Abstract
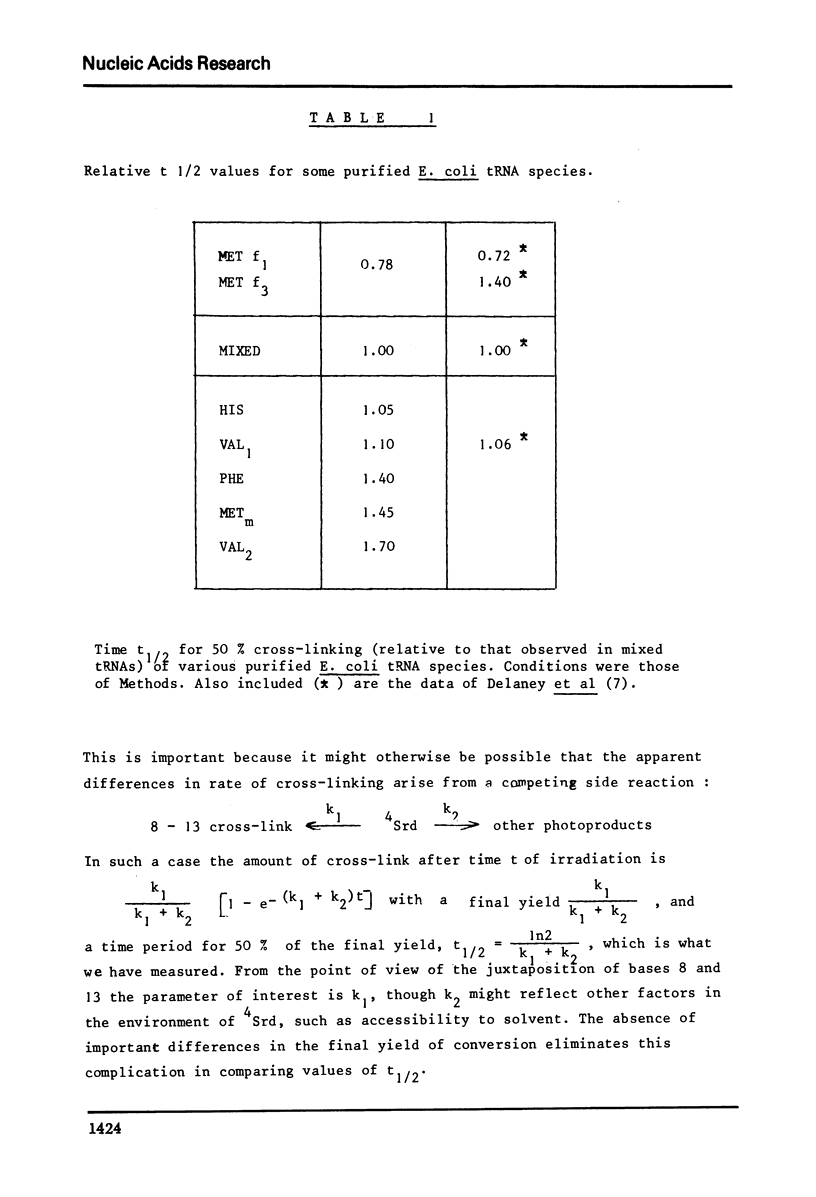
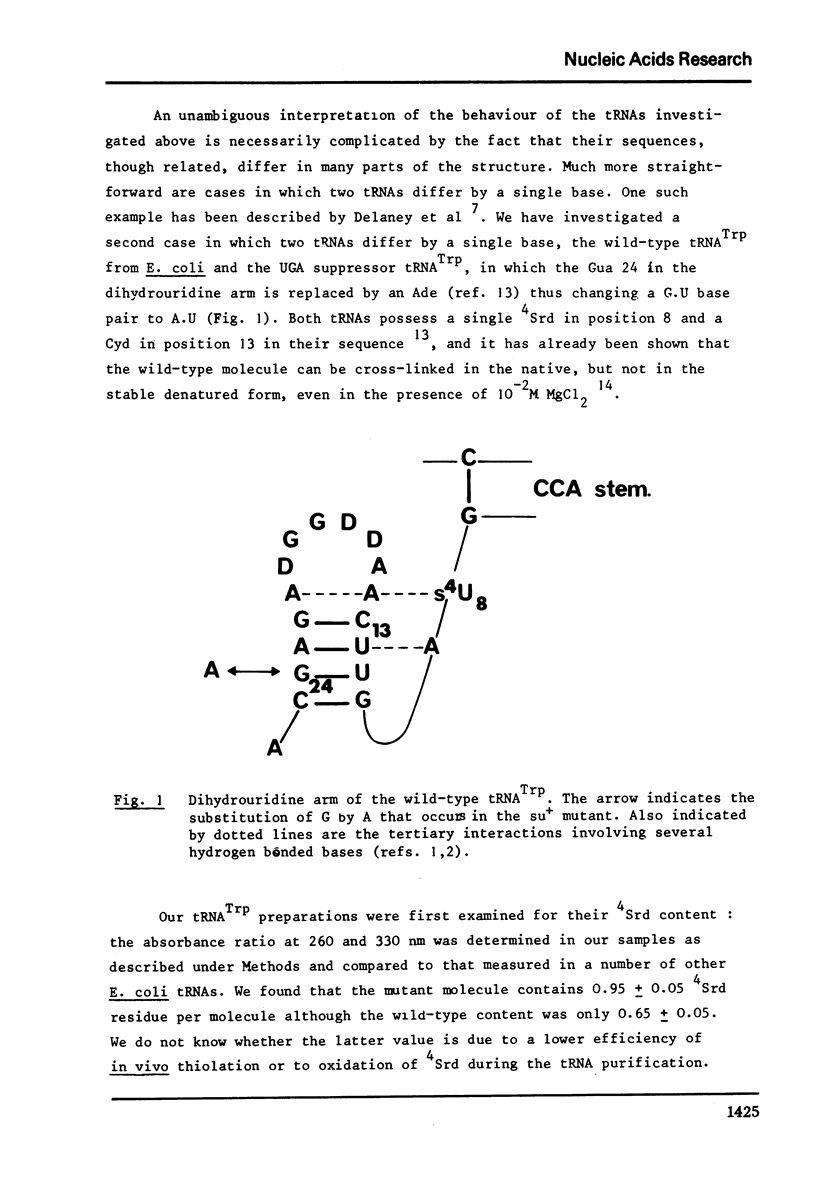
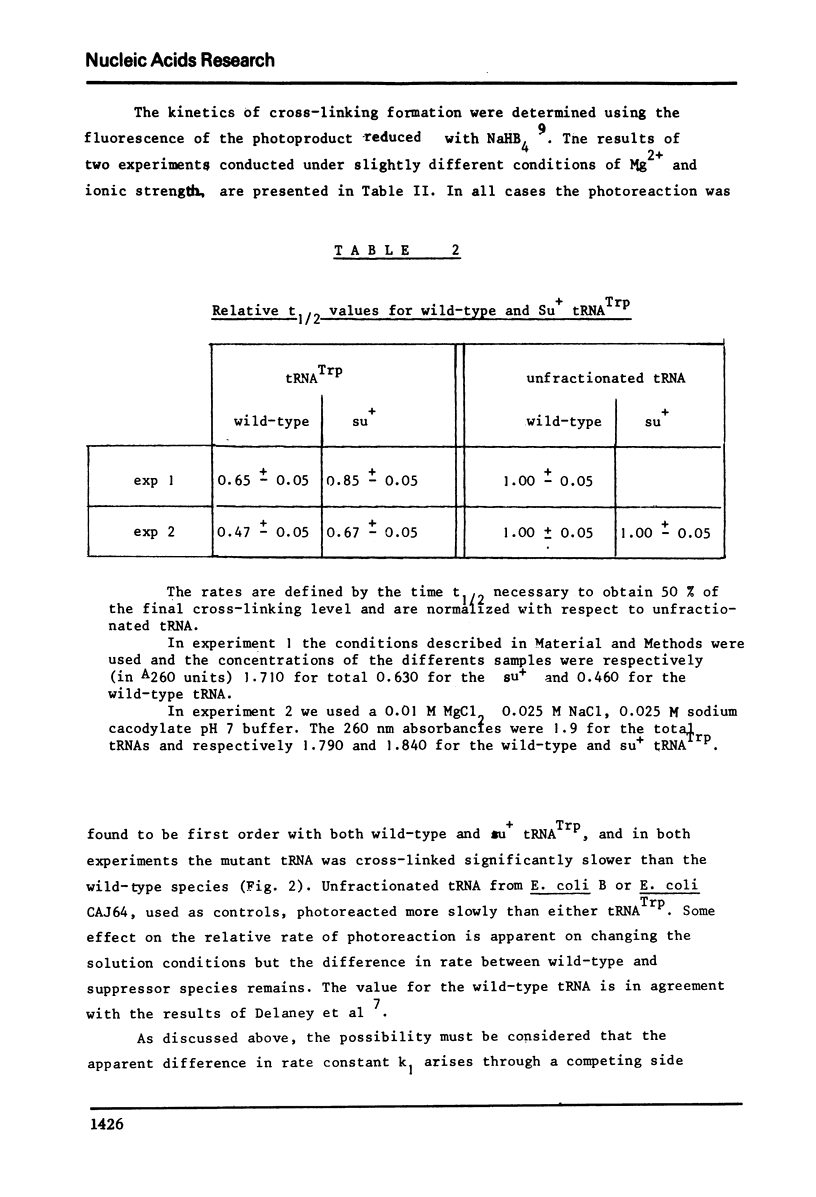
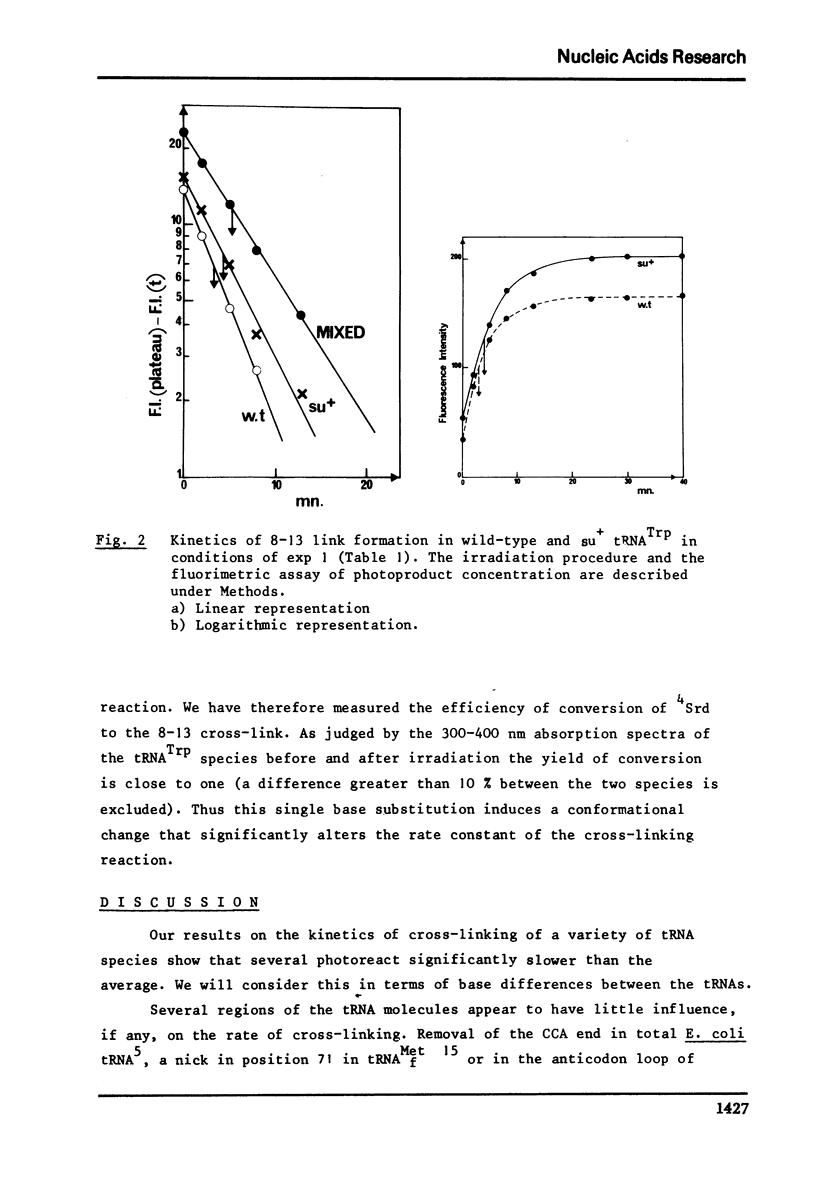
The conformation of ten purified tRNAs from Escherichia Coli has been investigated by means of the photo-induced cross-linking of 4Srd8 and Cyd13, which is sensitive to the juxtaposition of the two bases. Three tRNAs photo-react abnormally slowly; tRNAPhe, tRNAMet/m a and tRNAVal/2; a comparison with normally reacting species suggests that base 47 (Urd or modified Urd) is involved in a tertiary interaction in Class I tRNAs with the triplet 8, 14, 28. The UGA suppressor tRNATrp photoreacts significantly slower than the wild type. Thus the single base change Gua 24 to Ade induces a conformational change that alters the rate constant for the cross-linking reaction.
