Introduction
Intracranial hemorrhage refers to any bleeding within the intracranial vault, including the brain parenchyma and surrounding meningeal spaces. This article will focus on the acute diagnosis and management of primary non-traumatic intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH) in the emergency department.
Intracerebral Hemorrhage
Intracerebral hemorrhage, or ICH, is a devastating disease. The overall incidence of spontaneous ICH worldwide is 24.6 per 100,000 person-years with approximately 40,000 to 67,000 cases per year in the United States[1–3]. The 30-day mortality rate ranges from 35% to 52% with only 20% of survivors expected to have full functional recovery at 6 months.[3] Approximately half of this mortality occurs within the first 24 hours[4], highlighting the critical importance of early and effective treatment in the Emergency Department (ED).
Risk factors
A recent population-based meta-analysis showed that risk factors for ICH include male sex, older age, and Asian ethnicity.[1, 5] ICH is twice as frequent in low-to-middle income countries compared to high-income countries.[5] In the United States, several studies have shown that the incidence of ICH is greater in African Americans and Hispanics than in whites [6–8].
The most important risk factors for ICH include hypertension (HTN) and cerebral amyloid angiopathy (CAA). HTN-related ICH is more likely to occur in deep structures[9], and the risk of ICH increases with increasing blood pressure values[10]. CAA tends to occur in association with advanced age, and CAA-related ICH tends to occur in lobar regions.[11]
Other risk factors for ICH include:
-
a)
Alcohol intake: This risk appears to be dose-dependent, with a higher risk of ICH among those with a higher daily alcohol intake [10]. Acute changes in blood pressure during ingestion and withdrawal, effects on platelet function and coagulation, and dysfunction of the vascular endothelium may account for this risk. [12]
-
b)
Cholesterol: Low levels of total serum cholesterol are risk factors for ICH (in contrast to ischemic stroke, for which high cholesterol levels are a risk). [13]
-
c)
Genetics: The gene most strongly associated with ICH is the Apolipoprotein E (APOE) gene and its ε2 and ε4 alleles[14]. The presence of the ε2 allele was recently also linked to hematoma expansion [15].
-
d)
Anticoagulation: Oral anticoagulants are widely used as prophylaxis in patients with atrial fibrillation and other cardiovascular and prothrombotic states. The annual risk of ICH in patients taking warfarin ranges from 0.3 to 1.0% per patient-year with a significantly increased risk when the INR is >3.5[16].
-
e)
Drug abuse: Sympathomimetic drugs, such as cocaine, are risk factors for ICH, and patients actively using cocaine at the time of their ICH have significantly more severe presentations and worse outcomes[17].
Pathophysiology
Primary ICH is typically a manifestation of underlying small vessel disease. First, longstanding hypertension leads to hypertensive vasculopathy causing microscopic degenerative changes in the walls of small-to-medium penetrating vessels, which is known as lipohyalinosis[18]. Second, CAA is characterized by the deposition of amyloid-beta peptide (Aβ) in the walls of small leptomeningeal and cortical vessels[19]. Although the underlying mechanism leading to the accumulation of amyloid is still unknown, the final consequences are degenerative changes in the vessel wall characterized by the loss of smooth muscle cells, wall thickening, luminal narrowing, microaneurysm formation and microhemorrhages[20].
Following initial vessel rupture, the hematoma causes direct mechanical injury to the brain parenchyma. Perihematomal edema develops within the first 3 hours from symptom onset and peaks between 10 to 20 days.[21]Next, blood and plasma products mediate secondary injury processes including an inflammatory response, activation of the coagulation cascade, and iron deposition from hemoglobin degradation[21]. Finally, the hematoma can continue to expand in up to 38 percent of patients during the first 24 hours. [22]
Clinical presentation and diagnosis
The acute presentation of ICH can be difficult to distinguish from ischemic stroke. Symptoms may include headache, nausea, seizures and focal or generalized neurologic symptoms. Findings such as coma, headache, vomiting, seizures, neck stiffness and raised diastolic blood pressure increase the likelihood of ICH compared to ischemic stroke, but only neuroimaging can provide a definitive diagnosis [38].
Neuroimaging
Noncontrast computerized tomography
Noncontrast computerized tomography (CT) is the most rapid and readily available tool for the diagnosis of ICH [23] and remains the most commonly used technique in the ED. Besides providing the definitive diagnosis, CT may also show basic characteristics of the hematoma, such as: hematoma location, extension to the ventricular system, presence of surrounding edema, development of mass effect and midline shift.
A quick estimation of the hematoma volume can be rapidly performed in the ED with the validated ABC/2 technique[24] (Figure 1). The steps to follow using this technique are:
The CT slice with the largest area of hemorrhage is selected.
A is the largest hemorrhage diameter on the selected slice (in centimeters [cm]).
B is the largest diameter perpendicular to A on the same slice.
C is the approximate number of slices in which the hemorrhage is seen multiplied by the slice thickness (often 0.5cm slices).
A, B, and C are then multiplied and the product divided by 2.
Figure 1.
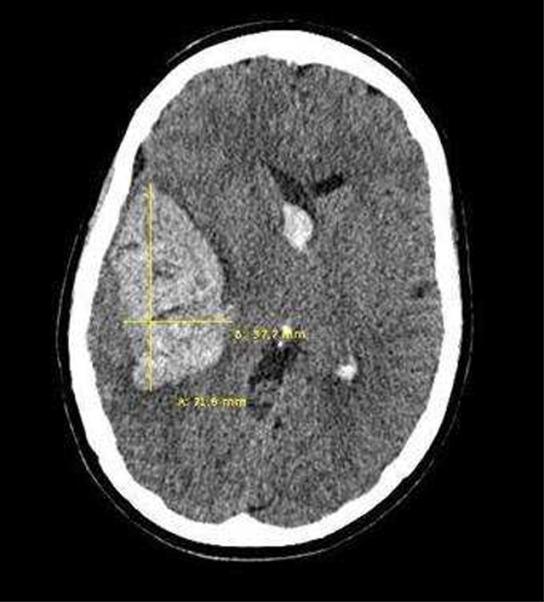
ABC/2 Technique
CT Angiography
CT Angiography (CTA) is gaining increasing acceptance as a diagnostic tool in the acute setting[25]. It is the most widely available, non-invasive technique for ruling out vascular abnormalities as secondary causes of ICH. The risk of acute nephropathy, if any, is likely quite low.[26] Up to 15% of patients with ICH will show an underlying vascular etiology on CTA, potentially changing acute management[27]. Finally, contrast extravasation seen on CTA images, also known as a “spot sign,” (Figure 2) is thought to represent ongoing bleeding and appears to mark those patients at highest risk of hematoma expansion, poor outcome and mortality.[28–31]
Figure 2.
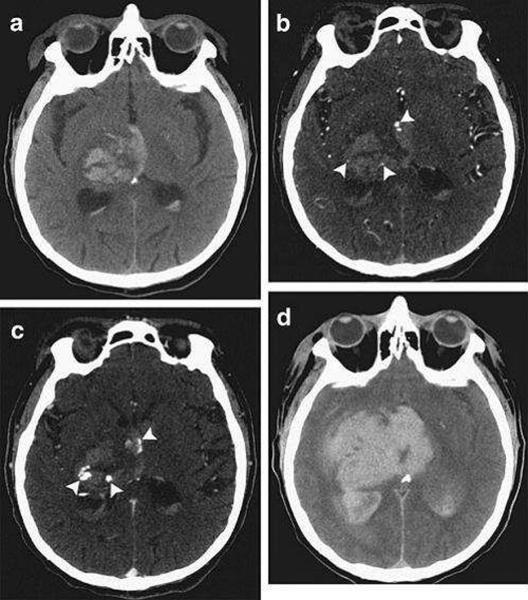
Computed Tomography (CT) and CT angiography of acute intracerebral hemorrhage. (A) Noncontrast CT shows a right thalamic intracerebral hemorrhage (24mL) with associated intraventricular hemorrhage (6mL). (B) CT angriography demonstrates 3 foci of contrast (spot signs) within the intracerebral hemorrhage (arrowheads) (C). Delayed CT angiography shows increased volume and changed morphology of the spot signs (arrowheads). (D) Noncontrast CT after 8 hours demonstrates expansion of the intracerebral hemorrhage (94ml) and intraventricular hemorrhage (82 mL). (Reproduced from [32])
MRI
MRI is equivalent to CT for the detection of acute ICH [32]. The imaging characteristics of ICH vary with time as the hemoglobin passes through different stages during the pathological process. In the acute phase, gradient recalled-echo (GRE) imaging techniques with T2*weighting are the best option to detect the presence of ICH [33]. MRI can also detect underlying secondary causes of ICH such as tumor and hemorrhagic transformation of ischemic stroke. Finally, for patients with poor kidney function or contrast allergies, the cerebral vasculature can be analyzed without contrast using Time-of-Flight MR angiography (MRA) [34].
