Abstract
The Russian wheat aphid, Diuraphis noxia (Kurdjumov), is an invasive insect pest that causes serious yield losses in bread wheat, Triticum aestivum L., durum wheat, T. turgidum L and barley, Hordeum vulgare L. Successful management of D. noxia has been achieved through resistant varieties via plant antixenosis (aphid non-preference), antibiosis (reduced aphid growth or fecundity), tolerance (plant compensatory growth after aphid feeding), or a combination of each. Previous phenotyping experiments revealed that plants of the variety Stoneham resist D. noxia damage via tolerance. In the present study, genes involved in upstream regulation of jasmonic acid (JA), salicylic acid (SA), ethylene (ET), auxin (AUX) and abscisic acid (ABA) biosynthetic pathways were monitored using qRT-PCR in Stoneham and susceptible Otis barley plants after D. noxia biotype 2 feeding. Results indicate that D. noxia tolerance in Stoneham plants is related to greater constitutive expression of JA-, ET- and AUX-biosynthetic pathway genes than in susceptible Otis plants, suggesting the possibility of immediate plant adjustments due to the stress of D. noxia feeding. There was limited induction of genes in the ET-(ACCS) and IAA (TDC) pathways in Stoneham tissues after D. noxia feeding. JA pathway genes upregulated in Otis tissues after D. noxia infestation failed to successfully defend Otis plants. AUX and ABA transcripts in Otis may be associated with developmental collapses resulting from source and sink adjustment failures.
Keywords: Diuraphis noxia, barley, biotypes, qRT-PCR, resistance, tolerance
Introduction
The Russian wheat aphid, Diuraphis noxia (Kurdjumov), is a devastating global pest of bread wheat, Triticum aestivum L., durum wheat, T. turgidum L. and barley, Hordeum vulgare L.1-3 The cumulative losses to all US small grain production due to D. noxia control, grain losses and lost community economic activity from 1986 to 1993 were valued at ~$1 billion.4 For US barley, D. noxia infestation of 0.7 million ha in 1992 caused losses of ~$18.5 million.5 In states east of the US Rocky Mountains, D. noxia has severely affected barley cultivation6 to the extent that until recently, producers in some areas were no longer attempting to grow barley.7 Soon after discovery of D. noxia in the US in 1986, an expedited effort to develop aphid-resistant wheat and barley cultivars began8 and continues today. Ten Dn (Diuraphis noxia resistance) genes from wheat9-13 and one gene from rye14 governing resistance to D. noxia have been characterized. The wheat cultivar ‘Halt’15 with resistance based on the single dominant gene Dn4 was introduced for cultivation, followed by ‘Prairie Red,’ ‘Yumar,’ ‘Prowers,’ ‘Prowers 99’16 and ‘Ankor’17 all of which express Dn4. Webster and others18 identified D. noxia resistance in barley that was transferred to the barley germplasms STARS-9301B and STARS-9577B, each containing two genes, Rdn1 and Rdn2, for resistance to D. noxia biotype 1.6,19 The barley varieties Stoneham, containing 9577B resistance, and Sidney, containing 9301B resistance, have since been released.7 Stoneham exhibits multiple categories of resistance to both D. noxia biotype 1 and biotype 2.20
Nevertheless, virulence has been documented in D. noxia populations in Africa, Europe, North America and South America,16,21-24 suggesting that additional breeding efforts are necessary for identifying resistance that exerts less selection pressure on D. noxia populations to delay biotype evolution. Diuraphis noxia feeds mainly on the adaxial surface of newly emerging leaves or within rolled leaves that result from D. noxia infestation. D. noxia feeding results in the breakdown leaf chloroplasts, leading to white, yellow, purple, or reddish-purple interveinal chlorosis on infested leaves.25 Symptomatic leaves have reduced photosynthetic efficiency, which leads to reduced vigor and increased sensitivity to environmental stresses. In young plants, heavy infestation causes tillers to become prostrate. In mature plants, infestations result in a failure of leaves of tillers to unfurl, trapping the flag leaf and causing it to curl inward.27 Both intercellular25 and intracellular27 penetration of mesophyll tissue of D. noxia-susceptible wheat plants may lead to dramatic shifts in phloem composition and create a nutritionally enhanced phloem diet28,29 rich in phloem-mobile mineral nutrients, amino acids and carbohydrates.30
Many factors affect the expression of arthropod resistance genes in plants. Painter31 partitioned plant resistance into the functional categories of antibiosis, non-preference (antixenosis) and tolerance. Antibiosis is a measure of the negative influence of the plant on the biology of an insect attempting to use that plant as a host31 and may be manifested as reduced insect body size and weight, prolonged development, reduced fecundity, or failure to pupate or emerge. Antixenosis (non-preference) resistance occurs when the plant acts as a poor host to an arthropod as a source of food, shelter, or an oviposition site. Antixenosis results in reduced colonization of a plant by arthropods, thus reducing damage losses caused by the pest.32,33
Tolerance resistance occurs when plants withstand or compensate for insect damage to yield significantly greater dry mass than susceptible plants under similar infestation.32,33 Tolerance raises plant economic injury levels and delays or negates the need for costly chemical control. The category of resistance expressed often determines the longevity of resistance under field conditions and may influence the occurrence of insect virulence.34 Therefore, the lack of selection pressure exerted by a tolerant accession on the insect population may delay development of virulence and is a desirable trait for incorporation into commercial cultivars for durable arthropod resistance. Despite these advantages, the use of plant tolerance in pest management is limited, due to a poor understanding of the mechanisms and genetics of plant tolerance.33
Experimental evidence obtained to date involving compatible and incompatible interactions between different species of plants and aphids has led to the identification of several types of plant resistance genes and plant insect defense response genes.35 Defense-signaling pathways dependent and independent of salicylate and jasmonate signaling molecules are involved in aphid-activated plant responses.35-38 Eliciting plant response molecules may originate directly from aphids or may be products of aphid endosymbiotic bacteria.39 Wheat and barley gene expression in response to D. noxia populations in North America and South Africa involves many plant sequences that may mediate antibiosis (hypersensitive defensive responses, cellular transport, Ca2+-influx, exocytosis) or tolerance (self-defense against toxins, proteolysis, chloroplast and mitochondria function, carbohydrate metabolism, cellular homeostasis).40-44
