Abstract
Salinity stress causes ionic stress (mainly from high Na+ and Cl- levels) and osmotic stress (as a result of inhibition of water uptake by roots and amplified water loss from plant tissue), resulting in cell death and inhibition of growth and ultimately adversely reducing crop productivity. In this report, changes in root nitric oxide content, shoot and root biomass, root H2O2 content, root lipid peroxidation, root cell death, root caspase-like enzymatic activity, root antioxidant enzymatic activity and root ascorbate and glutathione contents/redox states were investigated in maize (Zea mays L. cv Silverking) after long-term (21 d) salt stress (150 mM NaCl) with or without exogenously applied nitric oxide generated from the nitric oxide donor 2,2′-(Hydroxynitrosohydrazano)bis-ethane. In addition to reduced shoot and root biomass, salt stress increased the nitric oxide and H2O2 contents in the maize roots and resulted in elevated lipid peroxidation, caspase-like activity and cell death in the roots. Altered antioxidant enzymatic activities, along with changes in ascorbate and glutathione contents/redox status were observed in the roots in response to salt stress. The detrimental effects of salt stress in the roots were reversed by exogenously applied nitric oxide. These results demonstrate that exogenously applied nitric oxide confers salt stress tolerance in maize by reducing salt stress-induced oxidative stress and caspase-like activity through a process that limits accumulation of reactive oxygen species via enhanced antioxidant enzymatic activity.
Keywords: antioxidant enzyme activity, caspase-like activity, maize, nitric oxide, reactive oxygen species, salt stress
Introduction
Accumulation of salt (e.g., Na+ and Cl- ions from NaCl) in plant tissues to levels that limit water uptake by cells and interfere with normal metabolic functions in plant tissue can severely reduce plant growth and ultimately result in plant demise.1-3 One of the consequences of salt stress is excessive production of reactive oxygen species (ROS) such as the superoxide anion (O2-), hydrogen peroxide (H2O2) and the hydroxyl radical (OH˙), leading to oxidative damage (via peroxidation) to cellular macromolecules such as DNA, proteins and lipids in plants that cannot efficiently scavenge the excessive ROS.1,4,5 On the other hand, ROS can serve as signals that trigger antioxidant enzymatic activity so that excessive ROS can be efficiently scavenged to prevent their accumulation to toxic levels.6 Based on several lines of evidence (reviewed in ref. 4), abiotic stresses such as salt stress are thought to trigger excessive ROS generation, to which plants respond by augmenting their antioxidant defenses in order to maintain equilibrium between antioxidants and ROS (i.e. maintenance of redox homeostasis). Failure to maintain redox homeostasis would result in excessive accumulation of ROS to toxic levels, which would impose oxidative stress on the plant, for which the extent of lipid peroxidation is generally regarded as indicative of the severity of oxidative stress.4 Plants capable of triggering high levels of antioxidant enzyme activity and efficiently preventing excessive accumulation of ROS can thus be regarded as having high antioxidant capacity whereas those that cannot would be regarded as having lower antioxidant capacity.
Induction of lipid peroxidation by excessive ROS accumulation as a result of salt stress culminates in plant cell death if plant redox homeostasis cannot be maintained.4 Evidence from various laboratories demonstrates that the ROS-induced plant cell death can either be programmed cell death (PCD) resembling animal apoptotic cell death7-11 or non-apoptotic PCD.7 Recently, it has been shown that short-term salt stress causes apoptosis-like plant PCD whereas long-term salt stress induces non-apoptotic plant PCD.7 Central to the execution of ROS-induced cell death that is subsequent to both short- and long-term salt stress is the induction of elevated cysteine protease enzymatic activity (notably cysteine protease activity resembling caspase enzymatic activity) and DNA fragmentation during short-term salt stress7,8,10,11 and caspase-like cysteine protease enzymatic activity without DNA fragmentation during long-term salt stress.7
Identification of nitric oxide (NO) as a crucial determinant of the level of tolerance to salt stress in Atriplex centralasiatica seedlings12 consolidates the role of NO in mediating salt stress tolerance in plants. Several lines of research demonstrate that salt stress induces elevation of NO content in plant tissue in a pathway that involves nitric oxide synthase-like activity12-14 but there is also evidence that salt stress supresses NO biosynthesis.15 Furthermore, there is contradiction with regards to the timing of the onset and sustenance of the elevated NO content in response to salt stress because some reports show that salt induces elevation of NO content in plant tissue only a few hours after exposure to salt stress and NO content returns to basal levels within 8 h13; whereas some reports demonstrate that elevation of NO content is sustained in response to long-term salt stress up to at least 4 d during salt exposure.12 Furthermore, recent investigations have established that exogenously applied NO enhances plant tolerance against salt stress.13-15 Furthermore, it appears that the enhancement of plant tolerance against salt stress may be mediated in part by antioxidant enzymes that act to prevent oxidative stress.16-20
The conflicting reports on NO generation in response to salt stress prompted us to investigate the effect of long-term salt stress on NO content in maize roots, given the importance of NO in mediating plant tolerance against salt stress. Despite the evidence for a role of caspase-like activity in plant responses to salt stress and ROS-induced oxidative stress, the role of NO in regulating plant caspase-like activity in these responses has not been established. Furthermore, although the role of exogenously applied NO in enhancing salt stress tolerance and salt stress-induced oxidative stress tolerance in plants is partially understood, the majority of the reports focus on a subset of the plant antioxidant enzymes (e.g., there are no reports, in our knowledge, on the role of dehydroascorbate reductase in NO-mediated salt stress tolerance in plants) and antioxidant metabolites (such as the role of glutathione and ascorbate in NO-mediated salt stress tolerance) involved in the processes leading to NO-mediated salt stress tolerance in maize. Thus, to expand understanding of the molecular processes participating in NO-transduced salt stress tolerance, the influence of exogenously applied NO on caspase-like enzymatic activity in maize salt stress tolerance was investigated in this study along with the effects of exogenously applied NO on the enzymatic activity of various antioxidant enzymes and the content/redox status of ascorbate (As) and glutathione (GSH) under salt stress in maize seedlings.