Acute management
Airway
Patients with ICH are often unable to protect the airway. Endotracheal intubation may be necessary but this decision should be balanced against the risk of losing the neurologic examination. Rapid sequence intubation is typically the preferred approach in the acute setting. Pretreatment with lidocaine may be considered as it may blunt a rise in intracranial pressure (ICP) associated with intubation. Paralytic agents include succinylcholine, rocuronium or vecuronium, and for postintubation sedation, propofol is a reasonable choice given its short half-life. [35, 36]
Blood Pressure management
Elevated blood pressure (BP) is common in the acute setting after an ICH, and higher BP levels are associated with hematoma expansion and poor prognosis. However, it is not clear that reducing blood pressure improves outcomes[37]. While lowering BP may reduce the risk of expansion, it may theoretically also reduce cerebral perfusion. One randomized clinical trial found that lowering SBP to 140mmHg compared to 180mmHg reduced the risk of hematoma expansion but had no effect on outcomes[38]. A second trial found that rapid blood pressure lowering using intravenous Nicardipine appears safe but again showed no difference in outcomes[39]. Multiple clinical trials are currently ongoing to address this issue [40–42].
Until these trials clarify the role of BP management on hematoma expansion, expert guidelines from the American Heart Association/American Stroke Association (AHA/ASA) recommend BP treatment as in Table 1. [37] The European Stroke Initiative (EUSI) guidelines are similar (table 2) [43].
Table 1.
Recommended Guidelines from the AHA/ASA for Treating Elevated BP in Spontaneous ICH
|
SBP indicates systolic blood pressure;
MAP, mean arterial pressure. AHA/ASA, American Heart Association/American Stroke Association.
Adapted from [37]
Table 2.
Recommendations from EUSI for blood pressure management in ICH.
| Previous history of HTN | Gradually reduce MAP to <120 but >84 mm Hg; avoid a reduction of >20%. |
| BP limit is <180/105 mm Hg, if treatment is necessary target should be <160/100 mm Hg. | |
|
| |
| No history of HTN | Reduce MAP to 110 mm Hg. |
| BP limit is <160/95 mm Hg, if treatment is necessary target should be <150/90 mm Hg. | |
|
| |
| When increased ICP is present | Adapt MAP and BP limits to target a CPP of 60–70 mm Hg. |
EUSI guidelines for the management of blood pressure.
HTN= hypertension; MAP: mean arterial pressure; BP: blood pressure; ICP: intracranial pressure; CPP: cerebral perfusion pressure.
Adapted from [43].
In choosing medications to manage hypertension, intravenous antihypertensives with short half-lives should be considered as first-line therapy. The AHA recommends considering IV labetalol, nicardipine, esmolol, enalapril, hydralazine, sodium nitroprusside, or nitroglycerin[37]. The EUSI recommends IV labetalol, urapidil, sodium nitroprusside, nitroglycerin, or captopril [43].
Hemostatic therapy
It is tempting to consider that in a patient with ICH, acute hemostatic therapy will provide benefit. One phase III randomized trial in patients with no underlying coagulopathy found no clinical benefit from this approach [44]. As a result, current approaches to hemostasis are focused on correcting any underlying coagulopathies.
Oral anticoagulation
The most common class of agent used for oral anticoagulation is warfarin. Many authors believe that early action to rapidly correct the coagulopathy may prevent continued bleeding[45]. A number of therapeutic options are available for warfarin reversal.
As warfarin inhibits the vitamin K-dependent carboxylation of factors II, VII, IX, and X, vitamin K is a first line agent to restore these factors. Vitamin K given intravenously lowers the INR as early as 4 hours, but requires over 24 hours for full effect when used as monotherapy [46]. Vitamin K infusion at a dose of 5–10 mg should be started promptly and given slowly over 30 minutes.[47, 48]
While awaiting the effect of intravenous vitamin K, coagulation factors should be infused emergently. Fresh Frozen Plasma (FFP) contains all coagulation factors and is the most widely available and commonly used agent in the United States [49].Limitations include adverse events such as allergic reactions, potential transmission of infectious agents and transfusion-related acute lung injury (TRALI) [50].There is also significant time needed for its administration in actual practice, including time spent ordering, matching, thawing and delivering to the ED.[49]The dose of FFP ranges from 10 to 20 ml/kg of body weight. On average, the volume needed to correct the INR varies from 800–3500 ml, which may impose a significant volume load[51]. Early administration of coagulation factors maximizes the opportunity for early INR correction.[52
Prothrombin Complex Concentrates (PCCs) provide an alternate source of coagulation factors. PCCs contain coagulation factors prepared from pooled plasma. All available PCCs contain factors II, IX, and X, and some contain relevant amounts of factor VII and proteins C and S. In the United States there are currently two commercially available PCCs, Bebulin-VH Factor IX complex (Baxter, Westlake, CA)and Profilnine-SD (GrifolsBiologicals, Los Angeles, CA)[53]. Many other products are available in other countries, including Octaplex (Octapharma) and Beriplex (CSL Behring), which include clinically relevant amounts of all 4 vitamin K dependent factors, sometimes termed 4-factor PCCs to differentiate them from the other 3 factor PCCs [54]. PCC offers several advantages over FFP, including smaller infusion volume, faster time to INR correction, and lack of need for blood-type matching [53]. Thromboembolic (TE) events are potential complications of the use of PCC, although it is not clear that this risk (approximately 1.9%) is any different with FFP [55, 56].
Heparinoids
Heparin-related ICH is relatively rare, and data is sparse regarding appropriate treatment. One reasonable approach would be to reverse heparin with IV protamine sulfate at a dose of 1 to 1.5 mg per 100 units of heparin with a maximum dose of 50 mg [57].
Platelet function
The two major causes of platelet dysfunction are antiplatelet therapy and thrombocytopenia.
Antiplatelet agents use prior to an ICH is associated with a small increase in mortality, suggesting an opportunity for intervention.[58] The utility and safety of platelet transfusion in such patients is unknown, although some laboratory data suggest that such transfusions may improve platelet activity [59]. Platelet transfusion is therefore considered investigational by the AHA and is not recommended by the EUSI. The ongoing PATCH clinical trial will investigate whether platelet transfusions can improve outcome [60].
Additionally, it is not clear whether low platelets contribute to ongoing bleeding or worse outcome. Pending further data, current AHA recommendations are that patients with a severe thrombocytopenia should receive platelet transfusion.[37] A specific cutoff is not clarified; different groups use thresholds between 10,000 and 50,000 per microliter.
Novel antithrombotics
Recently, a number of new agents, such as factor Xa inhibitors Apixaban and Rivaroxaban and the direct thrombin inhibitor Dabigatran, have become available for stroke prevention [61–63]. There is no currently known antidote for reversal of these agents. Specific hemostatic agents such as rFVIIa and PCCs may be considered, though there is limited data on their use [64, 65]. For those cases related to Dabigatran use, a recently published expert recommendation states that the drug should be stopped immediately, supportive and symptomatic treatment should be initiated and, due to its renal excretion, aggressive diuresis and potential dialysis could be considered[66].
Intracranial pressure management
An increase in the intracranial pressure (ICP) may arise from the presence of intraventricular hemorrhage (IVH) and subsequent hydrocephalus, or from mass effect from a large hematoma or perihematomal edema. Currently, there are limited data regarding indications for ICP monitoring. Current guidelines from the AHA/ASA suggest that patients with a GCS score of ≤8, those with clinical evidence of transtentorial herniation, or those with significant IVH or hydrocephalus should be considered for ICP monitoring and treatment [37]. Cerebral perfusion pressure (CPP) can then be monitored, and recommendations are to maintain this between 50 to 70 mmHg.[27]
The initial management of elevated ICP should comprise simple measures such as elevation of the head of the bed, analgesia, and sedation. Medical options for ICP treatment include mannitol, hypertonic saline (ranging from 3% to 23.4%), and neuromuscular paralysis.[3, 67, 68] Barbiturates can be considered in refractory intracranial hypertension [35]. Although hyperventilation can produce a rapid decrease in the ICP, its effects are temporary, and its use should be reserved for impending herniation while awaiting surgical decompression.
Hyperglycemia management
Hyperglycemia measured at arrival in the ED is associated with worse outcome in both non-diabetic and diabetic patients.[69, 70] Declining glucose values after ICH are associated with a decreased risk of hematoma expansion and poor outcome, suggesting that early glucose control may improve outcomes.[45] Early evidence for this intervention comes from the QASC trial, in which patients with ICH and ischemic stroke were randomized to receive fever, hyperglycemia, and swallow screening, or not [71]. The intervention (including glucose management) lowered mortality and improved outcome. This highlights the need for careful glycemic control in the early phase.[37]
Temperature
The presence of fever is a common finding in patients with ICH, especially in those with IVH. Again, data from the QASC trial suggests lower mortality and improved outcome in those patients receiving fever control as part of a multidisciplinary approach [71]. Those with fever should undergo a thorough investigation to find a fever source if possible. [37]
Anemia
The presence of anemia is common in patients with ICH. It is present in up to 25% of cases at admission and is associated with larger hematoma volumes[72]. It also frequently develops during hospital stay.[73] Although current guidelines do not address this issue, a recent study found that packed red blood cell (PRBC) transfusion in these patients was associated with improved survival at 30 days[73]. Therefore, transfusion can be considered in such patients, although the ideal target hemoglobin level has not been determined.