Plant recognition of insect feeding probes and subsequent defense response gene activation through release of different elicitors initiates the expression of genes in different defense signaling pathways and is considered crucial to insect-plant interactions. Signaling pathways driven by jasmonic acid (JA), salicylic acid (SA), ethylene (ET), abscisic acid (ABA), giberellic acid, ROS and nitric oxide ultimately produce plant defense proteins and or secondary compounds. SA mediates localized plant tissue hypersensitive (HR) responses, systemic acquired resistance (SAR),36 and stimulates the expression of defense response genes, including pathogenesis-related (PR) proteins or PR genes with apoplastic localization. PR genes have been shown to be upregulated at the protein level in interactions between D. noxia on aphid-resistant wheat genotypes.45,46 Related experiments at the molecular level have provided different results, albeit with different D. noxia-resistant wheat genotypes containing different resistance genes.39,46
Genes putatively involved in JA biosynthesis and JA-mediated defense responses including 12-oxophytodienoate 10,11-reductase (OPDR), lipoxygenase (LOX) and cytochrome P450 are strongly induced by D. noxia feeding.40,44,47 Although the role of ET in plant induced defense responses of aphid feeding remains largely less explored, D. noxia feeding significantly increases ET production in foliage of aphid-resistant barley cultivars compared with susceptible cultivars.48 Genes encoding proteins involved in ET production or ET signaling (ACC oxidase, sterol ∇-7 reductase and ET-responsive elements) are also upregulated in aphid-resistant wheat infested with D. noxia.40,47
The involvement of the growth regulators ABA and giberellic acid in plant responses to aphid feeding is poorly documented. However, ABA is involved in plant response to biotic stresses,49,50 and gibberellic acid plays a role in plant defense response signaling by regulating β-1,3-glucanase release from aleurone cells.51 Sequences putatively involved in biosynthesis of ABA or giberellic acid or activated by ABA or giberellic acid signals (transketolase and aldehyde oxidase) are upregulated in D. noxia-infested resistant wheat leaf tissues.40,47
In our recent study, tolerance (reduced tissue dry weight loss) to D. noxia North American biotype 1 and the current predominant biotype—biotype 2—was observed in the D. noxia resistant barley cultivar Stoneham.20 This tolerance may be due to the activation of the biomass-accumulating metabolic genes primarily involved in cell repair and strengthening or photosynthesis regulation.35 In a functional genomic study, Gutsche and others43 found that the D. noxia biotype 1-tolerant barley variety Sidney expressed several genes associated with plant defense and ROS scavenging.52,53
An understanding of the functional genomic responses of barley plants tolerant to D. noxia feeding may provide information about the physiological mechanisms involved in plant tolerance responses and help identify phenotypic plant breeding characters linked to D. noxia tolerance. We hypothesized that feeding by D. noxia biotype 2 on D. noxia-tolerant barley plants activates defense signaling- and growth hormone-pathway genes when compared with susceptible plants. Thus, the objectives of the study were to identify differences between resistant Stoneham plants and susceptible Otis plants in constitutive and induced expression of representative genes in the JA-, SA-, ET-, IAA- and ABA-pathways.
Results
Salicylate pathway
In the salicylic acid biosynthetic pathway, there were no significant differences in isochorismate synthase (ICS) expression levels either between or within genotypes (Table 2). Although ICS expression was greater in Stoneham leaves at 120 hpi than at 3, 24, or 72 hpi, there was no difference in expression between Stoneham and susceptible Otis leaves at 120 hpi. In susceptible Otis leaves, MAPK kinase (MAPKK) expression did not differ between any hpi time points, but in Stoneham leaves MAPKK expression decreased over time, with significantly less expression at 120 hpi than at 0-, 24- or 72 hpi (Table 2). In addition, there were no differences between Otis and Stoneham in constitutive expression of either ICS (p < 0.46) or MAPKK transcripts (p < 0.07).
Table 2. Mean ± SE upregulation of isochorismate synthase (ICS) and mitogen-activated protein kinase kinase (MAPKK) in first and second leaves of Stoneham (aphid resistant) and Otis (aphid susceptible) barley plants after feeding by Diuraphis noxia biotype 2 for 3, 24, 72 or 120 h.
| Barley genotype |
Hours post infestation3 |
Mean ± SE log2 fold change1,2 |
|
|---|---|---|---|
| ICS | MAPKK | ||
| Otis |
0 (control) |
1.00 ± 0.00 |
1.00 ± 0.00 |
| |
3 |
0.37 ± 0.10 b |
0.84 ± 0.13 ab |
| |
24 |
1.17 ± 0.72 b |
0.94 ± 0.15 ab |
| |
72 |
0.58 ± 0.01 b |
0.72 ± 0.35 ab |
| |
120 |
2.73 ± 0.73 ab |
0.16 ± 0.05 b |
| Stoneham |
0 |
1.40 ± 1.16 b |
1.40 ± 0.42 a |
| |
3 |
0.25 ± 0.23 b |
0.89 ± 0.19 ab |
| |
24 |
0.32 ± 0.01 b |
1.04 ± 0.11 a |
| |
72 |
0.80 ± 0.38 b |
1.14 ± 0.17 a |
| 120 | 4.86 ± 1.14 a | 0.17 ± 0.02 b | |
Means within a column followed by the same letter are not significantly different (α > 0.05, Tukey’s HSD test).1 relative to susceptible Otis uninfested (0 h post post infestation) control.2 Fold change calculated as ΔΔCt = [(Ct for sample cDNA – Ct for control cDNA)GOI ] − [(Ct for sample cDNA – Ct for control cDNA)HK]. GOI = gene of interest. HK = UCE internal control gene. For MAPK kinase, fold change = [(1 + efficiency of GOI) –ΔCtGOI]/ [(1 + efficiency of HK) –ΔCtHK)] (Schmittgen and Livak 2008);3 a 15 min buffer period allowed for introduced aphids to settle onto leaves.
Jasmonate pathway
There were no significant differences in the PLDα1 or FAD3 content between Otis and Stoneham foliage at all post infestation times (Table 3). LOX2 upregulation also did not differ significantly between any post infestation times within Otis or Stoneham tissues, but expression in Stoneham leaves at 24 and 72 hpi was significantly greater than in Otis leaves (Table 3). AOS expression was significantly (p < 0.0001) greater in Otis plants at 72 hpi than in any other Otis-hpi combination and Stoneham leaves at 72 hpi (Table 3). The same trend was evident for OPR3 upregulation, with expression in Otis leaves significantly greater at 120 hpi than in any other Otis- or Stoneham-hpi combination
Table 3. Mean ± SE upregulation of phospholipase Dα1 (PLD α1), lipoxygenase 2 (LOX2), allene oxide synthase (AOS), allene oxide cyclase (AOC), Ω-3 fatty acid desaturase (FAD3) and 12-oxophytodienoate reductase 3 (OPR3) in first and second leaves of Stoneham (aphid resistant) and Otis (aphid susceptible) barley plants after feeding by Diuraphis noxia biotype 2 for 3, 24, 72 or 120 h.