Results
Salt stress and exogenously applied NO increase root nitric oxide content
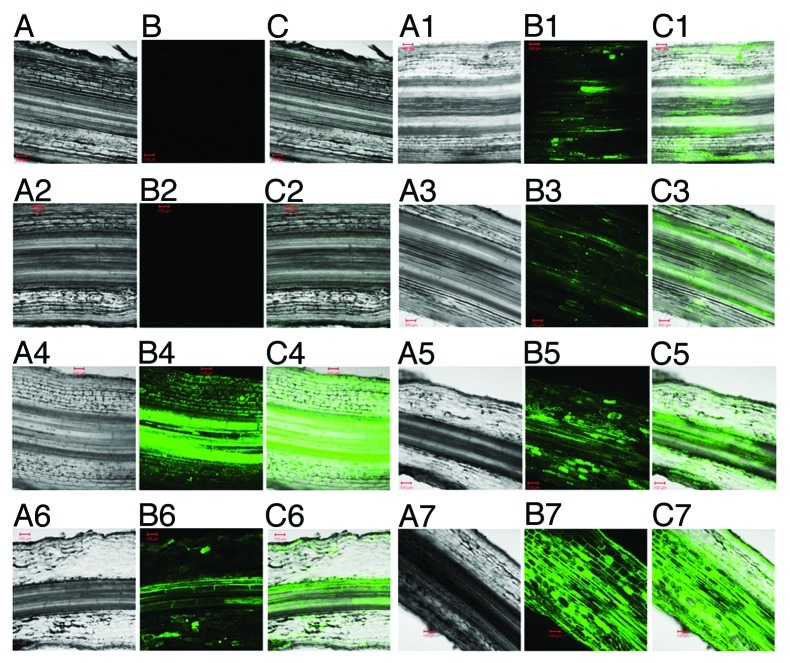
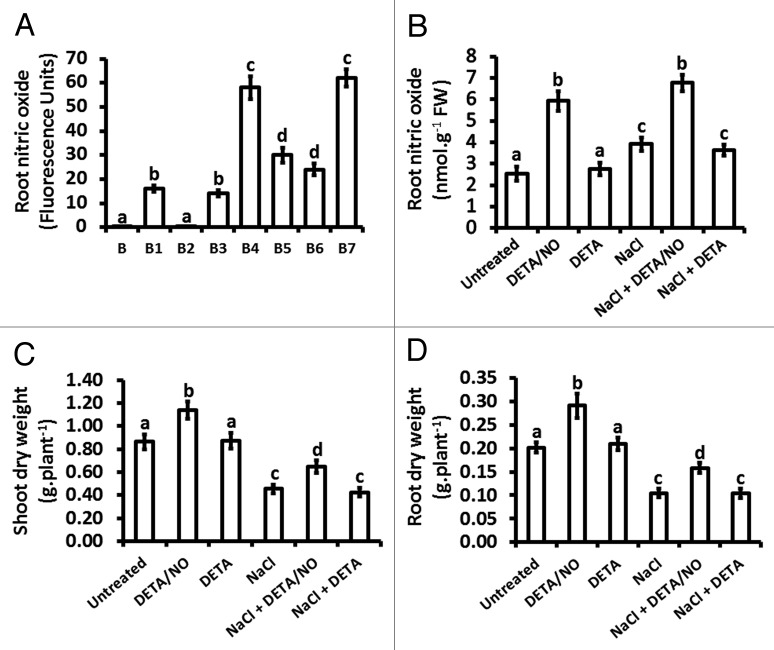
Exposure of maize to salt stress over a period of 21 d, as a result of treatment with 150 mM NaCl at three day intervals over the treatment period, induced an increase in root NO content (Fig. 1B5) in comparison to roots from untreated plants (Fig. 1B1). On the basis of fluorescence intensity, this salt stress-induced increase in root NO content was 2-fold higher than the root NO content of untreated plants (Fig. 2A) but the hemoglobin-based assay showed that this salt stress-induced increase in root NO content was 1.6-fold of the root NO content of untreated plants (Fig. 2B). Specificity of the fluorescence for NO was confirmed by examining fluorescence of the roots in the absence of the fluorescence-generating probe (4,5-diaminofluorescein diacetate; DAF-2DA) and also in the presence of the DAF-2DA after treatment of the roots with the NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), both of which showed extremely low levels of fluorescence (Figs. 1B, 1B2 and 2A).
Figure 1.
Detection of nitric oxide (NO) in maize roots using confocal microscopy. The effect of exogenously applied NO (10 μM DETA/NO) and salt stress (150 mM NaCl) on maize root NO content were measured. Bright field (A and A1–A7), fluorescence (B1–B7) and merged (C and C1–C7) micrographs of later root sections (100 μm) are shown. Root sections were obtained 21 d after treatment with NO and salt stress. DAF-2 DA fluorescent triazole derivative fluorescence was measured following pre-incubation with 400 μM cPTIO (B2), treatment with 10 μM DETA (B3), 10 μM DETA/NO (B4), 150 mM NaCl (B5), 10 μM DETA + 150 mM NaCl (B6), 10 μM DETA/NO + 150 mM NaCl (B7). As a control, autofluorescence at the same settings was also measured from roots, obtained from untreated plants, which were not stained with DAF-2 DA (B1). Micrographs are representative of three independent experiments. Red bar = 100 μm.
Figure 2.
NO content in maize roots and dry weights of shoots or roots in response to exogenously applied NO and NaCl treatment. NO content (A and B) were measured on maize plants treated at the V1 stage for a period of 21 d, with treatments done every three days. In panel A, fluorescence intensity was measured, using the AlphaEase FC imaging software, following pre-incubation with 400 μM cPTIO for 30 min (B2), treatment with 10 μM DETA (B3), 10 μM DETA/NO (B4), 150 mM NaCl (B5), 10 μM DETA + 150 mM NaCl (B6), 10 μM DETA/NO + 150 mM NaCl (B7), with autofluorescence intensity also measured from roots obtained from untreated plants without the DAF-2 DA fluorescence probe (B1). In panel B, No content was measured using a hemoglobin-based spectrophotometric assay. Biomass was also evaluated by measuring shoot (C) and root (D) dry weights at the end of the treatment period. Bars represent the means ± standard errors (SE) of three independent experiments with two plants used for each experiment to measure NO content and six plants per experiment for determining dry weights.
Treatment of maize with the NO donor 2,2′-(Hydroxynitrosohydrazano)bis-ethane (DETA/NO) over a period of 21 d at three day intervals over the treatment period resulted in an increase in root NO content (Fig. 1B4) in comparison to the NO content of roots from untreated plants (Fig. 1B1). The increase in root NO content resulting from treatment with DETA/NO was 3.8-fold (as estimated from fluorescence intensity) of the NO content of roots from untreated plants but the hemoglobin-based assay indicated a level that was 2.4-fold of NO content for the roots treated with DETA/NO in comparison to that of the roots from untreated plants. There was no statistically significant difference in root NO content between untreated plants and plants treated with a diethylenetriamine (DETA), which is chemically similar to DETA/NO except that it lacks the NO moiety and thus does not release NO (Figs. 1B3; 2A and B). Exposure of maize to a combination of NaCl and DETA/NO resulted in root NO content that was statistically similar to that observed for treatment with DETA/NO alone (Figs. 1B and 1B7; 2A and B). However, treatments in which a combination of NaCl and DETA was used led to root NO content that was statistically equivalent to the root NO content of plants treated only with NaCl (Figs. 1B5 and 1B6; 2A and B).
Noteworthy is the loss of the integrity of root morphology in response to NaCl (Fig. 1A5) and NaCl combined with DETA (Fig. 1A6) compared with root morphology of untreated (Fig. 1A and 1A1), DETA-treated (Fig. 1A3) and DETA/NO-treated (Fig. 1A4) plants. Such loss of root morphological integrity in treatments in which a combination of NaCl and DETA/NO (Fig. 1A7) is applied appears to be far less pronounced than that observed for the NaCl (Fig. 1A5) or the NaCl + DETA (Fig. 1A6) treatments.
Salt stress and exogenously applied NO alter shoot and root biomass
Dry weights were measured to determine the effect of the various treatments on shoot and root biomass, given that salt stress is known to retard plant growth.2,3 Treatment with DETA/NO resulted in increases in shoot and root dry weights that were 1.3-fold and 2.4-fold, respectively, of the dry weights of shoots and roots from untreated plants (Fig. 2C and D). On the other hand, DETA altered neither the shoot nor the root dry weights whereas salt stress caused reductions in shoot dry weight and root dry weight that were 0.5-fold and a 0.8-fold (respectively) of the corresponding dry weights of untreated plants (Fig. 2C and D). A combination of NaCl and DETA/NO increased the shoot dry weight to levels 1.4-fold of the shoot dry weight of plants treated with NaCl whereas the root dry weight increased to levels 1.5-fold of the shoot dry weight of plants treated with NaCl (Fig. 2C and D). Treatment of plants with a combination of NaCl and DETA resulted in shoot and root dry weights similar to those determined for treatments with NaCl alone (Fig. 2C and D).
