Abstract
Background
The human IGF2-P4 and H19 promoters are highly active in a variety of human cancers, while existing at a nearly undetectable level in the surrounding normal tissue. Single promoter vectors expressing diphtheria toxin A-fragment (DTA) under the control regulation of IGF2-P4 or H19 regulatory sequences (IGF2-P4-DTA and H19-DTA) were previously successfully used in cell lines, animal models and recently in human patients with superficial cell carcinoma of the bladder, pancreatic cancer and ovarian cancer (treated with H19-DTA). However this targeted medicine approach may be limited, as not all cancer patients express high levels of H19 and it requires prerequisite diagnostic test for H19. Hence, a double promoter DTA-expressing vector was created, carrying on a single construct two separate genes expressing the diphtheria toxin A-fragment (DTA), from two different regulatory sequences, selected from the cancer-specific promoters H19 and IGF2-P4.
Methods
H19 and IGF2-P4 gene expression was tested in cell lines of a broad spectrum of different carcinomas (bladder, pancreas, ovary, glioblastoma and HCC), by RT-PCR. The therapeutic potential of the double promoter toxin vector H19-DTA-(IGF2)-P4-DTA was tested in the different cancer cell lines.
Results
The double promoter vector exhibited superior inhibition activity compared to the single promoter expression vectors, in the different cancer cell lines furthermore, the double promoter vector H19-DTA-P4-DTA exhibited augmented-than-additive anti-cancer activity relative to single promoter expression vectors carrying either DTA sequence alone, when tested in a broad spectrum of tumor cells.
Conclusions
Our findings show that administration of H19-DTA-P4-DTA has the potential to reach tumor cells, deliver its intracellular toxin without targeting normal tissues, and thus may help reduce tumor burden, improve the quality of life of the patient; and prolong their life span. As H19 and IGF2 were expressed in a broad spectrum of different cancers, therefore we propose a double promoter expression approach for treating a variety of tumors expressing H19, IGF2, or both. According to this approach patients may be treated with a single double promoter expression toxin vector which is under the control of the IGF2 and H19 regulatory sequences, differentially expressed in those cancers. As majority of the tumor cells express H19, IGF2, or both, therefore the use of prerequisite diagnostic test will be unnecessary.
Keywords: IGF2, H19, pancreatic cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, diphtheria toxin A, targeted cancer therapy
Introduction
H19 and IGF2 (the human P3 and P4 promoters) are onco-fetal genes and are oncogenes [1-3], expressed in the fetus and/or shows aberrant allelic pattern of expression in a broad spectrum of tumors, but rarely in normal adult tissues [4-6]. Studies of various carcinomas (such as breast, bladder, pancreas, glioblastoma, ovary, hepatocellular and numerous other neoplasm) have demonstrated high expression levels of the H19 and IGF2 genes as compared to the normal tissue [7].
Based on early studies of our group and others, the transcriptional regulatory sequences of the H19 and IGF2 genes emerged as candidates for cancer targeted therapy.
H19 is a paternally-imprinted, oncofetal gene that encodes a RNA (with no protein product) acting as a “riboregulator” [8], which is expressed at substantial levels in embryonic tissues, in different human tumor types, and marginally or not expressed in the corresponding tissues of the adult [5,9].
The 67-aa IGF2 is a member of the insulin like growth factor family that is involved in cell proliferation and differentiation [10]. The human IGF2 gene contains 9 exons (E1–9) and 8 introns [10,11], and is transcribed from 4 different promoters (P1–P4) producing 4 different transcripts [11-13]. All four transcripts share a common coding region and a common 3.9kb 3-UTR, but variable 5-UTRs [11]. IGF2 is an imprinted gene that is almost exclusively expressed from the paternal allele [14-16]. The P3 and P4 promoters are the major IGF2 promoters during embryogenesis and tumor development, while P1 is exclusively active in adult liver tissue and P2 activity is rarely detected in adult human tissue [10]. Increased expression of IGF2 as a result of the loss of its imprinting is frequently seen in a variety of human tumors [16-18]. In addition, abnormal signal transduction and/or promoter activation was reported as a major mechanism for IGF2 over-expression in a variety of tumors including bladder carcinoma, hepatocellular carcinoma, breast cancer, ovarian cancer and prostate cancer [19-22]. The human H19 gene lies within 200kb downstream of the paternally expressed IGF2 gene at 11p.15.5. These two genes are frequently coordinately regulated, both in terms of their common expression pattern and are reciprocal imprinting. Enhancers located downstream of H19 stimulate transcription of both genes [23].
We have shown that IGF2 or H19 are significantly expressed in the majority of human bladder carcinomas [6,24]. Furthermore, we have recently shown that their combined expression is 100% [25]. Our group has previously reported the construction of a double promoter vector expressing diphtheria toxin A-chain gene, under the control of H19 and IGF2-P4 regulatory sequences (H19-DTA-P4-DTA). We showed that this construct was able to selectively kill tumor cell lines and inhibit tumor growth in vitro and in vivo in accordance to the transcriptional activity of the above-mentioned regulatory sequences [25].
The use of a double promoter DTA-expressing vector, carrying on a single construct two separate genes expressing the diphtheria toxin A-fragment (DTA), from two different regulatory sequences (H19 and IGF2-P4; ‘H19-DTA-P4-DTA’ vector) is highly novel. This novel approach, create a new family of plasmids regulated by two regulatory sequences, which in their natural genome position are both proximately located and are reciprocally imprinted. This is a new biology concept, which mimics the unique biology reciprocity relations phenomenon of IGF2 and H19.
