Abstract
SHORT-ROOT (SHR) is a key regulator of radial patterning and stem-cell renewal in the Arabidopsis root. Although SHR is expressed in the stele, its function in the vascular tissue was not recognized until recently. In shr, the protoxylem is missing due to the loss of expression of microRNA165A (miR165A) and microRNA166B (miR165B). shr is also defective in lateral root formation, but the mechanism remains unclear. To dissect the SHR developmental pathway, we recently have identified its direct targets at the genome scale by chromatin immunoprecipitation followed by microarray analysis (ChIP-chip). In further studies, we have shown that SHR regulates cytokinin homeostasis through cytokinin oxidase 3 and that this role of SHR is critical to vascular patterning in the root. In this communication we report that SHR also regulates miR165A and miR166B indirectly through its effect on cytokinin homeostasis. Although cytokinin is inhibitory to root growth, the root-apical-meristem defect in shr was not alleviated by reduction of endogenous cytokinin. These results together suggest that SHR regulates vascular patterning, but not root apical meristematic activity, through cytokinin homeostasis.
Keywords: SHORT-ROOT, arabidopsis, cytokinin, lateral root, meristem, miR165A, miR166B, pericycle, vascular patterning
As the major organ for water and inorganic-nutrient uptake, root is critically important for land-plant growth and development. The root increases its surface area by growing lengthwise through the mitotic activity of the root apical meristem and by branching through lateral or adventitious root formation. Because different cell types in the root have different functions, their cell-fate specification must be precisely regulated and proper ratios among them must be maintained.
A typical dicot primary root has more than seven cell types, which are arranged in two distinct patterns (Fig. 1A). The outer cell types, such as the epidermis, cortex, endodermis, and pericycle, form concentric rings. In the center of the root is the vascular tissue, which is composed of several cell types that are organized into separate domains of phloem and xylem. The pericycle surrounds the vascular tissue and, in most plant species, is the site of lateral root formation. The pericycle has long been regarded as a single cell type, but recent studies showed that it is composed of two populations of cells that differ both anatomically and functionally—one (XP) is associated with the xylem and the other (PP) adjoins the phloem.1 The XP cells have dense cytoplasm and can produce lateral roots.1 Cell division in the XP is induced by auxin but repressed by cytokinin.2 In contrast, the PP cells have large vacuoles, indicating a greater degree of differentiation, do not divide in the presence of auxin and are not involved in lateral root formation.1
Figure 1.
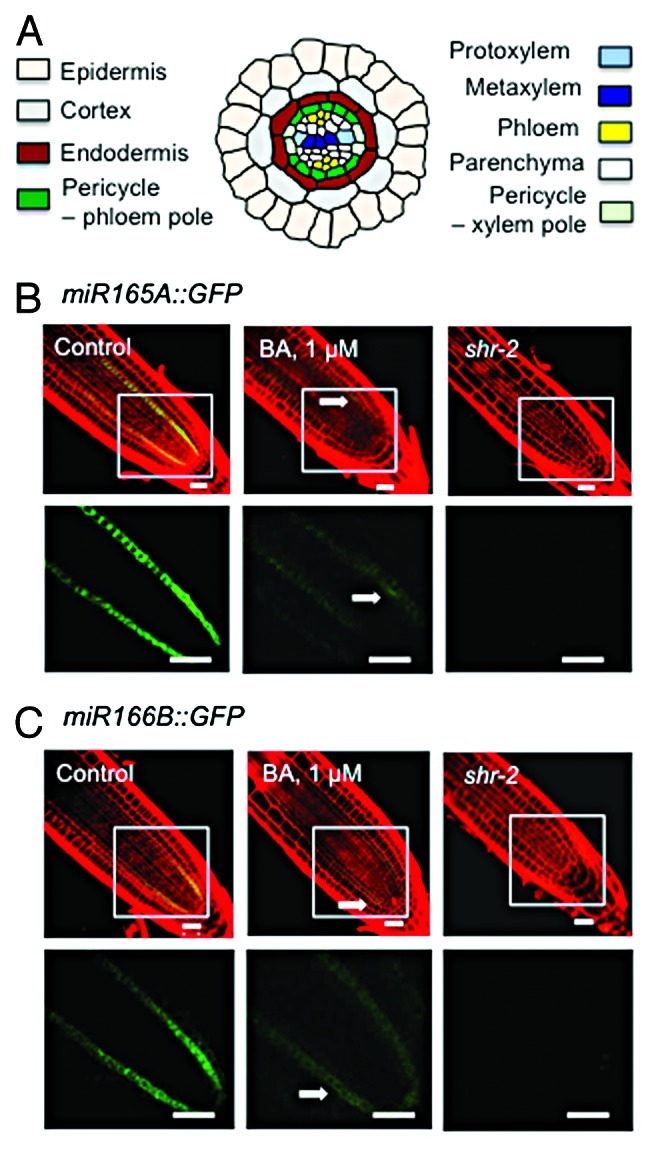
Cytokinin represses miR165A and miR166B expression. (A) Diagram of the cell pattern in the primary root of Arabidopsis thaliana. (B–C) Confocal microscopy image of miR165A::GFP and miR166B::GFP expression in wild-type root grown in normal medium or medium containing 1 μM 6-benzylaminopurine (a synthetic cytokinin) and in shr-2 mutant. The GFP signal in the framed area is shown at a higher magnification in the lower panel. Arrow marks residual expression. One-week-seedling roots were stained for 1 min with propidium iodide (10 μg/mL dissolved in water), and images were taken with a Zeiss LSM510 confocal microscope. Bars = 20 μm.
