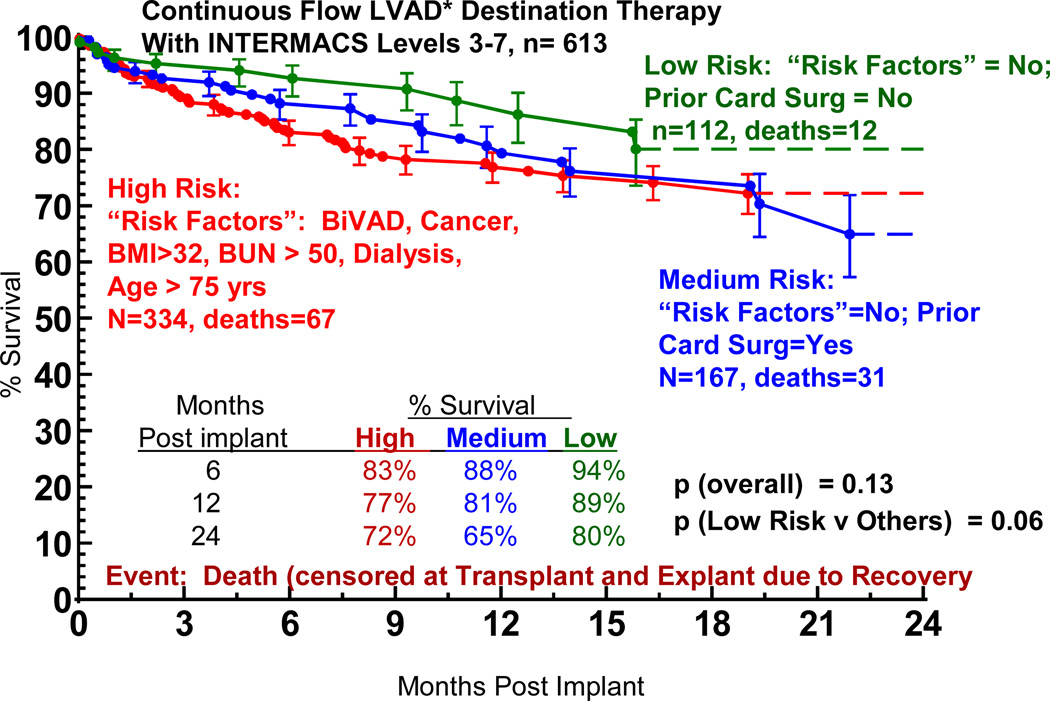
If we use the non-risk-adjusted 2 year transplant survival of 80% as an initial survival metric, generating solutions to the multivariate equation provides insight into patient subsets who would likely achieve the level of 2-year survival that might begin to compete with transplantation. Figure 17 depicts the age effect for a stable (INTERMACS level 3 or greater) patient with preserved renal function and without severe right ventricular failure who receives a continuous flow pump, stratified by the presence or absence of prior cardiac operations. For the patient with prior cardiac surgery, the 70% confidence limits overlap with a survival of at least 80% at 2 years for patients less than about 40 years of age; but for the patient without prior cardiac surgery survival is likely to be at least 80% at 2 years for patients age 62 or less. Figure 18 displays the solution to the multivariable equation for a 60 year old patient with preserved renal function and without right ventricular failure who receives a continuous flow pump, depicted for various BMIs stratified by the presence or absence of prior cardiac surgery. Overall, about 20% of the DT population experienced 2 year survival equal to or greater than the transplant reference line of 80% (Figure 19).
Figure 17.
Solutions to the multivariable equation (see Appendix 5). The nomograms are generated with variables set as indicated for 2 patients according to age at implant. The dashed lines indicate the 70% confidence limits around the solution curve. BMI, body mass index; Bi-VAD, biventricular support; BUN, blood urea nitrogen.
Figure 18.
Solutions to the multivariable equation (see Appendix 5). The nomograms are generated with variables set as indicated for 2 patients according to Body Mass Index. Depiction is as in Figure 17.
Figure 19.
Actuarial survival stratified by high, medium, and low risk patients. “Risk factors” include presence of biventricular support (BiVAD), previous cancer, body mass index (BMI) greater than 32, serum sodium less than 130, or blood urea nitrogen (BUN) greater than 50.