Full text
PDF


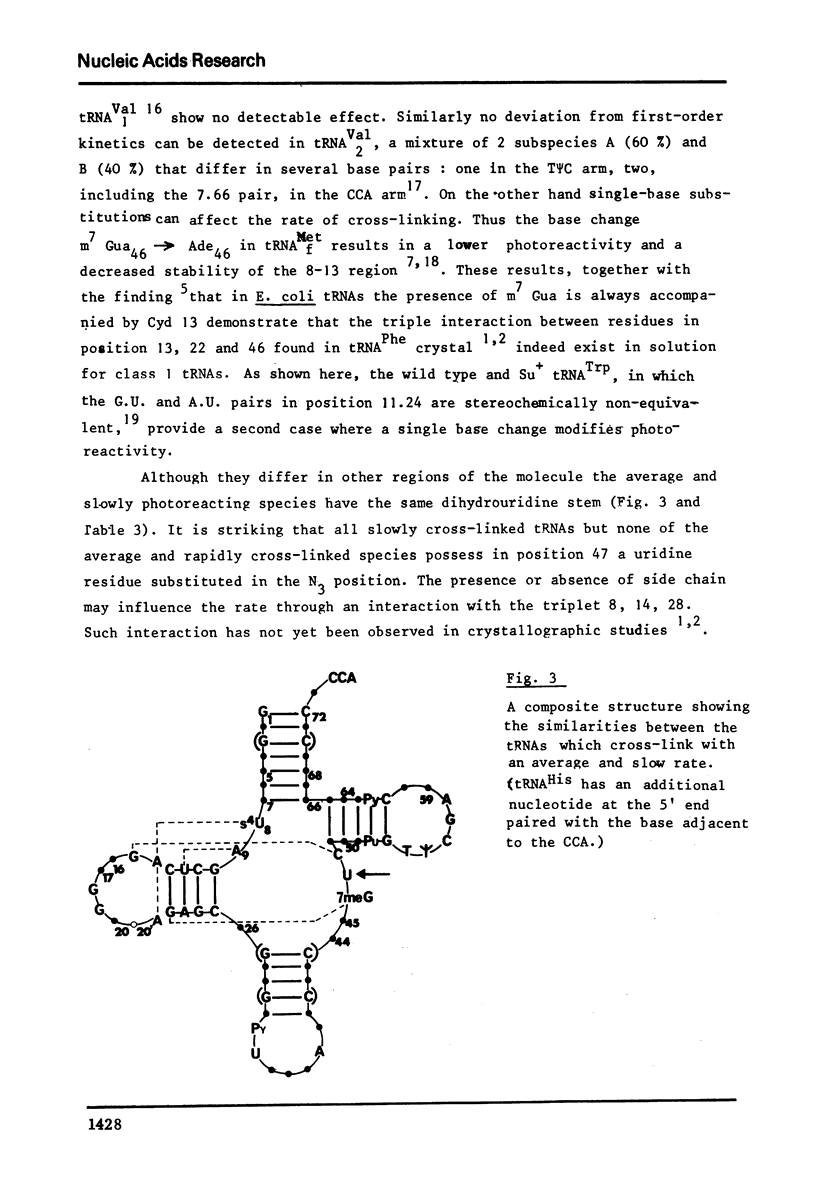
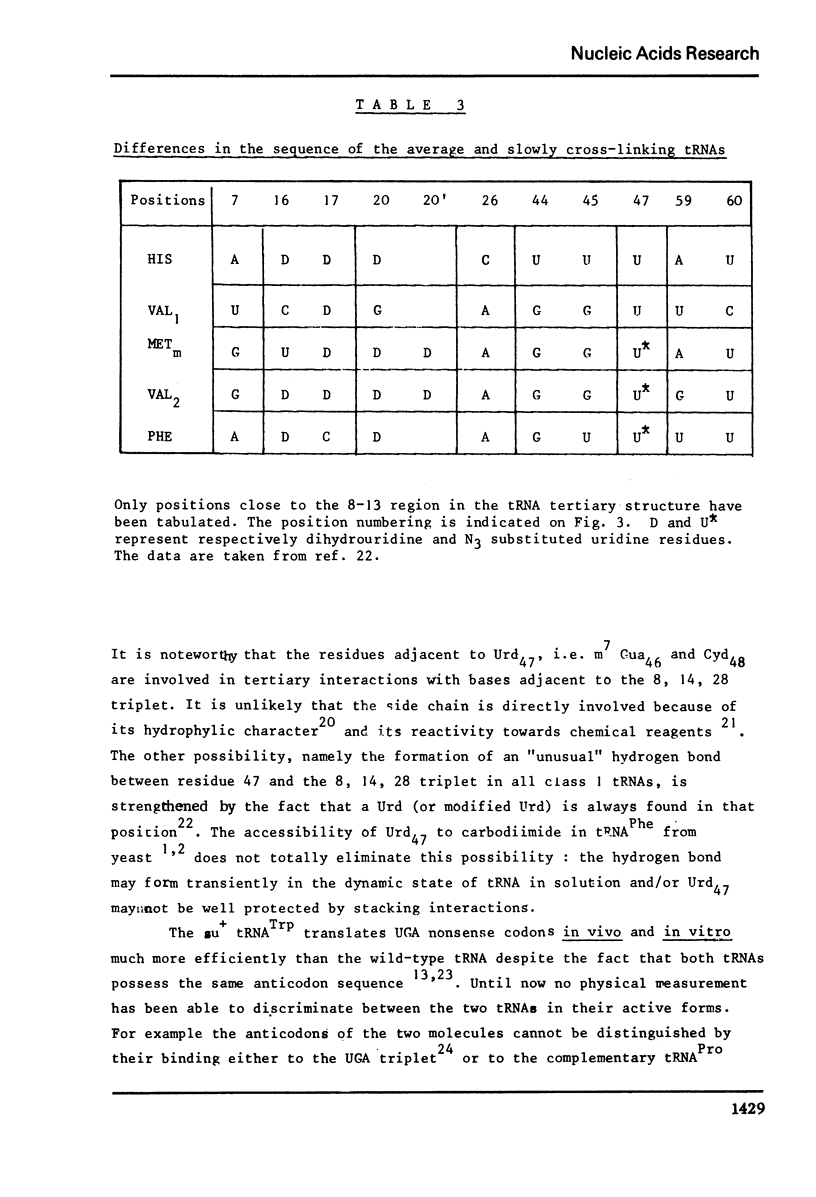


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrom D. E., Leonard N. J. Photoreaction of 4-thiouracil with cytosine. Relation to photoreactions in Escherichia coli transfer ribonucleic acids. Biochemistry. 1972 Jan 4;11(1):1–9. doi: 10.1021/bi00751a001. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H., Danchin A. Fluorescence of tryptophanyl-tRNA(Trp) from E. coli; An interaction between the indole and tRNA and its dependence on tRNA conformation. FEBS Lett. 1973 Mar 1;30(2):236–238. doi: 10.1016/0014-5793(73)80659-3. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H., Danchin A., Grunberg-Manago M. The effect of an intramolecular cross-link on reversible denaturation in tryptophan transfer ribonucleic acid from Escherichia coli. Biochemistry. 1973 Dec 18;12(26):5393–5399. doi: 10.1021/bi00750a023. [DOI] [PubMed] [Google Scholar]
- Carré D. S., Thomas G., Favre A. Conformation and functioning of tRNAs: cross-linked tRNAs as substrate for tRNA nucleotidyl-transferase and aminoacyl synthetases. Biochimie. 1974;56(8):1089–1101. doi: 10.1016/s0300-9084(74)80097-0. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Cole P. E., Hilbers C. W., Shulman R. G. The molecular mechanism of thermal unfolding of Escherichia coli formylmethionine transfer RNA. J Mol Biol. 1974 Jul 25;87(1):63–88. doi: 10.1016/0022-2836(74)90560-9. [DOI] [PubMed] [Google Scholar]