Exogenously applied NO reduces salt stress-induced oxidative damage
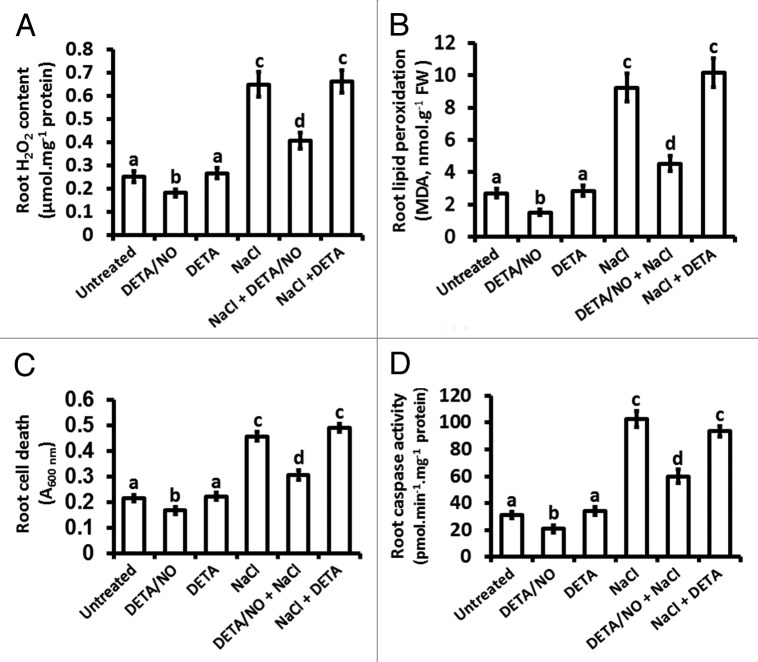
Several studies have established that salt stress leads to excessive accumulation of ROS, which cause oxidative stress in plant cells.4 The levels of H2O2 and the ameliorating effect of exogenously applied NO on the tolerance of maize against salt-induced oxidative damage was thus investigated by measuring the extent of malondialdehyde (MDA) accumulation, which is indicative of lipid peroxidation, in the various treatments. Roots from plants exposed to salt stress accumulated H2O2 to levels that were 2.6-fold of the H2O2 content of roots from untreated plants, whereas treatment with DETA/NO caused a reduction in root H2O2 levels to 0.7-fold compared with the H2O2 levels of roots from untreated plants, yet the H2O2 levels were similar between roots from DETA-treated and untreated plants (Fig. 3A). Treatment of maize with a combination of NaCl and DETA/NO reduced salt stress-induced H2O2 accumulation to 0.6-fold of the NaCl-treated plants whereas a combination treatment with NaCl and DETA did not alter the salt stress-induced H2O2 accumulation (Fig. 3A).
Figure 3.
Effect of nitric oxide and salt stress on H2O2 content, lipid peroxidation, cell viability and caspase-like activity in maize roots. H2O2 content (A), lipid peroxidation (B), cell death (C) and caspase-like enzymatic activity (D) were measured in maize roots after 21 d of treatment that was initiated at the V1 stage of vegetative growth. Treatments were with nutrient solution (UNTREATED) or nutrient solution supplemented to contain a final concentration of either 10 µM DETA/NO, 10 µM DETA, 150 mM NaCl, 10 µM DETA/NO + 150 mM NaCl and 10 µM DETA + 150 mM NaCl. Bars represent the means ± SE of three independent experiments with two separate plants used for each experiment.
No significant difference was observed between the MDA content of roots from untreated and DETA-treated plants but the MDA content of roots from plants treated with DETA/NO was 0.6-fold of the MDA content of roots from untreated plants (Fig. 3B). Treatment of maize with NaCl led to root MDA content that is 3.4-fold of root MDA from untreated plants and this increase was similar to that of roots from plants treated with a combination of NaCl and DETA (Fig. 3B). However, the salt stress-induced increase in MDA content was 0.5-fold in roots from plants treated with a combination of NaCl and DETA/NO in comparison to the MDA content of roots from plants treated with NaCl (Fig. 3B).
Salt stress-induced cell death and caspase-like activity are altered by exogenously applied NO
Earlier evidence suggests that salt stress induces plant caspase-like activity that participates in the orchestration of salt stress-induced PCD.7,10,11 It was thus investigated if exogenously applied NO can reverse the effects of salt stress on cell death and the associated caspase-like activity. Exogenously applied NO (as DETA/NO) decreased root cell death to 0.8-fold of the root cell death of untreated plants, but the cell death in roots from plants treated with DETA was similar to that of untreated plants (Fig. 3C). Induction of cell death by salt stress in roots was evident from the fact that Evans Blue uptake in these roots was 2.0-fold of the cell death observed in roots from untreated plants and this salt stress-induced increase in cell death was not altered by treating maize with a combination of NaCl and DETA (Fig. 3C). However, treatment with a combination of NaCl and DETA/NO reversed the effects of salt stress, as signified by the observation that exogenously applied NO reduced the extent of cell death to 0.7-fold of the cell death observed for the NaCl treatment (Fig. 3C).
Treatment of maize with DETA/NO reduced root caspase-like activity to levels that are 0.7-fold of those for roots from untreated plants but the caspase-like activity in response to treatment to DETA was similar to the caspase-like activity of roots in untreated maize (Fig. 3D). However, salt stress increased the level of caspase-like activity to 3-fold of the caspase-like activity observed in untreated plants and this salt stress-induced increase in caspase-like activity was similar to the increase seen for roots from plants treated with a combination of NaCl and DETA (Fig. 3D). Salt stress-induced caspase-like activity was alleviated by exogenously applied NO, as evident from a reduction of caspase-like activity to levels that are 0.6-fold of the levels observed for roots from NaCl-treated plants (Fig. 3D).
Exogenously applied NO modulates the responses of activity of antioxidant enzymes to salt stress
Alteration of the actvity of antioxidant enzymes in response to salt stress is well-documented in a variety of plant species1,4 and there is evidence that such responses are modulated by NO.16-20 However, the reports on the modulation of these antioxidant responses by NO are limited to short-term salt stress. The effect of NO on the antioxidant enzyme responses to long-term salt stress were thus investigated here.
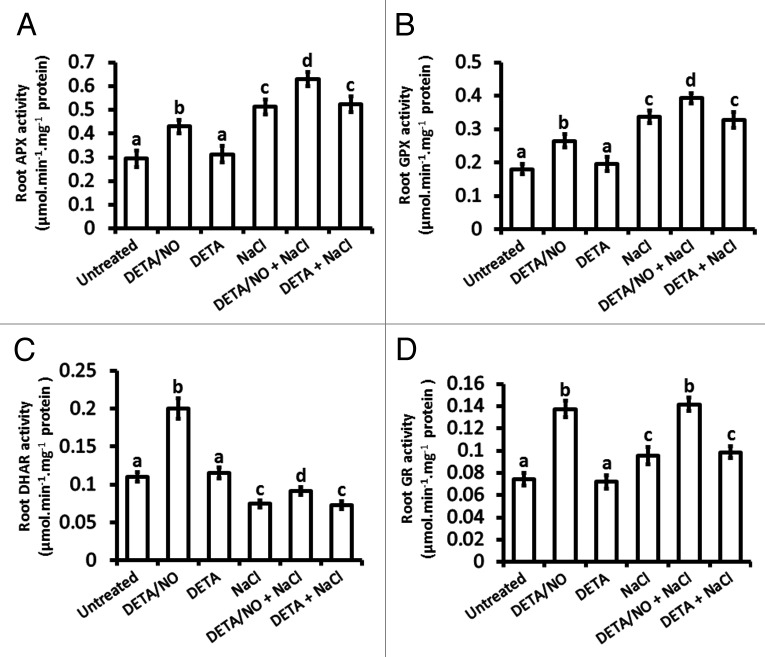
Ascorbate peroxidase (APX) activity in roots was elevated to levels 1.5-fold to those of untreated plants in response to treatment with the nitric oxide donor DETA/NO but no change in APX activity occurred in response to DETA in comparison to roots from untreated plants (Fig. 4A). On the other hand, the root APX activity in response to salt stress was induced to a slightly higher level than it was in response to exogenously applied NO, as indicated by a level that is 1.7-fold of the level seen for untreated plants (Fig. 4A). However, the combination of NaCl and DETA/NO caused the highest induction of root APX activity since this activity was 2.2-fold of the activity from untreated plants, whereas the combination of NaCl and DETA had a similar effect as NaCl alone (Fig. 4A). A similar trend of enzymatic activity was observed for root glutathione peroxidase (GPX) as it was for root APX activity (Fig. 4B).
