Abstract
Copper serves as an essential cofactor for a variety of proteins in all living organisms. Previously, we described a human gene (CTR1;SLC31A1) that encodes a high-affinity copper-uptake protein and hypothesized that this protein is required for copper delivery to mammalian cells. Here, we test this hypothesis by inactivating the Ctr1 gene in mice by targeted mutagenesis. We observe early embryonic lethality in homozygous mutant embryos and a deficiency in copper uptake in the brains of heterozygous animals. Ctr1−/− embryos can be recovered at E8.5 but are severely developmentally retarded and morphologically abnormal. Histological analysis reveals discontinuities and variable thickness in the basement membrane of the embryonic region and an imperfect Reichert's membrane, features that are likely due to lack of activity in the collagen cross-linking cupro-enzyme lysyl oxidase. A collapsed embryonic cavity, the absence of an allantois, retarded mesodermal migration, and increased cell death are also apparent. In the brains of heterozygous adult mice, which at 16 months are phenotypically normal, copper is reduced to approximately half compared with control littermates, implicating CTR1 as the required port for copper entry into at least this organ. A study of the spatial and temporal expression pattern of Ctr1 during mouse development and adulthood further shows that CTR1 is ubiquitously transcribed with highest expression observed in the specialized epithelia of the choroid plexus and renal tubules and in connective tissues of the eye, ovary, and testes. We conclude that CTR1 is the primary avenue for copper uptake in mammalian cells.
All living organisms require copper for growth and development (1–3). Equipped with a high redox potential, copper serves as a cofactor for proteins involved in a variety of biological reactions, such as photosynthesis and respiration, free radical eradication, connective tissue formation, iron metabolism, and neurological function. However, copper in excess of cellular needs mediates free radical production and direct oxidation of lipids, proteins, and DNA (1). Consequently, intracellular copper content is maintained by evolutionarily conserved cellular transport systems that regulate uptake, export, and intracellular compartmentalization (1, 4). In complex organisms such as mammals, the balance between copper necessity and toxicity is achieved both at the cellular level and at the tissue and organismal levels (reviewed in refs. 1–3).
Two inherited human disorders, X-linked Menkes disease and autosomal recessive Wilson disease, highlight the importance of intact cellular copper transport mechanisms (3). These two diseases are caused by mutations in distinct but highly homologous genes (ATP7A and ATP7B) encoding copper-transporting P-type ATPases that enable cellular egress of copper (5–10). In Menkes patients, a fatal defect in the ubiquitously expressed ATP7A gene leads to sequestration of copper in the intestine and kidney and a concomitant copper deficiency in the remaining parts of the body. In contrast, a defect in the ATP7B gene, whose expression is liver-specific, leads to a toxic build-up of copper in the liver and, eventually, in the brain. Mutations in the murine orthologs of these genes are also the bases for the mottled and toxic milk mouse mutants (11–13).
Whereas genetic evidence has established the obligate roles for ATP7A and ATP7B in mammalian copper export, no such evidence has been forthcoming for copper uptake. Until recently, ceruloplasmin had been widely regarded as the copper delivery protein (14), as 95% of copper in the circulation is complexed with this protein. However, the role of ceruloplasmin in copper uptake has been confounded by the description of aceruloplasminemic patients who suffer no copper imbalance (15, 16).
A likely candidate for copper uptake in mammals is CTR1. Previously, we identified the human gene CTR1 (SLC31A1) by complementation of a yeast mutant (ctr1) that is defective in high-affinity copper uptake (17, 18). Based on its copper-transporting function in a yeast assay, its sequence similarity to the yeast high-affinity copper uptake protein, and its ubiquitous expression, we hypothesized that CTR1 is required for copper uptake in mammalian cells (4). Indeed two recent studies, involving 64Cu uptake (19) and copper sensitivity (B.Z. and J.G., unpublished results) in CTR1-transfected lines, have confirmed that CTR1 transports copper in mammalian cells. However, evidence for a required role of CTR1 in mammalian copper uptake has not been forthcoming. No human diseases mapping to the CTR1 locus at 9q34 have a phenotype consistent with copper deficiency. Moreover, we ruled out CTR1 mutations in three classes of patients with “atypical” Menkes disease (Y.M.K. and S. Packman, unpublished data).