Auxin and cytokinin regulate not only lateral root formation but also root growth—auxin promotes, but cytokinin inhibits, root apical meristematic activity, thereby increasing and reducing the meristem size, respectively.3 Auxin and cytokinin also have opposite roles in vascular differentiation and patterning. On one hand, auxin induces xylem differentiation, whereas cytokinin promotes phloem specification. The ratio between their concentrations also determines the relative abundance of xylem and phloem.4,5 On the other hand, the high levels of auxin and cytokinin in the xylem and phloem respectively in turn instruct procambium cells to differentiate according to their position, thereby maintaining the continuity of the vascular tissue.4,5
Although the antagonistic roles of auxin and cytokinin in vascular tissue differentiation and patterning are well documented, only recently has light been shed on the molecular basis of this regulation. Bishopp et al.6 showed that cytokinin induces the expression of PIN-FORMED7 (PIN7) in procambium cells that are connected with phloem. PIN7 is an auxin transporter, and by exporting auxin to neighboring cells, it generates a region with a relatively high level of cytokinin (the PIN7 domain), which would assume phloem identity. In cells outside of the PIN7 domain, where the auxin level is relatively high, ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6) is induced. Because AHP6 is a pseudophosphotransfer protein that blocks cytokinin signal transduction, PIN7 is not expressed in the APH6 domain, which will maintain its relative high level of auxin and eventually differentiate into protoxylem. Although this model offers a molecular basis for the maintenance of vascular pattern, however, it does not explain how the vascular pattern is established de novo; nor does it account for the inhibitory effect of cytokinin on lateral root formation and xylem specification, because, according to this model, the AHP6 domain and the protoxylem would be maintained regardless of the level of cytokinin. Our recent study showing that SHR controls vascular patterning by regulating cytokinin homeostasis provides a critical piece to this puzzle.7
SHR was initially identified as a regulator of stem-cell renewal and ground-tissue patterning in the Arabidopsis root.8 It was subsequently found also to play a role in vascular tissue patterning and lateral root formation.9-11 Although much has been learned about the mechanisms by which SHR regulates ground tissue patterning,8,9,12-14 how SHR controls other aspects of root development is unclear. To dissect the mechanisms by which SHR controls root morphogenesis, we have determined the genomewide location of its direct targets by ChIP-chip.7 Through clustering analysis, we found that a large fraction of SHR targets are preferentially expressed in the pericycle and xylem. Using cell-type-specific GFP marker lines, we demonstrated that, in shr, the phloem and PP domains are expanded, whereas the xylem and XP domains are reduced in size. Despite the observation that auxin-signaling factors are among the list of SHR direct targets and a recent report that shr has an elevated level of auxin, the vascular patterning defect in shr is reproduced by cytokinin but not by auxin. The vascular patterning defect in shr is alleviated by overexpression of CKX1, a cytokinin-degradation enzyme.15 We found that, consistent with this result, cytokinin content is elevated in shr and SHR directly regulates CKX3. Remarkably, according to the RootMap,16 CKX3 is preferentially expressed in the protoxylem. These results suggest that, by activating CKX3, SHR produces a zone with a low level of cytokinin, therefore promoting xylem and XP specification. In shr or when exogenous cytokinin is present, this cytokinin minimum is disrupted, and the resulting high cytokinin would repress protoxylem and XP cell fate, consequently causing a defect in lateral-root formation. Because SHR is expressed from the globular stage of embryogenesis, SHR probably plays a key role in the initial set-up of cytokinin minimum and eventually the phloem and xylem domains.