In this study the therapeutic potential of the double promoter vector (‘H19-DTA-P4-DTA’) was further tested in a broad spectrum of tumor cell lines (glioblastoma, pancreatic ovarian, and hepatocellular carcinoma).
The results show very high inhibition activity of the double promoter vector in all tested cancer cell lines, as well as positive expression of H19 and IGF2 in these cells. Thus, indicating that the H19-DTA-P4-DTA construct has a high therapeutic potential and therefore could be a very promising candidate for targeted cancer therapy in a broad spectrum of tumors expressing H19, IGF2 or both.
Materials and methods
Cell culture
The human ovarian carcinoma cell lines (ES-2), human pancreatic cancer cell lines (CRL-1469), human hepatocellular carcinoma cell lines (Hep3B) and the glioma cell lines: human glioblastoma (U87 and A172) and mouse glioblastoma (GL261), were obtained from the American Type Culture Collection (ATCC; Rockville, MD). Cells were grown to confluence in a humidified incubator with 5% CO2 in polystyrene culture flasks and were maintained in DMEM-F12 (1:1) medium containing 10% fetal calf serum.
RNA isolation, cDNA synthesis and PCR
RNA was extracted from cell lines or frozen tissue blocks, using the RNA STAT-60™ Total RNA/mRNA isolation reagent, according to the manufacture’s instructions. The RNA was treated by RNAse-free DNAse I to eliminate any contaminating DNA. Total cDNA was synthesized from 2μg total RNA in 20μl reaction volume with 10ng/μl of the oligo-(dT) 15 primer and 10 units/μl M-MLV Reverse Transcriptase according to the manufacturer instructions. 2μl of cDNA samples were taken for the amplification of the different transcripts using the different primers. The amplification conditions were 95° C for 2 min, followed by 30 cycles of 94°C for 30 sec, 59°C for 45 sec and 72°C for 60 sec, and finally 72°C for 5 min. The PCR reactions were carried out in 25μl volumes in the presence of 6ng/μl of each of the forward and the reverse primers using 0.05 units/μl of Taq polymerase according to the kit instructions (Takara). The forward (5’-CCGGCCTTCCTGAACA) and reverse (5’-TTCCGATGGTGTCTTTGATGT) primers designed for the detection of H19 RNA are spanning exons 2-3 and from exon 5 respectively, in order to validate that the PCR product is of the H19 RNA transcript and not from the endogenous H19 gene. The primers designed for the detection of IGF2-P4 RNA were designed to bind at exon 6 (5’-TCCTCCTCCTCCTGCCCCAGCG), for the P4 transcript in the forward direction and the reverse primer (5’- CAGCAATGCAGCACGAGGCGAAGCC) was designed to bind the 3’ end of exon 7 and the 5’ end of exon 8 without the introns in between. The integrity of the cDNA was assayed by PCR analysis of the ubiquitous, cell cycle independent, histone variant, H3.3 [6]. The PCR products were separated by electrophoresis on 2% gel agarose, and detected by ethidium bromide dye.
Plasmid construction
The H19-Luc plasmid which contains the luciferase gene under the control of the human H19 promoter region from nucleotide –818 to + 14 was prepared as described [26]. The H19-Luc plasmid was digested with XbaI and NcoI, and the insert of the luciferase gene (luc) was replaced by the Diphtheria toxin A chain (DTA) coding region to yield the H19-DTA construct. The DTA gene was prepared from the pIBI30-DTA plasmid (kindly donated by Dr. Ian Maxwell, University of Colorado, USA). The human IGF2-P4 promoter from the Hup4 vector (described in [11]) (a kind gift from Prof. P.E. Holthuizen, University of Utrecht, The Netherlands) were constructed by GENEART into the pGL3 basic vector (Luc-1) (Promega, Madison, MI), which lacks any eukaryotic promoter and enhancer sequences and carries the Kanamycin resistance gene (insert 812 bp), using BstEII and Hind III restriction sites, resulting in the expression vector P4-Luc. The DTA containing vector P4-DTA was designed by replacing the luciferase gene in P4-Luc with the DTA gene between the XbaI and NcoI restriction sites. Each of the cloned promoters and the DTA gene were sequenced and compared to the published sequences of the gene bank. We constructed double promoter expression plasmids, carrying on a single construct two separate genes expressing the diphtheria toxin, from two different regulatory sequences: H19 and IGF2-P4 promoters (hereinafter "H19-DTA-P4-DTA"; depicted in Figure 1).
Figure 1.
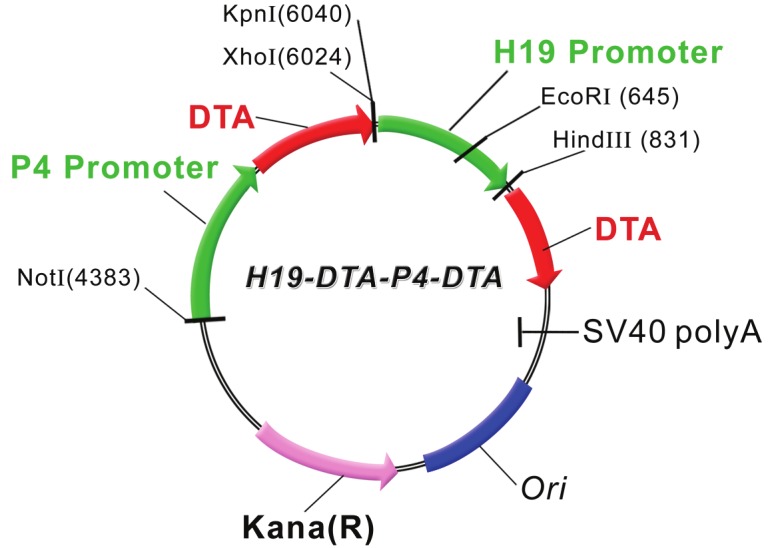
A schematic illustration depicting the construction of the double promoter H19-DTA-P4-DTA expression vector: The coding sequence of each DTA is under the transcriptional control of both H19 and IGF2-P4 promoter sequences, respectively, Kana (R) – kanamycin resistance gene.