Antiepileptics
Patients with ICH are at an increased risk of developing seizures; however, most of these events are subclinical electroencephalographic findings. Seizures are more common in lobar ICH and during the first 72 hours after admission.[74–76] The majority of patients develop a single episode of seizure during hospitalization, suggesting that those episodes are related to the pathophysiological processes that occur early after an ICH.[76] The use of prophylactic antiepileptic drugs (AEDs) in patients with ICH is a common practice, although it is not clear that the presence of seizures and/or the use of prophylactic AEDs affect short or long term outcome.[77, 78] Some studies have in fact reported an association between AEDs and worse outcome, although these patients were disproportionately exposed to phenytoin as the AED of choice. [79, 80]
Currently, the AHA/ASA recommends that AEDs should not be used routinely in patients with ICH. The only clear indications are the presence of clinical seizures or electrographic seizures in patients with a change in mental status. They also suggest that the use of continuous electroencephalography (EEG) monitoring should be considered in those patients with depressed mental status out of proportion to the degree of brain injury.[37]
Surgical interventions
External Ventricular Drain Placement
As described previously, some patients may benefit from ICP monitoring. External ventricular drain (EVD) placement not only provides the ability to monitor ICP but has the advantage of allowing therapeutic drainage of the CSF, which is valuable in patients with hydrocephalus.[81] The AHA recommends that ICP monitoring and treatment be considered in patients with a GCS score ≤ 8, those with clinical evidence of transtentorial herniation, or those with significant IVH or hydrocephalus.[82] The EUSI recommends considering continuous ICP monitoring in patients who need mechanical ventilation and recommend medical treatment of elevated ICP if clinical deterioration is related to increasing edema.[43]
Intraventricular thrombolysis
IVH occurs when ICH extends into the ventricles. It occurs in approximately 45% of ICH, more frequently in relatively large and deeply located (caudate nucleus and thalamus) hemorrhages [83]. The presence and the volume of IVH are correlated with poor prognosis in patients with ICH [84]. Although evacuation of an intraventricular clot is currently not routinely recommended, a recent study comparing the use of intraventricular rtPA to placebo showed that the use of rtPA was not only feasible and safe, but also showed a significantly greater rate of blood clot resolution[85]. In addition, a recent meta-analysis found that adding intraventricular fibrinolysis to EVD placement is associated with better functional outcome, [86] although no prospective randomized trial has evaluated this. The CLEAR III study, an ongoing phase III randomized clinical trial was designed to compare the effect on clinical outcome of the intraventricular use of rtPA compared to placebo (ClinicalTrials.gov # NCT00784134).
Hematoma evacuation
The role of surgical evacuation is to decrease mass effect related to the presence of blood, as well as to minimize secondary injury. The only clear recommendation for immediate surgical intervention is in patients with cerebellar hemorrhages with neurological deterioration, brainstem compression, and/or hydrocephalus from ventricular obstruction.[37] For these patients, emergency neurosurgical consultation should be obtained. However, it is less clear whether patients with supratentorial ICH will benefit. One large phase III clinical trial, the STICH trial, compared early hematoma evacuation with initial conservative treatment for patients with spontaneous supratentorial ICH[87]. This study showed no difference in outcome, suggesting that surgical evacuation provided no benefit. However, a subsequent subgroup analysis raised the possibility that those with hematomas ≤1 cm from the cortical surface (which are more easily accessible) might receive benefit [88]. This possibility is being evaluated in the ongoing STICH II trial [89]. The theoretical idea that hyperacute evacuation of the hematoma would be beneficial was not borne out when a study evaluating the effect of surgery within 4 hours was stopped due to a high rate of rebleeding.[90]
Minimally invasive surgery
The development of less invasive surgical techniques may decrease the risk of surgical complications. These techniques are showing promising results, particularly in deep hemorrhages where conventional surgery showed no benefit in the past [91]. Minimally invasive stereotactic puncture is reported to be safe and feasible and may lead to better long-term outcome and fewer complications when compared with conventional craniotomy[92] and conventional medical treatment[93, 94].
Prognosis
Multiple grading scores exist that allow for evidence-based risk stratification in the acute phase. First, the ICH score predicts 30-day mortality using features such as age, ICH volume and the presence of IVH, with higher score associated with worse outcome (Table 3) [95]. Second, the FUNC score (FUNCtional outcome risk stratification) predicts functional independence rather than mortality at 90-days (table 4) [96].The higher the FUNC score, the greater the chance of the patient recovering functional independence.
Table 3.
| Component | ICH score points |
|---|---|
|
| |
| GCS score | |
| 3–4 | 2 |
| 5–12 | 1 |
| 13–15 | 0 |
| ICH volume in cm3 | |
| ≥30 | 1 |
| <30 | 0 |
| IVH | |
| Yes | 1 |
| No | 0 |
| Infratentorial origin of ICH | |
| Yes | 1 |
| No | 0 |
| Age in years | |
| ≥80 | 1 |
| <80 | 0 |
ICH score, extracted from [95]
Table 4.
| Component | FUNC Score Points |
|---|---|
|
| |
| ICH volume, cm3 | |
| <30 | 4 |
| 30–60 | 2 |
| >60 | 0. |
| Age, years | |
| <70 | 2 |
| 70–79 | 1 |
| ≥80 | 0 |
| ICH location | |
| Lobar | 2 |
| Deep | 1 |
| Infratentorial | 0 |
| GCS score | |
| ≥9 | 2 |
| ≤8 | 0 |
| Pre-ICH cognitive impairment | |
| No | 1 |
| Yes | 0 |
| Total FUNC score | 0–11 |
FUNC score, extracted from [96].
There is some data that poor prognosis can lead to self-fulfilling prophecies of early death. Limiting care via early do not resuscitate (DNR) orders, withdrawal of care, or deferral of other life sustaining interventions is independently associated with both short- and long-term mortality after ICH, after controlling for clinical markers of disease severity, even in patients who do not specifically require defibrillation [97]. As such, new DNR orders or withdrawal of care are generally not recommended in the ED. The AHA recommends aggressive full care early after ICH onset with postponement of new DNR orders until at least the second full day of hospitalization [37].
Subarachnoid Hemorrhage
Subarachnoid hemorrhage (SAH) is defined by the extravasation of blood into the subarachnoid space. The most common cause of SAH is trauma;amongst the nontraumatic cases, rupture of an intracranial aneurysm is the leading cause, representing up to 85% of cases. This review will focus on aneurysmal subarachnoid hemorrhage (SAH).
Epidemiology
The overall incidence of SAH is between 9–20 per 100,000 person-years. SAH is more frequent in women, and the mean age of presentation is 55 years[98, 99]. In the U.S. the number of cases of SAH is 30,000 per year[100].
Risk Factors and Prognosis
Major risk factors associated with SAH are current and former history of smoking, hypertension and excessive alcohol intake[101]. Although one third of cases can be attributed to a current smoking status, this risk appears to rapidly disappear after a few years of smoking cessation[102]. Cocaine use is also associated with SAH, and these patients tend to be younger and have a worse outcome [103, 104]. First degree family history as well as some genetic conditions including autosomal dominant polycystic kidney disease, Marfan's syndrome and Ehlers-Danlos syndrome are also associated with an increased risk of SAH[105].
A recent meta-analysis reported that in a population without comorbidities, the prevalence of unruptured intracranial aneurysms (UIAs) is 3.2%[106]. Only a small percentage of these UIAs will rupture and cause an SAH. The risk of rupture is increased in cases of previous history of SAH, age older than 60, female gender and Japanese or Finnish descent. In addition, the risk is greater for aneurysms >10mm and those located in the posterior circulation.[107–109]
The introduction of surgical treatment options improved the prognosis of patients with SAH.[110]In a retrospective analysis before the introduction of endovascular treatment of SAH, the most important factors predicting poor outcome at three months were increasing age, worse admission grade on the World Federation of Neurological Surgeons (WFNS) grading scale (Table 6), the development of cerebral infarction and symptomatic vasospasm. Other factors included greater clot thickness on admission computed tomography (CT) scan, aneurysm rupture within the posterior circulation, intraventricular and intracerebral extension of the hematoma and higher systolic blood pressure on admission.[111]Analysis from the large International Subarachnoid Aneurysm Trial (ISAT)comparing neurosurgical clipping versus endovascular coiling showed similar results.[112]
Table 6.