Thus, to test our hypothesis that CTR1 is necessary for copper uptake, we generated and analyzed mice carrying a null mutation for Ctr1. We present genetic evidence that Ctr1 is essential for embryonic growth and development and is required for copper transport into the brain. We also report on the spatial and temporal expression pattern of Ctr1 during mouse development and adulthood, concluding that CTR1 contributes both a housekeeping and specialized epithelial transport function. The relationship between copper uptake as provided by CTR1 and copper export as provided by the Menkes/mottled copper-transporting ATPase is discussed.
Materials and Methods
Isolation of the Murine Ctr1 Gene.
We obtained and sequenced mouse-expressed sequence tags (EST) orthologous to human CTR1 from the Washington University–Howard Hughes Medical Institute mouse-EST project databases. We made primers to the mouse cDNA and used reverse transcriptase–PCR (Superscript, GIBCO) to obtain a full-length cDNA from adult mouse kidney. This fragment was subcloned into PCR Script (Stratagene), sequenced, and used as a probe for in situ hybridization and for isolation of full-length genomic clones from a 129/SvJ mouse library (Stratagene). Several genomic clones were recovered, and the Ctr1 gene was found to span 6 kb distributed over 4 exons.
Targeted Deletion of Ctr1.
To generate the targeting construct, we built a replacement vector in a multistep cloning process. We isolated a 2.3-kb BglII fragment 5′ of exon 1 of Ctr1, subcloned it into the BamHI site of pGEM-3Z (Promega), and released it by using flanking sites KpnI and SalI. We then cloned it into the KpnI–SalI sites of the pNeoZTK2 targeting vector (gift of R. Palmiter, University of Washington, Seattle). A 6.0-kb EcoRV fragment 3′ of exon 4 was cloned into the SmaI site of a modified pBR322 vector (unpublished data), creating SpeI and NotI sites. The 6.0-kb fragment was released by using SpeI and NotI and cloned into the SpeI–NotI sites of the pNeoZTK2 targeting vector.
The targeting construct was made linear with SstII and electroporated into JM1 129/SvJ embryonic stem cells as described (20). To identify embryonic stem (ES) cell clones containing targeted alleles of Ctr1, we assayed genomic DNA by PCR with a primer corresponding to a region 5′ of the BglII fragment and one inside the nlacZ gene. We confirmed the results by Southern blot with a 300-bp genomic probe located 3′ downstream of the 6.0-kb EcoRV fragment. The neoR gene introduces new HindIII and SpeI restriction enzyme sites, which were used to verify targeted clones by Southern blotting.
Two correctly targeted ES cell lines, 124 and 191, were used to generate two independent lines of heterozygous mice. Genotype was assessed routinely by PCR by using a pair of genomic primers (5′-CCGGAGTTGGTGATTCTAACTTTAG-3′ and 5′-CGATTCGCAGCGCATCGCCTTCTAT-3′) and a primer specific for neoR (5′-GATGATTGGAAAGGGAATCATATCT), yielding a 730-bp product for the wild-type allele and a 421-bp product for the mutant allele.
Genotypic Analysis of Mice and Embryos.
Genomic DNA was prepared from tails from F1 animals (21) and analyzed by both PCR and Southern blot, with identical results. F2 animals and embryos were analyzed by PCR using the primers named above. For embryos at age 8.5 days (E8.5) and older, a small piece of the yolk sac was dissected out and prepared as described (22). E6.5 and E7.5 embryos were photographed, and the extra-embryonic region was removed and the DNA prepared (21).
Histological Analyses and Immunohistochemistry.
Embryos in decidua were fixed for 12–14 h in 4% paraformaldehyde at 4°C. Embryos were dissected and embedded in JB-4 embedding medium following the manufacturer's protocol (Polysciences), and 5-μm sections were cut on a Sorvall brand JB-4A microtome. E6.5 and E7.5 embryos were dissected from decidua, and the extra-embryonic region was removed for genotyping; the embryonic region was embedded. Sections were immunostained with anti-collagen IV antibody (Chemicon) or with anti-matrix metalloproteinase-9 or gelatinase B (gift of Z. Werb, University of California, San Francisco) by standard procedures (23) with the substrate True Blue (Kirkegaard & Perry Laboratories) and counterstaining with Fast Red (Sigma). Histological sections were stained with Gill's No. 3 (Sigma) and 0.6% eosin Y (Sigma) in distilled water.
