Abstract
Large-scale protein-protein interaction studies recently demonstrated that the Arabidopsis TPL/TPR family of transcriptional co-repressors is involved in a broad range of developmental processes. TPL/TPRs predominantly interact with transcription factors that contain repression domain (RD) sequences. Interestingly, RDs reported in the literature are quite diverse in sequence, yet TPL/TPRs interact with proteins containing all of the known motifs. These data lead us to conclude that the TPL/TPRs act as general repressors of gene transcription in plants. To investigate this further, we examined interactions between TPL/TPR proteins encoded by the moss Physcomitrella patens genome and components of the auxin signaling pathway. As in Arabidopsis, moss TPL proteins interact with AUX/IAA and ARF proteins, suggesting that they act in both forms of ARF-mediated transcriptional repression. These data suggest that the involvement of TPL in auxin signaling has been conserved across evolution, since mosses and angiosperms diverged approximately 450 million years ago.
Keywords: Physcomitrella patens, TOPLESS, arabidopsis, co-repression, protein-protein interaction
Transcriptional co-repression is emerging as an important mechanism by which genes can be regulated. A major class of transcriptional co-repressors are the Gro/Tup1 proteins, which are conserved in eukaryotes.1 In plants, two families of Gro/Tup1 co-repressors have been identified: LEUNIG/LEUNIG_HOMOLOG (LUG/LUH) and the TOPLESS/TOPLESS-RELATED (TPL/TPR) groups.1 LUG, and its counterparts from other plant species, plays roles in vegetative and floral organ development, cell proliferation, meristem activity, embryo development and seed mucilage release.2-14 Similarly, TPL/TPRs have a range of reported functions, including roles in embryo development,15 auxin and jasmonic acid signaling,16,17 plant immunity18 and meristem fate.19,20 Recently, TPL/TPR proteins were implicated in additional biological processes, such as biotic and abiotic stress responses and the floral transition, by the establishment of a protein-protein interaction framework for this family of co-repressors from Arabidopsis.21,22 It was revealed that TPL/TPR proteins interact almost exclusively with transcription factors (TFs), many of which have previously been implicated in transcriptional repression.
Diverse Repression Domain Sequences Establish Interactions with TPL/TPRs
Prior to the TPL/TPR interactome framework,22 interaction data identified only TFs with the ERF-associated amphiphilic repression (EAR) domain (with amino acid sequence LxLxL)23 as TPL/TPR partners,16-18,20 suggesting that this specific motif was necessary for recruiting TPL/TPRs. Surprisingly though, the TPL/TPR interaction framework revealed that all of the previously characterized repression domains (RDs) (LxLxL; DLNxxP; R/KLFGV; TLxLF)21,22,24-27 were enriched among TFs that interact with TPL/TPR proteins, and were subsequently shown to be necessary for recruiting TPL/TPR.22 These findings demonstrate that the TPL co-repressors are able to interact with diverse short RD sequences. In addition, some interactors lacked any known RD, suggesting that undiscovered motifs may exist. For example, analysis of the interaction framework revealed putative novel RDs with similarity to the RLFGV sequence, one of which was also shown to be necessary for TPL interaction.22
While TFs were enriched within the TPL/TPR interaction framework, several uncharacterised proteins were also isolated that may represent novel transcription factors or adaptor proteins. Novel adaptors have the potential to broaden the range of TFs that can recruit the TPL/TPR proteins, including those without a RD. For example, TPL was shown to interact directly with EAR-containing JAZ proteins involved in jasmonic acid (JA) signaling.22,28 However, despite having a role in repression of JA-responsive genes, many JAZ proteins do not have a RD sequence. Recently, JAZ proteins were shown to associate with TPL/TPRs via the adaptor protein NINJA, which potentially allows TPL to be recruited by JAZ proteins lacking an RD.17
The Arabidopsis TPL/TPR interaction framework reveals that this family of co-repressors acts broadly throughout development. We predict that these factors work as general repressors of gene transcription and that they are part of the mechanism of context-specific switching between gene activation and repression.