| Barley genotype |
Hours post infestation3 |
Mean ± SE log2 fold change1,2 |
|||||
|---|---|---|---|---|---|---|---|
| PLDα1 | LOX2 | AOS | AOC | FAD3 | OPR3 | ||
| Otis |
0 (control) |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
| |
3 |
21.5 ± 0.5 a |
7.6 ± 4.2 cd |
1.3 ± 0.7 c |
3.9 ± 2.0 c |
0.7 ± 0.1 a |
13.7 ± 3.3 b |
| |
24 |
11.0 ± 1.1 a |
1.7 ± 0.6 d |
0.2 ± 0.1 c |
3.0 ± 1.4 c |
1.1 ± 0.3 a |
10.4 ± 8.0 b |
| |
72 |
11.5 ± 4.1 a |
9.5 ± 2.1 cd |
38.3 ± 5.6 a |
7.6 ± 6.4 bc |
0.7 ± 0.3 a |
20.8 ± 19.2 b |
| |
120 |
15.5 ± 7.0 a |
18.7 ± 0.5 bcd |
0.5 ± 0.5 c |
17.0 ± 2.9 abc |
1.3 ± 0.7 a |
120.6 ± 23.5 a |
| Stoneham |
0 |
20.4 ± 5.4 a |
24.1 ± 8.1 abc |
15.1 ± 6.5 bc |
12.4 ± 6.7 abc |
1.8 ± 1.0 a |
19.6 ± 4.3 b |
| |
3 |
28.1 ± 8.8 a |
16.9 ± 0.4 bcd |
8.9 ± 0.9 bc |
21.2 ± 2.1 ab |
0.3 ± 0.1 a |
31.3 ± 15.4 b |
| |
24 |
15.3 ± 2.1 a |
27.4 ± 2.0 ab |
25.7 ± 7.4 ab |
26.2 ± 5.1 a |
0.7 ± 0.3 a |
23.6 ± 3.5 b |
| |
72 |
10.3 ± 2.0 a |
39.2 ± 8.6 a |
0.8 ± 0.1 c |
7.9 ± 5.4 bc |
1.2 ± 0.3 a |
14.8 ± 2.5 b |
| 120 | 14.0 ± 1.4 a | 11.3 ± 0.6 bcd | 8.0 ± 7.1 bc | 8.0 ± 0.0 bc | 1.7 ± 0.5 a | 28.1 ± 5.5 b | |
Means within a column followed by the same letter are not significantly different (α > 0.05, Tukey’s HSD test).1 relative to susceptible Otis uninfested (0 h post post infestation) control.2 Fold change calculated as ΔΔCt = [(Ct for sample cDNA – Ct for control cDNA)GOI] − [(Ct for sample cDNA – Ct for control cDNA)HK]. GOI, gene of interest. HK, UCE internal control gene. A 15 min buffer period allowed for introduced aphids to settle onto leaves.
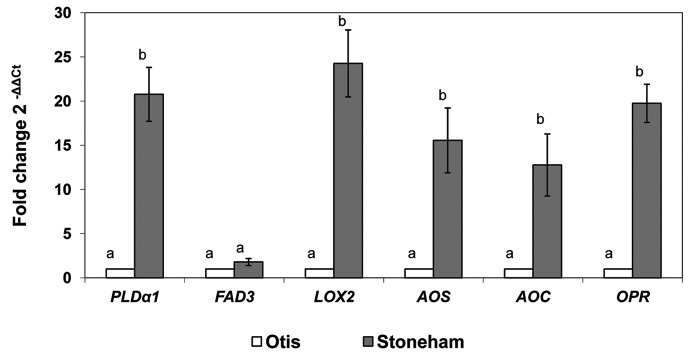
Within each genotype, there were no differences in AOC upregulation at any hpi time. (Table 3). Conversely, constitutive expression of all but one JA pathway gene (FAD3) was significantly greater in Stoneham than in Otis (Fig. 1). These included PLDα1 (p < 0.0006), LOX2 (p < 0.0009), AOS (p < 0.0074), AOC (p < 0.0154) and OPR (p < 0.0001) (Fig. 1). Constitutive expression was markedly greater in Stoneham than in Otis, and ranged from 12.8fold greater for AOC to 24.3fold greater for LOX2 (Fig. 1).
Figure 1.
Constitutive transcript levels (mean ± SE log2 fold change) of the jasmonate pathway genes phospholipase Dα1 (PLD α1), Ω-3 fatty acid desaturase (FAD3, lipoxygenase 2 (LOX2), allene oxide synthase (AOS), allene oxide cyclase (AOC) and 12-oxophytodienoate reductase 3 (OPR3) in two-leaf stage Stoneham and Otis barley plant tissues un-infested by Diuraphis noxia biotype 2. Fold change calculated as ΔΔCt = [(Ct for sample cDNA – Ct for control cDNA)GOI ] − [(Ct for sample cDNA – Ct for control cDNA)HK]. (Schmittgen et al. 2008);3 GOI, gene of interest. HK, UCE internal control gene. For each gene, means below a different letter differ significantly at p < 0.05 (PROC TTEST).
Ethylene pathway
There were no differences in expression of 1-aminocyclopropane-1-carboxylate oxidase (ACCO) between or within genotypes (Table 4). However, upregulation of 1-aminocyclopropane-1-carboxylate synthase (ACCS) was significantly (p < 0.0001) greater at 120 hpi in Otis tissues than at 3 hpi, and greater in Stoneham tissues at 0 or 72 hpi (Table 4). The opposite trend was true for constitutive expression of these ET pathway genes. ACCO constitutive expression was significantly greater (p < 0.0165) in Stoneham than Otis but there were no differences between varieties in constitutive expression of ACCS (p < 0.0602) (Fig. 2).
Table 4. Mean ± SE upregulation of genes from ethylene (ET), auxin-indole acetic acid (IAA) and abscisic acid (ABA) pathways in first and second leaves of Stoneham (aphid resistant) and Otis (aphid susceptible) barley plants after feeding by Diuraphis noxia biotype 2 for 3, 24, 72 or 120 h.