A double promoter control constructs was created, using the same strategy, expressing the luciferase reporter gene (‘H19-Luc-P4-Luc’). The double promoter expression plasmids were cloned by GENEART™, (Germany).
Transfection
Cationic polymer (jetPEI) transient transfection
The in vitro jetPEI™ transfection reagent compact the DNA into positively charged particles capable of interacting with anionic proteoglycans at the cell surface and entering cells by endocytosis. The transfection procedure was done as recommended by the manufacturer (Polyplus-transfection, France). A total of 0.1×1O6 cells/well were grown overnight in a twelve-well Nunc multidish (75mm). For each well, 2μg DNA and 4μl of the jetPEI (N/P =5) were diluted separately with 50μl of 150mM NaCl each, and vortex-mixed gently. The jetPEI solution was added at once to the DNA solution, the mixture was vortex-mixed for 10 seconds and the mixture was incubated for 15 minuets at room temperature. The 100μl jetPEI/DNA mixture was then applied drop-wise onto the serum containing medium of each well. The transfection experiment was stopped after 48 hours.
Luciferase activity
The cells were harvested and the luciferase activity was determined using the luciferase Assay System kit (Promega). The light output was measured using a Lumac Biocounter apparatus. The total protein content of the lysates was determined by the Bio-Rad protein assay reagent and the results were normalized to the total protein and expressed as Light units/μg protein. LucSV40 (Luc-4) was used as a positive control for the efficiency of transfection as it contains the SV40 promoter and enhancer, while Luc-1 that lacks any regulatory sequences was used as a negative control to determine the basal nonspecific luciferase expression, which was found to be negligible in all of the cell lines. All experiments were done in triplicates and the results expressed as mean and standard error.
Results
The possibility of using the double promoter strategy in a broad spectrum of cancers
The human IGF2-P4 and H19 regulatory sequences are highly active in a variety of human cancers. In order to confirm if the double promoter strategy could be applicable to a broader spectrum of cancer indications, we further studied the expression level of H19 and IGF2-P4 in different cancer cell lines. Furthermore we tested the activity of the double promoter vector, H19-DTA-P4-DTA, in different cancer cell lines.
The Expression of IGF2 and H19 in cell lines of different cancers determined by RT-PCR
A possible indication for H19 and IGF2-P4 promoter activity can be the expression of their specific transcripts. To evaluate the possible use of IGF2-P4 and H19 regulatory sequences for cancer targeted therapy, we determined the expression of IGF2-P4 and H19 transcripts by RT-PCR.
We determined the expression of H19 and IGF2 mRNA in a broad spectrum of cancer cell lines. Total RNA was extracted from the cell cultures and the expression of H19 and IGF2-P4 transcripts was detected by RT-PCR analysis in the human cancer cell lines: Hep3B (hepatocellular carcinoma), ES-2 (ovarian cancer) and CRL-1469 (pancreatic cancer), and in human glioma cell lines: A172 (glioblastoma), U87 (glioblastoma) and GL261 (mouse glioblastoma). The expression levels are shown in Figure 2.
Figure 2.
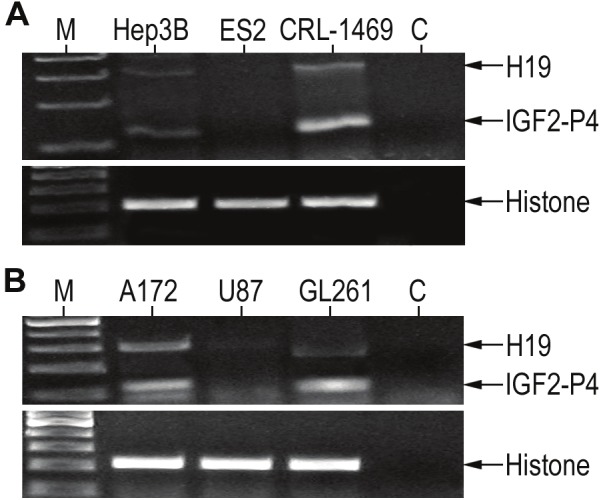
The expression of H19 and IGF2 in different cancer cell lines determined by RT-PCR: Shown are RT-PCR analyses of H19 (upper band) and IGF2-P4 (lower band) in human cancer cell lines of (A): Hep3B (hepatocellular carcinoma), ES-2 (ovarian cancer) and CRL-1469 (pancreatic cancer), and in human glioma cell lines (B): A172 ((glioblastoma), U87 (glioblastoma) and GL261 (mouse glioblastoma). ‘M’, 100bp DNA ladder, ‘C’, negative control. Lower figures, are RT-PCR product of histone internal control.
Enhanced in vitro activity of the double promoter H19-DTA-P4-DTA in different carcinoma cells
The activity of the double promoter construct H19-DTA-P4-DTA was tested in vitro by determining its ability to lyse different human carcinoma cell lines, relative to the activity of expression vectors carrying either sequence alone (single promoter constructs: H19-DTA or P4-DTA), in order to examine its activity in a broad spectrum of tumor cells.