Copper Measurement.
The brains, kidneys, small intestines, and livers were dissected from 6-week-old male mice. The tissue was weighed (ranging from 0.15 to 0.25 g) and digested in a Multiwave Microwave Digestor according to manufacturer's protocol (Perkin–Elmer). Copper content was measured by using an AAnalyst 100 Atomic Absorption spectrophotometer (Perkin–Elmer).
In Situ Hybridization.
Embryos and tissues were collected and fixed overnight in 4% paraformaldehyde, embedded in paraffin, and sectioned (8 μm) as described (24). We performed in situ hybridizations as described (24). Radiolabeled [33P]UTP Ctr1 sense and antisense probes were generated. Probes were hybridized to sagittal sections (8 μm) of wild-type E12.5, E14.5, E16.5, and E18.5 embryos and to wild-type sections (8 μm) from kidney, small intestine, brain, ovary, testes, and eye. Sections were subsequently washed, and a photographic emulsion (Kodak NBT3) was applied for 14 days. The emulsion was developed, counterstained with toulidine blue, and visualized by dark-field microscopy by using a Nikon Eclipse E800 microscope.
Results
Generation and Effects of the Ctr1 Null Mutation.
We generated a null mutation in Ctr1 by replacing the entire gene with a nlac/neoR cassette (Fig. 1A). This construct was electroporated into JM1 embryonic stem cells, and correctly targeted ES cells were discerned by Southern blot (Fig. 1B) and PCR analyses. Two independent lines (124 and 191) were used to produce chimeras that transmitted the targeted allele through the germ line. The F1 heterozygotes were generated by crossing the chimeras with C57BL/6 females. The F2 progeny from these mice were genotyped by Southern blot and PCR analyses (Fig. 1C). We analyzed a total of 989 F2 offspring and detected 429 wild-type and 560 heterozygous animals (Table 1). No homozygous mutants were recovered at birth.
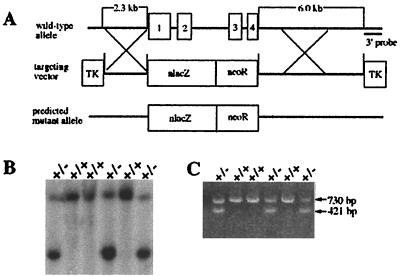
Figure 1.
Targeted disruption of Ctr1. (A) Schematic illustration of the Ctr1 gene, the targeting construct, and the predicted mutant allele. The four exons of Ctr1 are shown. Homologous recombination in the regions indicated X should result in the predicted mutant allele. (B) Southern blot verification of correctly targeted ES cell clones. Hybridization of SpeI-digested DNA from ES lines probed with a radiolabeled 3′ external probe reveals a targeting event as a pair of fragments. (C) PCR analysis of F1 heterozygous mouse genomic DNA using two pairs of primers, as described under Materials and Methods. The 730-bp fragment corresponds to the normal gene, and the 421-bp fragment is derived from the correctly targeted gene.
Table 1.
Genotype of offspring from heterozygous matings
| Ctr1+/+ | Ctr1+/− | Ctr1−/− | |
|---|---|---|---|
| Pups | 429 | 560 | 0 |
| E16.5 | 3 | 5 | 0 |
| E13.5 | 4 | 5 | 0 |
| E9.5 | 15 | 15 | 2 |
| E8.5 | 41 | 78 | 22 |
| E7.5 | 12 | 19 | 7 |
| E6.5 | 10 | 11 | 5 |
Data from lines 124 and 191 are comparable, and combined data are shown.
Examination of timed pregnancies uncovered Ctr1−/− embryos only during early stages of development (Table 1). No mutant embryos were found beyond embryonic day 9.5 (E9.5). All heterozygous embryos were phenotypically indistinguishable from wild-type, yet the observed genotypic distribution deviated from the expected Mendelian ratio of 1:2:1 in both embryos and viable pups. A possible explanation could be a selective advantage of wild-type over hemizygous mutant ovum or sperm, or of wild-type over heterozygous preimplantation embryos.
