Abstract
Background
The incidence of intraventricular hemorrhage (IVH) in very low birth weight infants can be used as an index of the quality of care in neonatal intensive care units as long as it is adjusted to reflect the infants’ risk profiles on admission to the unit, which may vary systematically from one institution to another. Adjustment for gestational, birth-related, and neonatological risk factors enables a fair comparison of IVH rates across neonatal intensive care units.
Methods
Data on 1782 neonates born at less than 32 weeks of gestation or weighing less than 1500 g at birth were retrieved from the 26 744 anonymous data sets collected in the Peri- and Neonatal Survey of the German state of Saxony in the years 2001–2005. An analysis of 30 putative risk factors with stepwise logistic regression analysis enabled the construction of a specific risk predictor for severe (grade 3–4) IVH. Risk-adjusted institutional incidence rates were then calculated.
Results
Five independent risk factors (low gestational age, low Apgar scores at 1 min, early infection, absence of pathological Doppler findings during pregnancy, and the use of tocolytic agents) were found to be relevant to the prediction of IVH. A risk predictor incorporating them was found to have a correct prediction rate (ROCAUC value) of 87.7%. The crude incidence of severe IVH in different institutions ranged from 1.92% to 15.02% (mean, 8.55%); after adjustment, the range was 5.14% to 11.58%. When the institutions studied were ranked in order of their incidence of IVH before and after adjustment for risk factors, individual institutions rose or fell by as many as 4 places in the ranking because of the adjustment.
Conclusion
These findings reveal the importance of adjusting the incidence of IVH in very low birth weight infants by the patients’ risk profiles to enable valid comparisons between institutions for the purpose of quality surveillance.
Among the 680 000 babies born in Germany every year, around 2100 perinatal intraventricular hemorrhages (IVHs) may be expected, potentially resulting in death, lasting damage, or impaired development. Extrapolating from the results of the peri- and neonatal data collection for Saxony in 2009 (http://www.slaek.de/36quali/80Downloads/2009/Neo_2009.pdf), around 470 severe cases of hemorrhage (grade 3–4) would be predicted just for the group of 8500 very low birth weight infants (less than 1500 g). All that survive must be expected to suffer long-term consequences in their neuromotor and cognitive development.
Although individual disposition varies, the cause of IVH must be seen in the complex of perinatal/obstetric and neonatal risk factors, which include structural, organizational, and subjective factors as well as medical ones. Not all of these can be measured objectively. Since the patient populations of the various neonatal facilities differ in their risk profile—that is, in regard to causes of disease, physical condition, and previous treatment and management—the interhospital IVH rates are bound to show differences (1– 3). It follows that these differences do not only represent differences in the quality of neonatal care. If the IVH rate is to function as a valid quality criterion of neonatal care – that is, as a performance indicator for interhospital quality comparisons – it needs to be adjusted for risk. This risk adjustment should take into account the infant’s preneonatal risk burden, upon which the neonatologist has no influence. Using a multivariate logistic regression analysis of merged data from perinatal and neonatal data collections, a predictor can be defined that will allow calculation of an estimated risk of occurrence of a target event (4– 6). By focusing on risk factors associated with pregnancy and birth and the ways in which they relate to IVH, the associated risk of IVH may be predicted. On this basis a risk-adjusted rate of incidence of IVH may be calculated. This reflects the outcome quality of neonatal units more reliably than an uncorrected rate of incidence. The aim of this article is to contribute to the valid assessment of neonatal outcomes and to ensuring robust qualitative comparison between hospitals.
Materials and methods
Data and data merging
The analysis is based on data from the peri- and neonatal data collected from all hospitals in Saxony in 2001–2005. Data merging and reduction to individual patients preserved anonymity and ensured data protection by using programmed match algorithms. Five control variables were used for matching: sex, date of birth, time of birth, gestational age (as originally recorded), and birth weight. Some deviations had to be tolerated; for birth weight, the tolerance was ±100 g. Deviations in the match variables were permitted for a maximum of one of these variables; multiple deviations did not in fact occur. Elimination of duplicate records due to transfer of neonates to another facility, and the required merging of all the individual data relating to those neonates, was done by reviewing all these individual data and merging them by hand. A record linkage rate of 90.1% was achieved, covering 1.16% of the neonate population.
Among a total of 26 744 neonatal datasets, there were 1910 datasets on infants with a birth weight lower than 1500 g or a gestational age of less than 32 completed weeks of gestation. Datasets of 116 infants who died within 4 days of birth without definite information about IVH and 19 datasets of infants who did not have a head ultrasound scan were excluded. There remained n = 1782 datasets, which formed the basis of the analysis. These included n = 168 (8.9%) from children transported from obstetric departments.
The study took into account ultrasound brain findings up until discharge, irrespective of the time of first observation or occurrence, as this is not recorded. Staging was according to Papile’s classification (7).
We analyzed the perinatal variables that are associated with the occurrence of IVH as a dependent variable or are closely related in time to delivery and postnatal vital signs (1, 8– 18), but that cannot be an expression or a result of neonatal interventions. To these were added, in the univariate analysis, some data on emergency medical care provided. However, these additional data were excluded from the multiple logistic regression to describe the risk predictor, because as neonatal treatment characteristics they are a part of pediatric quality of care and therefore cannot be included in the risk adjustment. Finally, a total of 30 covariables or complexes of variables (e.g., delivery risks, presentation, and mode of delivery) were identified (Table 1).