Consequently the protein synthesis inhibition activity of the H19-DTA-P4-DTA construct was tested in vitro in the following human cancer cells lines: Hep3B (hepatocellular carcinoma cells), ES-2 (ovarian cancer cells), CRL-1469 (pancreatic cancer cells), U87 (glioblastoma cells), A172 (glioblastoma cells) and GL261 (mouse glioblastoma cells).
Anti-tumor therapeutic activity was determined by measuring the inhibition of luciferase activity following co-transfection with LucSV40. Each cell line was co-transfected with 2μg of LucSV40 and H19-DTA, P4-DTA, or H19-DTA-P4-DTA in a dose-response manner at different concentrations (0.005mg - 0.05μg).
As seen in previous studies [25], the double promoter construct H19-DTA-P4-DTA exhibited far enhanced efficiency in lysing the cancer cell lines, relative to each of the single promoter constructs (Figure 3).
Figure 3.
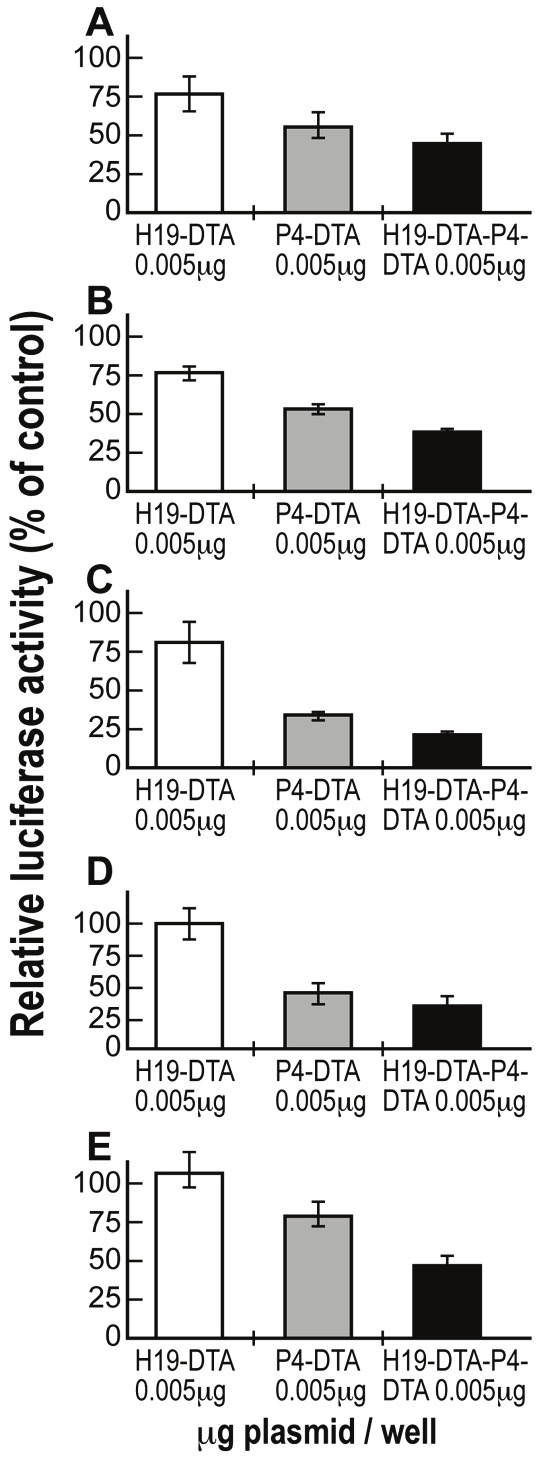
The enhanced activity of H19-DTA-P4-DTA vector in a spectrum of tumor cells: Protein synthesis inhibition activity of the H19-DTA (white), P4-DTA (gray) and H19-DTA-P4-DTA (black) vectors in Hep3B (A), ES-2 (B), CRL-1469 (C), U87 (D) and A172 (E) cells, was measured as a reduction of LucSV40 activity. Cells were co-transfected with 2μg of LucSV40, and with different concentrations (0.005mg - 0.05mg/well) of the DTA expressing vectors or with LucSV40 alone. Transfection experiments were stopped after 48 hours and luciferase activity was assessed. The decrease in LucSV40 activity was determined by comparison to the same cell type transfected with LucSV40 alone as a measure for cytotoxicity. The diverse effect of each vector at the lowest plasmid transfected concentration (0.005mg) is indicated.
Thus, the H19-DTA-P4-DTA double promoter expression vector exhibited significantly enhanced ability to lyse broad spectrum of tumor cells, relative to expression vectors carrying either sequence alone.
Augmented-than-additive protein synthesis inhibition activity of the H19-DTA-P4-DTA vector in different carcinoma cell lines
The presence of an augmented-than-additive protein synthesis inhibition activity of the double promoter construct H19-DTA-P4-DTA was tested in Hep3B, ES-2, CRL-1469 and U87 cells. The cells were co-transfected with 2μg of LucSV40 and either (a) the indicated concentrations (Figure 4) of single-promoter constructs H19-DTA + P4-DTA in combination, or (b) H19-DTAP4-DTA. The total amount of DNA co-transfected in samples receiving both single promoter constructs was therefore twice (0.005μg + 0.005μg) than the cells transfected only with H19-DTA-P4-DTA (only 0.005μg). Luciferase activity was determined and compared to that of cells transfected with LucSV40 alone. The double-promoter construct H19-DTA-P4-DTA exhibited enhanced efficiency in lysing the cancer cell lines, relative to the combined activity of both single promoter constructs (H19-DTA + P4-DTA), in Hep3B human liver cancer cells (Figure 4A). Very similar results were obtained in ES-2 human ovarian cancer cells (Figure 4B), CRL-1469 human pancreatic cancer cells (Figure 4C) and U87 human glioblastoma cells (Figure 4D).