DISCUSSION
Evolution of Destination Therapy
As early as 1991, the Institute of Medicine recognized the importance of detailed longitudinal follow-up data on patients receiving long-term MCS devices. In their report on “The Artificial Heart: Prototypes, Policies, and Patients”, the report supported development of a registry to follow mechanical circulatory support patients for the remainder of their lives. …”maintaining a Registry of recipients should be considered a routine aspect of this care. The committee recommends that NHLBI support long-term studies.” 6
After more than a decade of MCS as bridge-to-transplant therapy, the REMATCH TRIAL (conducted between 1998 and 2001)7, paved the way for FDA approval of the HeartMate XVE for DT in the US, followed by the CMS decision for Medicare coverage of DT in 2003. The prospect of widespread application of MCS as long-term therapy for patients with advanced heart failure provided the stimulus for the NHLBI to develop a platform for long-term follow-up of patients supported by MCS therapies. The resultant NHLBI contract laid the foundation for INTERMACS, as an extension of the earlier recommendations from the Institute of Medicine.
During the first 2 years of the INTERMACS experience, the landscape of approved device therapy was dominated by pulsatile technology, since continuous flow pumps were largely still under investigation in clinical trials.8 With the general recognition that the Heartmate XVE device was prone to device malfunction with the frequent need for reoperation within 12 to 18 months (see again Figure 6), few such pumps were being implanted for DT. With escalating enthusiasm for continuous flow technology in Europe and the U.S., many patients poorly suited for cardiac transplantation received a continuous flow pump (approved for BTT therapy in 2008) under the reimbursable approved indication of BTT.9 In reality, the transplant status of this group of patients was ambiguous, and MCS therapy was often applied as a strategy of “bridge-to-candidacy”. 9 Predictably, VADs implanted as DT represented less than 10% of devices entered into INTERMACS between 2006 and 2009.10 The marked increase in pumps implanted as DT in 2010 and 2011 (more than 30% of total implants)8 coincided with the approval of the HeartMate II continuous flow pump for Destination Therapy in January of 2010.
This rapid evolution toward continuously flow technology is poignantly reflected in the INTERMACS registry during the most recent era (see again Figure 2). Greater than 95 % of devices entered into INTERMACS in 2010 and 2011 were continuous flow pumps, and all DT devices were continuous flow devices.9 The general enthusiasm for continuous flow devices in DT strategies was also supported by the multi-institutional study of Park and colleagues.11
Reasons for Transplant Ineligibility
Despite the intended strategy of Destination Therapy as a “Permanent” therapy for advanced heart failure, the clinical reality is that few therapies are exclusively “either/or” for the duration of the heart failure patient’s life. Even with the most successful MCS, patients will be followed indefinitely with scheduled reevaluations to determine whether the device, heart transplantation, explant, or potentially other therapies provide the greatest opportunity going forward for the highest quality extended survival. The appreciation of possible neutralization of certain transplant contraindications following device implant was previously addressed by Kirklin and colleagues in categorizing such contraindications as “modifiable” OR “un-modifiable.”10 The challenge of clinical prescience regarding the reversibility of many co-morbid conditions is embodied in the designation “bridge-to-candidacy”, which is currently the most common device strategy (not an FDA approved indication) among implant recipients9 in INTERMACS.
Outcome of Patients with Initial DT Strategy
Despite the potential impurity of the designation DT as well as likely overlap (in terms of actually pursuing a course of long-term VAD therapy) with other strategy designations such as “Bridge-to-Candidacy”, this study was well-suited to critically analyze patients who received extended support with durable devices. Other studies have made inferences about “long-term” device therapy from patient populations8, 12,13 ultimately committed to transplantation. The “censoring” of patients at time of transplant introduces considerable uncertainty about long-term device potential, in that patients with major device complications (which would likely impact patient survival and quality of life if the device was retained) often undergo transplantation at an increased priority level, thereby potentially avoiding an unfavorable VAD outcome. This study had relatively few such patients, in that only about 10% of pulsatile device patients underwent transplantation during the study period, and less than 5% of continuous flow patients (see again Figures 4 and 5) were diverted to transplant.
Adverse Events
In the evaluation of patients potentially suitable for either cardiac transplantation or MCS DT, the expected life satisfaction during extended support will ultimately exert a major influence on therapy selection for both practitioner and patient. In this sense, therapy comparison and selection is confounded by the striking differences in the adverse event profiles of heart transplantation versus MCS. Whereas transplantation is challenged by the complications of rejection, infection, malignancy, and allograft vasculopathy 14 the adverse event profile of the MCS patient is dominated by device-related infection, bleeding events, right heart failure, central nervous system events, and device malfunction.9,15, 10. The concept of adverse event burden8 (see again Table 4) might provide a unifying method of expressing the overall adverse event impact of each therapy, but ultimately the patient will need to participate in therapy selection based, in part, on a subjective analysis of the relevant adverse event profile and how that might impact life satisfaction.