Figure 4.
Maize root antioxidant (APX, GPX, DHAR and GR) enzymatic activity in response to exogenously applied NO and salt stress. Total APX (A), GPX (B), DHAR (C) and GR (D) enzymatic activity were measured spectrophotometrically in roots of maize plants. Assays were done on maize plants that were treated at the V1 stage for a period of 21 d. Bars represent the means ± SE of three independent experiments on two separate plants for each experiment.
Treatment with exogenously applied NO (DETA/NO) induced root dehydroascorbate reductase (DHAR) actvity to levels that were 2-fold compared with those of untreated plants but DETA did not alter the DHAR activity (Fig. 4C). On the other hand, salt stress inhibited DHAR activity as it resulted in DHAR activity that is only 0.7-fold of the DHAR activity in roots from untreated plants (Fig. 4C). The salt stress-induced inhibition of DHAR activity was alleviated by exogenously applied NO but not by DETA, as indicated by the observation that the DHAR activity in roots from plants treated with a combination of NaCl and DETA/NO was 1.3-fold of the activity in roots from NaCl-treated plants and the DHAR activity of roots from plants treated with a combination of NaCl and DETA was similar to that of roots from NaCl-treated plants (Fig. 4C).
Glutathione reductase (GR) activity in roots was augmented by exogenously applied NO to levels that were 1.9-fold of roots from untreated plants, yet they were not altered by DETA in comparison to roots from untreated plants (Fig. 4D). However, the induction of root GR activity in response to salt stress was limited to a level that is only 1.3-fold of the level observed for untreated plants (Fig. 4D). However, the combination of NaCl and DETA/NO caused induction of root GR activity similar to that observed for exogenously applied NO, whereas the combination of NaCl and DETA had a similar effect as NaCl alone (Fig. 4D).
Exogenously applied NO improves ascorbate-glutathione redox homeostasis
Alteration of antioxidant enzyme activity by salt stress perturbs the favorable redox state of the antioxidants ascorbate (Asc) and glutathione (GSH), which is commonly indicated by a lower ratio of Asc to dehydroascorbate (DHAsc) and lower ratio of GSH to glutathione disulfide (GSSG) compared with the corresponding ratios in unstressed plants.1,4 On this basis, it was determined if the negative effects of long-term salt stress on these ratios are reversed by exogenously applied NO.
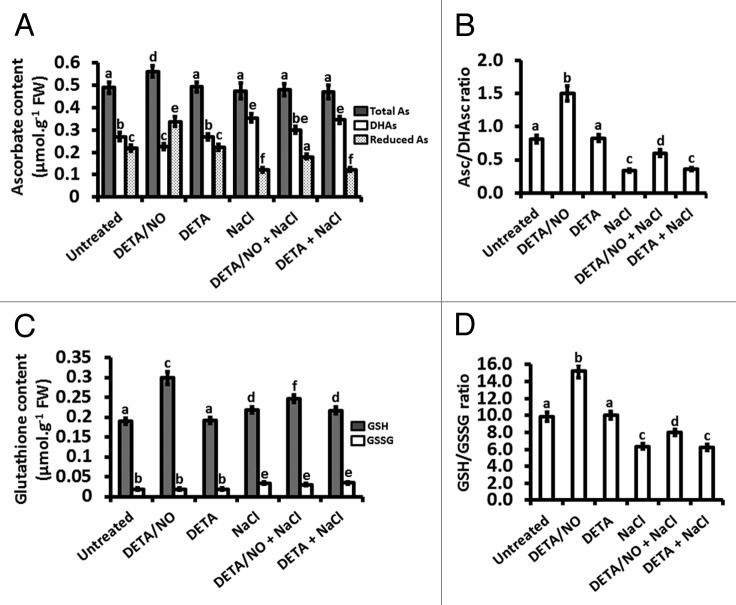
Root total Asc content was not altered in any of the treatments except the treatment with exogenously applied NO (DETA/NO), for which there was an increase in total Asc content compared with the total Asc of the root Asc content of untreated plants (Fig. 5A). DETA/NO reduced the level of root DHAsc compared with root DHAsc of untreated plants but DETA did not alter root DHAsc, whereas salt stress increased root DHAsc content compared with root DHAsc of untreated plants (Fig. 5A). Salt stress-induced accumulation of DHAsc was reversed by exogenously applied NO (the “DETA/NO + NaCl” treatment) but not by DETA, although the reversal did not bring the DHAsc content to the level of roots from untreated plants (Fig. 5A). The most pronounced changes were in the level of reduced Asc, for which DETA/NO increased the reduced Asc content of roots to levels that are 1.5-fold compared with those of roots from untreated plants whereas DETA did not alter the reduced Asc content of the roots and salt stress decreased the content of root reduced Asc to only 0.5-fold of the Asc content of roots from untreated plants (Fig. 5A). Exogenously applied NO reversed the salt stress-induced reduction in root reduced As content, as signified by an increase in root reduced Asc content (in the “DETA/NO + NaCl” treatment) to a level that is 1.5-fold of the reduced Asc content of roots from NaCl-treated plants, but nonetheless this reversal was not to the level of untreated plants (Fig. 5A). DETA did not reverse the effect of salt stress (in the “DETA + NaCl” treatment) on reduced Asc content (Fig. 5A). Analysis of the reduced Asc/DHAsc ratio in the roots showed that exogenously applied NO increases this ratio to 1.9-fold of the corresponding ratio in roots from untreated plants whereas salt stress decreases this ration to 0.4-fold of the ratio in roots from untreated plants (Fig. 5B). The decline in the reduced Asc/DHAsc ratio caused by salt stress was reversed by exogenously applied NO, as evident from the observation that the reduced Asc/DHAsc ratio in the “DETA/NO + NaCl” treatment was 1.8-fold of the corresponding ratio for the ‘NaCl’ treatment and the ratio for the “DETA + NaCl” treatment was similar to that of the ‘NaCl’ treatment (Fig. 5B).
Figure 5.
Effect of nitric oxide and salt-induced stress on the ascorbate-glutathione redox state. Asc content (A), Asc/DHAs ratios (B), GSH content (C) and GSH/GSSG ratios (D) were measured in maize plants treated for a period of 21 d, with treatments done every three days. Bars represent the means ± SE of three independent experiments for which two separate plants were used for each experiment.