Figure 4.
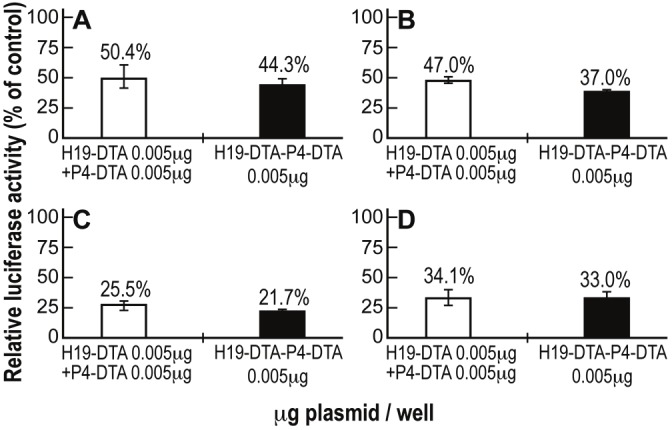
Augmented-than-additive activity of H19-DTA-P4-DTA in human different cancer cell lines: The additive effect of H19-DTA-P4-DTA vector was tested in Hep3B cells (A), ES-2 (B), CRL-1469 (C) and U87(D) cells transfected with only 0.005mg H19-DTA-P4-DTA (black) and compared to combination transfection of both vectors (white) H19-DTA + P4-DTA (which the total transfected concentration was therefore twice (0.005mg + 0.005mg).
Thus, H19-DTA-P4-DTA double promoter expression vector exhibited augmented-than-additive protein synthesis inhibition activity relative to expression vectors carrying either sequence alone when tested in a broad spectrum of tumor cells.
Discussion
The present study shows the successful use of a double promoter expressing vector, carrying on a single construct two separate DNA sequences expressing the diphtheria toxin A-fragment (DTA), from two different regulatory sequences, selected from the cancer-specific promoters H19 and IGF2-P4. This construct was used to transfect and to eradicate broad spectrum of tumor cells.
The H19 gene is an oncofetal RNA triggered by hypoxic stress [1]. H19 gene is highly expressed during embryogenesis, but shuts off in most tissues post-natally and re-expressed in a variety types of cancers, like bladder cancer [24], hepatocellular carcinoma [5], colorectal cancer [27], lung cancer [28], ovarian cancer [29], pancreatic cancer and in many other cancers [1].
Studies of various tumors (such as breast, bladder and hepatocellular carcinoma) have demonstrated high expression levels of the H19 gene as compared to the normal tissue [7]. Furthermore, in cancers of different etiologies and lineages aberrant expression in the allelic pattern was observed in some cases suggesting that H19 might play a role in promoting cancer progression, angiogenesis, and metastases [7].
The occurrences of loss of heterozygosity (LOH) as well as loss of imprinting (LOI) are major events related to cancer. LOI resulting in biallelic expression of the H19 gene, was found in various types of cancers [30].
IGF2 plays a fundamental role in human fetal growth displaying both tissue-specific and developmental regulation. As IGF2 promotes mitogenesis and inhibits apoptosis, the growth disorders that involve excessive growth, as well as malignant tumor cells accumulation could be attributable to over-expression of IGF2 [31]. Transgenic mice, over-expressing the IGF2 gene, developed spontaneous tumors at a high frequency [32-34], suggesting that overexpression of IGF2 may be involved not only in the progression of tumors but also in the initiation of neoplasia. IGF2 over-expression is significantly correlated to the increased tumor progression and proliferative activity as well as to decreased patient survival [34-36]. IGF2 overexpression in tumorigenesis is correlated to the induction of benign cellular proliferation, by preventing apoptosis, and later to the malignant hyper proliferation during tumor formation [37,38]. Increased expression of IGF2 is a common feature of both pediatric and adult malignancies [39], and mounting evidence implicates IGF2 as a major factor contributing to oncogenesis [29]. Cancer cells with a strong tendency to metastasize have higher expression of IGF2 [40]. Recent study has demonstrated that IGF2 represents a therapeutic target in ovarian cancer, particularly in the setting of Taxol resistance [41].
Therefore H19 and IGF2 regulatory sequences are expected to be good candidates for specifically inducing the expression of DTA in target tumor cells but not in cells of normal tissue. They are known to be differentially over-activated in various tumor types and to show no or minimum activity in the surrounding normal tissue [38,42].
Therefore, we applied an innovative approach using on a single construct more than one specific marker gene which are differentially expressed in tumor cells, for targeted cancer therapy.
Using a single promoter (e.g. an H19 promoter or an IGF2-P4 promoter) alone for expression of a cytotoxic gene presents several unresolved problems. For one, not every tumor cell of a given type of cancer is positive for expression via the H19 promoter or the IGF2-P4 promoter sequences. Moreover, using a single promoter (H19) expression vector, requires a prerequisite diagnostic test for H19, as not all tumor cells express H19 and therefore may not be suitable for this treatment.
Thus, such therapy could be limited in a sizable proportion of patients, even without accounting for tumor mutagenesis. Determination of responsiveness to such constructs would involve the costly and difficulty step of genotyping individual tumors.
Tumors are known to exhibit significant genomic instability and heterogeneity. Thus, even individuals with an H19-expressing tumor, for example, may contain some cancer cells that have downregulated or abrogated H19 expression via mutation. Therefore, expressing the cytotoxic gene from a single promoter in such patients may result in temporary and partial tumor regression that will rapidly be reversed when the cells containing these mutations survive and rapidly multiply.