Developmental defects in Ctr1−/− embryos are obvious on whole-mounted embryos (Fig. 2A). At days E6.5 and E7.5, Ctr1−/− embryos have a truncated embryonic region, and they appeared more fragile upon dissection of the decidua. Whereas littermates at E8.5 have proceeded into organogenesis, E8.5 mutant embryos retain large extra-embryonic regions, and the embryonic regions have atrophied. At E9.5, we recovered only two homozygous mutant yolk sacs, which lacked embryonic regions.
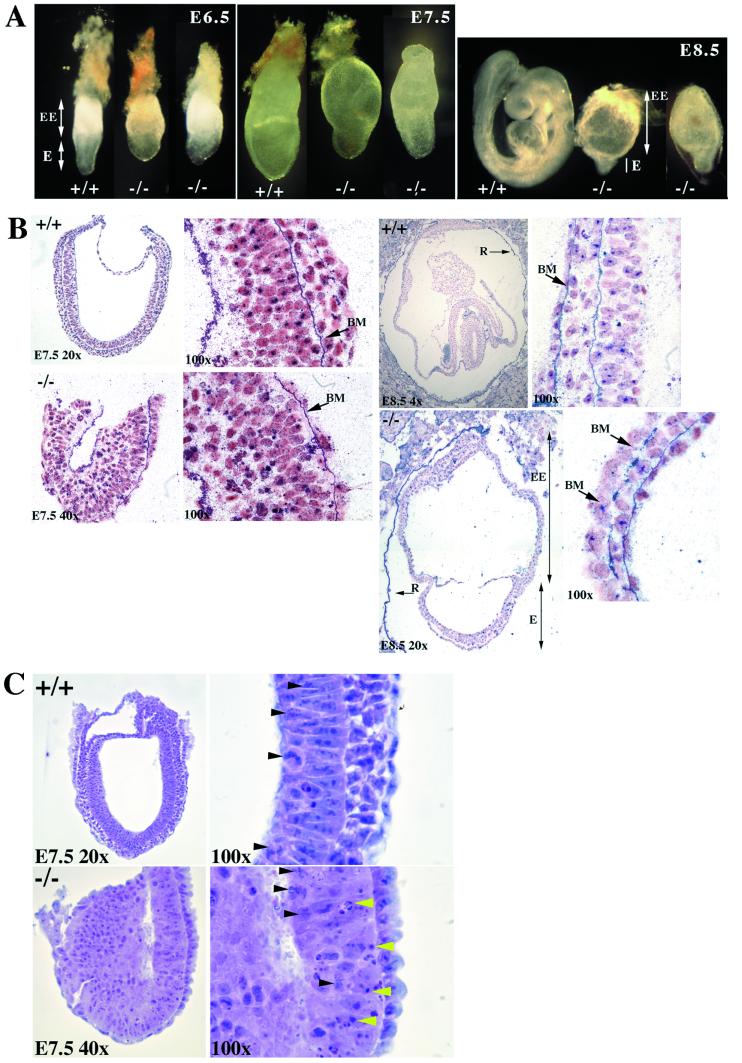
Figure 2.