Two microRNA genes, miR165A and miR166B, were recently shown to play a pivotal role in protoxylem and pericycle specification.12,17 It was also shown that SHR controls vascular patterning by direct activation of these genes in the endodermis, which moves into the stele to promote protoxylem differentiation.12 Because cytokinin level is elevated in shr and exogenous cytokinin causes a shr-like vascular patterning phenotype in wild-type root, we hypothesized that SHR also regulates miR165A and miR166B indirectly through cytokinin. To investigate this possibility, we examined roots that express the GFP reporter gene under the promoters of miR165A and miR166B, in the absence or presence of the synthetic cytokinin 6-benzylaminopurine (BA). As shown in Figure 1, in medium containing 1 μM BA, GFP fluorescence from the miR165A::GFP and miR166B::GFP reporter constructs was barely detectable, similar to that observed in shr roots. These results support the notion that SHR regulates miR165A and miR166B through its effect on cytokinin homeostasis.
SHR is essential not only for radial patterning but also for the maintenance of the root apical meristem. In shr, the quiescent center (QC) cells are exhausted early during postembryonic development and, as a consequence, the apical meristem is depleted and root growth ceases. Because cytokinin is inhibitory to root meristematic activity, the root growth defect in shr could be due to its high level of cytokinin. We first tested this hypothesis by overexpressing CKX1, a cytokinin oxidase, under the CaMV 35S promoter in shr. CKX1 was used in this study, because the 35S::CKX1 transgene has been shown to be able to reduce cytokinin content effectively in wildtype root.15 Although the vascular patterning phenotype in shr is rescued by the 35S::CKX1 transgene, as reported previously,7 root growth was not improved (Fig. 2A). We also tested the effect of pCRE1::CKX2, which expresses the CKX2 gene specifically in the stele, but again the root growth defect in shr was not rescued (not shown). Because root growth is determined by both mitotic activity in the apical meristem and longitudinal cell extension in the elongation zone, in order to assess the effect of cytokinin reduction on root meristematic activity in shr, we next compared the size of the root apical meristem in shr and shr overexpressing CKX1. As shown in Figure 2B, these plants had no difference in the size of their apical meristem, as indicated by the similar number of cortex cells along the longitudinal axis of the apical meristem (p = 0.82, t-test, n = 14). Although these results do not exclude a role for cytokinin in root growth in shr, they strongly suggest that other factors acting downstream of SHR play a major role in regulating root meristematic activity.
Figure 2.
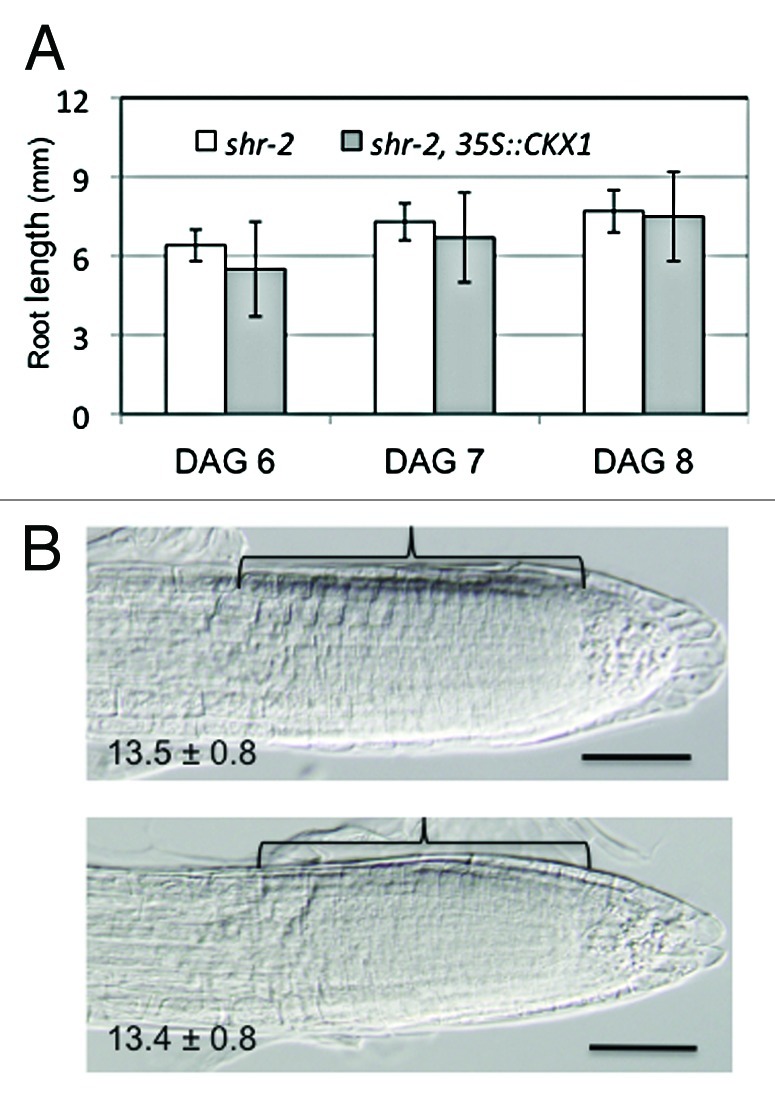
The root meristematic defect in shr is not rescued by reduction of cytokinin. (A) Root length of shr-2 and shr-2 expressing cytokinin oxidase 1 (35S::CKX1) at different time after seed germination. The error bars are standard deviations from measurements of 20 roots. p = 0.06, 0.2 and 0.8 respectively (t-test). DAG, days after germination. (B) Representative microscopic images of shr-2 (upper) and shr-2 35S::CKX1 (lower) roots, 7 d after germination. The value at the lower left corner is the number of ground tissue cells along the longitudinal axis of the meristem (bracket) and the standard deviations. p = 0.8 (t-test, n = 14). Bars = 50 μm.