- Favre A., Michelson A. M., Yaniv M. Photochemistry of 4-thiouridine in Escherichia coli transfer RNA1Val. J Mol Biol. 1971 May 28;58(1):367–379. doi: 10.1016/0022-2836(71)90252-x. [DOI] [PubMed] [Google Scholar]
- Favre A., Yaniv M. Introduction of an intramolecular flourescent pobe in E. coli tRNA(Val)(1). FEBS Lett. 1971 Oct 1;17(2):236–240. doi: 10.1016/0014-5793(71)80154-0. [DOI] [PubMed] [Google Scholar]
- Favre A., Yaniv M., Michelson A. M. The photochemistry of 4-thiouridine in Escherichia coli t-RNA Vał1. Biochem Biophys Res Commun. 1969 Oct 8;37(2):266–271. doi: 10.1016/0006-291x(69)90729-3. [DOI] [PubMed] [Google Scholar]
- Friedman S. Patterns of base modification in tRNA. Nat New Biol. 1973 Jul 4;244(131):18–20. doi: 10.1038/newbio244018a0. [DOI] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Högenauer G. Binding of UGA to wild type and suppressor tryptophan tRNA from E. coli. FEBS Lett. 1974 Mar 1;39(3):310–312. doi: 10.1016/0014-5793(74)80137-7. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Suddath F. L., Quigley G. J., McPherson A., Sussman J. L., Wang A. H., Seeman N. C., Rich A. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science. 1974 Aug 2;185(4149):435–440. doi: 10.1126/science.185.4149.435. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Bierbaum J. Use of photochemically induced cross-linking as a conformational probe of the tertiary structure of certain regions in transfer ribonucleic acid. Biochemistry. 