Phenotypic analysis of wild-type and mutant embryos. (A) Whole-mount gross morphology of wild-type and Ctr1−/− embryos at E6.5, E7.5, and E8.5. The embryos are oriented such that the embryonic region (E) is located at the bottom and the extra-embryonic structures are on the top (EE). All embryos were genotyped by PCR after photography. Identical phenotypes were observed in both the 124 and 191 lines, and wild-type embryos were indistinguishable from heterozygotes. Photographs were taken on a Nikon SMZ 800 dissecting microscope. E6.5 embryos were photographed at ×6.5, E7.5 at ×5, and E8.5 at ×4 with a ×2.5 projection lens. (B) Histological examination of sectioned embryos. E7.5 and E8.5 wild-type and mutant were stained with anti-collagen IV staining and counterstained with Fast Red. At E7.5, the basement membrane (BM) of the mutant embryo appears intact. At E8.5, the mutant extra-embryonic region (EE) and underdeveloped embryonic region (E) are seen. Note the Reichert's membrane (R) is broken in the E8.5 mutants but intact in the wild-type E8.5. At ×100, the basement membrane is intact in the wild type, but gaps along the basement membrane are apparent in the mutant, as indicated by the arrows. (C) Hematoxylin and eosin staining of sagittal sections of E7.5 wild-type and mutant embryos. Only the embryonic region is shown because the upper extra-embryonic region was removed for genotyping by PCR. At ×100 in the mutant, areas of apoptosis can be seen (indicated by yellow arrowheads); however, mitosis is unaffected (indicated by black arrowheads). In the wild-type normal section at ×100, mitosis can be seen as indicated by the black arrowheads.
We confirmed that Ctr1 was inactivated in the mutant embryos by in situ hybridization. At E8.5, Ctr1 is expressed in wild-type E8.5 embryos, but the transcript is absent in the mutant embryos (results not shown).
Histology of Ctr1−/− Embryos.
Examination of sectioned embryos uncovered more specific defects (Fig. 2B). At E7.5, the cavity of the mutant embryos is compressed compared with that of their littermates, and the embryos have not undertaken gastrulation. By collagen IV immunostaining, the basement membrane between the ectoderm and endoderm appears intact; however, the endoderm is sparse in the mutant, and the mesoderm is not apparent. Hematoxylin and eosin staining of E7.5 sections (Fig. 2C) shows cell death in the embryonic region in Ctr1−/− compared with their wild-type littermates, but the number of mitotic cells seems to be unaffected.
By E8.5, development of the Ctr1−/− embryos is greatly retarded, approximating normal E7.5 in size and architecture (Fig. 2B). In all mutant embryos examined, the allantois is absent, and the Reichert's membrane is fragile and discontinuous. This fragility could account for the hemorrhaging we observed in whole-mount mutant embryos on days E7.5 and E8.5 because of invasion of maternal blood (data not shown). Collagen IV immunostaining shows that the basement membrane of the embryonic region is discontinuous and variable in thickness compared with the intact basement membrane in their littermates. However, the chorion and the basement membrane of the extra-embryonic region appear normal, as does the ectoplacental cone, as evidenced by immunostaining with an antibody against matrix metalloproteinase-9 or gelatinase B (results not shown).
Analysis of Heterozygous Animals.
Like the heterozygous embryos, heterozygous adults are indistinguishable from their wild-type littermates. Body weight, fertility, litter size, and lifespan appear normal, and no gross morphological or physiological abnormalities are apparent in the 15 months we have followed these animals.
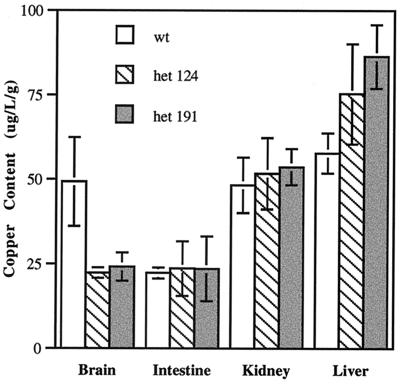
We asked whether any effect on copper homeostasis could be discerned in the heterozygous animals. By atomic absorption spectrophotometry, we compared the levels of copper in four organs taken from 6-week-old wild-type and heterozygous animals. Copper is decreased in the brains of the heterozygotes by ≈50% compared with control littermates (Fig. 3). No significant difference was observed in the gut, kidney, or liver, although copper levels in the liver tissue varied widely. These findings demonstrate that copper content is reduced in at least one tissue of the heterozygous animals and strongly implicate CTR1 as the threshold for control of copper uptake in the brain.
Figure 3.
Copper content of brain, small intestine, kidney, and liver from heterozygous and wild-type 6-week-old male littermates. Six heterozygous mice from each independent line 124 and 191 and four wild-type littermate mice were analyzed. Digested tissue samples were assayed three times as normalized to wet weight of tissue. The measurements were averaged, and the standard deviation for each was determined by using the MICROSOFT EXCEL program.