The Role of TPL in Auxin Signaling has been Conserved in Land Plants
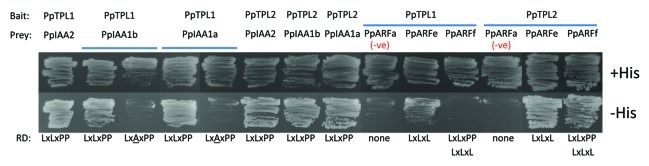
In angiosperms, the auxin response is controlled by AUX/IAA proteins and the auxin response factor (ARF) group of TFs. In Arabidopsis, it was shown that, in the absence of auxin, TPL interacts with AUX/IAA proteins to prevent activation of auxin responsive genes by activating ARFs.16 We were interested to learn whether the role of TPL in auxin signaling is an ancient mechanism conserved in plants. The moss Physcomitrella patens diverged from the angiosperms approximately 450 million years ago, and examination of its genome reveals that it encodes many components of the auxin signaling pathway, including three AUX/IAA proteins (PpIAA1A, PpIAA1B, PpIAA2) and 15 ARF proteins.29 The moss genome also encodes Gro/Tup1 co-repressor proteins including 2 TPL proteins (PpTPL1 and 2),29 and 4 putative LUG-like factors (Fig. 1). First we investigated whether the TPL-AUX/IAA protein-protein interactions are conserved in moss using well-established protocols.22 Our data reveals that both PpTPL1 and PpTPL2 interact with all three moss AUX/IAA proteins (Fig. 2). Moss AUX/IAA proteins have a modified EAR-like motif (LxLxPP),29 the mutation of which disrupts the interaction with TPL (Fig. 2). Critically, this provides the first biological evidence that LxLxPP is necessary for recruiting PpTPLs, suggesting LxLxPP could act as a repression domain in moss. Second, we examined interactions between PpTPL1/2 and moss ARF proteins. ARFs fall into two broad classes—those that activate gene transcription and those that directly repress it.30 The Arabidopsis TPL/TPR interaction framework revealed that TPL interacts directly with repressive ARFs, suggesting that the TPL co-repressors function in both forms of ARF-mediated repression. Here we examined whether moss TPL proteins also interact with repressive ARFs. Although all putative repressive ARFs were tested in yeast two-hybrid experiments, only interactions with two were identified. PpTPL2 interacts with both PpARFe (Pp1s339_47V6.1) and PpARFf (Pp1s279_9V6.1), while PpTPL1 only interacts with PpARFe. PpARFe and PpARFf have recognizable RD sequences at similar positions within the proteins. One other moss ARF has a known RD (Pp1s280_7V6.1), but at a different site in the protein, and no interaction with PpTPL1/2 was detected. Phylogenetic analyses show that PpARFe and PpARFf cluster with the Arabidopsis ARF10, 16 and 17 proteins.29 These proteins group separately from the activating ARFs and have a short middle region similar to the ARF repressors, but distinct from that of the activating ARFs, which is longer and often Q-rich.29,30 It is interesting to note that ARF17 was identified among the Arabidopsis TPL/TPR interactors,21,22 suggesting that this group of ARFs have the potential to act as repressors, and that these TPL-ARF interactions may be evolutionarily conserved.
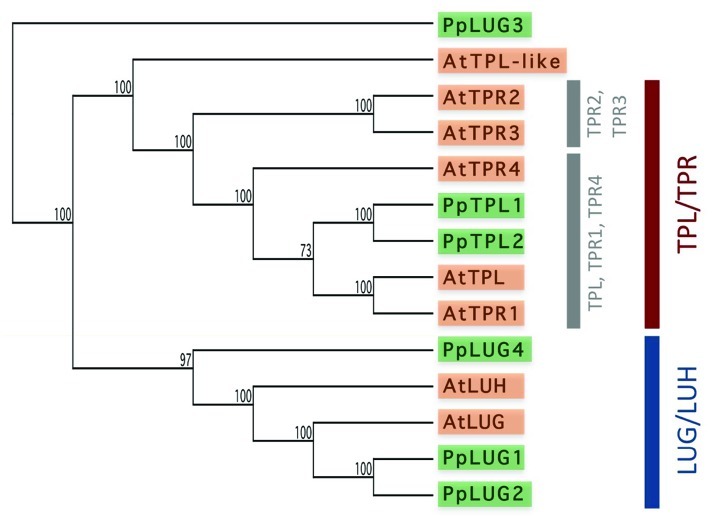
Figure 1.
Phylogenetic relationship of Arabidopsis and moss TPL and LUG proteins. An UPGMA tree, showing the relationships among putative TPL and LUG family proteins from Arabidopsis and P. patens, was generated from ClustalW alignments of full-length proteins using the MacVector software suite. Arabidopsis proteins are highlighted in orange, and P. patens proteins in green. The TPL/TPR and LUG/LUH clades are highlighted. Note that the moss TPL sequences (PpTPL1 and PpTPL2) group with the Arabidopsis TPL, TPR1 and TPR4 proteins, suggesting that this might be the ancestral clade and that the TPR2/TPR3 clade diverged later in the angiosperms, or that the TPR2/TPR3 genes were lost from the moss genome. AtTPL, At1g15750; AtTPR1, At1g80490; AtTPR2, At3g16830; AtTPR3, At5g27030; AtTPR4, At3g15880; AtTPL-like, At2g25420; AtLUG, At4g32551; AtLUH, At2g32700; PpTPL1, Pp1s99_260V6.1; PpTPL2, Pp1s316_34V6.1; PpLUG1, Pp1s371_17V6.1; PpLUG2, predicted from Pp1s371_10V6.1 and Pp1s371_13V6.1; PpLUG3, Pp1s45_33F3.1; PpLUG4, Phypa_162589.
