Abstract
The relationship between thyroid and breast diseases has been documented, but the clinical significance of Graves’ disease and breast cancer is unclear. We present a young patient with a history of Graves’ disease who developed a multicentric infiltrating ductal carcinoma of the breast several months after discontinuing her treatment with propylthiouracil. Early-onset breast cancer in women without a family history of early breast cancer may be related to hyperthyroidism. The relationship between thyroid hormone and estrogen is discussed.
Keywords: Graves’ disease, thyroid hormone, estrogen, breast cancer
Introduction
The relationship between diseases of the breast and thyroid has been previously reported [1]. Thyroid hormone is known to positively influence cell growth and dysplasia in breast cancer cell lines as well as patients with breast disease. Only a limited number of cases exist in which patients with Graves’ disease develop an early-onset breast cancer [2,3], however data suggests there is a higher incidence of breast cancer in patients with other autoimmune thyroid diseases, specifically Hashimoto’s thyroiditis [1,4]. In this brief communication, we report a young female patient with a history of Graves’ disease who presented with the acute-onset development of a multicentric infiltrating ductal carcinoma following discontinuation of her treatment for Graves’. The cellular and molecular interactions between thyroid hormone and estrogen are discussed in detail.
Case report
A thirty year-old African American gravida 6, para 3 female was referred for evaluation of two lumps in her left breast that she noticed in the past month. Her past medical history is only significant for Graves’ disease, diagnosed four years prior to her presentation, for which she had been treated with propylthiouracil (PTU). Her disease was medically controlled with PTU during this time, but medical management does not eliminate the underlying pathology of Graves’ disease. The patient electively discontinued her treatment due to financial reasons three months prior to her presentation with multiple breast masses. She reported no family history of ovarian or colon cancer, although her maternal grandmother underwent a mastectomy at 75 years of age for breast cancer.
She was afebrile and normotensive at initial presentation. Physical exam confirmed three mobile masses in her left breast. She displayed no thyromegaly, tracheal deviation, or discrete palpable nodules. There was no palpable lymphadenopathy in central, lateral or posterior compartments of neck. Her TSH level was low at 0.280 mIU/mL (normal = 0.340-4.820 mIU/mL) and free T4 was 0.83 ng/dL (normal = 0.77-1.61 ng/dL). Mammogram demonstrated four highly suspicious masses of the left breast (Figure 1), and ultrasound determined mass sizes ranging from 0.5 to 2.0 cm in diameter. Breast MRI confirmed these measurements (Figure 2). There was no evidence of abnormalities of the right breast on physical and radiological examinations. Additionally, there was no palpable or radiologically suspicious axillary lymphadenopathy bilaterally. Core needle biopsies of each of the four left breast masses revealed infiltrating ductal carcinoma. A unilateral radical mastectomy with sentinel node excision was performed. The final pathology report demonstrated a 2.3cm focus of an infiltrating ductal carcinoma with 2 histologically identical satellite breast lesions measuring 1.4 and 0.8 cm in size. Three sentinel nodes were excised, 2 of which were positive for micrometastases measuring 0.4 and 0.5 mm. The overlying skin and nipple did not show evidence of malignancy and all surgical margins were free of tumor invasion. Immunostaining of the excised breast tissue was significant for human epidermal growth factor receptor 2 (Her2 receptor) positivity. Estrogen and progesterone receptor staining of this tissue sample were negative. Ki-67 proliferation index was quantified at 25%. As of the time of submission of manuscript, the patient has completed 3 of 6 cycles of treatment with a Taxotere, Carboplatin, and Herceptin (TCH) chemotherapy regimen. She is expected to carry out the remaining 3 cycles of the TCH regimen and will be referred for radiation therapy. She will also be considered for endocrine therapy.
Figure 1.
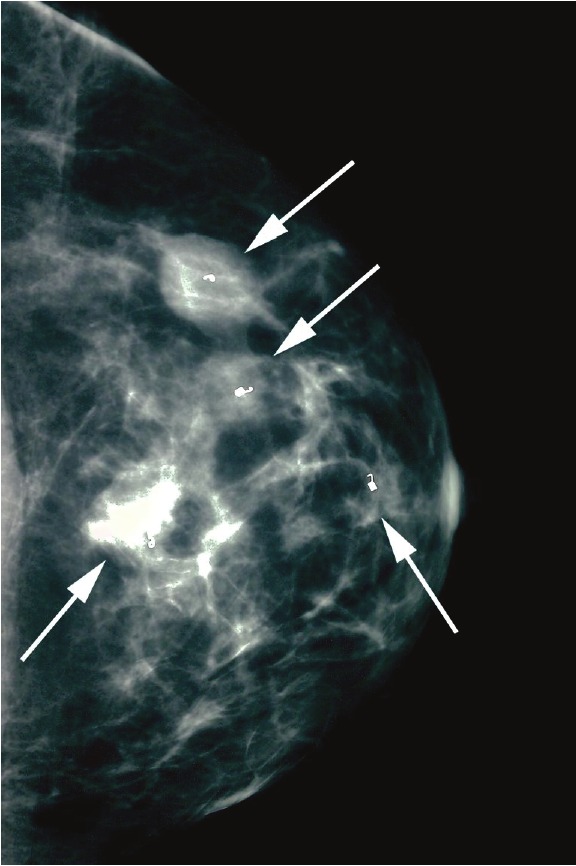
Patient mammogram demonstrating craniocaudal view of the left breast with four biopsy markers in place (arrows). Areas of concern appear whiter than surrounding tissue.