Table 1. Analyzed risk variables from the peri- and neonatal data collection for the occurrence of intraventricular hemorrhage (IVH).
| Perinatal data | Neonatal data | |
|
|
|
*1Not included in multivariate analysis
The interval from rupture of membranes to delivery was not taken into account because it is not described in the perinatal data collection form, and neither were transport and transfer modalities, because the pediatrician influences decisions on these in ways that cannot be measured. The same applies to the CRIB (Critical Risk Index for Babies) score, which included markers of physical status in the first 12 hours of life.
Some numeric raw data were categorized (e.g., Apgar scores, cervical width, pH values, base deficit).
The study, including its ethical aspects, was approved by the steering committee of the Saxony State Medical Association.
Statistical methods
For details of the statistical methods used, including the steps by which the risk-adjusted values were calculated, the reader is referred to the eBox and previous publications (4, 5).
eBOX. Statistical method.
The univariate description of the candidate variables for the multivariate predictor include, in addition to the data relating to incidence (N) in the variable categories, the odds ratio (OR) and the p value of the chi square test for each category with reference to a chosen reference category.
The multivariate predictor is the result of a stepwise choice of model for a statistically optimal logistic regression model. It includes the quantitative relationships of the risk variables that determine the dependent variable. This allows individual and hospital-specific risk profiles to be determined and the event probability—in this case the expected incidence rate of intraventricular hemorrhage (IVH)—to be calculated. The actually observed incidence rate is then corrected by the difference between the mean raw incidence rate of all hospitals and the incidence rate predicted by the predictor (= the hospital’s “permitted” rate) (hospital-specific incidence rate [IR] + [total IR for Saxony – predicted hospital-specific IR] = risk-adjusted IR). The adjusted incidence rate determined in this way equates to the delivery-associated differences between the patient profiles.
The confidence intervals of the predicted hospital-specific incidence rates are calculated from the Wald confidence limits using the estimated error of the predictor averaged out for all the cases at one hospital. The individual case-related estimated errors in turn derive via the error propagation law from the estimated error of the linear predictor in the individual case. The widths of the confidence intervals for the risk-adjusted incidence rates are identical to those of the predicted hospital-specific risks, since only the logit model is regarded as containing errors, whereas the patients are counted not as a sample, but as the total population. The multivariate predictor was subjected to comprehensive validation testing. This included the Hosmer-Lemeshow test for goodness of fit, Wald tests of the regression coefficients, and estimating the area under the curve of the receiver operating characteristic (ROC) curve by simple reclassification (C statistic) and bootstrap cross-validation. Validation testing also involved inclusion of cluster effects on a trial basis using correlated intrahospital residuals and selecting and comparing suitable categorizations of the quantitative variables. As in all explanatory statistical regression models of this kind, despite their proven validity, the conclusions that may be drawn are not of a causal nature, but only suitable for prediction and the construction of hypotheses. All analyses were carried out using SAS (http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#titlepage.htm).
The statistical methods are described in detail in previous publications (4, 5).
Results
The results of the statistical analysis presented here relate to infants with severe IVH (Papile grades 3 and 4). The numbers of all documented IVHs are shown in Table 2. The particular risk carried by very low birth weight infants is visible in their relatively high incidence rates of bleeding. In terms of absolute numbers, IVHs in infants closer to maturity are also significant.
Table 2. Incidence of IVH for all neonatal treatment cases and for infants <1500 g or <32 weeks’ gestation categorized according to severity, with associated mean gestational age (full weeks) and birth weight (g).
| Grade of hemorrhage (Papile classification) | Total n = 26 609 | <1500 g or <32 gw n = 1782 |
| n grade 1 | 417 (1.57%) | 112 (6.29%) |
| 34.4 ± 4.1 weeks | 29.1 ± 2.5 weeks | |
| 2314 ± 926 g | 1195 ± 391 g | |
| n grade 2 | 143 (0.54%) | 93 (5.22%) |
| 30.3 ± 5.1 weeks | 27.1 ± 2.4 weeks | |
| 1630 ± 944 g | 1048 ± 390 g | |
| n grades 3 + 4 | 193 (0.73%) | 151 (8.47%) |
| 28.3 ± 4.9 weeks | 26.1 ± 2.3 weeks | |
| 1294 ± 885 g | 886 ± 311 g | |
| n grades 1–4 | 753 (2.83%) | 356 (19.97%) |
| 37.3 ± 3.2 weeks | 29.3 ± 2.7 weeks | |
| 2947 ± 822 g | 1255 ± 392 g |
IVH, intraventricular hemorrhage; GW, gestational week; n, number
No significant change in incidence rates was observed over the period of the years under analysis.
Univariate analysis
Univariate analysis produced significant correlations between a number of risk factors and the occurrence of severe IVH (Table 3 and eTable). In addition to low gestational age and low birth weight, a cervical width on admission of more than 3 cm, intrapartum tocolysis, breech presentation with vaginal delivery, perinatal infection or early sepsis, low Apgar scores, and requirement for resuscitation are factors that increase risk. On the other hand, completed antenatal corticosteroid therapy (to promote fetal lung maturation), a pathological Doppler result (pathological flow pattern in the uterine artery, umbilical artery, or the fetal middle cerebral artery), planned cesarean section, and a prepartum hospital stay of more than 1 day showed a risk-reducing effect. Statistically, male sex was not shown to be a risk factor.