Therefore the use of double promoter expressing vectors is highly novel. Tumor cells can express high levels of H19 and IGF2, or only one of those genes. That way, majority of the tumor cells could efficiently express the diphtheria toxin.
This novel approach, create a new family of plasmids regulated by two regulatory sequences, which in their natural genome position are both proximately located and are reciprocally imprinted. This is a novel biology concept, which mimics the unique biology reciprocity relations phenomenon of IGF2 and H19.
In vitro superior therapeutic effect in a broad spectrum of cancers
In order to determine if the double promoter strategy could be applicable to broader spectrum of cancer indications we further studied the expression of H19 and IGF2 in a broad spectrum of tumor cells. Furthermore we tested the protein synthesis inhibition activity of the double promoter vector, H19-DTA-P4-DTA, in different cancer cells.
Superior activity of the double promoter construct, H19-DTA-P4-DTA (Figure 3) relative to the single promoter constructs (H19-DTA, or P4-DTA), was demonstrated in the following human cancer cells lines: Hep3B (hepatocellular carcinoma cells), ES-2 (ovarian cancer), U87 (glioblastoma), A172 (glioblastoma) and CRL-1469 (pancreatic cancer).
Thus, H19-DTA-P4-DTA expression vector consistently exhibited significantly superior activity when tested in a broad spectrum of tumor cells, relative to expression vectors carrying either sequence alone. The consistency of these results across each of these cancer cell lines demonstrates the superior ability of the H19-DTA-P4-DTA construct in broad spectrum of tumor cells expressing H19, IGF2, or both.
The protein synthesis inhibition activity of the DTA expressing vectors (Figure 3), does correlate with the H19 and IGF2-P4 RNA expression levels (Figure 2) in those cells, except ES-2 cell line in which RNA could not be detected. This exception could be explained by possible inhibitory elements upstream the endogenous regulatory sequences which do not appear in the cloned promoters and therefore their activity is not correlated.
Similarly, in our previous srudies, H19 RNA could not be detected in T24P (human bladder cancer cell lines). However, H19 expression was detected in tumors developed in nude mice following inoculation of T24P cells, correspondingly to the case of ES-2 cells.
This occurrence may results from the major role IGF2 and H19 play in tumor development. Both genes contribute to the initiation of neoplasia and for tumor growth directly or via secondary routes such as angiogenesis, uncontrolled cells proliferation and inhibition of apoptosis. Moreover, IGF2 exerts its effects in autocrine and paracrine manner [31,43] which contributes to its significant expression in vivo, in tumors.
The reciprocally imprinted IGF2 and H19 genes are expressed during embryonal life and are downregulated postnatally. Therefore IGF2, as well as H19, has been long thought to be an ideal tumor marker [44]. Thus the use of such tumor markers, by expressing toxin only in cancer cells, presents distinguish targeted therapy approach.
In vitro augmented-than-additive activity
In vitro additive activity of the double promoter vector H19-DTA-P4-DTA (Figure 4) was exhibited in Hep3B, ES-2, U87 and CRL-1469 human cancer cells lines.
Thus, H19-DTA-P4-DTA vector exhibited superior efficiency in lysing the cancer cell lines, relative to the combined activity of both single promoter constructs (H19-DTA + P4-DTA).
It should be stressed that the superior activity was demonstrated even though the total amount of DNA co-transfected in samples receiving both single promoter constructs (H19-DTA + P4-DTA) was therefore twice (0.005μg + 0.005μg) than the cells transfected only with H19-DTA-P4-DTA (0.005μg).
Overall, the double promoter vector H19-DTA-P4-DTA exhibited augmented-than-additive anticancer activity relative to single promoter expression vectors carrying either DTA sequence alone, when tested in a broad spectrum of tumor cells.
The consistency of these results across each of the different cancer cell lines demonstrates the superior ability of H19-DTA-P4-DTA double promoter construct in cancer in general.
As H19 and IGF2 are expressed at very high levels in a broad spectrum of different cancers, therefore we propose a double promoter expression approach for treating a variety of tumors expressing H19, IGF2, or both. According to this approach patients may be treated with a single double promoter expression toxin vector which is under the control of the IGF2 and H19 regulatory sequences, differentially expressed in those cancers.
As the majority of the tumor cells express H19, IGF2, or both, therefore the use of prerequisite diagnostic test (such as in-situ hybridization) will be unnecessary.
Although this is a preliminary study, our working hypothesis is that administration of H19-DTA-P4-DTA has the potential to reach tumor cells, deliver its intracellular toxin without targeting normal tissues, and thus may help reduce tumor burden, improve the quality of life of the patients; and prolong their life span. This suggested approach should be further studied in appropriate animal models.