Figure 2.
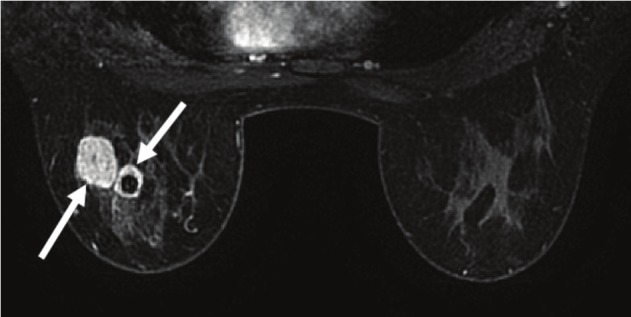
Craniocaudal view of patient's breast MRI showing two independent focal densities representing malignantprocesses in the left breast (arrows). The surrounding benign breast tissue appears dark in comparison.
Discussion
The interaction between thyroid hormone and estrogen-sensitive tissues, notably the breast, has been well documented [5-8]. The association between breast cancer in patients with autoimmune thyroid disease has also been described in case reports and larger studies [1]. While autoimmune and hyperthyroid disease states are associated with an increase in the risk of a primary breast cancer, hypothyroidism has also be associated with a decreased risk of primary breast carcinoma [9]. This suggests that it may be simply the hyperthyroid state that increases the risk for a primary breast malignancy, rather than any underlying autoimmune thyroid process.
Poorly controlled levels of either steroid hormone are known to produce malignant changes in a variety of tissue subtypes. At the molecular level, the similar downstream signaling pathways and heteromeric nature of steroid hormone receptors allow considerable cross-talk and may provide an explanation for patients with Graves’-associated breast changes-as well as an explanation for estrogen-associated thyroid changes (Figure 3). It is known that estrogen and thyroid hormones activate the same mitogen-activated protein kinase (MAPK) and other non-nuclear signaling pathways [7]. Alone, this MAPK and its associated extracellular signal-regulated kinase 1/2 (ERK 1/2) already mobilize the cytoplasmic machinery necessary to stimulate cell growth and division. Additionally, triiodothyronine (T3) has been shown to induce a cascade of events which ultimately upregulate estrogen response element (ERE)-mediated gene transcription in breast cancer cells dose-dependently [5,10]. Even in physiologically normal breast tissue, ERE-mediated gene transcription is responsible for the majority of cell growth and division. The response of breast cancer cells to T3 from these studies was also observed to be attenuated with the co-administration of an estrogen antagonist. These data suggest the role of T3 as a competitive inhibitor of the estrogen receptor. By an alternative mechanism, T3 and T4 are also thought to stimulate the αvβ3 integrin receptor, and ultimately active the downstream ERα [6]. Although each of these results presented here are limited by the fact that they were demonstrated only in estrogen receptor-positive cell lines, they may explain the increased risk of breast cancer in patients with poorly controlled hyperthyroid disease (perhaps even in estrogen receptor-negative primary breast cancers). If one considers all these points of interaction, it is likely that there is no singular mechanism explaining the interaction between thyroid hormone and estrogen, but that there are multiple points of overlap in their signaling pathways. Because of such significant overlap, much of which cannot be discussed in a manuscript of this length, there may be a role for thyroid hormone in the pathogenesis of estrogen receptor-negative breast cancer. This area of clinical research deserves further investigation.
Figure 3.