World Federation of Neurological Surgeons grading scale
| WFNS Grade | GCS | Motor Deficit |
|---|---|---|
|
| ||
| 1 | 15 | Absent |
| 2 | 13 to 14 | Absent |
| 3 | 13 to 14 | Present |
| 4 | 7 to 12 | Present or absent |
| 5 | 3 to 6 | Present or absent |
Extracted from [146]
Pathophysiology
Aneurysms are more common at the bifurcation of the arteries located on the base of the brain, especially the large arteries that form the circle of Willis.[113] Hemodynamic factors that contribute to the formation and growth of aneurysms are wall shear stress (WSS) and hydrostatic and transmural pressures. High WSS is encountered at the branch points of cerebral arteries and long-term exposure to this could trigger vessel wall remodeling through interaction with the endothelium and the secretion of factors such as nitric oxide (NO) and endothelial growth factors. Hydrostatic and transmural pressures produce a mechanical stretch of the wall that induces upregulation of certain molecules such as endothelin-1 B receptors (ETBR) that further affect vascular smooth muscle cells by promoting apoptosis.[114] Although the mechanism of formation and growth of aneurysms is now partially understood, it is still unclear what leads to aneurysmal rupture.
Clinical Presentation
The characteristic complaint of patients with SAH is a severe headache of acute onset. This headache is commonly described as being “the worst headache of my life” and with the highest intensity at onset. Although it is frequently accompanied by other symptoms, headache can be the only complaint in up to 40% of patients. [115] Recently, a prospective study found that the following clinical characteristics represent the highest risk of belonging to a case of SAH: age>40 years, associated neck pain or stiffness, witnessed loss of consciousness, onset with exertion, vomiting, arrival by ambulance, and blood pressure above 160/100.[116]
A subgroup of patients develops “warning signs” before the index SAH. The most common warning sign is again headache, which is of moderate intensity and less severe than those described in SAH. This is commonly referred to as “sentinel headache” or “warning leak” and may be associated with a small leakage of blood into the subarachnoid space or a small bleed into the aneurysmal wall. A thorough evaluation is warranted in these cases, since an SAH can develop up to 110 days later.[117, 118]
Physical examination may demonstrate neck stiffness and meningismus. [119]Although not specific, funduscopic evaluation may reveal subhyaloid, vitreous or intraretinal hemorrhage (which are known as Terson's syndrome) which is associated with higher mortality[120]. These eye findings may be found with any intracranial bleeding and are believed to be associated with sudden increase in intracranial pressure. Focal neurological deficits may also be found, and can be related to nerve compression by the aneurysm, intraparenchymal extension of the bleeding, or vasospasm (which typically occurs later in the course).
Diagnosis
Up to 1 in 20 SAH patients are missed during initial evaluation[121]. A high index of suspicion and a low threshold for performing diagnostic studies are key factors in making the diagnosis of SAH.
The gold standard diagnostic approach has been to initially perform a non-contrast CT scan of the brain followed by a lumbar puncture (LP) and analysis of the cerebrospinal fluid (CSF) when the CT is negative. Recently, however, some groups have suggested that current generation multislice CT scanners, as well as the availability of CT angiography in the acute setting, may offer opportunities to selectively defer LP.[122][123].
Computed Tomography
When there is clinical suspicion of SAH, the initial test of choice is a noncontrast CT scan. The sensitivity of the CT scan to detect SAH is maximal within the first 24 hours after the bleed and then decreases with time. The volume of blood in the subarachnoid space and the resolution of the scanner also influence the CT detection rate[124]. In fact, a recent multicenter prospective study of 3132 patients found that of those undergoing current generation CT within 6 hours of symptom onset, the sensitivity was 100% (95% confidence interval, 97–100%) with a negative predictive value of 100% (95% confidence interval, 99.5%–100%)[123]. Noncontrast CT also provides information regarding the volume of blood, extension to the cerebral parenchyma, the presence of hydrocephalus and the potential location of the aneurysm. Blood located in the interhemispheric fissure and the surrounding sulci has high probability of coming from an anterior cerebral or anterior communicating artery aneurysm, while blood in the posterior aspect of the Sylvian fissure is probably related to a middle cerebral artery aneurysm. (Figure 3)[124–126]
Figure 3.
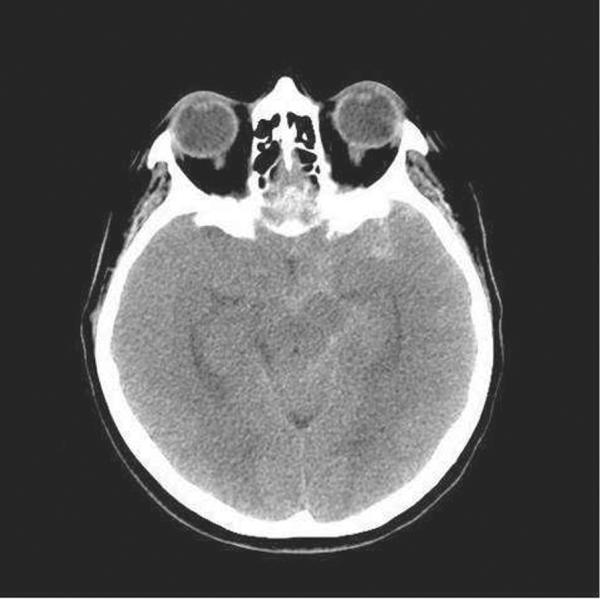
Subarachnoid Hemorrhage in the left Sylvian fissure, sulci of the left hemisphere, and along the left and central aspect of the suprasellar cistern, left ambient cistern, and interpenduncular cistern.
CT Angiography
CTA is a fast, noninvasive and readily available method to screen for the presence of aneurysm [127]. A recent meta-analysis showed that CTA has a pooled sensitivity of ~98% to detect aneurysm with sensitivities ranging from 86 to 100% [128]. Aneurysm detection rates are related to the experience of the reviewer and aneurysmal size. Pooled specificity of CTA in this analysis reached 100% with a range of 50 – 100% [128]. Also, three dimensional CTA may be as sensitive and specific as digital subtraction angiography (DSA)for the detection of aneurysms (Figure 4) [129]. As a result, patients with a negative CT/CTA have a less than 1% likelihood of aneurysmal SAH[130]. The Neurocritical Care Society recommends preferential use of CTA as an exploratory approach when it is readily available and of high technical quality over DSA if an immediate intervention is not planned[131].
Figure 4.
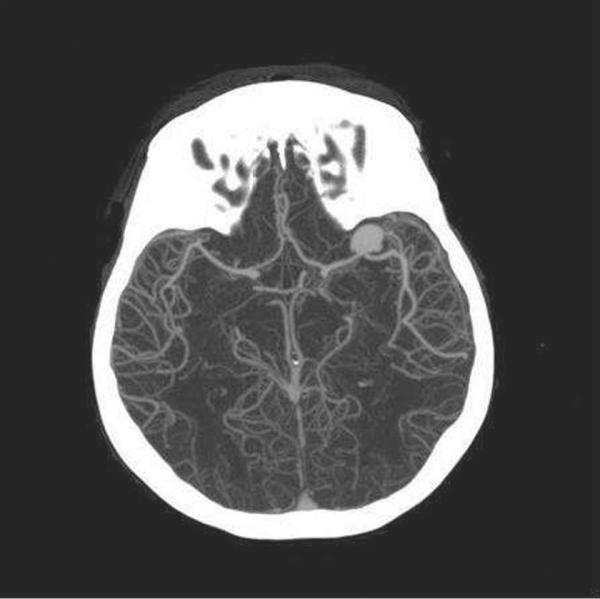
3D reconstruction CTA on the same patient from Figure 3. An aneurysmal sac is appreciated at the distal M1 segment of the left middle cerebral artery (MCA)
Lumbar Puncture
Lumbar Puncture (LP) is considered effectively 100% sensitive for detection of blood in the subarachnoid space, and it is recommended in all patients undergoing a workup for SAH with a negative CT[132–134]. CSF characteristics of SAH include an elevated opening pressure, presence of erythrocytes or red blood cells (RBCs), and xanthochromia. CSF should be visually inspected for the presence of xanthochromia, a term that refers to the yellow aspect of the CSF attributable to the formation of bilirubin from the breakdown of hemoglobin in the CSF [135].
Special consideration should be given to the use of spectrophotometry in CSF analysis for the detection of bilirubin. The use of this technique is strongly advocated in the United Kingdom (UK), where the rate of visual assessment of the cerebrospinal fluid fell to 6%, while the use of spectrophotometry rose to 94% in recent years[136]. This method has been shown to have ~ 100% sensitivity for the detection of bilirubin in patients with SAH, but with a low specificity [137, 138]. In the United States however, the majority of centers use visual inspection instead[139]. Some authors recommend spectrophotometry if available in those cases where visual inspection yields doubtful results [140].
Digital Subtraction Angiography
Digital subtraction angiography (DSA) allows for direct visualization of the cerebral vasculature and remains the gold standard for detecting aneurysms. This diagnostic tool requires a dedicated neurointerventional team and provides an opportunity for therapeutic interventions as well as diagnosis.
MRI
MRI is rarely used to diagnose SAH in the ED as availability is limited, and logistical barriers to its use are much higher than with CT. Blood is not easily detectable in T1-weighted and T2-weighted MRI sequences in the acute setting likely because the generation of deoxyhemoglobin with paramagnetic properties is delayed in the subarachnoid space[141]. However, the sensitivity of fluid attenuation inversion recovery (FLAIR) sequences is comparable to that of the CT in the acute phase of an SAH, and potentially superior in the subacute phase[142, 143].