Survival
The pace of progress in effective extended MCS has been well-chronicled since the original REMATCH publication cited a 2-year survival less than 25% with the Heartmate XVE as DT.7 Subsequent reports with the same pump technology reported 2-year survival of nearly 60%.11 With continuous flow technology as bridge-to-transplant therapy, 1 year survival improved from 68% in the original Heartmate II BTT trial12 to 73% in the mid-trial update.11 In the subsequent Heartmate II DT Trial 2-year survival improved from 58% in the early trial experience to 63% for the mid-trial group.11 The clear survival benefit of continuous flow technology over pulsatile pumps for DT was documented in the INTERMACS analysis of 2011.10 Other studies indicate that survival improvements have continued despite little change in overall patient risk profile over the past 4 years.9 The trajectory of incremental survival documented in this analysis is consistent with improvements in the field of MCS over the past 5–8 years.
Risk Factors for DT
The New York Heart Association Class IV designation has become the major descriptor of heart failure level sufficiently severe to warrant device therapy or transplantation, but it is well recognized that this encompasses different levels of clinical decompensation. The current analysis identified patients in cardiogenic shock to be at higher risk for mortality with DT, a finding noted early in the INTERMACS experience.8 This finding was facilitated by the INTERMACS subclassification of New York Heart Association Class IV for heart failure, in which 5 INTERMACS profiles describe the various stages of NYHA Class IV (see again Appendix 3). After the first year of data collection, it was noted that over 40% of patients receiving durable devices were in acute decompensation (Level 1, cardiogenic shock). With the appreciation of the important increment of mortality associated with implantation under such unstable conditions, the proportion of patients receiving implants in shock fell to under 15% in 2010.9 Currently, increasing proportions of patients are currently implanted in the lower INTERMACS levels of NYHA Class IV.
Right ventricular dysfunction severe enough to require RVAD support was the greatest predictor of subsequent mortality, which has been a consistent finding in prior INTERMACS analyses.2,8,9,10 Precise definitions of various degrees of RV dysfunction have proved challenging, and have been recently modified to better reflect clinical correlates (see Appendix). Even the identification of severe RV failure by the need for RVAD support is rather subjective, since the specific trigger for initiating RVAD placement differs among experienced VAD surgeons. The importance of identifying patients at risk for severe RV failure is evident, since such patients on the transplant list would be poor candidates for triage to DT VAD therapy. INTERMACS has viewed RV dysfunction to be such a critical issue that a scientific task force is being assembled to address the issues of identification, prevention, and management of the failing right ventricle during LVAD support.
Quality of Life
The findings of improved quality of life (QOL) indicators as judged by the EQ5D instrument are consistent with improvements in QOL and functional status using the Minnesota Living with Heart Failure Questionnaire.17 and the Kansas City Cardiomyopathy Questionnaire18 in the Heartmate II device trial.19, 20 Other studies have shown substantial improvement in the 6-minute walk test post implant.20. Similar positive findings were reported by Park and colleagues.11 Although a myriad of studies have documented improvement in QOL and functional outcome indicators after heart transplantation21,22 few studies have directly compared transplantation to MCS.23
Survival Justification to Triage from Transplant to DT Therapy
The solutions to the risk factor model provide insight into the types of patients who, depending on their co-morbidities for cardiac transplantation, might warrant consideration for triage to Destination Therapy MCS with continuous flow technology. The profile of such patients would currently include preserved renal function, absence of severe RV failure, stable cardiovascular function on inotropic support, and BMI suitable BMI for transplant. Currently, the model does not justify triage to DT if the patient has undergone previous cardiac operations except for relatively young patients, a group less likely to select long term pump support unless they were highly sensitized. Although this model would not yet support tapping into the large cohort of patients with prior cardiac operations, details of that decision-making process would depend on risk factor modeling within the transplant population to seek subsets whose predicted 2 year survival might be less than 80%. Realizing that inferences are being drawn from a group of patients considered ineligible for cardiac transplantation, the stratified actuarial depiction in Figure 20 suggests that nearly 20% of the actual DT patient population achieved survival that would be competitive with the “average” transplant patient.