Table 3. Excerpt from the analysis of the univariate relationship between risk variables and grade 3+4 intraventricular hemorrhage (IVH) (a detailed presentation is given in the eTable).
| Variable | n total | n IVH | OR | p | |||
| Birth weight 750–1000 g | 291 (16%) | 49 (17%) | 10.5 | 0.0045 | |||
| Reference: >2000 g | 53 (3%) | 1 (1.9%) | 1 | ||||
| Gestational age 24–25 weeks | 163 (9.1%) | 59 (36%) | 52.8 | <0.0001 | |||
| Reference: >31 weeks | 282 (16%) | 3 (1.1%) | 1 | ||||
| Cervical width ≥ 4cm | 310 (17.4%) | 57 (36%) | 3.26 | <0.0001 | |||
| Reference: <4cm | 1455 (81.6%) | 94 (6.5%) | 1 | ||||
| Pathological Doppler result | 326 (18%) | 12 (3.7%) | 0.36 | 0.0006 | |||
| Reference: no pathological Doppler result | 1456 (82%) | 139 (9.5%) | 1 | ||||
| Breech presentation vaginal | 39 (2.2%) | 13 (33%) | 4.02 | 0.0002 | |||
| C section | 285 (16%) | 14 (4.9%) | 0.41 | 0.0069 | |||
| Reference: vaginal cephalic | 280 (16%) | 31 (11%) | 1 | ||||
| 1 min Apgar: 0.1 | 78 (4.5%) | 20 (26%) | 8.43 | <0.0001 | |||
| 2–5 | 493 (28%) | 83 (17%) | 4.95 | <0.0001 | |||
| Reference: 6–10 | 1171 (67%) | 46 (3.9%) | 1 | ||||
| Infection <3 days | 146 (8.2%) | 38 (26%) | 4.74 | <0.0001 | |||
| Reference: no infection | 1636 (9.2%) | 113 (6.9%) | 1 | ||||
n, number of infants; OR, odds ratio; p, p value chi square tes
eTable. Univariate correlations between risk variables and severe intraventricular hemorrhage (IVH) (grades 3 + 4) in comparison to the corresponding reference category.
| Variable | n total | IVH grades 3 + 4 | |||
| n | OR*1 | p value*2 | |||
| Antepartum tocolysis | No | 824 (46%) | 71 (8.6%) | 1 (Ref.) | |
| Yes | 958 (54% | 80 (8.4%) | 0.97 | 0.8408 | |
| Intrapartum tokolysis | No | 1219 (68%) | 91 (7.5%) | 1 (Ref.) | 0.0245 |
| Yes | 563 (32%) | 60 (11%) | 1.48 | ||
| Antepartum maintenance tocolysis | None | 828 (46%) | 71 (8.6%) | 1 (Ref.) | |
| 1 day | 230 (13%) | 30 (13%) | 1.60 | 0.0413 | |
| 2–4 days | 355 (20%) | 29 (8.2%) | 0.95 | 0.8181 | |
| 5–9 days | 160 (9%) | 12 (7.5%) | 0.86 | 0.6537 | |
| 10 days or more | 209 (12%) | 9 (4.3%) | 0.48 | 0.0388 | |
| Lung maturation therapy | No | 470 (26%) 1312 (74%) | 56 (12%) | 1 (Ref.) | |
| Yes | 95 (7.2%) | 0.58 | 0.0018 | ||
| Cervical width on admission | <4 cm | 1455 (82%) | 94 (6.5%) | 1 (Ref.) | |
| %ge;4 cm | 310 (17%) | 57 (18%) | 3.26 | <0.0001 | |
| Data missing | 17 (1%) | 0 (0%) | |||
| Pathological Doppler result | No | 1456 (82%) | 139 (9.5%) | 1 (Ref.) | |
| Yes | 326 (18%) | 12 (3.7%) | 0.36 | 0.0006 | |
| Presentation | Mode | ||||
| Normal cephalic | Vaginal | 280 (16%) | 31 (11%) | 1 (Ref.) | |
| Breech | Vaginal | 39 (2.2%) | 13 (33%) | 4.02 | 0.0002 |
| No information | Vaginal | 30 (1.7%) | 4 (13%) | 1.24 | 0.7099 |
| Normal cephalic | Planned cesarean | 581 (33%) | 30 (5.2%) | 0.44 | 0.0016 |
| Abnormal cephalic | Planned cesarean | 7 (0.4%) | 0 (0%) | ||
| Breech | Planned cesarean | 285 (16%) | 14 (4.9%) | 0.41 | 0.0069 |
| Other | Planned cesarean | 559 (31%) | 59 (11%) | 0.95 | 0.8195 |
| Data missing | 1 (0.1%) | 0 (0%) | |||
| Base deficit <-12 | No | 1445 (96%) | 115 (8.0%) | 1(Ref) | |
| Yes | 53 (3.5%) | 10 (19%) | 2.69 | 0.0048 | |
| Data missing | 284 (16%) | 26 (9.2%) | 1.17 | 0.5006 | |
| pH <7.1 | No | 1610 (95%) | 123 (7.6%) | 1(Ref) | |
| Yes | 79 (4.7%) | 15 (19%) | 2.8 | 0.0003 | |