On the basis of all these observations, we suggest that SHR regulates vascular patterning, but not apical meristematic activity, in the Arabidopsis root through cytokinin homeostasis.
Acknowledgments
This work was supported by a set-up fund from Florida State University to H.C. The authors thank A.B. Thistle (Florida State University) for careful editing of this manuscript and Ji-Young Lee (Boyce Thompson Institute) for the miR165A::GFP and miR166B::GFP lines.
Glossary
Abbreviations:
- BA
6-benzylaminopurine
- miR
microRNA
- XP
xylem-associated pericycle
- PP
phloem-associated pericycle
- ChIP
chromatin immunoprecipitation
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19118
References
- 1.Parizot B, Laplaze L, Ricaud L, Boucheron-Dubuisson E, Bayle V, Bonke M, et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2008;146:140–8. doi: 10.1104/pp.107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Mo X, Shou H, Wu P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol. 2006;47:1112–23. doi: 10.1093/pcp/pcj082. [DOI] [PubMed] [Google Scholar]
- 3.Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol. 2007;17:678–82. doi: 10.1016/j.cub.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Mähönen AP, Bishopp A, Higuchi M, Nieminen KM, Kinoshita K, Törmäkangas K, et al. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311:94–8. doi: 10.1126/science.1118875. [DOI] [PubMed] [Google Scholar]
- 5.Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 2000;14:2938–43. doi: 10.1101/gad.189200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishopp A, Help H, El-Showk S, Weijers D, Scheres B, Friml J, et al. A mutually inhibitory interaction between auxin and cytokinin specifies vascular pattern in roots. Curr Biol. 2011;21:917–26. doi: 10.1016/j.cub.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Cui H, Hao Y, Kovtun M, Stolc V, Deng XW, Sakakibara H, et al. Genome-wide direct target analysis reveals a role for SHORT-ROOT in root vascular patterning through cytokinin homeostasis. Plant Physiol. 2011;157:1221–31. doi: 10.1104/pp.111.183178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–67. doi: 10.1016/S0092-8674(00)80865-X. [DOI] [PubMed] [Google Scholar]
- 9.Levesque MP, Vernoux T, Busch W, Cui H, Wang JY, Blilou I, et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLoS Biol. 2006;4:e143. doi: 10.1371/journal.pbio.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner J, Donner TJ, Scarpella E. Simultaneous activation of SHR and ATHB8 expression defines switch to preprocambial cell state in Arabidopsis leaf development. Dev Dyn. 2011;240:261–70. doi: 10.1002/dvdy.22516. [DOI] [PubMed] [Google Scholar]
- 11.Yu NI, Lee SA, Lee MH, Heo JO, Chang KS, Lim J. Characterization of SHORT-ROOT function in the Arabidopsis root vascular system. Mol Cells. 2010;30:113–9. doi: 10.1007/s10059-010-0095-y. [DOI] [PubMed] [Google Scholar]
- 12.Carlsbecker A, Lee J-Y, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, et al. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature. 2010;465:316–21. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–5. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 14.Sozzani R, Cui H, Moreno-Risueno MA, Busch W, Van Norman JM, Vernoux T, et al. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature. 2010;466:128–32. doi: 10.1038/nature09143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–50. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–6. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 17.Miyashima S, Koi S, Hashimoto T, Nakajima K. Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development. 2011;138:2303–13. doi: 10.1242/dev.060491. [DOI] [PubMed] [Google Scholar]