If such triage decisions did occur, a paradigm shift of this magnitude in the arena of advanced heart failure would certainly accelerate the pace of device implants and potentially affect listing criteria at major heart transplant centers as well as allocation algorithms. Major challenges to ensure truly informed patient decisions regarding device therapy versus transplantation would further confound decisions in this evolving platform of heart failure care.
Of course the primary metric would be survival, but survival at what interval? Is predicted 2 year survival of sufficient duration for such a paradigm shift? In great measure the answer depends on the “expected” trajectory of the survival curve beyond 2 years, since the possibility appears remote that patients and providers will await 5 more years of patient follow-up, a randomized trial of VAD versus transplant, or other longitudinal analyses before beginning the “shift”. Societal and healthcare “impatience” is already evident with the “REVIVE IT” Trial, which allocates long-term device therapy possibly before the patient would normally be considered for transplantation
Another imponderable in this therapeutic allocation process is the clear knowledge the patients with a DT device can always be reconsidered for cardiac transplantation if device complications or other impairments in quality of life tip the balance toward transplantation. Other unintended consequences may occur: the whole priority allocation for other patients awaiting transplantation may be affected, and hospitals without expertise in transplant surgery and medicine may preferentially (and possibly inappropriately) offer devices rather than transplant to transplant-eligible patients.
Once this “magic metric” of survival (for example, 80% at 2 years) is accepted for devices in the advanced heart failure community, other metrics which majorly impact the quality of patient existence will assume center stage in our ongoing analyses. For just that reason, quality of life and functional outcome indicators represents a major INTERMACS focus in future analyses.
In considering specific patients for triage from transplantation to device therapy, the presence of non-cardiac comorbidities are known to impair long-term survival after cardiac transplantation, as has been quantified in an analysis from the Cardiac Research Database.24 Another important group of patients for potential triage are those who are highly sensitized with circulating anti-HLA antibodies. The opportunity for destination therapy could provide an important benefit for these sensitized patients who frequently endure long waiting times while continuing to experience advanced heart failure symptoms. Ultimately, the triage of these higher risk transplant subsets could increase the survival utility of the precious and limited resource of donor hearts.
Study Limitations
Inferences from this database have important strengths and weaknesses. The strengths include the scope and rigor of INTERMACS. As an NHLBI initiative INTERMACS engages NIH oversight, follows OSMB surveillance, and requires performance standards of participating institutions. The power of INTERMACS is further enhanced by the CMS mandate for data submission on all approved and consented durable mechanical circulatory devices implanted at Centers approved for DT therapy, formal definitions and processes for adjudication of adverse events, dedicated electronic data submission, compulsive data monitoring, and a hospital auditing process, all of which raise INTERMACS close to the rigor of a clinical trial. Indeed, the quality and completeness of data collection has been of sufficient quality that the FDA has embraced the use of INTERMACS patients as “concurrent controls” in at least 1 pivotal clinical device trial,25 and the INTERMACS platform has been held in high regard by the NHLBI.1
However, important study limitations relate to the knowledge that INTERMACS does not represent a complete sample of DT patients. Follow-up is not available for patients who do not provide informed consent, which represent about 9.6% of available patients with FDA approved devices. One could speculate about whether unconsented patients represent a higher risk group of patients. Furthermore, all patients who receive DT therapy as part of a clinical device trial are excluded from INTERMACS. Finally, maximal followup of continuous flow DT patients is only now exceeding 2 years, generating uncertainty about truly long-term survival.
Conclusions
In conclusion, 1) Destination Therapy represents an increasing LVAD application and currently accounts for nearly 1/3 of overall MCS activity in the U.S.; 2) Evolution from pulsatile to continuous flow technology has dramatically improved one and two year survival; 3) Destination Therapy is not appropriate for patients with rapid hemodynamic deterioration or cardiogenic shock; 4) The presence of severe right ventricular failure is a contraindication for destination therapy; 5) Important subsets of DT patients now enjoy survival which may be competitive with heart transplantation out to about two years, and 6) Future studies will focus on transplant eligible subsets who may benefit from informed discussions about mechanical circulatory support as an alternative long-term option.
ACKNOWLEDGMENTS
The authors wish to acknowledge the skilled participation of Ms. Susan Meyers in all areas of the analyses and Ms. Peggy Holmes in the manuscript preparation.