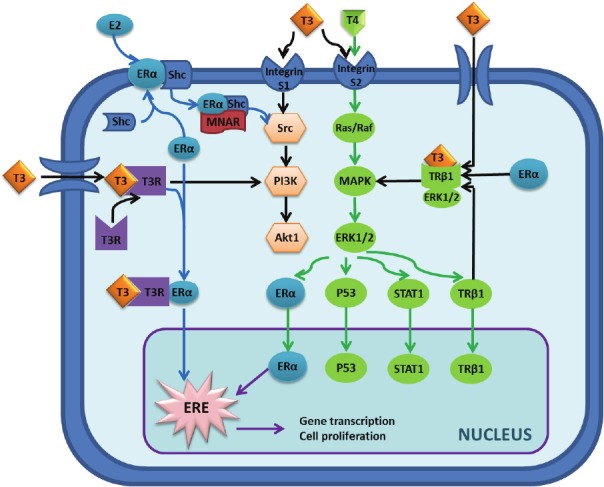
Overview of the molecular interaction between estrogen and thyroid hormone. The interaction can be broken down into nuclear and non-nuclear signaling pathways. Non-nuclear pathway: Binding of estrogen to estrogen receptor α (ERα) can activate the ERα-shc complex, thereby allowing the Modulator of Nongenomic Actions of the Estrogen Receptor (MNAR) to bind and ultimately activate the src tyrosine kinase. Src can then phosphorylate/activate the Ras/Raf g-protein or phosphoinositol 3-kinase (PI3K) protein to induce cellular proliferation pathways via Akt or Mitogen Activated Protein Kinase (MAPK), respectively. MAPK exerts its effects by phosphorylating/activating Extracellular Signal-Regulated Kinase 1/2 (ERK1/2), which amplifies its effects via a host of other signaling molecules-only several of which are illustrated here. At the same time, triiodothyronine (T3) and tetraiodothyronine (T4) are producing similar effects. These two hormones have been shown to stimulate different subunits of the αvβ3 integrin receptor, S1 and S2, which produces the same signal cascade utilized by estrogen involving PI3K and MAPK. T3 also exerts its effects in estrogen-sensitive tissues by translocating into the cell where it can bind to its cytoplasmic receptor and heteromerize with ERα as shown. From there, this ERα and thyroid receptor complex can either activate MAPK or translocate to the nucleus to exert its transcriptional effects. Nuclear pathway: Once inside the nucleus, the downstream effectors of both thyroid hormone and estrogen (ERα, p53, STAT1, and thyroid hormone receptor β1) can effect transcriptional changes to inhibit apoptosis as well as induce cellular proliferation. Specifically, the activated ERα (which can either be phosphorylated by ERK1/2 or heteromerized with thyroid hormone receptor) can stimulate the Estrogen Response Element (ERE) to alter the transcriptional levels of key proteins involved in cell proliferation and survival.
Conclusion
We propose that the development of a multifocal breast cancer in our patient is likely an adverse sequela of her history of Graves’ disease. Although this patient has a family history of breast cancer in her maternal grandmother, the authors believe that the underlying pathogenesis of this patient’s breast cancer is not likely to be related to the pathogenesis of her maternal grandmother’s cancer due to the vast difference in age of cancer onset. Without adequate suppression of our patient’s hyperthyroidism, her early-onset multifocal breast cancer may have resulted from the estrogenic effects of thyroid hormone on proliferating breast tissue or from some other, less understood autoimmune phenomenon. Despite the fact that immunostaining for estrogen receptor was negative in our patient’s breast tissue specimen, alternative mechanisms can cause this, as discussed above, in which thyroid hormone may induce downstream signals similar to the way estrogen signaling has been shown to modulate breast cancer. Whether the malignant changes in our patient began prior to her diagnosis of Graves’ disease or during the course of her treatment cannot be determined. Perhaps earlier detection of her thyroid disease might have deterred this aberrant breast process. It is imperative that physicians recognize the clinical significance of the interactions between thyroid hormone and estrogen in these hormonally-responsive tissues.
References
- 1.Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F, Cecchetti D, Martino E, Pinchera A. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab. 1996;81:990–994. doi: 10.1210/jcem.81.3.8772562. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JM, Gorman CA, Elveback LR, Wentz JR. Incidence of malignant neoplasms of all types of patients with Graves' disease. Arch Intern Med. 1978;138:944–947. [PubMed] [Google Scholar]
- 3.Radical mastectomy in a patient with coexistent Graves' disease. Am J Surg. 1976;132:110–111. doi: 10.1016/0002-9610(76)90303-2. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Maruchi N. Breast cancer in patients with Hashimoto's thyroiditis. Lancet. 1975;2:1119–1121. doi: 10.1016/s0140-6736(75)91006-5. [DOI] [PubMed] [Google Scholar]
- 5.Hall LC, Salazar EP, Kane SR, Liu N. Effects of thyroid hormones on human breast cancer cell proliferation. J Steroid Biochem Mol Biol. 2008;109:57–66. doi: 10.1016/j.jsbmb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145:3265–3272. doi: 10.1210/en.2004-0308. [DOI] [PubMed] [Google Scholar]
- 7.Dinda S, Sanchez A, Moudgil V. Estrogenlike effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene. 2002;21:761–768. doi: 10.1038/sj.onc.1205136. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Jeyakumar M, Bagchi MK. Ligand-dependent cross-talk between steroid and thyroid hormone receptors. Evidence for common transcriptional coactivator(s) J Biol Chem. 1996;271:14825–14833. [PubMed] [Google Scholar]
- 9.Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, Hsu L, Krishnamurthy S, Theriault RL, Hortobagyi GN. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103:1122–1128. doi: 10.1002/cncr.20881. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira CR, Brentani MM. Triiodothyronine mimics the effects of estrogen in breast cancer cell lines. J Steroid Biochem Mol Biol. 1996;59:271–279. doi: 10.1016/s0960-0760(96)00117-3. [DOI] [PubMed] [Google Scholar]