| Barley genotype |
Hours post infestation3 |
Mean ± SE log2 fold change1,2 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
|
Ethylene pathway |
Indole-3-acetic acid (auxin) pathway |
Abscisic acid pathway |
||||
| ACCO | ACCS | TDC | T5M | Amidase | NCED | P5CS1 | ||
| Otis |
0 (control) |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
1.00 ± 0.0 |
| |
3 |
1.8 ± 0.4 b |
4.3 ± 1.6 c |
42.9 ± 36.4 b |
12.6 ± 0.6 bc |
1.1 ± 0.6 a |
1.1 ± 1.0 b |
1.1 ± 0.5 b |
| |
24 |
1.4 ± 0.5 b |
21.9 ± 1.1 bc |
394.9 ± 9.7 b |
8.0 ± 3.1 c |
0.9 ± 0.6 a |
0.9 ± 0.8 b |
0.8 ± 0.2 b |
| |
72 |
1.8 ± 0.8 b |
25.3 ± 7.9 bc |
2340.3 ± 512.1 b |
12.3 ± 0.3 bc |
1.1 ± 0.5 a |
0.8 ± 0.5 b |
1.4 ± 0.3 ab |
| |
120 |
1.4 ± 0.6 b |
301.7 ± 122.2 ab |
29238.6 ± 4991.3 a |
98.7 ± 2.4 a |
1.8 ± 1.0 a |
10.7 ± 0.3a |
4.9 ± 2.3 a |
| Stoneham |
0 |
3.9 ± 2.0 ab |
3.0 ± 2.2 c |
21.7 ± 8.8 b |
12.9 ± 2.2 bc |
0.7 ± 0.4 a |
1.2 ± 1.2 b |
1.0 ± 0.6 b |
| |
3 |
4.5 ± 0.1 ab |
19.1 ± 16.8 bc |
24.4 ± 7.7 b |
16.8 ± 4.1 b |
1.1 ± 0.6 a |
0.5 ± 0.4 b |
1.0 ± 0.1 b |
| |
24 |
2.2 ± 0.1 b |
44.8 ± 7.7 bc |
291.0 ± 137.2 b |
15.8 ± 1.2 bc |
0.8 ± 0.4 a |
0.7 ± 0.5 b |
0.8 ± 0.6 b |
| |
72 |
6.1 ± 0.6 a |
11.6 ± 2.6 c |
551.7 ± 80.8 b |
10.95 ± 1.1 bc |
0.9 ± 0.3 a |
0.9 ± 0.8 b |
1.5 ± 1.1 ab |
| 120 | 2.6 ± 0.6 b | 463.0 ± 177.0 a | 5118.7 ± 1242.0 b | 12.3 ± 0.3 bc | 1.6 ± 0.0 a | 8.7 ± 3.1 a | 2.0 ± 0.2 ab | |
Means within a column followed by the same letter are not significantly different (α > 0.05, Tukey’s HSD test).1 relative to susceptible Otis uninfested (0 h post post infestation) control.2 Fold change calculated as ΔΔCt = [(Ct for sample cDNA – Ct for control cDNA)GOI] − [(Ct for sample cDNA – Ct for control cDNA)HK]. (Schmittgen and Livak 2008);3 GOI = gene of interest. HK = UCE internal control gene. A 15 min buffer period allowed for introduced aphids to settle onto leaves.
Figure 2.
Constitutive transcript levels (mean ± SE log2 fold change) of ethylene [ACCO and ACCS] and auxin-indole acetic acid [TDC, T5M, amidase] pathway genes in two-leaf stage Stoneham and Otis barley plant tissues un-infested by Diuraphis noxia biotype 2. Fold change calculated as ΔΔCt = [(Ct for sample cDNA – Ct for control cDNA)GOI] − [(Ct for sample cDNA – Ct for control cDNA)HK]. (Schmittgen and Livak 2008);3 GOI = gene of interest. HK = UCE internal control gene. For each gene, means below a different letter differ significantly at p < 0.05 (PROC TTEST).
Indole-3-acetic acid (Auxin) pathway
The trend in expression observed for ACCS was also evident for tryptophan decarboxylase (TDC) and tryptophan 5-monooxygenase (T5M). Upregulation of both TDC and T5M was significantly (p < 0.0001) greater at 120 hpi in Otis tissues than in Otis or Stoneham at any other hpi time point (Table 4). There were no differences in expression of amidase between or within genotypes. Constitutive expression of both TDC and T5M was significantly greater in Stoneham than Otis (p < 0.0015 for TDC, p < 0.0005 for T5M) but there were no differences between varieties in constitutive expression of amidase (p < 0.0927) (Fig. 2).
Abscisic acid pathway
The trend in expression of 9-cis-epoxycarotenoid dioxygenase (NCED) was similar for each barley genotype, with expression significantly (p < 0.0001) greater at 120 hpi than at all other post infestation times within genotypes. The same was true for δ 1-pyrroline-5-carboxylate synthetase 1 (P5CS1) in Otis, but not in Stoneham, where there were no differences between any post infestation times (Table 4). There were no differences evident between the resistant and susceptible varieties in constitutive levels of these transcripts (data not shown).
Discussion
Manifestation of plant resistance to insects is a complex plant-insect interaction displayed as antibiosis, antixenosis, tolerance and or combinations of these categories of resistance. Numerous plant biochemical pathways regulating plant defense, development and metabolism are simultaneously or sequentially involved in these interactions. Tolerance resistance to insects is unique in terms of the plant’s ability to withstand damage accrued by insect injury and further compensate for damage through growth and developmental activities. Plant tolerance responses are of specific interest since tolerance does not normally elicit the occurrence on insect virulence. We hypothesized that plant hormone genes associated with defense- and developmental pathway-signaling are significantly altered in response to D. noxia feeding in the transcriptome of D. noxia-tolerant plants. Our results from qRT- PCR assays monitoring key genes participating in biosynthetic pathways of these hormones support this hypothesis. However, their induction by D. noxia does not appears to condition resistance. In addition, the resulting expression of tolerance plant phenotypes may also be related to transcriptome changes that lead to the down stream synthesis of proteins functioning in plant tolerance. Upon insect challenge and attack, a series of phased cellular plant responses begin locally that may eventually result in systemic responses. At the onset of D. noxia feeding, plant cell wall components participate in insect defense responses to perceive insect elicitor signals prior to JA or SA signaling.35 Signal transduction in aphid-induced plant responses is a focus of interest,54 involving interactions of both SA and JA regulated pathways. However, the SA pathway appears less effective in eliciting the defenses of barley plants against aphids, including D. noxia,40,47 than in wheat, where evidence exists that SA is involved in wheat resistance responses to D. noxia.55
Our results also demonstrated a pattern of defense gene upregulation as opposed to downregulation - a similar occurrence in other plant-aphid interaction systems.35,54 However in D. noxia-tolerant barley, induction of SA- (ICS1, MAPKK) and JA pathway transcripts (PLDα1, LOX2, AOS, AOC, FAD3, OPR3) appears to have little if any involvement in D. noxia resistance. Similar results with D. noxia on wheat have been observed for SA transcripts but not JA transcripts.44,47,56 The lack of SA signaling in barley may be indicative of conservation measures used by tolerant plants to expend fewer metabolic costs toward D. noxia defense.
An additional contrast of our results to those of previous studies is that although we observed several JA pathway genes to be induced to greater levels in susceptible plants than in resistant plants by D. noxia feeding, most of these genes were constitutively expressed at greater levels in tolerant plants than in resistant plants. As such, the lack of induction of these genes suggests that barley tolerance to D. noxia does not involve JA-mediated induced defense responses, but instead that tolerance relies on constitutive expression of JA-transcripts in D. noxia tolerant plants (Fig. 1).