Both exogenously applied NO and NaCl increased GSH content in the roots but the increase in GSH was more pronounced in response to exogenously applied NO (1.6-fold of the GSH content of roots from untreated plants) than in response to salt stress (1.1-fold of the root GSH content from untreated plants), whereas DETA resulted in root GSH content similar to that observed in untreated plants while the combination treatment with both DETA and NaCl had the same effect as salt stress alone (Fig. 5C). Furthermore, supplementation of the plants with exogenous NO during salt stress, as indicated by the observation that the ‘DETA/NO + NaCl’ treatment increased the GSH content to levels that were 1.1-fold of the NaCl treatment (Fig. 5C). GSSG content was not altered in any of the treatments except treatments that involved NaCl (salt stress alone, salt stress in combination with DETA/NO, salt stress in combination with DETA), for which all the treatments involving NaCl caused an increase (approximately 1.8-fold of the GSSG content of roots from untreated plants for the “NaCl”, “DETA/NO + NaCl” and “DETA + NaCl” treatments) in GSSG content compared with untreated plants (Fig. 5C). The GSH/GSSG ratio was higher (1.6-fold of the ratio of roots from untreated plants) in roots from plants treated with exogenous NO than roots from untreated plants, whereas DETA did not alter the ratio in comparison to that from untreated plants (Fig. 5D). On the other hand, salt stress reduced the ratio to a level that is 0.6-fold of the ratio for roots from untreated plants and the reduction in the ratio was not reversed by supplementation of the salt stressed plants with DETA (Fig. 5D). However, supplementation of the NaCl treatment (a salt stress-inducing treatment) with exogenously applied NO (as DETA/NO) reversed the effect of salt stress on the GSH/GSSG ratio as it resulted in a ratio that was 1.3-fold of the ratio observed for the roots from salt-stressed plants, which in other words was 0.8-fold of the ratio from untreated plants vs. the salt stress treatment that had a ratio 0.6-fold of the ratio for roots from untreated plants (Fig. 5D).
Discussion
We have analyzed the responses of maize to long-term (21 d) exposure to salt stress and exogenously applied nitric oxide using 150 mM NaCl to induce salt stress and 10 µM DETA/NO as the source of exogenously applied NO. The experimental conditions (a final concentration of 150 mM NaCl and/or 10 µM DETA/NO applied every 3 d for 21 d) were based on our own preliminary studies that showed that 150 mM NaCl caused an upsurge in root NO content within 3 d of treatment with NaCl whereas exogenously applied DETA/NO (single dose) caused elevation of root NO content, which could be detected 3 d after application and 150 mM NaCl also caused elevated (albeit less than that seen for DETA/NO) NO content in roots and this could be detected 3 d after application of NaCl (unpublished). Furthermore, several studies have reported the effects of lower (25, 50, 100 and 200 mM NaCl) salt concentration on maize and other species in relation to effects on biomass and other physiological parameters in maize and other species and also in relation to the effects of exogenous NO on salt stress tolerance for periods shorter (ranging from 2 h, 2 d, 4 d and up to 9 d) than 21 d.21-26 From our preliminary results, we also established that the detrimental effects of a final concentration of 250 mM NaCl applied at three days intervals to maize for a total period of 21 d leads to extensive tissue damage that is not reversed by exogenously applied NO (unpublished). For these reasons, we chose 150 mM NaCl applied at intervals of three days for a total period of 21 d (regarded here as long-term since it is the longest salt stress treatment reported in literature in relation to the effect of NO on plant salt stress tolerance to date).
Based on both fluorescence intensity and spectrophotometric measurements to detect the levels of NO produced in the various treatments involving NaCl and NO, our results suggest that long-term exposure to DETA/NO leads to release of NO into maize tissue and this elevates NO content in maize roots and also that long-term treatment with NaCl elevates NO levels content in maize roots. However, the increase in maize root NO content in response to salt stress is far less pronounced than the increase caused by exogenously applied NO. The fact that the fluorescence intensity observed for samples that were not stained with DAF-2 DA (a NO probe) was extremely low (almost undetectable), together with a similar observation for samples that were stained with DAF-2 DA after removal of endogenous NO by the NO scavenger cPTIO, confirms that the fluorescence detected in the treatments was specifically arising from NO. Furthermore, given that both the flourometric and spectrophotometric measurement of NO show no significant difference between untreated and DETA-treated samples, we conclude that DETA does not influence nitric oxide content in maize roots and is thus an appropriate control for plant treatments involving DETA/NO.
The first indication that exogenously applied NO may improve salt stress tolerance is that salt stress caused loss of root normal morphology whereas this was reversed by exogenously applying NO simultaneously with the salt stress treatment, but in-depth investigation on root tissue structure is required to fully appreciate the effect of salt stress on root morphology and the role of NO in negating that effect, given that our analysis on this effect was superficial. Nonetheless, analysis of shoot and root dry weights shows that exogenously applied NO alleviates the loss of biomass caused by salt stress because these dry weights were higher under salt stress in the presence of exogenously applied NO than under salt stress in the absence of exogenously applied NO, whereas DETA did not have any alleviating effect on the salt stress-induced loss of shoot and root biomass. This is further evidence that exogenously applied NO improves maize performance under salt stress.
The poor growth performance of maize under salt stress can be attributed to excessive accumulation of ROS because H2O2 levels increased drastically in response to salt stress and this corresponded to reduced dry weights in plants subjected to salt stress. This view is supported by the observation that extensive cell death occurred in response to salt treatment and this trend observed for cell death corresponded to that seen for lipid peroxidation. It is thus plausible to suggest that salt stress induces excessive accumulation of H2O2 and the resulting excessive ROS levels destabilize the cell membrane, leading to cell death and loss of biomass. The fact that exogenously applied NO reduced H2O2 content in plants treated with DETA/NO alone and in plants treated simultaneously with DETA/NO and NaCl; together with the fact that such treatments reversed the extent of lipid peroxidation, cell death and biomass loss; implies that exogenously applied NO is involved in improving maize tolerance to salt stress by regulating H2O2 accumulation and cell death. Such effects are not seen with DETA, confirming that the ameliorating effects on salt stress tolerance in the DETA/NO treatments are conferred by the exogenously applied NO. On the basis that the trend seen for cell death corresponded well with the trend observed for caspase-like activity across the treatments described here, it is likely that excessive H2O2 accumulation and the ensuing macromolecular peroxidation resulting from salt stress triggers caspase-like activity that elicits a PCD pathway, leading to the observed loss in biomass. Determination of PCD-specific events, such as DNA fragmentation and cytochrome c release, in the set of treatments described here form part of our near-future investigations to establish if the responses are mediated via a PCD pathway. Noting that exogenously applied NO reduced the caspase-like activity associated with salt stress; we suggest that enhanced scavenging of ROS such as H2O2, together with a mechanism to restrict caspase-like activity, are part of the molecular processes through which exogenously applied NO improves plant tolerance to salt stress. In fact, it will be interesting to establish if cystatin actvity is involved in the improvement of plant salt stress tolerance via a pathway mediated by NO since cystatins act to inhibit caspase-like actvity and are implicated in abiotic stress responses in plants27 and NO appears to regulate the expression of at least one class of cystatin.28
Involvement of ROS scavenging in the NO-mediated long-term salt stress tolerance in maize is supported by the enhancement of antioxidant enzymatic activity in the treatments described here. APX, GPX and GR activities increased in response to both exogenously applied NO on its own and salt stress alone but the salt stress-induced increase in these enzymatic activities was significantly lower than the increase in the enzymatic activities seen for treatments where salt stress was applied in combination with exogenously applied NO. Given that H2O2 levels remained high in the salt stressed maize and were attenuated in the ‘NaCl + DETA/NO’ treatment, together with a much more pronounced increase in APX, GPX and GR activities in the ‘NaCl + DETA/NO’ treatment, it hypothesized that the increase in APX and GPX activity in response to NaCl is inadequate to counteract the excessively high levels of H2O2 that accumulate in response to salt stress while the increase in GR actvity under salt stress in insufficient to cater for efficient regeneration of GSH. The result of such inefficiency in the antioxidant system is accelerated cell death under salt stress. Supplementing the salt-treated plants with NO would thus be thought to induce sufficient APX and GPX activity to efficiently reduce H2O2 to levels that are less damaging to the plant, albeit not down to the levels of untreated plants.