| Data missing | 93 (5.2%) | 13 (14%) | 1.96 | 0.0283 | |
| Emergency cesarean section | No | 1644 (92%) 1 | 133 (8.1%) | 1 (Ref.) | |
| Yes | 38 (7.7%) | 18 (13%) | 1.70 | 0.0448 | |
| Predelivery hospital stay | Up to 1 day | 865 (49%) | 91 (11%) | 1 (Ref.) | |
| More than 1 day | 917 (51%) | 60 (6.5%) | 0.60 | 0.0026 | |
| 1-min Apgar | 0, 1 | 78 (4.5%) | 20 (26%) | 8.43 | <0.0001 |
| 2–5 | 493 (28%) | 83 (17%) | 4.95 | <0.0001 | |
| 6–10 | 1171 (67%) | 46 (3.9%) | 1 (Ref.) | ||
| Data missing | 40 (2.2%) | 2 (5.0%) | 1.29 | 0.7326 | |
| 5-min Apgar | 0, 1 | 7 (0.4%) | 2 (29%) | 5.74 | 0.0191 |
| 2–5 | 149 (8.6%) | 42 (28%) | 5.64 | <0.0001 | |
| 6–10 | 1582 (91%) | 103 (6.5%) | 1 (Ref.) | ||
| Data missing | 44 (2.5%) | 4 (9.1%) | 1.44 | 0.4960 | |
| 10-min Apgar | 0, 1 | 4 (0.2%) | 1 (25%) | 3.97 | 0.1984 |
| 2–5 | 47 (2.7%) | 16 (34%) | 6.15 | <0.0001 | |
| 6–10 | 1678 (97%) | 130 (7.7%) | 1 (Ref.) | ||
| Data missing | 53 (3.0%) | 4 (7.5%) | 0.97 | 0.9572 | |
| Weight, g | <750 | 215 (12%) | 59 (27% | 19.67 | <0.0001 |
| 750–<1000 | 291 (16%) | 49 (17%) | 10.53 | 0.0045 | |
| 1000–<1500 | 852 (48%) | 36 (4.2%) | 2.29 | 0.4042 | |
| 1500–<2000 | 371 (21%) | 6 (1.6%) | 0.85 | 0.8855 | |
| ≥2000 | 53 (3.0%) | 1 (1.9%) | 1 (Ref.) | ||
| Gestational age, weeks | <24 | 25 (1.4%) | 11 (44%) | 73.07 | <0.0001 |
| 24–25 | 163 (9.1%) | 59 (36%) | 52.76 | <0.0001 | |
| 26–27 | 252 (14%) | 36 (14%) | 15.50 | <0.0001 | |
| 28–29 | 382 (21%) | 34 (8.9%) | 9.09 | <0.0001 | |
| 30–31 | 678 (38%) | 8 (1.2%) | 1.11 | 0.8776 | |
| ≥32 | 282 (16%) | 3 (1.1%) | 1 (Ref.) | ||
| Pediatrician present before delivery | No | 79 (4.4%) | 8 (10%) | 1.23 | 0.5894 |
| Yes | 1703 (96%) | 143 (8.4%) 1 (Ref.) | |||
| Sex | Male | 917 (51%) | 87 (9.5%) | 1 (Ref.) | |
| Female | 865 (49%) | 64 (7.4%) | 0.76 | 0.1136 | |
| Periventricular leukomalacia | No | 1740 (98%) | 135 (7.8%) | 1 (Ref.) | |
| Yes | 42 (2.4%) | 16 (38%) | 7.32 | <0.0001 | |
| Intubation | No | 1011 (57%) | 25 (2.5%) | 1 (Ref.) | |
| Yes | 771 (43%) | 126 (16%) | 7.70 | <0.0001 | |
| Volume replacement | No | 1469 (82%) | 97 (6.6%) | 1 (Ref.) | |
| Yes | 313 (18%) | 54 (17%) | 2.95 | <0.0001 | |
| Mask ventilation | No | 1048 (59%) | 71 (6.8%) | 1 (Ref.) | |
| Yes | 734 (41%) | 80 (11%) | 1.68 | 0.0021 | |
| Delivery before hospital admission | No | 1774(100%) | 151 (8.5%) | 1 (Ref.) | |
| Yes | 8(0.4%) | 0 (0%) | |||
| Multiple births | None | 1315 (74%) | 120 (9.1%) | 1 (Ref.) | |
| Twin | 403 (23%) | 28 (6.9%) | 0.74 | 0.1729 | |
| Triplet | 64 (3.6%) | 3 (4.7%) | 0.49 | 0.2238 | |
| Infection <3 days | No | 1636 (92%) | 113 (6.9%) | 1 (Ref.) | |
| Yes | 146 (8.2%) | 38 (26%) | 4.74 | <0.0001 | |
| Asphyxia | No | 1540 (86%) | 96 (6.2%) | 1 (Ref.) | |
| Yes Without HIE*4 | 222 (12%) | 45 (20%) | 3.82 | <0.0001 | |
| Yes With HIE | 20 (1.1%) | 10 (50%) | 15.04 | <0.0001 | |
*1OR, odds ratio of the category in question in contrast to the reference category;
*2 raw p values of the local chi square test of the category in question in contrast to the reference category, without statistical adjustment for multiple tests;
*3hypoxic-ischemic encephalopathy; Ref., reference
Of the risk factors on the infant’s side associated with IVH rates, only the 1-minute Apgar score, which could not be influenced by the pediatrician, was included in the multivariate analysis.