Ethylene transcripts mediate plant stress and pathogen responses57,58 and in rice plants, ACCO and ACCS are upregulated in response to feeding by the brown planthopper, Nilaparvata lugens (Stal).59 In addition to regulating plant growth and development, abscisic acid (ABA) also mediates environmental stress responses.60 Rapid ABA biosynthesis occurs in response to dehydration61 and is closely correlated with loss of cell turgor.62 D. noxia infestation causes upregulation of numerous transcripts related to ABA- and ET-signaling pathways in resistant wheat plants, but no such transcripts are upregulated in D. noxia-susceptible plants.40,47 These results strongly suggest a role for ABA- and ET-signaling pathway genes in D. noxia tolerance. Our results with barley however, indicate that ET-genes mediate resistance via constitutive expression, as opposed to induction. Though uniquely upregulated in susceptible plants, induction of ABA- and ET-signaling pathway genes failed to prevent D. noxia biotype 2 phloem removal and expression of the susceptible phenotype. Yet the same ET-genes were highly constitutively expressed in tolerant Stoneham barley plants (Fig. 2, Table 4).
Auxin is produced largely in apical leaf regions (the D. noxia feeding site) and transported via shoots and roots to regulate root growth.63,64 As suggested by Boyko and others40 and Kawano,65 auxin pathway signaling may be associated with accelerated production of auxin-related transcripts in aphid susceptible plants to enhance ROS production. In D. noxia-susceptible wheat plants, ~9% of the upregulated transcriptome involves auxin signaling and is absent in resistant plants.47 Increased root auxin content in drought stressed plants is attributed to polar transport process toward roots.66
Auxin and ET are also intimately linked and production of one stimulates production of the other.67,68 Susceptible Otis plants exhibit leaf rolling upon D. noxia attack, a physiological symptom associated with dehydration. Thus, upregulation of ET- and IAA- pathway genes may be an attempt by plants to mitigate D. noxia-induced dehydration.
ET biosynthetic pathway genes exhibited similar patterns of upregulation in D. noxia tolerant and susceptible plants. Interestingly, ABA biosynthetic pathway genes were upregulated belatedly in Otis but displayed little temporal variation in expression in resistant Stoneham plants. Finally, the TDC and T5M IAA genes were upregulated to extraordinarily high levels in Otis plants, and of these, only TDC was belatedly expressed in Stoneham plants.
Differential display studies involving wheat genotypes possessing different genes associated with antibiosis (Dn1) and tolerance (Dn2) resistance to D. noxia demonstrate clear genomic differences in responses of plants with different types of resistance. Genotypes possessing tolerance regulate transcripts involved in cell homeostasis, chloroplast proteins and energy conservation, whereas genotypes possessing antibiosis regulate transcripts involved in elicitor recognition, ion flux, oxidative stress detoxification, toxin production and ubiquitin-mediated protein degradation.41,69
Our detection of significantly greater constitutive expression of JA-, ET- and IAA-pathway genes in resistant Stoneham plants has not been demonstrated previously in cereal plants. Although JA is involved in diverse biological processes in plants, it serves a unique role in seed germination, root growth, leaf senescence and stomatal opening.70 Ellis and others71 and Ellis and Turner72 showed that constitutive expression of JA or ET in Arabidopsis plays a role in resistance to the green peach aphid, Myzus persicae (Sulzer) and plant pathogens. In related studies, Leon-Reyes and others73 showed that constitutively higher expression of JA and ET led to increased JA-dependent defenses in Arabidopsis. Constitutive activation of the JA pathway also enhances production of secondary metabolites in tomato.74 Auxin regulates the expression of genes associated with hormone biosynthesis, catabolism and signaling75 and modulates defense and development. Auxin also promotes degradation of a family of transcriptional repressors (Aux/IAA) that bind to auxin response factors (ARFs) and inhibit the transcription of auxin response genes.76 Auxin-mediated attenuation of Arabidopsis defense has been reported previously.77
In our experiments, the heightened constitutive expression of JA and ET biosynthetic pathway genes in D. noxia-tolerant Stoneham barley tissues may help ameliorate stress associated with D. noxia feeding immediately after attack, through adjustments in stomatal opening and root growth. Root growth compensation in Stoneham to D. noxia damage was well established in our earlier studies.20 Increased constitutive expression of IAA genes in Stoneham foliage may be associated with regulating production of other hormones. During post D noxia infestation, the level of IAA expression was extremely high in Otis plants, potentially enhancing the expression of susceptibility symptoms, as reported in several previous studies of plant-pathogen interactions.78-80
Our results identify unique differences in molecular responses of the D. noxia-tolerant barley variety Stoneham to D. noxia feeding. Expression data suggest that rather than induced SA- and JA defense signals, tolerant plants use constitutively expressed growth and developmental pathway genes involving ET- and IAA-signals to respond to D. noxia biotype 2 herbivory.
Materials and Methods
Plant and insect culture
Seed of the D. noxia biotype 2 (RWA2), susceptible barley cultivar Otis and the resistant barley cultivar Stoneham (Otis*4/STARS 9577B)19 were obtained from Dr. Frank B. Peairs, Department of Bioagricultural Sciences and Pest Management, Colorado State University, Fort Collins, CO (seeds.colostate.edu/CSGA/documents/SpringDir2009.pdf).
D. noxia biotype 2 collected from wheat fields near Biggsdale, CO (via the USDA- ARS Plant Science Research Laboratory at Stillwater, OK) were cultured in the greenhouse on susceptible ‘Jagger’ wheat plants at Kansas State University for use in the experiments. The identity of D. noxia biotype 2 was verified in diagnostic plant differential greenhouse assays at Stillwater, OK, and Manhattan, KS. Voucher specimen no. 176 (D. noxia biotype 2) is deposited with the Kansas State University Museum of Entomological and Prairie Arthropod Research.
Plant-insect interactions
Experiments were conducted in May 2009 in a growth chamber (Percival Scientific Inc., Model E-30B) at 22 ± 2°C, 40 - 50% RH and a 14:10 [L:D] h photoperiod, corresponding to optimal conditions for plant growth and insect development. Barley plants were grown in 16.5-cm-diameter plastic pots with Pro-Mix Bx potting mix (Premier ProMix). Seven days after sowing, two-leaf stage Otis and Stoneham plants were selected with similar growth condition by size and appearance.
Plants were organized in a completely randomized design with a factorial treatment design that included two barley genotypes, two D. noxia biotype 2 infestation levels (0 and 30 per plant) and five plant tissue harvest times (0, 3, 24, 72 and 120 h post infestation). For infested treatments, 30 fourth instar D. noxia biotype 2 nymphs were equally introduced onto the first and second leaf blades of each infested plant. A 15 min buffer period was allowed for introduced aphids to settle at each time point. Each of the infested and non-infested plants was enclosed in organdy fabric 100 mesh flexible cages (30 cm diameter × 45cm height) fastened by rubber bands to the base of pots and left undisturbed inside the growth chamber. Three biological replicates of two plants per replicate were used to collect the required plant tissues.