Further evidence supporting the involvement of exogenously applied NO in the enhancement of plant tolerance to salt stress via regulation of antioxidant enzyme activity is demonstrated by the observation that long-term salt stress inhibited DHAR activity and exogenously applied NO significantly relieved the salt-induced inhibition on DHAR activity, although the relief of the salt-induced inhibition on DHAR actvity by NO was not sufficient to bring the actvity back to the level of untreated plants. This further raises the possibility that As consumption is elevated, probably as a result of heightened oxidation of ascorbate because of augmented APX activity, under salt stress and the regeneration of reduced As under these conditions is restricted because of the inhibition of DHAR activity under salt stress. The fact that exogenously applied NO relieved the salt stress-induced DHAR activity implies that efficient regeneration of reduced Asc ensures better availability of this reductant to sustain APX activity at levels sufficient to scavenge H2O2 and thus reduce cellular damage caused by this ROS. In fact, results presented here show that exogenously applied NO increased the total Asc pool and the reduced Asc content in the absence of salt stress whereas it decreased the oxidized As content in the absence of salt stress. However, salt stress increased the oxidized Asc content and decreased the reduced Asc content. This is evident from the ratio of Asc to reduced Asc. This implies that salt stress places the plant tissue in an unfavorable (oxidized) redox state with regards to Asc and exogenously applied NO improves the redox state by pushing the redox balance toward a more reduced state in terms of Asc. Furthermore, exogenously applied NO also elevated the total GSH pool in the absence of salt stress, suggesting elevated GSH biosynthesis (which has previously been demonstrated29 or enhanced GR activity (which has been demonstrated in this study). However, salt stress increased the GSSG content substantially but increased the GSH content only moderately; yet the exogenously applied NO augmented the GSH content in the presence of salt to levels considerably higher than salt stress alone. The effect of exogenously applied NO on GSH in the presence of salt resulted in improved GSH/GSSG ratios compared with the poor ratios under salt stress in the absence of exogenously applied NO. This implies that salt stress places the plant tissue in an unfavorable redox state also with regards to GSH and exogenously applied NO improves the redox state by channelling the redox balance toward a more reduced state in terms of GSH, likely via both enhanced GSH biosynthesis and sufficiently augmented GR activity.
We hypothesize that NO decreases the accumulation of ROS by enhancing their scavenging through elevation of antioxidant enzyme activity and improving the redox state of ascorbate and glutathione. This would imply that the reduction in ROS levels translate to less cellular macromolecular (DNA, protein and lipid) oxidation (and thus less oxidative damage to the cell). Together with restrained caspase-like actvity (which translates to less caspase-induced cell death), the reduction of oxidative damage to the cells implies that more cells would be viable to support optimal metabolism to promote optimal growth, resulting in improved shoot and root biomass in the presence of exogenously applied NO. Given that the content of NO in roots from plants treated with NaCl increased by 1.6-fold of the NO content in roots from untreated plants whereas the NO content of roots from plants treated simultaneously with a combination of NaCl and NO increased by 2.8-fold of the content in roots from untreated plants, in other words the NO content in roots from plants treated with a combination of NaCl and NO was ± 70% higher than the NO content in roots from plants treated only with NaCl. We thus propose that the amount of NO resulting from treatment with NaCl is not sufficient to reverse the detrimental effects of NaCl on plant metabolism and biomass whereas the supplementation of NO (as exogenously applied NO in the form of an NO donor) to the plants under the NaCl stress leads to NO content far above the level that the plant can accumulate under NaCl stress. For our treatments, this level appears to be ± 70% above the level generated under NaCl stress. The hypothesis would thus be that a certain minimum NO content is required in the plant to counteract the negative effects of salt stress and this minimum is not met by the elevated NO level that the plant naturally makes in response to salt stress but can only be achieved by intervention (in this case exogenously supplying NO to the plant) to raise the NO content to levels that are significantly higher than those attained by the plant on its own in response to NaCl stress. Genetic engineering to enhance the ability of the plant to produce higher NO content in response to NaCl stress than the content that the plant produces without the genetic enhancement could be a potential strategy to improve plant performance under salinity stress.
In conclusion, exogenously applied nitric oxide improves maize tolerance to long-term salt stress by inducing elevated antioxidant enzyme activity to maintain redox homeostasis; resulting in restricted H2O2 accumulation, limited macromolecular (lipid) peroxidation and constrained caspase-like actvity that limit the extent of salt stress-induced cell death. The roles of NO in conferring salt stress tolerance in maize, as reported here, strengthens the foundation on which genetic engineering of crop plants for regulated nitric oxide biosynthesis under salt stress can be used to improve plant performance in saline soils.
Materials and Methods
Plant growth
Maize (Zea mays L. cv Silverking) seeds (donated by Capstone Seeds Pty Ltd) were surface-sterilized in 0.35% sodium hypochlorite for 10 min and then rinsed four times with sterile distilled water. Seeds were imbibed in sterile distilled water for 30 min and sown in 2 L of pre-soaked (distilled water) filtered silica sand (98% SiO2, Rolfes® Silica, Brits), in 19.5 cm diameter plastic pots. The sand was kept moist by irrigating it with distilled water during germination. Germinated seedlings (one plant per pot) were grown on a 25/19°C day/night temperature cycle under a 16/8 h light/dark cycle, at a photosynthetic photon flux density of 300 µmol photons.m−2.s−1 during the day phase. Plants were supplied with nutrient solution [1mM K2SO4, 2 mM MgSO4, 5 mM CaCl2, 5 mM KNO3, 10 mM NH4NO3, 1 mM K2HPO4 buffer at pH 7.2, 5 µM H3BO3, 5 µM MnSO4, 1 µM ZnSO4, 1 µM CuSO4, 2 µM Na2MoO4, 1 µM CoSO4, 100 µM Fe-NaEDTA and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) at pH 7.2] at the V1 stage (when the collar of the first true leaf is visible). Plants at the V1 stage which were of similar height were selected for all experiments.
Treatment of plants
One week after the plants had reached the V1 stage, control plants were supplied with nutrient solution every third day. For treatments, the nutrient solution was supplemented with the following final concentrations: 150 mM NaCl, 10 μM DETA/NO (a nitric oxide donor), 10 μM DETA (contains no NO moiety and thus serves as a control for NO treatments), a combination of 150 mM NaCl with 10 μM DETA/NO and finally a combination of 150 mM NaCl with 10 μM DETA. Treatments or nutrient solution (200 ml per pot) were applied to each plant directly to the sand at the base of the stem of the plant in the pot every three days.
Analysis of growth
After 21 d of treatment, plants were removed from the sand, being careful to avoid any loss of shoots and roots during the up-rooting of the plants and scored for dry weight of the shoots and roots.