Multivariate analysis
Because some data were missing, the data for the multivariate analysis were reduced from 1782 to 1742 data pairs with 149 severe IVHs. Of the many relevant univariate correlations between risk variables and the occurrence of IVH, most were shown to be superfluous by multivariate logistic regression with calculation of a predictor for the occurrence of IVH. Severe IVH can be predicted with an accuracy of 87.7% on the basis of five variables (Table 4).
Table 4. Multivariate predictor for grade 3–4 intraventricular hemorrhage (IVH) on the basis of n = 149 IVH cases among 1742 datasets.
| Variable | Regression coefficient | OR | 95% CI | p value | |
| Intercept | –5.8446 | ||||
| Gestational age <26 weeks | 3.5218 | 33.844 | 16.735 – 68.443 | <0.0001 | |
| 26–27 | 2.3550 | 10.538 | 5.154 – 21.545 | <0.0001 | |
| 28–29 | 2.0245 | 7.572 | 3.746 – 15.304 | <0.0001 | |
| 30–32 (reference) | |||||
| P63 1-min Apgar <6 | 0.9572 | 2.604 | 1.737 – 3.904 | <0.0001 | |
| N16 Early sepsis <3 days | 0.9347 | 2.546 | 1.581 – 4.101 | 0.0001 | |
| P35.3 No pathological Doppler result | 0.7966 | 2.218 | 1.142 – 4.309 | 0.0187 | |
| P44.2 Intrapartum tocolysis | 0.6496 | 1.915 | 1.285 – 2.853 | 0.0014 | |
Validation of this logistic regression model: p = 0.59 in Hosmer-Lemeshow test; area under the ROC: AUC = 0.877 (simple reclassification) or AUC = 0.861 (bootstrap cross-validation); Nagelkerke’s R2 = 0.44.
*1 P(perinatal) and N (neonatal), data from the perinatal/neonatal data collection form; ROC, receiver operating characteristic; AUC, area under the curve; OR, odds ratio
The predictor for severe IVH is based on all 1742 incident and nonincident cases recruited from 9 hospitals where severe IVH occurred and 14 hospitals where it did not occur. The predictor includes the following variables: gestational age, pathological Doppler result, low 1-minute Apgar score (with a risk-increasing cutoff value <6), perinatal infection, and a delivery requiring the use of tocolysis as relevant independent risk factors. Presentation and mode of delivery are not taken into account in predicting IVH.
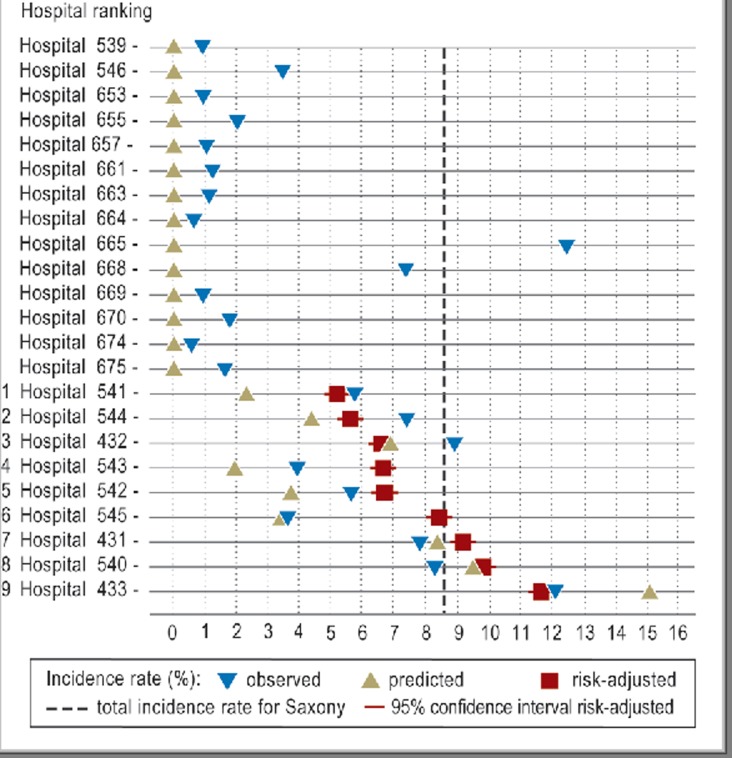
Grade 3 or 4 IVHs occurred in 9 of the 23 hospitals with infants with birth weight below 1500 g or less than 32 weeks’ gestational age. Their incidence rate for severe IVH form the basis for a hospital rank order that is markedly changed by risk adjustment (Table 5, Figure). Shifts by up to four places occur (average: 2.33 places).