At each post-infestation tissue collection time, plants were carefully removed and the number of colonizing insects was ensured by gentle removal of insects from plants using a feather touch painting brush. Plants were then quickly washed with DEPC treated double distilled water and cut at the collar region with a razor and frozen in liquid nitrogen independently.
RNA isolation and cDNA synthesis
Total RNA was extracted from frozen whole plant tissue using TRI® reagent (Molecular Research Center, Inc.) following the manufacturer's instructions and further purified using Qiagen RNeasy column (Qiagen). Total RNA was treated with DNase I (Sigma) and then spectrometrically quantified using Nanodrop ND-1000® (Fisher Scientific). First strand cDNA was synthesized by reverse transcription of 5 µg of purified total RNA using Superscript™ III Reverse Transcriptase (Invitrogen) according to manufacturer’s instructions.
Defense and developmental pathway genes and primer design
The expressed sequence tags (ESTs) of genes representing different pathways were downloaded from the Barley1 GeneChip.81www.affymetrix.com/analysis/netaffx/xmlquery.affx?netaffx=netaffx4_annot through NetAffx Query. Each EST was represented by multiple sequences and specific ESTs were selected based on their origin from barley (first priority), wheat (second priority) or Arabidopsis, rice, or tomato. The list included six JA pathway genes (PLDα1, LOX2, AOS, AOC, FAD3, OPR3), two SA pathway genes (ICS, MAPKK), two ET pathway genes (ACCO, ACCS), three indole acetic acid (auxin) pathway genes (TDC, T5M, amidase) and two abscisic acid pathway genes (NCED, P5CS1) (Table 1). Sequences representing different unigenes were selected using the unigene database. Specific primers were designed for selected gene sequences using Beacon Designer® software (Premier Biosoft International) under SYBR Green Design, BLAST searched for cross-homology to complete sequences in the barley genome and tested for template structure to avoid formation of primer secondary structures. Primers were purchased from Integrated DNA Technologies, Coralville, IA. The barley ubiquitin conjugating enzyme (UCE) was used as an internal reference gene43,82 and primers were designed using the same method.
Table 1. Affymetrix Barley1 Genechip ESTs representing jasmonic acid (JA), salicylic acid (SA), ethylene (ET), abscisic acid (ABA) and auxin (IAA) biosynsthetic pathway genes used to design RT-PCR primer sequences.
| Gene | Barley 1 Contig | Barley 1 Source | Primer Sequences |
|---|---|---|---|
|
ICS |
8434_AT |
Arabidopsis thaliana |
F 5′TTGGAAAAGGATATAGCACTTTAG3′ R 5′GCAAACTGAGATGCTTTGAG 3′ |
|
MAPKK |
24896_AT |
Oryza sativa |
F 5′GCACCACCAGACCAGTTC3′ R 5′TCGTGAAGTAGTCAGAGAGATC3′ |
|
PLDα1 |
3043_AT |
enzyme |
F 5′CCATCCTCACCACATAGATTGC3′ R 5′CACAAGTTCTGAATCACCAAAGG3′ |
|
FAD3 |
9168_S_AT |
Triticum aestivum |
F 5′GAGACATAATCTACTACCAAACTG3′ R 5′TCCACCTGCTTGAATTGC3′ |
|
LOX2 |
2306_S_AT |
Hordeum vulgare |
F 5′GTGGATGAGTGGAACAAC3′ R 5′CGCCTAGTTGAGTTACAC3′ |
|
AOS |
3097_AT |
Hordeum vulgare |
F 5′GGCACCAAGGTTGAGTTC3′ R 5′CGGTGTAAGGATCGTTGC3′ |
|
AOC |
CEB0020D05R2_S_AT |
Oryza sativa |
F 5′CACCGAGCCACACGCATG3′ R 5′GCAACACACGGAGATTCATTCAAC3′ |
|
OPR3 |
9556_AT |
Lycopersicon esculentum |
F 5′TACACCGACTACCCGTTCC3′ R 5′CCCAAACCCATCTACCATCAC3′ |
|
ACCO |
13312_AT |
Arabidopsis thaliana |
F 5′CGAGACACAAATTAAGAAGTTC3′ R 5′TGAGTAGCTAGAGCAAGTG3′ |
|
ACCS |
15816_AT |
Triticum aestivum |
F 5′GCTGGTGCATACATGGATG3′ R 5′CCGTAAACAAGCAAAACAAAG3′ |
|
TDC |
11623_AT |
enzyme |
F 5′GTCAACCGCCTTCTAATGG3′ R 5′GCTGGTAGTCTTCTTGATGAG3′ |
|
TMO |
4820_S_AT |
Arabidopsis thaliana |
F 5′CACGAAGATGATAAACTGATGAAC3′ R 5′GACAAGAGACTGGATTAATTGAAC3′ |
| Amidase |
14937_AT |
Oryza sativa |
F 5′CTGGCTATCTCACCTGTTAG3′ R 5′GCTCGCATTATCTTCTCAAG3′ |
|
NECD |
4988_AT |
enzyme |
F 5′GGTGATGGACGCACAGTC3′ R 5′GCACCATTCTGTTGATCTACTAG3′ |
|
P5CS-1 |
4853_AT |
enzyme |
F 5′AGTGTGCCGATACTATTAAGC3′ R 5′CTTCAAGAGCAAGCAAAACC3′ |
| UCE | SMEM0014A16R2_S_AT | enzyme |
F 5′CCATCCGAACATCAATAGCAATG3′ R 5′GGTAAGCAGCGAGCAGATTG3′ |
Quantitative real-time PCR (qRT-PCR)
qRT-PCR reactions were performed using an iCycler iQ Real-Time PCR machine (Bio-Rad Laboratories). Each qRT-PCR reaction consisted of 12.5 µl 2x iQ SYBR Green Supermix (Bio-Rad), 1 µl each of forward and reverse primers (10 µM) and 10.5 µl of cDNA as template in a final reaction volume of 25 µl. Each PCR plate included non-infested controls for making comparisons and calculations of different genes at different time points. Each plate carried two technical replicates of each cDNA biological replicate and the UCE internal control gene. Two biological replicates were used because there was little difference in standard errors of the biological replicates 1 and 2 when analyzed in qRT-PCR, and because previous gene expression studies of wheat response to D. noxia herbivory40,47 used a two biological replicate, two technical replicate protocol.
The qRT-PCR amplification protocol was 95°C for 15 min and 40 cycles of 95°C for 30 sec, 52, 54, 58 or 60°C for 15 sec (primer-dependent) and 72°C for 40 sec each. The threshold was determined as the first cycle above the background fluorescence in which all samples were in the exponential amplification phase. After the amplification step, the machine continued to proceed for dissociation (melt) curve analysis in order to determine if only one product was amplified during annealing and amplification. A single peak in the dissociation curve implied that only the gene of interest was amplified. For the dissociation curve, PCR conditions were 95°C for 1 min, 55°C for 1 min and increase in set point temperature after cycle 2 by 0.5°C for each cycle at every 10 sec.