Nitric oxide detection by confocal laser scanning microscopy (CLSM)
Measurement of NO was performed with the NO probe DAF-2DA, using a modified method.30 Fresh root sections (150 μm thick) were incubated in loading buffer [0.1 mM CaCl2, 10 mM KCl, 10 mM Tris(hydroxymethyl)aminomethane hydrochloride (TRIS-HCl), pH 7.2] and DAF-2DA at a final concentration of 20 μM for 1 h in the dark at 25°C, followed by washing with loading buffer three times for 15 min each time. For control to determine autofluorescence, root sections were incubated in loading buffer devoid of DAF-2DA. For control to determine if the fluorescence obtained resulted specifically from NO, root sections were first incubated in loading dye devoid of DAF-2DA in the presence of 400 μM cPTIO for 30 min, followed by three washes with loading dye in the absence of DAF-2D before incubation in loading dye containing DAF-2D. NO-induced fluorescence was recorded using a Zeiss confocal laser-scanning microscope (LSM 510 META; excitation at 488 nm, emission at 515 nm). Pixel intensities estimated as the level of fluorescence of DAF-2T were calculated using the AlphaEase FC imaging software (Alpha Innotech Corporation).
Evaluation of cell viability
A modified method was followed for the cell viability assays (i.e., after 21 d from the first treatment).31 Briefly, fresh root tissue (100 mg per treatment per plant) from two different plants of each of the treatments was harvested and stained at room temperature with 0.25% (w/v) Evans Blue dye for 15 min. The roots were washed for 45 min in distilled water to remove surface-adsorbed dye, followed by extraction of the Evans Blue stain (taken up by dead root cells) from root tissue using 1% (w/v) SDS, after incubation for 1 h at 55°C. Absorbance of the extract was measured at 600 nm to determine the level of Evans Blue up-take by the root tissue.
Preparation of protein extracts
Extracts were obtained from maize roots by grinding the root tissue into a fine powder in liquid nitrogen and homogenizing 500 mg of the tissue with either 1 ml of homogenizing buffer [40 mM K2HPO4, pH 7.4, 1 mM EDTA (EDTA), 5% (w/v) polyvinylpyrrolidone (PVP) molecular weight = 40 000] for determination of NO content, antioxidant enzymatic activities and caspase-like activity, or 1 ml of 10% trichloroacetic acid (TCA) for H2O2 content, lipid peroxidation, ascorbate, dehydroascorbate, glutathione and glutathione disulfide. The resulting homogenates were centrifuged at 12,000 X g for 15 min and the supernatants were used for biochemical assays. Protein concentrations for all assays were measured in the extracts (prepared with homogenizing buffer) as instructed for the RC DC Protein Assay Kit 11 (Bio-Rad Laboratories).
Spectrophotometric quantification of NO content
NO content was measured by slight modification of the hemoglobin-based assay.32 Briefly, protein extracts were incubated with 100 U of catalase and 100 U of superoxide dismutase for 10 min, followed by addition of oxyhemoglobin to a final concentration of 10 μM. The mixture was incubated for 2 min, followed by measurement of NO content by following the conversion of oxyhemoglobin to methemoglobin spectrophotometrically at 401 and 421 nm.
Measurement of H2O2 content and lipid peroxidation levels
H2O2 content was determined based on an adapted method.33 The reaction mixture contained 75 µl of the TCA extract, 5 mM K2HPO4 at pH 5.0 and 0.5 M KI. Samples were incubated at 25°C for 20 min and absorbance readings of the samples were taken at 390 nm. H2O2 content was calculated based on a standard curve constructed from the absorbance (A390 nm) of H2O2 standards. Products of lipid peroxidation (reflected by MDA content) were estimated.34 For the MDA measurements, 1 ml of TCA extract was taken and 4 ml 0.5% 2-thiobarbituric acid in 20% TCA was added. The mixture was heated for 30 min at 95°C and then cooled in ice for 10 min. The specific absorbance of products was read at 532 nm and nonspecific background-absorbance at 600 nm. The concentration of MDA was calculated using a molar extinction coefficient 155 mM−1 cm−1.
Determination of caspase-like activity
For cysteine protease activity determination, 1 mM phenylmethylsulfonyl fluoride (PMSF) and 10 mM β-mercaptoethanol were added to the protein extract (prepared with homogenizing buffer) to inhibit other classes of proteases (e.g., serine proteases) in the homogenate prior to incubation of the mixture at 37°C for 5 min (cysteine protease inhibition by PMSF is reversed in the presence of β-mercaptoethanol but inhibition of other classes of proteases by PMSF is not reversed by β-mercaptoethanol). Subsequently, 0.5 mM N-Acetyl-Asp-Glu-Val-Asp-p-Nitroanilide (Ac-DEVD-pNA; Sigma-Aldrich), which is a caspase-specific substrate, was added to the reaction mixture and incubated at 37°C for 60 min. Caspase-like cysteine protease activity was determined by measuring absorbance at 405 nm every 20 min, using the extinction coefficient of 9.6 mM−1 cm−1 for p-nitroaniline.
Assays for antioxidant enzyme activities
For all antioxidant enzyme activity assays, proteins were prepared using the homogenizing buffer. APX (EC1.11.1.11) activities were measured using a modified method.35 In summary, the root extracts (extracts supplemented with ascorbate to a final concentration of 2 mM) were added to the assay buffer (50 mM K2HPO4, pH 7.0, 0.1 mM EDTA, 50 mM ascorbate). The reaction was initiated with 1.2 mM H2O2 in a final reaction volume of 200 µl and APX activity was calculated based on the change in absorbance at 290 nm using the extinction co-efficient of 2.8 mM−1cm−1.
For total glutathione peroxidase (GPX, EC 1.11.1.9) activity, a modified method was used.36 The reaction mixture contained 50 µg of protein extract, 50 mM K2HPO4, pH 7.0, 1 mM EDTA, 2 mM glutathione (GSH), 0.1 mM nicotinamide adenine dinucleotide phosphate (NADPH), 2.5 units of glutathione reductase and 90 µM H2O2. GPX activity was calculated based on the change in absorbance at 340 nm resulting from the oxidation of NADPH in the reaction, using the extinction coefficient of 6.2 mM−1cm−1 for NADPH.
Dehydroascorbate reductase (DHAR, EC. 1.8.5.1) activity was measured according to a modified method.37 The reaction mixture contained: 100 mM K2HPO4 pH 6.3, 1 mM dehydroascorbate (DHAsc), 2 mM GSH and 50 µg of enzyme extract. The DHAR activity was monitored by following the formation of ascorbate at 265 nm for 5 min and calculated using the extinction coefficient of 14 mM−1cm−1.
Glutathione reductase (GR, EC. 1.6.4.2) activity was determined by following the rate of NADPH oxidation at 340 nm.38 The assay mixture contained: 0.2 mM NADPH, 0.5 mM GSSG, 1 mM EDTA in 100 mM K2HPO4 pH 7.8 and 50 μg of enzyme extract in a 200 µl reaction. GR activity was calculated based on the oxidation of NADPH in the reaction, using the extinction coefficient of 6.2 mM−1cm−1.
Evaluation of the Ascorbate and Glutathione pool
For the purpose of evaluating the Asc or DHAsc and GSH or GSSG pool for the treatments highlighted, TCA extracts were used. Ascorbate (Asc) and oxidized ascorbate (DHAsc) were measured,39 and the redox state of ascorbate was expressed as the ratio of Asc to DHAsc. The levels of glutathione (GSH) and glutathione disulfide (GSSG) were estimated,40 and the glutathione redox state was expressed as the ratio of GSH to GSSG.
Statistical analysis
All experiments were performed three times independently. Samples from different replicates were stored separately at -80°C until analyzed and results were given as mean ± standard deviation. For statistical analysis, One-way analysis of variance (ANOVA) test was used for all data and means were compared according to the Tukey-Kramer test at 5% level of significance, using GraphPad Prism 5.03 software.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the University of the Western Cape and the National Research Foundation (South Africa).