Figure 2.
Protein-protein interactions between moss TPL proteins and components of the auxin signaling pathway. Interactions were tested between PpTPL1/2 bait proteins and moss AUX/IAA or ARF prey proteins in yeast two-hybrid assays. Yeast containing the bait and prey constructs were plated on media that selects for protein-protein interactions (minimal media minus histidine + 2.5mM 3-AT; -His) or control media without selection (+His). Yeast growth in the –His row indicates interaction between the bait and prey proteins listed at the top of the figure. Negative controls for interactions, a moss ARF protein lacking a repression domain (RD) are indicated (-ve). Putative RD sequences present in the appropriate AUX/IAA or ARF protein are listed along the bottom of the panel. Mutated RD residues are underlined. PpTPL1, Pp1s99_260V6.1; PpTPL2, Pp1s316_34V6.1; PpIAA1a, AB061222; PpIAA1b, Pp1s184_21V6.1; PpIAA2, Pp1s73_11V6.1; PpARFa, Pp1s14_392V6.1; PpARFe, Pp1s339_47V6.1; PpARFf, Pp1s279_9V6.1
In angiosperms, the functional study of activating transcription factors is often hampered by genetic redundancy. One approach to overcome this is to generate chimeric TFs fused to the EAR motif that act as dominant repressors.31 The use of chimeric TF repressors has not yet been reported in moss. However, our discovery that the LxLxPP motif can recruit PpTPL1/2 proteins suggests that chimeric TFs fused to the LxLxPP sequence may represent a specific and robust tool for such studies in moss.
Our data shows for the first time that the involvement of TPL in auxin signaling has been conserved since moss and angiosperms diverged. Conservation of the repression mechanism used by the auxin signaling pathway, demonstrates an early adoption of the TPL/TPR co-repressor system in plant evolution. Data from Arabidopsis suggests that TPL/TPRs were co-opted into many biological processes, placing these co-repressors at the center of plant development. It will now be important to compare the TPL/TPR interactome in moss to the Arabidopsis framework and to determine which developmental processes require its activity in moss. To help with the dissection of biological processes dependent on the extensive co-repressor-TF interaction framework, we have initiated a PLAnt Corepressor Interaction Database (placid.leeds.ac.uk). The aim of this database will be to collate interactions between transcription factors and co-repressors across different plant species.
Acknowledgments
This work was supported by grants from the BBSRC and the Gatsby Charitable Foundation. We thank Simon Wright and Fabio Fedi for their assistance with the yeast two-hybrid experiments reported here.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19283
References
- 1.Liu Z, Karmarkar V. Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci. 2008;13:137–44. doi: 10.1016/j.tplants.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z, Meyerowitz EM. LEUNIG regulates AGAMOUS expression in Arabidopsis flowers. Development. 1995;121:975–91. doi: 10.1242/dev.121.4.975. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Wang S, Huang H. LEUNIG has multiple functions in gynoecium development in Arabidopsis. Genesis. 2000;26:42–54. doi: 10.1002/(SICI)1526-968X(200001)26:1<42::AID-GENE7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Cnops G, Jover-Gil S, Peters JL, Neyt P, De Block S, Robles P, et al. The rotunda2 mutants identify a role for the LEUNIG gene in vegetative leaf morphogenesis. J Exp Bot. 2004;55:1529–39. doi: 10.1093/jxb/erh165. [DOI] [PubMed] [Google Scholar]
- 5.Navarro C, Efremova N, Golz JF, Rubiera R, Kuckenberg M, Castillo R, et al. Molecular and genetic interactions between STYLOSA and GRAMINIFOLIA in the control of Antirrhinum vegetative and reproductive development. Development. 2004;131:3649–59. doi: 10.1242/dev.01205. [DOI] [PubMed] [Google Scholar]
- 6.Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z. Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci U S A. 2004;101:11494–9. doi: 10.1073/pnas.0403055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks RG, Liu Z, Fischer RL. SEUSS and LEUNIG regulate cell proliferation, vascular development and organ polarity in Arabidopsis petals. Planta. 2006;224:801–11. doi: 10.1007/s00425-006-0264-6. [DOI] [PubMed] [Google Scholar]
- 8.Gregis V, Sessa A, Colombo L, Kater MM. AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell. 2006;18:1373–82. doi: 10.1105/tpc.106.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitaraman J, Bui M, Liu Z. LEUNIG_HOMOLOG and LEUNIG perform partially redundant functions during Arabidopsis embryo and floral development. Plant Physiol. 2008;147:672–81. doi: 10.1104/pp.108.115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregis V, Sessa A, Dorca-Fornell C, Kater MM. The Arabidopsis floral meristem identity genes AP1, AGL24 and SVP directly repress class B and C floral homeotic genes. Plant J. 2009;60:626–37. doi: 10.1111/j.1365-313X.2009.03985.x. [DOI] [PubMed] [Google Scholar]