Table 5. Incidence rates of grade 3 + 4 intraventricular hemorrhage (IVH) in the basic data for Saxony (total = 149/1742 = 8.55%) and ranking of the neonatal units/care level 1–3 before and after adjustment (rank categories take into account the statistical uncertainty in the adjustment).
| Hospital/level | Observed incidence rate | Predicted incidence rate | Predicted deviation from Saxony | Adjusted incidence rate | 95% CI | Raw ranking | Adjusted rank category |
| 14 Hospitals/3 | 0/102 | 2.46 | – 6.09 | ||||
| 541/2 | 2/87 = 2.30 % | 5.71 | –2.84 | 5.14 | 4.86–5.43 | 2 | 1 |
| 544/2 | 1/23 = 4.35 % | 7.34 | –1.21 | 5.56 | 5.28–5.85 | 5 | 1 |
| 432/1 | 32/469 = 6.82 % | 8.85 | –0.30 | 6.52 | 6.24–6.81 | 6 | 2 |
| 543/2 | 1/52 = 1.92 % | 3.89 | – 4.67 | 6.59 | 6.31–6.88 | 1 | 2 |
| 542/2 | 2/54 = 3.70 % | 5.61 | – 2.94 | 6.65 | 6.36–6.93 | 4 | 2 |
| 545/2 | 2/59 = 3.39 % | 3.59 | – 4.97 | 8.36 | 8.07–8.64 | 3 | 3 |
| 431/1 | 28/337 = 8.31 % | 7.75 | – 0.80 | 9.11 | 8.82–9.40 | 7 | 4 |
| 540/2 | 5/53 = 9.43 % | 8.23 | – 0.32 | 9.76 | 9.47–10.04 | 8 | 5 |
| 433/1 | 76/506 = 15.02 % | 11.99 | 3.44 | 11.58 | 11.30–11.87 | 9 | 6 |
| SD = 7.38 | Mean number of places moved = 2.33 | ||||||
SD, standard deviation; CI, confidence interval
Figure.
Incidence rates of severe intraventricular hemorrhage (IVH) (grade 3–4) before (▼) and after (■) risk adjustment together with predicted values (▲) based on the multivariate predictor, calculated according to the obstetric risk profile
The predicted incidence rate is related to the risk factor burden of the patient population at the time when the neonatologist takes over care, but must not be regarded as a criterion of obstetric quality. That requires a separate analysis.
Discussion
Univariate analysis
The univariate analysis gave results that largely agree with the existing literature (1, 8– 19). This is the case for the correlations of delivery mode, infection, Doppler results, neonatal adaptation, and postnatal treatment measures related to these. In addition to gestational age, the variables most closely related to IVH risk were a low 1-minute Apgar score (<6), followed by early infection. This Apgar score is the one that most reliably reflects the acute effects of birth on brain function and is regarded as an important risk indicator for IVH (17, 20). Looking at the relationship between mode of delivery and IVH, the present analysis shows an advantage for infants born by planned cesarean section compared to those born vaginally or after unplanned/emergency section. A lower risk of IVH in infants born after elective cesarean section is also reported in the literature (9, 15). As to the outcome, however, the delivery-related conditions accompanying or giving rise to the mode of delivery are more significant (16).
Some studies refer to the significance of cervical width at the time of delivery and of contraction activity for the risk of IVH (9, 16, 20). Unfortunately, neither of these risk factors is depicted in the perinatal data collection. Cervical width on admission to hospital was recorded, but not cervical width at the time when delivery was ended by cesarean section. Nevertheless, the univariate analysis by the present authors shows that a cervical width ≥4 cm correlates closely with IVH. This supports the assumption that this finding at the time when abdominal delivery is ended has significance for the prognosis of the infant.
In contrast to other reports (21), we did not find a higher risk for male sex. The larger number of male infants compared to female infants with IVH corresponded to the higher proportion of males among preterm infants.
Multivariate analysis
Multivariate logistic regression considerably reduced the number of independent predictor variables relevant to the calculation of a predictor for IVH. The reason for this is that a risk factor that statistically accompanies another factor, because the former correlates with the latter, is superfluous for statistical prediction; it makes no additional contribution toward explaining the dependent variables (5, 22).
The result of the multivariate logistic regression shows that the main predictor of the risk of IVH, as is known, is gestational age, but that other factors that describe the obstetric situation and the infant’s adaptation in a complex and indirect way also have predictive value. Although the mode of delivery is not contained as a predictive factor in the risk predictor, this does not mean that it is etiologically insignificant.
The predictor variables for high-grade IVH include low 1-minute Apgar score, perinatal infection, and early sepsis. The multivariate predictor includes in addition the absence of pathological Doppler findings during pregnancy and use of tocolysis during labor as effective risk factors, but these are not necessarily to be regarded as causative factors.
Low Apgar scores and infections are already known as risk factors and are plausible as such (12, 17, 20), because it is possible to assign a causative role to them in the pathogenetic pathway. The same is not true of other predictor variables, which are to be seen as surrogates for other factors that do have a causative effect. For example, pathological Doppler results during pregnancy prompt specific preventative steps in medical care and have significance for management of the birth. This underlines the importance of targeted surveillance and check-ups during pregnancy and birth and, of course, afterwards as well (23).
The significance of tocolysis as an independent predictor variable might be explained by the fact that this variable captures cases with advanced obstetric findings in mothers admitted as inpatients or before surgical interruption of labor (e.g., where the cervix is widely dilated and contractions are effective with hypoxic fetal heart rate features). These factors are under discussion in the literature as important risk variables associated with the brain lesion (9, 15, 19), and were also shown by our univariate analysis to be relevant risk variables. This is a good example of a possible explanation of variables that are proved statistically to be predictive, but cannot be directly causally associated with the target event.