Efficiency ranged from 97.6 to 102.6% for all primers except for MAPK kinase (89.6). Relative gene expression was calculated using the 2 -ΔΔCt method83 with BioRad Gene Expression Macro Version 1.1, where ΔΔCt = [(Ct for sample cDNA − Ct for control cDNA)GOI] − [(Ct for sample cDNA − Ct for control cDNA)HK]. GOI was the gene of interest and HK was the UCE internal control gene. For MAPK kinase, fold change was calculated as [(1 + efficiency of GOI) –ΔCtGOI] / [(1 + efficiency of HK) –ΔCtHK)]. When relative gene expression was calculated using the 2 -ΔΔCt method, the time 0 uninfested Otis control gave a fold change value of 1. There was no difference across replicates and hence no standard error.
Statistical analysis
Treatment differences in transcript accumulation of induced plant responses were assessed for significance after conducting ANOVA using PROC GLM.84 Gene expression in susceptible (Otis) plants and tolerant (Stoneham) plants was determined by comparing mean expression values of plants in different RWA hpi treatments to non-infested control plants of each variety. Differences in constitutive levels of transcript expression between non-infested Otis and Stoneham plants (0 h infestation) were determined by PROC TTEST.84
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This research was supported funding by a Kansas Crop Improvement Association grant to C.M.S. and facilities from Kansas State University and from a Government of India DBT Specialized Biotechnology Training Associateship to M.M. Contribution no. 11–106-J from the Kansas Agricultural Experiment Station. This research was performed in the Gene Expression Facility at Kansas State University, which is supported through the National Science Foundation grant, DBI 0421427.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19139
References
- 1.Jones JW, Byers JR, Butts RA, Harris JL. A new pest in Canada: Russian wheat aphid, Diuraphis noxia (Mordvilko) (Homoptera: Aphididae) Can Entomol. 1989;121:623–4. doi: 10.4039/Ent121623-7. [DOI] [Google Scholar]
- 2.Kovalev OV, Poprawski TJ, Stekolshchikov AV, Vereshchagina AB, Gandrabur A. Diuraphis aizenberg (Hom., Aphididae): key to apterous viviparous females, and review of Russian language literature on the natural history of Diuraphis noxia (Kurdjumov, 1913) J Appl Entomol. 1991;112:425–36. doi: 10.1111/j.1439-0418.1991.tb01076.x. [DOI] [Google Scholar]
- 3.Blackman RL, Eastop VF. Aphids on the World’s Crops. An Identification and Information Guide. 2nd Ed. Chichester: Wiley, 2000:1-476. [Google Scholar]
- 4.Morrison WP, Peairs FB. Response model concept and economic impact. In: Quisenberry SS, Peairs FB, eds. A Response Model for an Introduced Pest-The Russian Wheat Aphid. Lanham, MD: Thomas Say Publication in Entomology. Entomological Society of America, 1998:1-11. [Google Scholar]
- 5.Legg A, Amosson S. Economic impact of the Russian wheat aphid in the western United States. Great Plains Agricultural Council Publication. 1991–1992;147:1993. [Google Scholar]
- 6.Mornhinweg DW, Porter DR, Webster JA. Inheritance of Russian wheat aphid resistance in spring barley germplasm line STARS-9577B. Crop Sci. 2002;42:1891–3. doi: 10.2135/cropsci2002.1891. [DOI] [Google Scholar]
- 7.Bregitzer P, Mornhinweg DW, Jones BL. Resistance to Russian wheat aphid damage derived from STARS 9301B protects agronomic performance and malting quality when transferred to adapted barley germplasm. Crop Sci. 2003;43:2050–7. doi: 10.2135/cropsci2003.2050. [DOI] [Google Scholar]
- 8.Souza EJ. Host plant resistance to Russian wheat aphid (Homoptera: Aphididae) in wheat and barley. In: Quisenberry SS, Peairs FB, eds. A Response Model for an Introduced Pest-The Russian Wheat Aphid. Lanham, MD: Thomas Say Publication in Entomology. Entomological Society of America, 1998:122-47. [Google Scholar]
- 9.Marais GF, Du Toit F. A monosomic analysis of Russian wheat aphid resistance in the common wheat PI 292994. Plant Breed. 1993;111:246–8. doi: 10.1111/j.1439-0523.1993.tb00636.x. [DOI] [Google Scholar]
- 10.Schroeder-Teeter S, Zemetra RS, Schotzko DJ, Smith DJ, Rafi M. Monosomic analysis of Russian wheat aphid (Diuraphis noxia) resistance in Triticum aestivum line PI 137739. Euphytica. 1994;74:117–20. doi: 10.1007/BF00033775. [DOI] [Google Scholar]
- 11.Ma ZQ, Saidi A, Quick JS, Lapitan NLV. Genetic mapping of Russian wheat aphid resistance genes Dn2 and Dn4 in wheat. Genome. 1998;41:303–6. [Google Scholar]
- 12.Liu XM, Smith CM, Gill BS, Tolmay V. Microsatellite markers linked to six Russian wheat aphid resistance genes in wheat. Theor Appl Genet. 2001;102:504–10. doi: 10.1007/s001220051674. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Smith M, Gill S. Identification of microsatellite markers linked to Russian wheat aphid resistance genes Dn4 and Dn6. Theor Appl Genet. 2002;104:1042–8. doi: 10.1007/s00122-001-0831-y. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GR, Papa D, Peng J, Tahir M, Lapitan NLV. Genetic mapping of Dn7, a rye gene conferring resistance to the Russian wheat aphid in wheat. Theor Appl Genet. 2003;107:1297–303. doi: 10.1007/s00122-003-1358-1. [DOI] [PubMed] [Google Scholar]
- 15.Quick JS, Ellis GE, Normann RM, Stromberger JA, Shanahan JF, Peairs FB, et al. Registration of ‘Halt’ wheat. Crop Sci. 1996;36:210. [Google Scholar]
- 16.Haley SD. Winter wheat breeding update from CSU. Agronomy News 20(5). Cooperative Extension Service, Fort Collins, CO: Colorado State University, 2000. [Google Scholar]
- 17.Haley SD, Quick JS, Johnson JJ, Peairs FB, Stromberger JA, Clayshulte SR, et al. Registration of ‘Anchor’ wheat. Crop Sci. 2004;44:1025–6. doi: 10.2135/cropsci2004.1025a. b. [DOI] [Google Scholar]
- 18.Webster JA, Baker CA, Porter DR. Detection and mechanisms of Russian wheat aphid (Homoptera:Aphididae) resistance in barley. J Econ Entomol. 1991;84:669–73. [Google Scholar]
- 19.Mornhinweg DW, Porter DR, Webster JA. Registration of STARS-9577B Russian wheat aphid resistant barley germplasm. Crop Sci. 1999;39:882–3. doi: 10.2135/cropsci1999.0011183X003900030063x. [DOI] [Google Scholar]
- 20.Murugan M, Khan SA, Cardona PS, Orozco GV, Viswanathan P, Reese J, et al. Variation of resistance in barley against biotypes 1 and 2 of the Russian wheat aphid (Hemiptera: Aphididae) J Econ Entomol. 2010;103:938–48. doi: 10.1603/EC09396. [DOI] [PubMed] [Google Scholar]
- 21.Basky Z. Biotypic and pest status differences between Hungarian and South African populations of Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae) Pest Manag Sci. 2003;59:1152–8. doi: 10.1002/ps.750. [DOI] [PubMed] [Google Scholar]
- 22.Haley SD, Peairs FB, Walker CB, Rudolph JB, Randolph TL. Occurrence of new Russian wheat aphid biotype in Colorado. Crop Sci 2004a;.44:1589-92.