Emergency Management
Airway management
The initial management of an SAH does not differ from other medical emergencies, and airway management is similar to that described previously for ICH.
Neurological exam
During the initial evaluation, a neurological exam should be performed and documented. Clinical grading scales that mark the severity of SAH include the Hunt and Hess scale (table 5) and the World Federation of Neurological Surgeons (WFNS) grading scale (Table 6). The Hunt and Hess scale was originally designed to evaluate the operative risk of patients and to aid at deciding the best timing for neurosurgical intervention[144], but it is now widely known and accepted as a predictor of outcome. It is based on the level of severity of clinical signs with a correlation with poor outcome with a higher grade. The WFNS grading scale uses the GCS score and group them into five grades but also takes into account the presence of a motor neurological deficit. Although widely used as predictors of outcome, their value has been challenged by a recent review[145].
Table 5.
Hunt and Hess grading scale
| Grade | Criteria |
|---|---|
|
| |
| 1 | Asymptomaticor minimal headacheand slight nuchal rigidity |
| 2 | Moderate to severe headache, nuchalrigidity, no neurological deficit other than cranial nerve palsy |
| 3 | Drowsiness, confusion, or mild focal neurologic deficit |
| 4 | Stupor, moderate to severe hemiparesis,possibly early decerebrate rigidity |
| 5 | Deep coma, decerebrate rigidity |
Adapted from [144]
Medical Management
Blood pressure management
When considering an optimal BP goal, an appropriate balance should be maintained. Hypotension may theoretically increase the risk of ischemia, while elevated BP raises the concern for aneurysmal rupture and rebleeding. Current guidelines recommend that hypotension should be avoided and that treatment of hypertension should be initiated until the aneurysm is secure only with extreme BP values when the MAP is >110 mm Hg aiming at maintaining a good cerebral perfusion pressure (CPP). The recommended agents to lower BP are nicardipine, labetalol and esmolol[131, 147].
Seizure prophylaxis
To date, no randomized controlled trial has evaluated the benefits of the prophylactic use of AEDs in patients with SAH. The incidence of seizure varies extensively in the literature [148]. Risk factors for the development of onset seizures include poor Hunt and Hess score, acute hydrocephalus, cerebral ischemia and large volume of subarachnoid blood[131, 149, 150].
Non-convulsive seizures and non-convulsive status epilepticus may occur after SAH, leading clinicians to recommend continuous electroencephalogram (cEEG) monitoring in poor Hunt and Hess grade patients[151]. Current guidelines recommend considering the use of routine AEDs especially in patients at higher risk, and using an alternative to phenytoin which has been linked to a poor prognosis[131]. Commonly used agents include levetiracetam, valproate, and fosphenytoin (it is unclear whether this shares the same possible negative effects as phenytoin).
Glycemic control
Both elevated and low glucose levels are associated with worse outcome after SAH. Hypoglycemia is associated with vasospasm, infarction and more disability at 3 months.Hyperglycemia on admission and during hospitalization is also associated with poor outcome and short-term mortality[152–154]. Although a specific target glucose level has not been established, it is currently recommended to avoid hypoglycemia (serum glucose <80 mg/dl) and maintain maximum values below 200 mg/dl[131].
Temperature
Fever is common after SAH. Fever at admission as well as its presence during hospitalization are associated with poor outcome [155]. A possible infectious etiology should always be investigated, although this is uncommon during initial presentation. Medical and physical interventions can be used as therapeutic measures to reduce fever. Currently, it is recommended to initiate therapy with antipyretic agents, such as acetaminophen when fever is present [131]. Physical surface or intravascular cooling devices should be used only when antipyretics fail and close monitoring and treatment of shivering should be started[131].
Vasospasm and Delayed Cerebral Ischemia
Vasospasm and delayed cerebral ischemia (DCI) are deadly complications of SAH associated with poor outcome. Angiographic vasospasm occurs in up to 70% of cases[156]. Symptomatic vasospasm, associated with new focal neurological findings and/or deterioration in the level of consciousness, occurs in approximately 30% of cases[157]. DCI can occur days after the index SAH and can lead to neurological deterioration and focal neurological deficits[158]. One preventive measure is the use of oral nimodipine (60 mg by mouth or nasogastric tube (NGT) every 4 hours for 21 days) and should be initiated soon after diagnosis of SAH[159]. Once vasospasm occurs, a range of medical and interventional therapies are available to maintain adequate cerebral perfusion [131]. One recognized therapy is the “triple-H therapy”, characterized by hypervolemia through volume expansion, hypertension with BP augmentation, and hemodilution aimed at reducing blood viscosity [160]. Currently, the Neurocritical Care Society recommends that euvolemia be pursued and that the routine use of hemodilution should be reserved for cases of erythrocythemia[131]. For BP augmentation, a stepwise approach is recommended and an SBP goal of >160 mmHg or a MAP > 120 mmHg is a reasonable approach [131, 161].
Aneurysm repair
Patients with SAH require emergency neurosurgical and/or endovascular consultation. There are currently at least two options for the acute treatment of a ruptured aneurysm: endovascular coiling or surgical clipping. Treatment of a recently ruptured aneurysm reduces the rate of rebleeding, and the benefit is related to the time to treatment initiation[162]. Current guidelines recommend that surgical clipping or endovascular coiling should be performed to reduce the rate of rebleeding after aneurysmal SAH, and these procedures should be performed early in the disease course. [131, 147]
The selection of the most appropriate intervention depends on a range of characteristics including age, clinical status and medical comorbidities. Aneurysm characteristics, such as location, shape, and size, are taken into consideration as well, which highlights the value of a specialized multidisciplinary group to provide care and decision-making. Some expert consensus groups recommend that SAH be preferentially managed at high volume centers (defined as those centers with greater than 60 cases of SAH per year)[131].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.van Asch CJJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. The Lancet Neurology. 2010;9(2):167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar MI, Freeman WD. Spontaneous intracerebral hemorrhage. Semin Neurol. 2010;30(5):555–64. doi: 10.1055/s-0030-1268865. [DOI] [PubMed] [Google Scholar]
- 3.Broderick J, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults. Stroke. 2007;38(6):2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 4.Elliott J, Smith M. The Acute Management of Intracerebral Hemorrhage: A Clinical Review. Anesthesia & Analgesia. 2010;110(5):1419–1427. doi: 10.1213/ANE.0b013e3181d568c8. [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology. 2009;8(4):355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 6.Labovitz DL, et al. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65(4):518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 7.Ayala C, et al. Racial/Ethnic Disparities in Mortality by Stroke Subtype in the United States, 1995–1998. American Journal of Epidemiology. 2001;154(11):1057–1063. doi: 10.1093/aje/154.11.1057. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi AI, Giles WH, Croft JB. Racial differences in the incidence of intracerebral hemorrhage. Neurology. 1999;52(8):1617. doi: 10.1212/wnl.52.8.1617. [DOI] [PubMed] [Google Scholar]
- 9.Matsukawa H, et al. Factors associated with lobar vs. non-lobar intracerebral hemorrhage. Acta Neurologica Scandinavica. 2011 doi: 10.1111/j.1600-0404.2011.01615.x. no-no. [DOI] [PubMed] [Google Scholar]
- 10.Ariesen MJ, et al. Risk Factors for Intracerebral Hemorrhage in the General Population. Stroke. 2003;34(8):2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 11.Maia LF, Mackenzie IRA, Feldman HH. Clinical phenotypes of Cerebral Amyloid Angiopathy. Journal of the Neurological Sciences. 2007;257(1–2):23–30. doi: 10.1016/j.jns.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Badjatia N, Rosand J. Intracerebral Hemorrhage. The Neurologist. 2005;11(6):311–324. doi: 10.1097/01.nrl.0000178757.68551.26. [DOI] [PubMed] [Google Scholar]
- 13.Wieberdink RG, et al. Serum Lipid Levels and the Risk of Intracerebral Hemorrhage: The Rotterdam Study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(12):2982–2989. doi: 10.1161/ATVBAHA.111.234948. [DOI] [PubMed] [Google Scholar]