References
- 1.Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abulail R, Hochberg A, Galun E. The H19 noncoding RNA is essential for human tumor growth. PLoS ONE. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matouk IJ, Mezan S, Mizrahi A, Ohana P, Abu-Lail R, Fellig Y, Degroot N, Galun E, Hochberg A. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim Biophys Acta. 2010;1803:443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Hu JF, Daniels M, Shiran H, Zhou X, Yan H, Lu H, Zeng Z, Wang Q, Li T, Hoffman AR. A methylated oligonucleotide inhibits IGF2 expression and enhances survival in a model of hepatocellular carcinoma. J Clin Invest. 2003;111:265–273. doi: 10.1172/JCI15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariel I, Lustig O, Schneider T, Pizov G, Sappir M, De-Groot N, Hochberg A. The imprinted H19 gene as a tumor marker in bladder carcinoma. Urology. 1995;45:335–338. doi: 10.1016/0090-4295(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 5.Ariel I, Miao HQ, Ji XR, Schneider T, Roll D, de Groot N, Hochberg A, Ayesh S. Imprinted H19 oncofetal RNA is a candidate tumour marker for hepatocellular carcinoma. Mol Pathol. 1998;51:21–25. doi: 10.1136/mp.51.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayesh B, Matouk I, Ohana P, Sughayer MA, Birman T, Ayesh S, Schneider T, de Groot N, Hochberg A. Inhibition of tumor growth by DT-A expressed under the control of IGF2 P3 and P4 promoter sequences. Mol Ther. 2003;7:535–541. doi: 10.1016/s1525-0016(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 7.Matouk I OP, Ayesh S, Sidi A, Czerniak A, de Groot N, Hochberg A. The oncofetal H19 RNA in human cancer, from the bench to the patient. Cancer Therapy. 2005;3:249–266. [Google Scholar]
- 8.Erdmann VA, Barciszewska MZ, Szymanski M, Hochberg A, de Groot N, Barciszewski J. The non-coding RNAs as riboregulators. Nucleic Acids Res. 2001;29:189–193. doi: 10.1093/nar/29.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariel I, Ayesh S, Perlman EJ, Pizov G, Tanos V, Schneider T, Erdmann VA, Podeh D, Komitowski D, Quasem AS, de Groot N, Hochberg A. The product of the imprinted H19 gene is an oncofetal RNA. Mol Pathol. 1997;50:34–44. doi: 10.1136/mp.50.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engstrom W, Shokrai A, Otte K, Granerus M, Gessbo A, Bierke P, Madej A, Sjolund M, Ward A. Transcriptional regulation and biological significance of the insulin like growth factor II gene. Cell Prolif. 1998;31:173–189. doi: 10.1111/j.1365-2184.1998.tb01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holthuizen PE, Cleutjens CB, Veenstra GJ, van der Lee FM, Koonen-Reemst AM, Sussenbach JS. Differential expression of the human, mouse and rat IGF-II genes. Regul Pept. 1993;48:77–89. doi: 10.1016/0167-0115(93)90337-8. [DOI] [PubMed] [Google Scholar]
- 12.de Pagter-Holthuizen P, Jansen M, van der Kammen RA, van Schaik FM, Sussenbach JS. Differential expression of the human insulin-like growth factor II gene. Characterization of the IGF-II mRNAs and an mRNA encoding a putative IGF-II-associated protein. Biochim Biophys Acta. 1988;950:282–295. doi: 10.1016/0167-4781(88)90124-8. [DOI] [PubMed] [Google Scholar]
- 13.Holthuizen P, van der Lee FM, Ikejiri K, Yamamoto M, Sussenbach JS. Identification and initial characterization of a fourth leader exon and promoter of the human IGF-II gene. Biochim Biophys Acta. 1990;1087:341–343. doi: 10.1016/0167-4781(90)90010-y. [DOI] [PubMed] [Google Scholar]
- 14.Ekstrom TJ, Cui H, Li X, Ohlsson R. Promoter-specific IGF2 imprinting status and its plasticity during human liver development. Development. 1995;121:309–316. doi: 10.1242/dev.121.2.309. [DOI] [PubMed] [Google Scholar]
- 15.Giannoukakis N, Deal C, Paquette J, Goodyer CG, Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- 16.Ohlsson R, Franklin G. Normal development and neoplasia: the imprinting connection. Int J Dev Biol. 1995;39:869–876. [PubMed] [Google Scholar]
- 17.Morison IM, Reeve AE. Insulin-like growth factor 2 and overgrowth: molecular biology and clinical implications. Mol Med Today. 1998;4:110–115. doi: 10.1016/s1357-4310(97)01197-0. [DOI] [PubMed] [Google Scholar]
- 18.Wu HK, Squire JA, Catzavelos CG, Weksberg R. Relaxation of imprinting of human insulin-like growth factor II gene, IGF2, in sporadic breast carcinomas. Biochem Biophys Res Commun. 1997;235:123–129. doi: 10.1006/bbrc.1997.6744. [DOI] [PubMed] [Google Scholar]
- 19.Bae SK, Bae MH, Ahn MY, Son MJ, Lee YM, Bae MK, Lee OH, Park BC, Kim KW. Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res. 1999;59:5989–5994. [PubMed] [Google Scholar]
- 20.Lu L, Katsaros D, Wiley A, Rigault de la Longrais IA, Puopolo M, Schwartz P, Yu H. Promoter-specific transcription of insulin-like growth factor-II in epithelial ovarian cancer. Gynecol Oncol. 2006;103:990–995. doi: 10.1016/j.ygyno.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Mineo R, Fichera E, Liang SJ, Fujita-Yamaguchi Y. Promoter usage for insulin-like growth factor-II in cancerous and benign human breast, prostate, and bladder tissues, and confirmation of a 10th exon. Biochem Biophys Res Commun. 2000;268:886–892. doi: 10.1006/bbrc.2000.2225. [DOI] [PubMed] [Google Scholar]