Expression of Ctr1 in Embryos and Adult Organs.
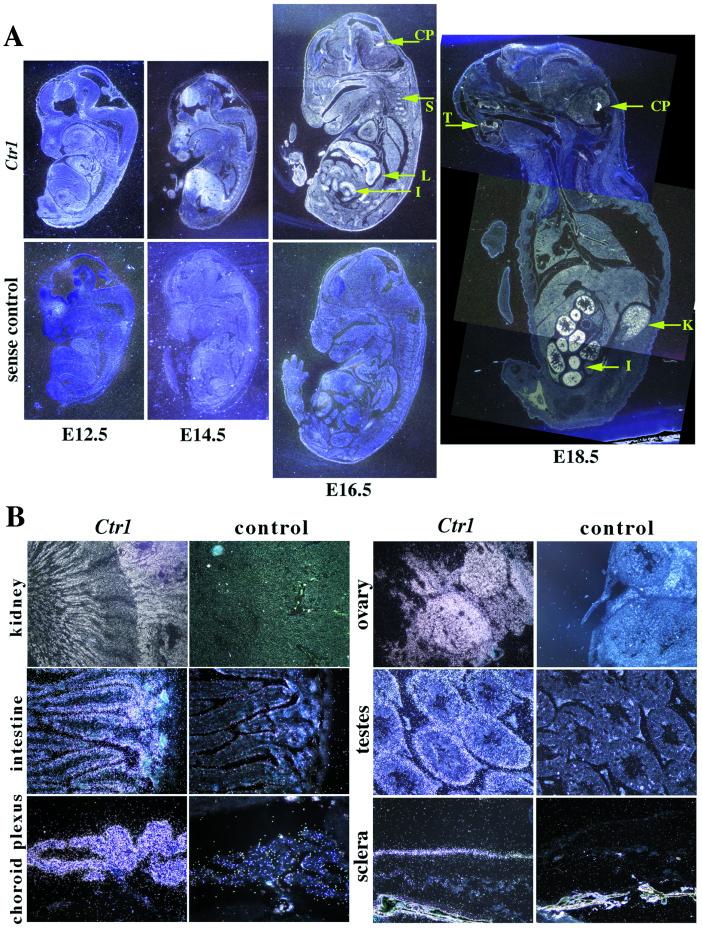
In the present study, the effect of the Ctr1 null mutation so early in development precludes our understanding its role in specific tissues later in development and in the adult. Although previous Northern analyses demonstrated developmental expression of Ctr1 from E6.5 to adult (Y.M.K., unpublished results, and ref. 25), in situ hybridization provides an elaboration of these findings. Although diffuse expression continues during embryogenesis, by E14.5 it becomes enhanced in particular tissues including the liver, somites, and the choroid plexus, the site of cerebral spinal fluid synthesis in the brain (Fig. 4A). By day E18.5, expression in the intestine, kidney, and choroid plexus is pronounced and also appears in the developing tooth buds.
Figure 4.
In situ hybridization of Ctr1 in wild-type embryos and adult tissues. (A) Temporal and spatial expression of Ctr1 in the developing mouse. At E14.5, expression can be observed in the forebrain, the liver, the nasal regions, and the somites. By E16.5, Ctr1 expression is ubiquitous, with high expression in the liver (L), intestine (I), somites (S), and choroid plexus (CP). By E18.5 expression is more restricted to the choroid plexus (CP), kidney (K), intestine (I), and tooth buds (T). (B) Expression of Ctr1 in adult tissues. Ctr1 is expressed in the tubules in the inner cortex and inner medulla of the kidney, the villi of the small intestine, the choroid plexus of the brain, the stroma of the ovary, the seminiferous tubules of the testes, and the sclera of the eye. Sections were photographed under dark-field illumination. The E12.5 embryo was photographed at ×4, the E14.5, at ×2, and the E16.5 and E18.5 at ×1. The kidney was photographed at ×4, the ovary, testes, and eye at ×20, and the intestine and choroid plexus at ×40.