Risk adjustment and hospital ranking
The statistically calculated predictor of the individual and, following on from that, the specific hospital risk for the occurrence of severe IVH may be regarded as valid and accurate. This is shown by its high accuracy rate, measured as the area under the curve (AUC) of the receiver operating characteristic (ROC). Simply put, this describes the percentage of true-positive and true-negative predictions, which is 87.8%. This now puts us in a position to compare the outcome quality of neonatal units in terms of the important quality indicator IVH in a risk-adjusted manner—thus enabling a fair comparison between hospitals and with the national average. One particular advantage of logistic regression as a risk adjustment technique is the possibility of detailed intrahospital analysis of conditions of care and to draw conclusions from this analysis. Hospitals whose risk-adjusted incidence rate is markedly higher than the national average cannot explain this away in their interpretation of results by referring to peculiarities in their patient population, because with this technique those peculiarities are already compensated for. The gap between risk-adjusted incidence rates and the national average may therefore be taken as a reliable measure of differences in the quality of neonatal care.
The predictor contains variables that characterize the condition of the infant at the point at which it comes under the care of the neonatologist. Clearly, gestational age has the highest predictive power as measured by the odds ratio. However, compared with the odds ratio in univariate analysis, as a multivariate confounder-adjusted predictor gestational age loses some of that power. From this it is clear that the conclusions to be drawn from a quality comparison based solely on outcome standardization according to gestational age or birth weight are limited. Added to that is the reduced reliability of conclusions where patient numbers and event incidences are low; this matters less with a multivariate predictor.
From 2001 to 2005 the incidence rates of severe IVH in hospitals in Saxony ranged between 1.92% and 15.02%. This variance is explained not just by differences in the quality of obstetric and neonatal care, but also by differences in hospitals’ patient populations in terms of underlying diseases, number of weeks of pregnancy, social and age profile, quality of antenatal care—that is, in terms of factors that influence prematurity. The model presented here for risk-adjusted quality assessment of neonatal care takes account of risk characteristics associated with delivery with the aim of better reflecting the performance of neonatal care units, and in a way that allows comparisons. The remaining variance of incidence rates after risk adjustment, which is from 5.14% to 11.58%, is considerably lower, and in view of the predictor’s 87.7% accuracy may be regarded as a good approximation of differences in quality. This corrected variance may be taken as a representative measure of differences in neonatal care. The risk adjustment results in a new hospital ranking with hospitals shifting by an average of 2.33 places (1 to 4 places for 9 hospitals).
The risk adjustment also makes it possible, when appropriate, to calculate the existing potential for avoiding undesired events and working toward change in a targeted way. Approaches to this would include, on the obstetric side, optimizing the baseline situation of the infant, and, on the neonatal side, further improving care.
That there is scope for improvement is shown by results from other federal states in Germany, such as Baden-Württemberg and Berlin, which achieved rates of severe IVH of 6.6% and 4.4% respectively during the same period (24)—although this comparison is only conditionally valid because the results are not risk adjusted.
A Germany-wide risk adjustment for IVH rates as a quality indicator of neonatal care is not possible using the neonatal data collection form alone, without merging data from the perinatal form. Because of its design and the level of detail it is intended to collect, the neonatal data collection form contains no relevant information associated with the delivery that would assist in this task. With the introduction of data merging at a national level in 2011, we are now in a situation that allows risk-adjusted quality assessment within the frame of hospital external quality assurance.
The model presented here has been tested and shown to be robust, and is suitable for use throughout Germany for a variety of purposes.
Key Messages.
The incidence rates of intraventricular hemorrhage (IVH) of all grades of severity in very premature infants (birth weight <1500 g or birth before 32 weeks’ gestational age) can be as high as 40%, depending on the profile of the patient population and the quality of care. Incidence rates of severe (grade 3–4) IVH range up to more than 10%.
A valid interhospital comparison of neonatal outcome quality requires that account should be taken of the varying risk profiles of the patients at the point where they pass into the care of the neonatologist.
A risk-adjusted rate of incidence for IVH can be estimated for each hospital on the basis of a multiple logistic regression analysis of obstetric/perinatal risk factors.
Hospital rankings for the incidence of severe IVH change considerably as a result of risk adjustment.
Risk-adjusted presentation of outcomes is an important step toward robust interhospital quality comparison and structured dialogue.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Dipl.-Math. Friedrich and Dipl.-med. Kaiser have received honoraria from the Saxony State Medical Association. Prof. Dr. rer. nat. Koch has received honoraria from the Saxony State Medical Association and the TU Dresden Medical School.