Glossary
Abbreviations:
- Ac-DEVD-pNA
Acetyl-Asp-Glu-Val-Asp-p-nitroanilide
- ANOVA
one-way analysis of variance
- APX
ascorbate peroxidase
- Asc
ascorbate
- cPTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- DAF-2DA
4,5-diaminofluorescein diacetate
- DETA
diethylenetriamine
- DETA/NO
2,2′-(hydroxynitrosohydrazano)bis-ethane
- DHAR
dehydroascorbate reductase
- DHAsc
dehydroascorbate
- EDTA
ethylenediaminetetraacetic acid
- GPX
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- GSSG
glutathione disulfide
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MDA
malondialdehyde
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- PCD
programmed cell death
- PMSF
phenylmethylsulfonyl fluoride
- PVP
polyvinylpyrrolidone
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- TCA
trichloroacetic acid
- Tris-HCl
Tris(hydroxymethyl)aminomethane hydrochloride
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18967
References
- 1.Abogadallah GM. Antioxidative defense under salt stress. Plant Signal Behav. 2010;5:369–74. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005;444:139–58. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 3.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–81. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 4.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–30. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:463–99. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- 6.Miller G, Shulaev V, Mittler R. Reactive oxygen signaling and abiotic stress. Physiol Plant. 2008;133:481–9. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 7.Andronis EA, Roubelakis-Angelakis KA. Short-term salinity stress in tobacco plants leads to the onset of animal-like PCD hallmarks in planta in contrast to long-term stress. Planta. 2010;231:437–48. doi: 10.1007/s00425-009-1060-x. [DOI] [PubMed] [Google Scholar]
- 8.Affenzeller MJ, Darehshouri A, Andosch A, Lütz C, Lütz-Meindl U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J Exp Bot. 2009;60:939–54. doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadjev I, Stone JM, Gechev TS. Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol. 2008;270:87–144. doi: 10.1016/S1937-6448(08)01403-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Li X, Liu Y, Zhao X. Salt stress induces programmed cell death in Thellungiella halophila suspension-cultured cells. J Plant Physiol. 2010;167:1145–51. doi: 10.1016/j.jplph.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Zuppini A, Gerotto C, Baldan B. Programmed cell death and adaptation: two different types of abiotic stress response in a unicellular chlorophyte. Plant Cell Physiol. 2010;51:884–95. doi: 10.1093/pcp/pcq069. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Yin H, Yang L, Xie Z, Liu X. Differential salt tolerance in seedlings derived from dimorphic seeds of Atriplex centralasiatica: from physiology to molecular analysis. Planta. 2011;233:859–71. doi: 10.1007/s00425-010-1347-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YY, Wang LL, Liu YL, Zhang Q, Wei QP, Zhang W-H. Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta. 2006;224:545–55. doi: 10.1007/s00425-006-0242-z. [DOI] [PubMed] [Google Scholar]
- 14.Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004;134:849–57. doi: 10.1104/pp.103.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao MG, Tian QY, Zhang WH. Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol. 2007;144:206–17. doi: 10.1104/pp.107.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molassiotis A, Tanou G, Diamantidis G. NO says more than ‘YES’ to salt tolerance: Salt priming and systemic nitric oxide signaling in plants. Plant Signal Behav. 2010;5:209–12. doi: 10.4161/psb.5.3.10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi QH, Ding F, Wang XF, Wei M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem. 2007;45:542–50. doi: 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Tanou G, Molassiotis A, Diamantidis G. Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J Plant Physiol. 2009;166:1904–13. doi: 10.1016/j.jplph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Wu X, Zhu W, Zhang H, Ding H, Zhang JH. Exogenous nitric oxide protects against salt-induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicom esculentum Mill.) Acta Physiol Plant. 2010 doi: 10.1007/s11738-010-0648-x. In Press. [DOI] [Google Scholar]
- 20.Zheng CF, Dong JG, Liu FL, Dai TB, Liu WC, Jing Q, et al. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ Exp Bot. 2009;67:222–7. doi: 10.1016/j.envexpbot.2009.05.002. [DOI] [Google Scholar]
- 21.Zörb C, Schmitt S, Neeb A, Karl S, Linder M, Schubert S. The biochemical reaction of maize (Zea mays L.) to salt stress is characterized by a mitigation of symptoms and not by a specific adaptation. Plant Sci. 2004;167:91–100. doi: 10.1016/j.plantsci.2004.03.004. [DOI] [Google Scholar]
- 22.Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W. Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta. 2006;224:545–55. doi: 10.1007/s00425-006-0242-z. [DOI] [PubMed] [Google Scholar]
- 23.Shi Q, Ding F, Wang X, Wei M. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem. 2007;45:542–50. doi: 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Zhang F, Guo J, Yang Y, Li B, Zhang L. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004;134:849–57. doi: 10.1104/pp.103.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanou G, Molassiotis A, Diamantidis G. Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J Plant Physiol. 2009;166:1904–13. doi: 10.1016/j.jplph.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Uchida A, Jagendorf AT, Hibino T, Takabe T, Takabe T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002;63:515–23. doi: 10.1016/S0168-9452(02)00159-0. [DOI] [Google Scholar]
- 27.Zhang X, Liu S, Takano T. Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol. 2008;68:131–43. doi: 10.1007/s11103-008-9357-x. [DOI] [PubMed] [Google Scholar]
- 28.Belenghi B, Acconcia F, Trovato M, Perazzolli M, Bocedi A, Polticelli F, et al. AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. Eur J Biochem. 2003;270:2593–604. doi: 10.1046/j.1432-1033.2003.03630.x. [DOI] [PubMed] [Google Scholar]
- 29.Innocenti G, Pucciariello C, Le Gleuher M, Hopkins J, de Stefano M, Delledonne M, et al. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta. 2007;225:1597–602. doi: 10.1007/s00425-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 30.Corpas FJ, Barroso JB, Carreras A, Quirós M, León AM, Romero-Puertas MC, et al. Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol. 2004;136:2722–33. doi: 10.1104/pp.104.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanevas N, Sunohara Y, Matsumoto H. Characterization of reactive oxygen species involved oxidative damage in Hapalosiphon species crude extract-treated wheat and onion roots. Weed Biol Manage. 2007;7:172–7. doi: 10.1111/j.1445-6664.2007.00253.x. [DOI] [Google Scholar]
- 32.Murphy ME, Noack E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994;233:240–50. doi: 10.1016/S0076-6879(94)33027-1. [DOI] [PubMed] [Google Scholar]
- 33.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 34.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 35.Asada K. Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol. 1984;105:422–9. doi: 10.1016/S0076-6879(84)05059-X. [DOI] [Google Scholar]
- 36.Drotar A, Phelps P, Fall R. Evidence for glutathione peroxidase activities in cultured cells. Plant Sci. 1985;42:35–40. doi: 10.1016/0168-9452(85)90025-1. [DOI] [Google Scholar]
- 37.Arrigoni O, De Gara L, Tommasi F, Liso R. Changes in ascorbate system during seed development of Vicia faba L. Plant Physiol. 1992;99:235–8. doi: 10.1104/pp.99.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esterbauer H, Grill D. Seasonal variation of glutathione and glutathione reductase in needles of Picea abies. Plant Physiol. 1978;61:119–21. doi: 10.1104/pp.61.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kampfenkel K, Van Montagu M, Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem. 1995;225:165–7. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- 40.Smith IK. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985;79:1044–7. doi: 10.1104/pp.79.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]