These observations were confirmed and extended in adult organs (Fig. 4B). Ctr1 expression is plentiful in a variety of epithelial-derived tissues, including the renal convoluted tubules, the intestinal villi, and the choroid plexus. Expression is also abundant throughout connective tissue, including the stroma of the ovarian cortex, the wall of the seminiferous tubules in the testes, and the sclera of the eye. Liver, lung, spleen, mammary gland, and placenta show more diffuse expression (data not shown).
Discussion
We present genetic evidence for the essential role of the copper transporter CTR1 in mouse development. In particular, we see early embryonic lethality associated with a Ctr1 null mutation. We find approximately half the copper content in brains of heterozygous animals, implicating CTR1 as required for copper uptake in at least this organ. Our expression studies also strongly suggest a ubiquitous role for CTR1 in copper uptake in the developing and adult animal, coupled with a more specialized role in epithelial cells and in connective tissue. We conclude that CTR1 is required for copper uptake in mammalian cells.
Our findings on the distribution of Ctr1 expression and of in utero lethality in the mutant are reminiscent of those for the Menkes/mottled copper-transporting ATPase, a protein that serves as a ubiquitous copper exporter (2, 24). Like the Ctr1 null mutant, many alleles at the mottled locus (Atp7a), including dappled and tortoiseshell, die in utero (26–28). The abundant expression of both Ctr1 and Atp7a in the choroid plexus supports this structure as an important copper port for the brain (24, 29, 30). The brain may be particularly vulnerable to reduced CTR1 levels, as evidenced by our atomic absorption studies, because copper might pass first through the choroid plexus and subsequently be taken up by the neurons and glia themselves. Neurological degeneration is the primary cause for demise in Menkes patients (3), and female carriers of Menkes disease also exhibit neurological symptoms (31). It will be of interest to continue to follow the Ctr1+/− mice for neurological findings.
Although the effects of copper deficiency in the early embryo are likely to be pleiotrophic, our data highlight the importance of copper for the development of the basement membrane and the Reichert's membrane. Gastrulation does not proceed without stable cross-linking of connective tissue proteins, and this reaction requires the cupro-enzyme lysyl oxidase (32). Thus, the absence of the mesoderm and the allantois, a mesoderm-derived structure, could be secondary to the defect in the basement membrane; however, a direct effect of copper deficiency on mesodermal migration is possible.
That lysyl oxidase may be particularly vulnerable to copper insufficiency is supported by occipital horn syndrome patients who suffer only from connective tissue defects, yet have mutations in the Menkes ATP7A gene. Indeed, Ctr1 is highly expressed in the connective tissues in the sclera of the eye, the stroma of the ovary, and the seminiferous tubules of the testes.
The finding of embryonic lethality in the Ctr1 mutant may provide an explanation for the lack of obvious candidates of human diseases attributable to mutations in CTR1. Many human mutations in CTR1 may be sufficiently deleterious to result in fetal demise.
Acknowledgments
We thank Richard Palmiter for the pNeoZTK2 vector. We thank Margaret Flannery and Juanito Meneses for excellent technical advice, Martha Gunthorpe and Hernan Consengco for technical assistance, and Zena Werb, Seymour Packman, and members of our laboratory for helpful discussions and support. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. J.G. is an investigator with the Howard Hughes Medical Institute.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 6543.