References
- 1.Jensen A, Klingmüller V, Künzel W, Sefkow S. Das Hirnblutungsrisiko bei Früh- und Reifgeborenen. Geburtsh Frauenheilk. 1992;52:6–20. doi: 10.1055/s-2007-1022943. [DOI] [PubMed] [Google Scholar]
- 2.Paneth N, Pinto-Martin J, Gardiner J, et al. Incidence and timing of germinal matrix/intraventricular hemorrhage in low birth weight infants. Am J Epidemiol. 1993;137:1167–1176. doi: 10.1093/oxfordjournals.aje.a116619. [DOI] [PubMed] [Google Scholar]
- 3.Synnes AR, Macnab YC, Qiu Z, et al. Neonatal intensive care unit characteristics affect incidence of severe intraventricular hemorrhage. Med Care. 2006;44:754–759. doi: 10.1097/01.mlr.0000218780.16064.df. [DOI] [PubMed] [Google Scholar]
- 4.Gmyrek D, Koch R, Vogtmann Ch, Kaiser A, Friedrich A. Risikoadjustierte Qualitätsbeurteilung am Beispiel der neonatalen Spätinfektion. Z Evid Fortbild Qual Gesundh Wesen. 2011;105:124–132. doi: 10.1016/j.zefq.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Koch R, Gmyrek D, Vogtmann Ch. Risikoadjustierte Qualitätsbeurteilung in Perinatalzentren ausgehend von der Perinatal- und Neonatalerhebung in Sachsen. Z Geburtshilfe Neonatol. 2005;209:210–218. doi: 10.1055/s-2005-916244. [DOI] [PubMed] [Google Scholar]
- 6.Simpson JM, Evans N, Gibbered RW, Heuchan AM, Henderson-Smart DJ. Australian and New Zealand Neonatal Network, School of Public Health analysing differences in clinical outcomes between hospitals. Qual Saf Health Care. 2003;12:257–262. doi: 10.1136/qhc.12.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 8.Heuchan AM, Evans N, Henderson-Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995-97. Arch Dis Child Fetal Neonatal Ed. 2002;86:F86–F90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoegberg U, Holmgren PA. Infant mortality of very preterm infants by mode of delivery, institutional policies and maternal diagnosis. Acta Obstet Gynecol Scand. 2007;86:693–700. doi: 10.1080/00016340701371306. [DOI] [PubMed] [Google Scholar]
- 10.How H, Houry J, Sibai BM. Cervical dilatation on presentation for preterm labor and subsequent preterm birth. Am J Perinatol. 2009;26:1–6. doi: 10.1055/s-0028-1090586. [DOI] [PubMed] [Google Scholar]
- 11.Ment L, Oh W, Philip A, et al. Risk factors for early intraventricular hemorrhage in low birth weight infants. J Pediatr. 1992;121:776–783. doi: 10.1016/s0022-3476(05)81915-8. [DOI] [PubMed] [Google Scholar]
- 12.O´Shea M, Savitz DA, Hage ML, Feinstein KA. Prenatal events and the risk of subependymal/intraventricular hemorrhage in very low birth weight neonates. Paediatr Perinat Epidemiol. 1992;6:352–362. doi: 10.1111/j.1365-3016.1992.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 13.Osborn D, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Murphy D, Sellers S, Mackenzie I, Yudkin P, Johson A. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet. 1995;346:1449–1454. doi: 10.1016/s0140-6736(95)92471-x. [DOI] [PubMed] [Google Scholar]
- 15.Qiu H, Paneth N, Lorenz JM, Collins M. Labor and delivery factors in brain damage, disabling cerebral palsy and neonatal death in low-birth-weight infants. Am J Obstet Gynecol. 2003;189:1143–1149. doi: 10.1067/s0002-9378(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 16.Riskin A, Riskin-Mashiah S, Bader D, et al. Delivery mode and severe intraventricular hemorrhage in single, very low birth weight, vertex infants. Obstet Gynecol. 2008;112:21–28. doi: 10.1097/AOG.0b013e31817cfdf1. [DOI] [PubMed] [Google Scholar]
- 17.Salafia C, Minor V, Rosenkrantz T, et al. Maternal, placental, and neonatal association with early germinal matrix/intraventricular hemorrhage in infants born before 32 weeks gestation. Am J Perinatol. 1995;12:429–436. doi: 10.1055/s-2007-994514. [DOI] [PubMed] [Google Scholar]
- 18.Shaver D, Bada H, Korones SH, et al. Early and late intraventricular hemorrhage: the role of obstetric factors. Obstet Gynecol. 1992;80:831–837. [PubMed] [Google Scholar]
- 19.Vela-Huerta MM, Amador-Licona M, Medina-Ovando N, Aldana-Valenzuela C. Factors associated with early severe intraventricular hemorrhage in very low birth weight infants. Neuropediatrics. 2009;40:224–227. doi: 10.1055/s-0030-1248249. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Dorn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26:419–424. doi: 10.1055/s-0029-1214237. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed MA, Aly H. Male gender is associated with intraventricular hemorrhage. J Pediatrics. 2010;125:e333–e339. doi: 10.1542/peds.2008-3369. [DOI] [PubMed] [Google Scholar]
- 22.Schneider A, Hommel G, Blettner M. Linear regression analysis—part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107(44):776–782. doi: 10.3238/arztebl.2010.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obladen M, Metzte B, Henrich W, Aktas A, Chernik C, Schulz-Baldes A. Interdisciplinary surveillance of intraventricular hemorrhage associated conditions in infants <1000g. Acta Paediatrica. 2008;997:731–737. doi: 10.1111/j.1651-2227.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 24.Schulz-Baldes A, Misselwitz B, Metze B, Obladen M Ärztekammer Berlin. Projekte 2005 Qualitätssicherung und Qualitätsmanagement. Berlin: 2006. 6 Jahre Berliner Neonatalerhebung: Entwicklungen in Gesamtberlin seit 1998; pp. 17–40. [Google Scholar]