- 14.Biffi A, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Annals of Neurology. 2010;68(6):934–943. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brouwers HB, et al. Apolipoprotein E Genotype Predicts Hematoma Expansion in Lobar Intracerebral Hemorrhage. Stroke. 2012 doi: 10.1161/STROKEAHA.111.643262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty ML. Anticoagulant-Associated Intracerebral Hemorrhage. Semin Neurol. 2010;30(05):565, 572. doi: 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Schild S, et al. Intracerebral Hemorrhage in Cocaine Users. Stroke. 2010;41(4):680–684. doi: 10.1161/STROKEAHA.109.573147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30(3):536–50. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Vinters H. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18(2):311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Annals of Neurology. 2011;70(6):871–880. doi: 10.1002/ana.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42(6):1781–6. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brott T, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Panagos PD, Jauch EC, Broderick JP. Intracerebral hemorrhage. Emerg Med Clin North Am. 2002;20(3):631–55. doi: 10.1016/s0733-8627(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 24.Kothari RU, et al. The ABCs of Measuring Intracerebral Hemorrhage Volumes. Stroke. 1996;27(8):1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 25.Delgado Almandoz JE, Romero JM. Advanced CT Imaging in the Evaluation of Hemorrhagic Stroke. Neuroimaging clinics of North America. 2011;21(2):197–213. doi: 10.1016/j.nic.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Oleinik A, et al. CT Angiography for Intracerebral Hemorrhage Does Not Increase Risk of Acute Nephropathy. Stroke. 2009;40(7):2393–2397. doi: 10.1161/STROKEAHA.108.546127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delgado Almandoz JE, et al. Diagnostic Accuracy and Yield of Multidetector CT Angiography in the Evaluation of Spontaneous Intraparenchymal Cerebral Hemorrhage. American Journal of Neuroradiology. 2009;30(6):1213–1221. doi: 10.3174/ajnr.A1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein JN, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68(12):889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, et al. Contrast Extravasation on CT Predicts Mortality in Primary Intracerebral Hemorrhage. American Journal of Neuroradiology. 2008;29(3):520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallevi H, et al. The Spot Sign in Intracerebral Hemorrhage: The Importance of Looking for Contrast Extravasation. Cerebrovascular Diseases. 2010;29(3):217–220. doi: 10.1159/000267842. [DOI] [PubMed] [Google Scholar]
- 31.Li N, et al. Contrast Extravasation on Computed Tomography Angiography Predicts Clinical Outcome in Primary Intracerebral Hemorrhage. Stroke. 2011;42(12):3441–3446. doi: 10.1161/STROKEAHA.111.623405. [DOI] [PubMed] [Google Scholar]
- 32.Kidwell CS, et al. Comparison of MRI and CT for Detection of Acute Intracerebral Hemorrhage. JAMA: The Journal of the American Medical Association. 2004;292(15):1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 33.Smith SD, Eskey CJ. Hemorrhagic Stroke. Radiologic clinics of North America. 2011;49(1):27–45. doi: 10.1016/j.rcl.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 34.DeLano MC, DeMarco JK. 3.0 T versus 1.5 T MR Angiography of the Head and Neck. Neuroimaging clinics of North America. 2006;16(2):321–341. doi: 10.1016/j.nic.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Rincon F, Mayer S. Clinical review: Critical care management of spontaneous intracerebral hemorrhage. Critical Care. 2008;12(6):237. doi: 10.1186/cc7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein J, Gilson A. Critical Care Management of Acute Intracerebral Hemorrhage. Current Treatment Options in Neurology. 2011;13(2):204–216. doi: 10.1007/s11940-010-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgenstern LB, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke. 2010;41(9):2108–2129. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson CS, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. The Lancet Neurology. 2008;7(5):391–399. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 39.Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38(2):637–48. doi: 10.1097/CCM.0b013e3181b9e1a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi A, Palesch Y. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) II: Design, Methods, and Rationale. Neurocritical Care. 2011;15(3):559–576. doi: 10.1007/s12028-011-9538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delcourt C, et al. The second (main) phase of an open, randomised, multicentre study to investigate the effectiveness of an intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT2) International Journal of Stroke. 2010;5(2):110–116. doi: 10.1111/j.1747-4949.2010.00415.x. [DOI] [PubMed] [Google Scholar]
- 42.Butcher K, et al. The Intracerebral Haemorrhage Acutely Decreasing Arterial Pressure Trial: ICH ADAPT. Int J Stroke. 2010;5(3):227–33. doi: 10.1111/j.1747-4949.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 43.Steiner T, et al. Recommendations for the management of intracranial haemorrhage - part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis. 2006;22(4):294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 44.Mayer SA, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 45.Aguilar MI, et al. Treatment of Warfarin-Associated Intracerebral Hemorrhage: Literature Review and Expert Opinion. Mayo Clinic proceedings. Mayo Clinic proceedings. Mayo Clinic. 2007;82(1):82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- 46.Watson HG, et al. A comparison of the efficacy and rate of response to oral and intravenous Vitamin K in reversal of over-anticoagulation with warfarin. Br J Haematol. 2001;115(1):145–9. doi: 10.1046/j.1365-2141.2001.03070.x. [DOI] [PubMed] [Google Scholar]
- 47.Goodnough LT, Shander A. How I treat warfarin-associated coagulopathy in patients with intracerebral hemorrhage. Blood. 2011;117(23):6091–6099. doi: 10.1182/blood-2010-11-316075. [DOI] [PubMed] [Google Scholar]
- 48.Recommendations for the Management of Intracranial Haemorrhage – Part I: Spontaneous Intracerebral Haemorrhage. Cerebrovascular Diseases. 2006;22(4):294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 49.Goldstein J, Rosand J, Schwamm L. Warfarin Reversal in Anticoagulant- Associated Intracerebral Hemorrhage. Neurocritical Care. 2008;9(2):277–283. doi: 10.1007/s12028-008-9049-z. [DOI] [PubMed] [Google Scholar]
- 50.O'Shaughnessy DF, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 51.Steiner T, Rosand J, Diringer M. Intracerebral Hemorrhage Associated With Oral Anticoagulant Therapy. Stroke. 2006;37(1):256–262. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein JN, et al. Timing of Fresh Frozen Plasma Administration and Rapid Correction of Coagulopathy in Warfarin-Related Intracerebral Hemorrhage. Stroke. 2006;37(1):151–155. doi: 10.1161/01.STR.0000195047.21562.23. [DOI] [PubMed] [Google Scholar]
- 53.Bershad E, Suarez J. Prothrombin Complex Concentrates for Oral Anticoagulant Therapy-Related Intracranial Hemorrhage: A Review of the Literature. Neurocritical Care. 2010;12(3):403–413. doi: 10.1007/s12028-009-9310-0. [DOI] [PubMed] [Google Scholar]
- 54.Holland L, et al. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion. 2009;49(6):1171–1177. doi: 10.1111/j.1537-2995.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 55.Dentali F, et al. Safety of prothrombin complex concentrates for rapidanticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost. 2011;106(3):429–38. doi: 10.1160/TH11-01-0052. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein JN, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care. 2009;10(1):28–34. doi: 10.1007/s12028-008-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gurol M, Greenberg S. Management of intracerebral hemorrhage. Current Atherosclerosis Reports. 2008;10(4):324–331. doi: 10.1007/s11883-008-0050-y. [DOI] [PubMed] [Google Scholar]
- 58.Thompson BB, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology. 2010;75(15):1333–42. doi: 10.1212/WNL.0b013e3181f735e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naidech AM, et al. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocrit Care. 2012;16(1):82–7. doi: 10.1007/s12028-011-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Gans K, et al. Patch: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurology. 2010;10(1):19. doi: 10.1186/1471-2377-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Granger CB, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 62.Patel MR, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 63.Connolly SJ, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 64.van Ryn J, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103(6):1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 65.Eerenberg ES, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 66.Watanabe M, Siddiqui FM, Qureshi AI. Incidence and management of ischemic stroke and intracerebral hemorrhage in patients on dabigatran etexilate treatment. Neurocrit Care. 2012;16(1):203–9. doi: 10.1007/s12028-011-9591-y. [DOI] [PubMed] [Google Scholar]
- 67.Rangel-Castillo L, Gopinath S, Robertson CS. Management of Intracranial Hypertension. Neurologic clinics. 2008;26(2):521–541. doi: 10.1016/j.ncl.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helbok R, et al. Effect of mannitol on brain metabolism and tissue oxygenation in severe haemorrhagic stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2011;82(4):378–383. doi: 10.1136/jnnp.2009.198754. [DOI] [PubMed] [Google Scholar]