- 11.Stahle MI, Kuehlich J, Staron L, von Arnim AG, Golz JF. YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell. 2009;21:3105–18. doi: 10.1105/tpc.109.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bui M, Lim N, Sijacic P, Liu Z. LEUNIG_HOMOLOG and LEUNIG regulate seed mucilage extrusion in Arabidopsis. J Integr Plant Biol. 2011;53:399–408. doi: 10.1111/j.1744-7909.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, DeBowles D, Esfandiari E, Dean G, Carpita NC, Haughn GW. The Arabidopsis transcription factor LUH/MUM1 is required for extrusion of seed coat mucilage. Plant Physiol. 2011;156:491–502. doi: 10.1104/pp.111.172023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker M, Tehseen M, Doblin MS, Pettolino FA, Wilson SM, Bacic A, et al. The transcriptional regulator LEUNIG_HOMOLOG regulates mucilage release from the Arabidopsis testa. Plant Physiol. 2011;156:46–60. doi: 10.1104/pp.111.172692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long JA, Woody S, Poethig S, Meyerowitz EM, Barton MK. Transformation of shoots into roots in Arabidopsis embryos mutant at the TOPLESS locus. Development. 2002;129:2797–806. doi: 10.1242/dev.129.12.2797. [DOI] [PubMed] [Google Scholar]
- 16.Szemenyei H, Hannon M, Long JA. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science. 2008;319:1384–6. doi: 10.1126/science.1151461. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez AC, et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature. 2010;464:788–91. doi: 10.1038/nature08854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z, Xu F, Zhang Y, Cheng YT, Wiermer M, Li X, et al. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc Natl Acad Sci U S A. 2010;107:13960–5. doi: 10.1073/pnas.1002828107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kieffer M, Stern Y, Cook H, Clerici E, Maulbetsch C, Laux T, et al. Analysis of the transcription factor WUSCHEL and its functional homologue in Antirrhinum reveals a potential mechanism for their roles in meristem maintenance. Plant Cell. 2006;18:560–73. doi: 10.1105/tpc.105.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallavotti A, Long JA, Stanfield S, Yang X, Jackson D, Vollbrecht E, et al. The control of axillary meristem fate in the maize ramosa pathway. Development. 2010;137:2849–56. doi: 10.1242/dev.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PlaCID. leeds.ac.uk – Plant Co-repressor Interaction Database: a developing compendium of experimentally derived co-repressor interactions.
- 22.Causier B, Ashworth M, Guo W, Davies B. The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol. 2012;158:423–38. doi: 10.1104/pp.111.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohta M, Matsui K, Hiratsu K, Shinshi H, Ohme-Takagi M. Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell. 2001;13:1959–68. doi: 10.1105/TPC.010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsui K, Umemura Y, Ohme-Takagi M. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. Plant J. 2008;55:954–67. doi: 10.1111/j.1365-313X.2008.03565.x. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis WUSCHEL is a bifunctional transcription factor that acts as a repressor in stem cell regulation and as an activator in floral patterning. Plant Cell. 2009;21:3493–505. doi: 10.1105/tpc.109.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M, Ohme-Takagi M. A novel group of transcriptional repressors in Arabidopsis. Plant Cell Physiol. 2009;50:970–5. doi: 10.1093/pcp/pcp048. [DOI] [PubMed] [Google Scholar]
- 27.Kagale S, Links MG, Rozwadowski K. Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol. 2010;152:1109–34. doi: 10.1104/pp.109.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arabidopsis Interactome Mapping Consortium Evidence for network evolution in an Arabidopsis interactome map. Science. 2011;333:601–7. doi: 10.1126/science.1203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, et al. The evolution of nuclear auxin signalling. BMC Evol Biol. 2009;9:126–41. doi: 10.1186/1471-2148-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453–60. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuda N, Matsui K, Ikeda M, Nakata M, Oshima Y, Nagatoshi Y, et al. CRES-T, an effective gene silencing system utilizing chimeric repressors. Methods Mol Biol. 2011;754:87–105. doi: 10.1007/978-1-61779-154-3_5. [DOI] [PubMed] [Google Scholar]