- 22.Sohda T, Yun K, Iwata K, Soejima H, Okumura M. Increased expression of insulin-like growth factor 2 in hepatocellular carcinoma is primarily regulated at the transcriptional level. Lab Invest. 1996;75:307–311. [PubMed] [Google Scholar]
- 23.Leighton PA, Saam JR, Ingram RS, Stewart CL, Tilghman SM. An enhancer deletion affects both H19 and Igf2 expression. Genes Dev. 1995;9:2079–2089. doi: 10.1101/gad.9.17.2079. [DOI] [PubMed] [Google Scholar]
- 24.Ariel I, Sughayer M, Fellig Y, Pizov G, Ayesh S, Podeh D, Libdeh BA, Levy C, Birman T, Tykocinski ML, de Groot N, Hochberg A. The imprinted H19 gene is a marker of early recurrence in human bladder carcinoma. Mol Pathol. 2000;53:320–323. doi: 10.1136/mp.53.6.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amit D, Hochberg A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J Transl Med. 2010;8:134. doi: 10.1186/1479-5876-8-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohana P, Kopf E, Bibi O, Ayesh S, Schneider T, Laster M, Tykocinski M, de Groot N, Hochberg A. The expression of the H19 gene and its function in human bladder carcinoma cell lines. FEBS Lett. 1999;454:81–84. doi: 10.1016/s0014-5793(99)00780-2. [DOI] [PubMed] [Google Scholar]
- 27.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 28.Kaplan R, Luettich K, Heguy A, Hackett NR, Harvey BG, Crystal RG. Monoallelic upregulation of the imprinted H19 gene in airway epithelium of phenotypically normal cigarette smokers. Cancer Res. 2003;63:1475–1482. [PubMed] [Google Scholar]
- 29.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, Nichols TD, Marks JR, Berchuck A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4:283–292. doi: 10.1158/1541-7786.MCR-05-0138. [DOI] [PubMed] [Google Scholar]
- 30.Ariel I, Lustig O, Oyer CE, Elkin M, Gonik B, Rachmilewitz J, Biran H, Goshen R, de Groot N, Hochberg A. Relaxation of imprinting in trophoblastic disease. Gynecol Oncol. 1994;53:212–219. doi: 10.1006/gyno.1994.1118. [DOI] [PubMed] [Google Scholar]
- 31.Pavelic K, Bukovic D, Pavelic J. The role of insulin-like growth factor 2 and its receptors in human tumors. Mol Med. 2002;8:771–780. [PMC free article] [PubMed] [Google Scholar]
- 32.Bates P, Fisher R, Ward A, Richardson L, Hill DJ, Graham CF. Mammary cancer in transgenic mice expressing insulin-like growth factor II (IGF-II) Br J Cancer. 1995;72:1189–1193. doi: 10.1038/bjc.1995.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853–857. doi: 10.1038/sj.onc.1206188. [DOI] [PubMed] [Google Scholar]
- 34.Rogler CE, Yang D, Rossetti L, Donohoe J, Alt E, Chang CJ, Rosenfeld R, Neely K, Hintz R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J Biol Chem. 1994;269:13779–13784. [PubMed] [Google Scholar]
- 35.Kawamoto K, Onodera H, Kondo S, Kan S, Ikeuchi D, Maetani S, Imamura M. Expression of insulin-like growth factor-2 can predict the prognosis of human colorectal cancer patients: correlation with tumor progression, proliferative activity and survival. Oncology. 1998;55:242–248. doi: 10.1159/000011858. [DOI] [PubMed] [Google Scholar]
- 36.Takanami I, Imamuma T, Hashizume T, Kikuchi K, Yamamoto Y, Yamamoto T, Kodaira S. Insulin-like growth factor-II as a prognostic factor in pulmonary adenocarcinoma. J Surg Oncol. 1996;61:205–208. doi: 10.1002/(SICI)1096-9098(199603)61:3<205::AID-JSO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Christofori G, Naik P, Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 38.Li SL, Goko H, Xu ZD, Kimura G, Sun Y, Kawachi MH, Wilson TG, Wilczynski S, Fujita-Yamaguchi Y. Expression of insulin-like growth factor (IGF)-II in human prostate, breast, bladder, and paraganglioma tumors. Cell Tissue Res. 1998;291:469–479. doi: 10.1007/s004410051016. [DOI] [PubMed] [Google Scholar]
- 39.Foulstone E, Prince S, Zaccheo O, Burns JL, Harper J, Jacobs C, Church D, Hassan AB. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J Pathol. 2005;205:145–153. doi: 10.1002/path.1712. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 41.Huang GS, Brouwer-Visser J, Ramirez MJ, Kim CH, Hebert TM, Lin J, Arias-Pulido H, Qualls CR, Prossnitz ER, Goldberg GL, Smith HO, Horwitz SB. Insulin-like growth factor 2 expression modulates Taxol resistance and is a candidate biomarker for reduced disease-free survival in ovarian cancer. Clin Cancer Res. 2010;16:2999–3010. doi: 10.1158/1078-0432.CCR-09-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fichera E, Liang S, Xu Z, Guo N, Mineo R, Fujita-Yamaguchi Y. A quantitative reverse transcription and polymerase chain reaction assay for human IGF-II allows direct comparison of IGF-II mRNA levels in cancerous breast, bladder, and prostate tissues. Growth Horm IGF Res. 2000;10:61–70. doi: 10.1054/ghir.2000.0141. [DOI] [PubMed] [Google Scholar]
- 43.Kawamoto K, Onodera H, Kan S, Kondo S, Imamura M. Possible paracrine mechanism of insulin-like growth factor-2 in the development of liver metastases from colorectal carcinoma. Cancer. 1999;85:18–25. doi: 10.1002/(sici)1097-0142(19990101)85:1<18::aid-cncr3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Biran H, Ariel I, de Groot N, Shani A, Hochberg A. Human imprinted genes as onco-developmental markers. Tumour Biol. 1994;15:123–134. doi: 10.1159/000217882. [DOI] [PubMed] [Google Scholar]