- 69.Stead L, et al. Emergency Department Hyperglycemia as a Predictor of Early Mortality and Worse Functional Outcome after Intracerebral Hemorrhage. Neurocritical Care. 2010;13(1):67–74. doi: 10.1007/s12028-010-9355-0. [DOI] [PubMed] [Google Scholar]
- 70.Kimura K, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. Journal of the Neurological Sciences. 2007;255(1–2):90–94. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Middleton S, et al. Implementation of evidence-based treatment protocols to manage fever, hyperglycaemia, and swallowing dysfunction in acute stroke (QASC): a cluster randomised controlled trial. Lancet. 2011;378(9804):1699–706. doi: 10.1016/S0140-6736(11)61485-2. [DOI] [PubMed] [Google Scholar]
- 72.Kumar MA, et al. Anemia and hematoma volume in acute intracerebral hemorrhage. Critical Care Medicine. 2009;37(4):1442–1447. doi: 10.1097/CCM.0b013e31819ced3a. 10.1097/CCM.0b013e31819ced3a. [DOI] [PubMed] [Google Scholar]
- 73.Sheth KN, et al. Packed Red Blood Cell Transfusion and Decreased Mortality in Intracerebral Hemorrhage. Neurosurgery. 2011;68(5):1286–1292. doi: 10.1227/NEU.0b013e31820cccb2. 10.1227/NEU.0b013e31820cccb2. [DOI] [PubMed] [Google Scholar]
- 74.Claassen J, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69(13):1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- 75.Vespa PM, et al. Acute seizures after intracerebral hemorrhage. Neurology. 2003;60(9):1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 76.De Herdt V, et al. Early seizures in intracerebral hemorrhage. Neurology. 2011;77(20):1794–1800. doi: 10.1212/WNL.0b013e31823648a6. [DOI] [PubMed] [Google Scholar]
- 77.Passero S, et al. Seizures after Spontaneous Supratentorial Intracerebral Hemorrhage. Epilepsia. 2002;43(10):1175–1180. doi: 10.1046/j.1528-1157.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 78.Reddig RT, Nixdorf KE, Jensen MB. The prophylactic use of an antiepileptic drug in intracerebral hemorrhage. Clinical Neurology and Neurosurgery. 2011;113(10):895–897. doi: 10.1016/j.clineuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naidech AM, et al. Anticonvulsant Use and Outcomes After Intracerebral Hemorrhage. Stroke. 2009;40(12):3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 80.Messé S, et al. Prophylactic Antiepileptic Drug Use is Associated with Poor Outcome Following ICH. Neurocritical Care. 2009;11(1):38–44. doi: 10.1007/s12028-009-9207-y. [DOI] [PubMed] [Google Scholar]
- 81.Smith M. Monitoring Intracranial Pressure in Traumatic Brain Injury. Anesthesia & Analgesia. 2008;106(1):240–248. doi: 10.1213/01.ane.0000297296.52006.8e. [DOI] [PubMed] [Google Scholar]
- 82.Morgenstern LB, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–29. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hallevi H, et al. Intraventricular hemorrhage. Neurology. 2008;70(11):848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuhrim S, et al. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Critical Care Medicine. 1999;27(3):617–621. doi: 10.1097/00003246-199903000-00045. [DOI] [PubMed] [Google Scholar]
- 85.Naff N, et al. Low-Dose Recombinant Tissue-Type Plasminogen Activator Enhances Clot Resolution in Brain Hemorrhage. Stroke. 2011;42(11):3009–3016. doi: 10.1161/STROKEAHA.110.610949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaberel T, et al. Intraventricular Fibrinolysis Versus External Ventricular Drainage Alone in Intraventricular Hemorrhage. Stroke. 2011 doi: 10.1161/STROKEAHA.111.615724. [DOI] [PubMed] [Google Scholar]
- 87.Mendelow AD, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. The Lancet. 2005;365(9457):387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 88.Mendelow AD, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 89.Mendelow AD, et al. Surgical Trial in Lobar Intracerebral Haemorrhage (STICH II) Protocol. Trials. 2011;12(1):124. doi: 10.1186/1745-6215-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morgenstern LB, et al. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56(10):1294–1299. doi: 10.1212/wnl.56.10.1294. [DOI] [PubMed] [Google Scholar]
- 91.Tan SH, et al. Hypertensive basal ganglia hemorrhage: a prospective study comparing surgical and nonsurgical management. Surgical Neurology. 2001;56(5):287–292. doi: 10.1016/s0090-3019(01)00561-4. [DOI] [PubMed] [Google Scholar]
- 92.Zhou H, et al. A prospective controlled study: Minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurology. 2011;11(1):76. doi: 10.1186/1471-2377-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W-Z, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. International Journal of Stroke. 2009;4(1):11–16. doi: 10.1111/j.1747-4949.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 94.Marquardt G, et al. Basal ganglia haematomas in non-comatose patients: subacute stereotactic aspiration improves long-term outcome in comparison to purely medical treatment. Neurosurgical Review. 2005;28(1):64–69. doi: 10.1007/s10143-004-0355-4. [DOI] [PubMed] [Google Scholar]
- 95.Hemphill JC, 3rd, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 96.Rost NS, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke. 2008;39(8):2304–9. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 97.Zahuranec DB, et al. Early care limitations independently predict mortality after intracerebral hemorrhage. Neurology. 2007;68(20):1651–7. doi: 10.1212/01.wnl.0000261906.93238.72. [DOI] [PubMed] [Google Scholar]
- 98.van Gijn J, Rinkel GJE. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(2):249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 99.de Rooij NK, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(12):1365–1372. doi: 10.1136/jnnp.2007.117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zacharia BE, et al. Epidemiology of Aneurysmal Subarachnoid Hemorrhage. Neurosurgery clinics of North America. 2010;21(2):221–233. doi: 10.1016/j.nec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Feigin VL, et al. Risk Factors for Subarachnoid Hemorrhage. Stroke. 2005;36(12):2773–2780. doi: 10.1161/01.STR.0000190838.02954.e8. [DOI] [PubMed] [Google Scholar]
- 102.Anderson CS, et al. Active and Passive Smoking and the Risk of Subarachnoid Hemorrhage. Stroke. 2004;35(3):633–637. doi: 10.1161/01.STR.0000115751.45473.48. [DOI] [PubMed] [Google Scholar]
- 103.Broderick JP, et al. Major Risk Factors for Aneurysmal Subarachnoid Hemorrhage in the Young Are Modifiable. Stroke. 2003;34(6):1375–1381. doi: 10.1161/01.STR.0000074572.91827.F4. [DOI] [PubMed] [Google Scholar]
- 104.Vannemreddy P, et al. Influence of cocaine on ruptured intracranial aneurysms: a case control study of poor prognostic indicators. Journal of Neurosurgery. 2008;108(3):470–476. doi: 10.3171/JNS/2008/108/3/0470. [DOI] [PubMed] [Google Scholar]
- 105.Ferro J, Canhão P, Peralta R. Update on subarachnoid haemorrhage. Journal of Neurology. 2008;255(4):465–479. doi: 10.1007/s00415-008-0606-3. [DOI] [PubMed] [Google Scholar]
- 106.Vlak MHM, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. The Lancet Neurology. 2011;10(7):626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 107.Wermer MJH, et al. Risk of Rupture of Unruptured Intracranial Aneurysms in Relation to Patient and Aneurysm Characteristics. Stroke. 2007;38(4):1404–1410. doi: 10.1161/01.STR.0000260955.51401.cd. [DOI] [PubMed] [Google Scholar]
- 108.Wiebers DO. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. The Lancet. 2003;362(9378):103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 109.Ishibashi T, et al. Unruptured Intracranial Aneurysms. Stroke. 2009;40(1):313–316. doi: 10.1161/STROKEAHA.108.521674. [DOI] [PubMed] [Google Scholar]
- 110.Lerch C, et al. Specialized neurocritical care, severity grade, and outcome of patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006;5(2):85–92. doi: 10.1385/ncc:5:2:85. [DOI] [PubMed] [Google Scholar]
- 111.Rosengart AJ, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2315–21. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 112.Risselada R, et al. Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: results from the International Subarachnoid Aneurysm Trial (ISAT) Eur J Epidemiol. 2010;25(4):261–6. doi: 10.1007/s10654-010-9432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly ME, Dodd R, Steinberg GK. Chapter 39 Subarachnoid hemorrhage. In: Marc F, editor. Handbook of Clinical Neurology. Elsevier; 2008. pp. 791–808. [DOI] [PubMed] [Google Scholar]
- 114.Penn DL, Komotar RJ, Sander Connolly E. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. Journal of Clinical Neuroscience. 2011;18(11):1435–1438. doi: 10.1016/j.jocn.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 115.Polmear A. Sentinel headaches in aneurysmal subarachnoid haemorrhage: what is the true incidence? A systematic review. Cephalalgia. 2003;23(10):935–41. doi: 10.1046/j.1468-2982.2003.00596.x. [DOI] [PubMed] [Google Scholar]
- 116.Perry JJ, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: prospective cohort study. BMJ. 2010;341:c5204. doi: 10.1136/bmj.c5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shinohara Y. Hemorrhagic stroke syndromes: clinical manifestations of, intracerebral and subarachnoid hemorrhage. In: Marc F, editor. Handbook of Clinical Neurology. Elsevier; 2008. pp. 577–594. [DOI] [PubMed] [Google Scholar]