1973 May 8;12(10):1977–1984. doi: 10.1021/bi00734a022. [DOI] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Robertus J. D., Ladner J. E., Finch J. T., Rhodes D., Brown R. S., Clark B. F., Klug A. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature. 1974 Aug 16;250(467):546–551. doi: 10.1038/250546a0. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Lührmann R., Gassen H. G. On the mRNA induced conformational change of AA-tRNA exposing the T-pse-C-G sequence for binding to the 50S ribosomal subunit. Biochem Biophys Res Commun. 1974 Feb 4;56(3):807–814. doi: 10.1016/0006-291x(74)90677-9. [DOI] [PubMed] [Google Scholar]
- Siddiqui M. A.Q., Ofengand J. The 3'-terminal hexanucleotide, CAACCA, is not essential for fragment recombination or cross-linking in E. coli tRNA(fMet). FEBS Lett. 1972 May 1;22(2):169–174. doi: 10.1016/0014-5793(72)80036-x. [DOI] [PubMed] [Google Scholar]
- Siddiqui M. A.Q., Ofengand J. The function of pseudouridylic acid in transfer ribonucleic acid. Photochemical and chemical modification of formylmethionine tRNA of E. Coli. FEBS Lett. 1971 Jun 10;15(2):105–110. doi: 10.1016/0014-5793(71)80033-9. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Sequence relationship of three valine acceptor tRNAs from Escherichia coli. Nat New Biol. 1971 Sep 22;233(38):113–114. doi: 10.1038/newbio233113a0. [DOI] [PubMed] [Google Scholar]