DISCLOSURE STATEMENT
This work was sponsored by the national institutes of health, national Heart, Lung and Blood Institute (NHLBI), Registry of Mechanical Circulatory Support Devices for End-Stage Heart Failure (INTERMACS). Contract No. HHSN268200548198C.
Dr. Kirklin is Princple Investigator for INTERMACS
Dr. Pagani has a research contract with the NHLBI and HeartWare for the REVIVE-IT trial and HeartWare for the ENDURANCE trial. All contracts are managed by the University of Michigan.
Dr. Naftel receives research funding from Thoratec but no salary. He is a consultant for HeartWare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIO
- 1.Miller MA, Ulisney K, Baldwin JT, et al. INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support): A New Paradigm for Translating Registry Data Into Clinical Practice. Journal of American College of Cardiology. 2010;56(9):738–740. doi: 10.1016/j.jacc.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS Database for Durable Devices for Circulatory Support: First Annual Report. J Heart Lung Transplant. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Warner-Stevenson L, Kirklin JK, Pagani FD, et al. INTERMACS Profiles of Advanced Heart Failure: First Definition. J Heart Lung Transp. 2009;28:535–541. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 4.McGiffin DC, Naftel DC, Kirklin JK, et al. Predicting Outcome After Listing for Heart Transplantation in Children: Comparison of Kaplan-Meier and Parametric Competing Risk Analysis. J Heart Lung Transplant. 1997;16:713–722. [PubMed] [Google Scholar]
- 5.Stehlik J, Edwards lB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Heart Transplant Report-2011. J Heart Lung Transplant. 2011;30(10):1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Institutes of Medicine. Washington, DC: The National Academies Press; 1991. The artificial heart: prototypes, policies and patients. [PubMed] [Google Scholar]
- 7.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345(20):1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29(1):1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirklin JK, Naftel DC, Kormos RL, et al. The Fourth INTERMACS Annual Report 4000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Kormos RL, et al. Third INTERMACS Annual Report: The evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in Advanced Heart Failure Patients with Left Ventricular Assist Devices for Destination Therapy. Circ Heart Fail. 2012;5:241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 12.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Eng J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 13.Starling RC, Naka Y, Boyle AJ, et al. Results of the post-US Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Young JB, McGiffin DC, et al. Heart Transplantation. Philadelphia, PA: Churchill Livingston Publishers; 2002. published. [Google Scholar]
- 15.Aissaoui N, Borgermann J, Gummert J, et al. HeartWare continuous-flow ventricular assist device thrombosis: The Bad Oeynhausen experience. J Thorac Cardiovasc Surg. 2012;143:e37–e39. doi: 10.1016/j.jtcvs.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Pagani FD, Miller LW, Russell SD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 17.Rector TS, Kubo SH, Cohn JN, et al. Validity of the Minnesota Living with Heart Failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. 1993;71:1106–1107. doi: 10.1016/0002-9149(93)90582-w. [DOI] [PubMed] [Google Scholar]
- 18.Spertus J, Peterson E, Conard MW, et al. Monitoring clinical changes in patients with heart failure: A comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Starling RC. Improved quanity and quality of life: A winning combination to treat advanced heart failure. J Am Coll Cardiol. 2010;55:1835–1836. doi: 10.1016/j.jacc.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiology. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Grady KL, Jalowiec, White-Williams C, et al. Predictors of quality of life in patients at one year after heart transplantation. J Heart Lung Transplant. 1999;18(3):202–210. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 22.Grady KL, Naftel DC, White-Williams, et al. Predictors of Quality of Life at 5 to 6 Years After Heart Transplantation. J Heart Lung Transplant. 2005;24(9):1431–1439. doi: 10.1016/j.healun.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Kugler C, Malehsa D, Tegtbur U, et al. Health-related quality of life and exercise tolerance in recipients of heart transplants and left ventricular assist devices: A prospective, comparative study. J Heart and Lung Transplant. 2011;30(2):204–210. doi: 10.1016/j.healun.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Kirklin JK, Naftel DC, et al. Mechanical Circulatory Support: Registering a Therapy in Evolution. Circulation-Heart Failure. 2008;1:200–205. doi: 10.1161/CIRCHEARTFAILURE.108.782599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aaronson KD, Slaughter MS, McGee E, et al. Evaluation of the HeartWare® HVAD Left Ventricular Assist Device System for the Treatment of Advanced Heart Failure: Results of the ADVANCE Bridge to Transplant Trial. Circulation. 2010;122:2216. [Google Scholar]