- 118.Suarez JI, Tarr RW, Selman WR. Aneurysmal Subarachnoid Hemorrhage. New England Journal of Medicine. 2006;354(4):387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- 119.van Gijn J, Kerr RS, Rinkel GJE. Subarachnoid haemorrhage. The Lancet. 2007;369(9558):306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 120.Hassan A, et al. Terson's Syndrome. Neurocritical Care. 2011;15(3):554–558. doi: 10.1007/s12028-011-9555-2. [DOI] [PubMed] [Google Scholar]
- 121.Vermeulen MJ, Schull MJ. Missed Diagnosis of Subarachnoid Hemorrhage in the Emergency Department. Stroke. 2007;38(4):1216–1221. doi: 10.1161/01.STR.0000259661.05525.9a. [DOI] [PubMed] [Google Scholar]
- 122.McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 17(4):444–51. doi: 10.1111/j.1553-2712.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 123.Perry JJ, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011;343:d4277. doi: 10.1136/bmj.d4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Provenzale J, Hacein-Bey L. CT evaluation of subarachnoid hemorrhage: a practical review for the radiologist interpreting emergency room studies. Emergency Radiology. 2009;16(6):441–451. doi: 10.1007/s10140-009-0824-8. [DOI] [PubMed] [Google Scholar]
- 125.Karttunen AI, et al. Value of the quantity and distribution of subarachnoid haemorrhage on CT in the localization of a ruptured cerebral aneurysm. Acta Neurochirurgica. 2003;145(8):655–661. doi: 10.1007/s00701-003-0080-8. [DOI] [PubMed] [Google Scholar]
- 126.Tryfonidis M, et al. The value of radio-anatomical features on non-contrast CT scans in localizing the source in aneurysmal subarachnoid haemorrhage. Clinical Anatomy. 2007;20(6):618–623. doi: 10.1002/ca.20475. [DOI] [PubMed] [Google Scholar]
- 127.Hoh BL, et al. Results of a prospective protocol of computed tomographic angiography in place of catheter angiography as the only diagnostic and pretreatment planning study for cerebral aneurysms by a combined neurovascular team. Neurosurgery. 2004;54(6):1329–40. doi: 10.1227/01.neu.0000125325.22576.83. discussion 1340-2. [DOI] [PubMed] [Google Scholar]
- 128.Westerlaan HE, et al. Intracranial Aneurysms in Patients with Subarachnoid Hemorrhage: CT Angiography as a Primary Examination Tool for Diagnosis— Systematic Review and Meta-Analysis. Radiology. 2011;258(1):134–145. doi: 10.1148/radiol.10092373. [DOI] [PubMed] [Google Scholar]
- 129.Prestigiacomo CJ, et al. Three dimensional CT angiography versus digital subtraction angiography in the detection of intracranial aneurysms in subarachnoid hemorrhage. J Neurointerv Surg. 2010;2(4):385–9. doi: 10.1136/jnis.2010.002246. [DOI] [PubMed] [Google Scholar]
- 130.McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 2010;17(4):444–51. doi: 10.1111/j.1553-2712.2010.00694.x. [DOI] [PubMed] [Google Scholar]
- 131.Diringer M, et al. Critical Care Management of Patients Following Aneurysmal Subarachnoid Hemorrhage: Recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocritical Care. 2011;15(2):211–240. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 132.Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53(5):365–72. doi: 10.1136/jnnp.53.5.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Edlow JA, et al. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2008;52(4):407–36. doi: 10.1016/j.annemergmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 134.Edlow JA. What are the unintended consequences of changing the diagnostic paradigm for subarachnoid hemorrhage after brain computed tomography to computed tomographic angiography in place of lumbar puncture? Acad Emerg Med. 2010;17(9):991–5. doi: 10.1111/j.1553-2712.2010.00840.x. discussion 996-7. [DOI] [PubMed] [Google Scholar]
- 135.Roost KT, et al. The formation of cerebrospinal fluid xanthochromia after subarachnoid hemorrhage. Neurology. 1972;22(9):973. doi: 10.1212/wnl.22.9.973. [DOI] [PubMed] [Google Scholar]
- 136.Petzold A, Keir G, Sharpe LT. Spectrophotometry for Xanthochromia. New England Journal of Medicine. 2004;351(16):1695–1696. doi: 10.1056/NEJM200410143511627. [DOI] [PubMed] [Google Scholar]
- 137.Wood MJ, Dimeski G, Nowitzke AM. CSF spectrophotometry in the diagnosis and exclusion of spontaneous subarachnoid haemorrhage. Journal of Clinical Neuroscience. 2005;12(2):142–146. doi: 10.1016/j.jocn.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 138.Perry JJ, et al. Should Spectrophotometry Be Used to Identify Xanthochromia in the Cerebrospinal Fluid of Alert Patients Suspected of Having Subarachnoid Hemorrhage? Stroke. 2006;37(10):2467–2472. doi: 10.1161/01.STR.0000240689.15109.47. [DOI] [PubMed] [Google Scholar]
- 139.Edlow JA, Bruner KS, Horowitz GL. Xanthochromia. Archives of Pathology & Laboratory Medicine. 2002;126(4):413–415. doi: 10.5858/2002-126-0413-X. [DOI] [PubMed] [Google Scholar]
- 140.Linn FHH, et al. Visual inspection versus spectrophotometry in detecting bilirubin in cerebrospinal fluid. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(10):1452–1454. doi: 10.1136/jnnp.2004.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. The Lancet Neurology. 2008;7(3):256–267. doi: 10.1016/S1474-4422(08)70041-3. [DOI] [PubMed] [Google Scholar]
- 142.Mitchell P, et al. Detection of subarachnoid haemorrhage with magnetic resonance imaging. Journal of Neurology, Neurosurgery & Psychiatry. 2001;70(2):205–211. doi: 10.1136/jnnp.70.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.U-King-Im JM, et al. Current diagnostic approaches to subarachnoid haemorrhage. European Radiology. 2005;15(6):1135–1147. doi: 10.1007/s00330-005-2665-5. [DOI] [PubMed] [Google Scholar]
- 144.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 145.Rosen D, MacDonald R. Subarachnoid hemorrhage grading scales. Neurocritical Care. 2005;2(2):110–118. doi: 10.1385/NCC:2:2:110. [DOI] [PubMed] [Google Scholar]
- 146.Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. Journal of Neurosurgery. 1988;68(6) doi: 10.3171/jns.1988.68.6.0985. null. [DOI] [PubMed] [Google Scholar]
- 147.Bederson JB, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 148.Bederson JB, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 149.Choi K-S, et al. Seizures and Epilepsy following Aneurysmal Subarachnoid Hemorrhage : Incidence and Risk Factors. J Korean Neurosurg Soc. 2009;46(2):93–98. doi: 10.3340/jkns.2009.46.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pinto A, Canhão P, Ferro J. Seizures at the onset of subarachnoid haemorrhage. Journal of Neurology. 1996;243(2):161–164. doi: 10.1007/BF02444009. [DOI] [PubMed] [Google Scholar]
- 151.Claassen J, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocritical Care. 2006;4(2):103–112. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- 152.Naidech A, et al. Moderate Hypoglycemia is Associated With Vasospasm, Cerebral Infarction, and 3-Month Disability After Subarachnoid Hemorrhage. Neurocritical Care. 2010;12(2):181–187. doi: 10.1007/s12028-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 153.Lee S-H, et al. Effects of admission glucose level on mortality after subarachnoid hemorrhage: A comparison between short-term and long-term mortality. Journal of the Neurological Sciences. 2008;275(1–2):18–21. doi: 10.1016/j.jns.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 154.Kruyt ND, et al. Hyperglycemia and Clinical Outcome in Aneurysmal Subarachnoid Hemorrhage. Stroke. 2009;40(6):e424–e430. doi: 10.1161/STROKEAHA.108.529974. [DOI] [PubMed] [Google Scholar]
- 155.Fernandez A, et al. Fever after subarachnoid hemorrhage. Neurology. 2007;68(13):1013–1019. doi: 10.1212/01.wnl.0000258543.45879.f5. [DOI] [PubMed] [Google Scholar]
- 156.Schmidt JM, et al. Frequency and clinical impact of asymptomatic cerebral infarction due to vasospasm after subarachnoid hemorrhage. Journal of Neurosurgery. 2008;109(6):1052–1059. doi: 10.3171/JNS.2008.109.12.1052. [DOI] [PubMed] [Google Scholar]
- 157.Frontera JA, et al. Defining Vasospasm After Subarachnoid Hemorrhage. Stroke. 2009;40(6):1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 158.Washington C, Zipfel G, Hemorrhage T.P.i.t.I.M.-d.C.C.o.t.C.C.M.o.S. Detection and Monitoring of Vasospasm and Delayed Cerebral Ischemia: A Review and Assessment of the Literature. Neurocritical Care. 2011;15(2):312–317. doi: 10.1007/s12028-011-9594-8. [DOI] [PubMed] [Google Scholar]
- 159.Rabinstein AA, Lanzino G, Wijdicks EFM. Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. The Lancet Neurology. 2010;9(5):504–519. doi: 10.1016/S1474-4422(10)70087-9. [DOI] [PubMed] [Google Scholar]
- 160.Treggiari M, Hemorrhage P.i.t.I.M.-d.C.C.o.t.C.C.M.o.S. Hemodynamic Management of Subarachnoid Hemorrhage. Neurocritical Care. 2011;15(2):329–335. doi: 10.1007/s12028-011-9589-5. [DOI] [PubMed] [Google Scholar]
- 161.Lee K, Lukovits T, Friedman J. “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage. Neurocritical Care. 2006;4(1):68–76. doi: 10.1385/NCC:4:1:068. [DOI] [PubMed] [Google Scholar]
- 162.Investigators* TC. Rates of Delayed Rebleeding From Intracranial Aneurysms Are Low After Surgical and Endovascular Treatment. Stroke. 2006;37(6):1437–1442. doi: 10.1161/01.STR.0000221331.01830.ce. [DOI] [PubMed] [Google Scholar]