References
- 1.Harris E D. Proc Soc Exp Biol Med. 1991;196:130–140. doi: 10.3181/00379727-196-43171b. [DOI] [PubMed] [Google Scholar]
- 2.Bull P C, Cox D W. Trends Genet. 1994;10:246–252. doi: 10.1016/0168-9525(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 3.Danks D M. In: The Metabolic Basis of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 2. New York: McGraw–Hill; 1995. pp. 2211–2235. [Google Scholar]
- 4.Vulpe C, Packman S. Annu Rev Nutr. 1995;15:293–322. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- 5.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Nat Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 6.Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco A P. Nat Genet. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- 7.Mercer J F, Livingston J, Hall B, Paynter J A, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, et al. Nat Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 8.Bull P C, Thomas G R, Rommens J M, Forbes J R, Cox D W. Nat Genet. 1993;5:327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Heiny M E, Gitlin J D. Biochem Biophys Res Commun. 1993;197:271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- 10.Tanzi R E, Petrukhin K, Chernov I, Pellequer J L, Wasco W, Ross B, Romano D M, Parano E, Pavone L, Brzustowicz L M, et al. Nat Genet. 1993;5:344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 11.Levinson B, Vulpe C, Elder B, Martin C, Verley F, Packman S, Gitschier J. Nat Genet. 1994;6:369–373. doi: 10.1038/ng0494-369. [DOI] [PubMed] [Google Scholar]
- 12.Mercer J F, Grimes A, Ambrosini L, Lockhart P, Paynter J, Dierick H, Glover T W. Nat Genet. 1994;6:374–378. doi: 10.1038/ng0494-374. [DOI] [PubMed] [Google Scholar]
- 13.Theophilos M B, Cox D W, Mercer J F. Hum Mol Genet. 1996;5:1619–1624. doi: 10.1093/hmg/5.10.1619. [DOI] [PubMed] [Google Scholar]
- 14.Linder M C, Hazegh-Azam M. Am J Clin Nutr. 1996;63:797–811. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida K, Furihata K, Takeda S, Nakamura A, Yamamoto K, Hiyamuta S, Ikeda S, Shimizu N, Yanagisawa N. Nat Genet. 1995;9:267–272. doi: 10.1038/ng0395-267. [DOI] [PubMed] [Google Scholar]
- 16.Harris Z L, Takahashi Y, Miyajima H, Serizawa M, MacGillivray R T, Gitlin J D. Proc Natl Acad Sci USA. 1995;92:2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou B, Gitschier J. Proc Natl Acad Sci USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dancis A, Haile D, Yuan D S, Klausner R D. J Biol Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- 19.Moller L B, Petersen C, Lund C, Horn N. Gene. 2000;257:13–22. doi: 10.1016/s0378-1119(00)00394-2. [DOI] [PubMed] [Google Scholar]
- 20.Qiu M, Bulfone A, Ghattas I, Meneses J J, Christensen L, Sharpe P T, Presley R, Pedersen R A, Rubenstein J L. Genes Dev. 1995;9:2523–2538. doi: 10.1101/gad.9.20.2523. [DOI] [PubMed] [Google Scholar]
- 21.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 23.Tebbs R S, Flannery M L, Meneses J J, Hartmann A, Tucker J D, Thompson L H, Cleaver J E, Pedersen R A. Dev Biol. 1999;208:513–529. doi: 10.1006/dbio.1999.9232. [DOI] [PubMed] [Google Scholar]
- 24.Kuo Y M, Gitschier J, Packman S. Hum Mol Genet. 1997;6:1043–1049. doi: 10.1093/hmg/6.7.1043. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Prohaska J R, Dagenais S L, Glover T W, Thiele D J. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 26.Philips R J S. Genet Res. 1961;2:290–295. [Google Scholar]
- 27.Miller J. X-Linked Traits: A Catalogue of Loci in Nonhuman Mammals. Cambridge: Cambridge Univ. Press; 1990. pp. 115–125. [Google Scholar]
- 28.Cecchi C, Biasotto M, Tosi M, Avner P. Hum Mol Genet. 1997;6:425–433. doi: 10.1093/hmg/6.3.425. [DOI] [PubMed] [Google Scholar]
- 29.Murata Y, Kodama H, Abe T, Ishida N, Nishimura M, Levinson B, Gitschier J, Packman S. Pediatr Res. 1997;42:436–444. doi: 10.1203/00006450-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Nishihara E, Furuyama T, Yamashita S, Mori N. NeuroReport. 1998;9:3259–3263. doi: 10.1097/00001756-199810050-00023. [DOI] [PubMed] [Google Scholar]
- 31.Gerdes A M, Tonnesen T, Horn N, Grisar T, Marg W, Muller A, Reinsch R, Barton N W, Guiraud P, Joannard A, et al. Clin Genet. 1990;38:452–459. doi: 10.1111/j.1399-0004.1990.tb03612.x. [DOI] [PubMed] [Google Scholar]
- 32.Rucker R B, Kosonen T, Clegg M S, Mitchell A E, Rucker B R, Uriu-Hare J Y, Keen C L. Am J Clin Nutr. 1998;67:996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]