- 23.Porter DR, Baker CA, El-Bouhssini M. Resistance in wheat to a new North American-Russian wheat aphid biotype. Plant Breed. 2005;124:603–4. doi: 10.1111/j.1439-0523.2005.01165.x. [DOI] [Google Scholar]
- 24.Tolmay VL, Lindeque RC, Prinsloo GJ. Preliminary evidence of a resistance-breaking biotype of the Russian wheat aphid, Diuraphis noxia (Kurdjumov) (Homoptera: Aphididae) in South Africa. Afr Entomol. 2007;15:228–30. doi: 10.4001/1021-3589-15.1.228. [DOI] [Google Scholar]
- 25.Fouche A, Verhoeven RL, Hewitt PH, Walters MC, Kriel CF, De Jager J. Russian aphid (Diuraphis noxia) feeding damage on wheat, related cereal and a Bromus grass species, In: Walters MC, ed. Progress in Russian wheat aphid (Diuraphis noxia Mordw.) research in the Republic of South Africa. Republic of South Africa: Department of Agriculture Technical Communication 191, 1984:22-33. [Google Scholar]
- 26.Walters MC, Penn F, Du Toit F, Botha TC, Aalbersberg K, Hewitt PH, et al. The Russian wheat aphid, farming in South Africa, Leafl Series, Wheat C3. Pretoria: Government Printer, 1980. [Google Scholar]
- 27.Saheed SA, Liu L, Jonsson L, Botha CEJ. Xylem—as well as phloem—sustains severe damage due to feeding by the Russian wheat aphid. S Afr J Bot. 2007;73:593–9. doi: 10.1016/j.sajb.2007.05.008. [DOI] [Google Scholar]
- 28.Burd JD, Elliott NC. Changes in chlorophyll a fluorescence induction kinetics in cereals infested with Russian wheat aphid. J Econ Entomol. 1996;89:1332–7. [Google Scholar]
- 29.Telang A, Sandstrom J, Dyreson E, Moran NA. Feeding damage by Diuraphis noxia results in a nutritionally enhanced phloem diet. Entomol Exp Appl. 1999;91:403–12. doi: 10.1046/j.1570-7458.1999.00508.x. [DOI] [Google Scholar]
- 30.Macedo TB, Higley LG, Ni X, Quisenberry SS. Light activation of Russian wheat aphid-elicited physiological responses in susceptible wheat. J Econ Entomol. 2003;96:194–201. doi: 10.1603/0022-0493-96.1.194. [DOI] [PubMed] [Google Scholar]
- 31.Painter RH. Insect Resistance in Crop Plants. New York, NY: MacMillan, 1951. [Google Scholar]
- 32.Pedigo LP. Entomology and Pest Management. 3rd ed. Saddle River, NJ: Prentice-Hall, 1999. [Google Scholar]
- 33.Smith CM. Plant Resistance to Arthropods - Molecular and Conventional Approaches. Dordrecht, The Netherlands: Springer, 2005. [Google Scholar]
- 34.Gallun RL. Genetic inter-relationship between host plants and insects. J Environ Qual. 1972;1:259–65. doi: 10.2134/jeq1972.00472425000100030011x. [DOI] [Google Scholar]
- 35.Smith CM, Boyko EV. Mini Review: The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol Exp Appl. 2006;122:1–16. doi: 10.1111/j.1570-7458.2006.00503.x. [DOI] [Google Scholar]
- 36.Walling LL. The myriad plant responses to herbivores. J Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 37.Kaloshian I. Gene-for-gene disease resistance: bridging insect pest and pathogen defense. J Chem Ecol. 2004;30:2419–38. doi: 10.1007/s10886-004-7943-1. [DOI] [PubMed] [Google Scholar]
- 38.Goggin FL. Plant-aphid interactions: Molecular and ecological perspectives. Curr Opin Plant Biol. 2007;10:1–10. doi: 10.1016/j.pbi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Miles PW. Aphid saliva. Biol Rev Camb Philos Soc. 1999;74:41–85. doi: 10.1017/S0006323198005271. [DOI] [Google Scholar]
- 40.Boyko EV, Smith CM, Vankatappa KT, Bruno JM, Deng Y, Starkey SR, et al. The molecular basis of plant gene expression during aphid invasion: wheat Pto- and Pti-like sequences modulate aphid-wheat interaction. J Econ Entomol. 2006;99:1430–45. doi: 10.1603/0022-0493-99.4.1430. [DOI] [PubMed] [Google Scholar]
- 41.Schultz T. Profiling of wounding and Diuraphis noxia induced transcripts in hexaploid wheat using cDNA-AFLP analysis. University of Pretoria: M. Sc. dissertation.
- 42.Van Eck L, Lapitan NLV, Botha A-M. Transcriptional regulation in wheat results in distinct modes of resistance to Diuraphis noxia 18th Biennial International Plant Resistance to Insects (IPRI) Workshop, 10-13 February 2008. Fort Collins, CO: Colorado State University, 2008. [Google Scholar]
- 43.Gutsche A, Heng-Moss T, Sarath G, Twigg P, Xia Y, Lu G, et al. Gene expression profiling of tolerant barley in response to Diuraphis noxia (Hemiptera: Aphididae) feeding. Bull Entomol Res. 2009;99:163–73. doi: 10.1017/S0007485308006184. [DOI] [PubMed] [Google Scholar]
- 44.Liu X, Meng J, Starkey S, Smith CM. Wheat gene expression is differentially affected by a virulent Russian wheat aphid biotype. J Chem Ecol. 2011;37:472–82. doi: 10.1007/s10886-011-9949-9. [DOI] [PubMed] [Google Scholar]
- 45.Van Der Westhuizen AJ, Qian XM, Botha AM. β-1,3-glucanases in wheat and resistance to the Russian wheat aphid. Physiologia Plantarum 1998;.103:125-131.
- 46.Van Der Westhuizen AJ, Qian XM, Botha AM. Differential induction of apoplastic peroxidase and chitinase activities in susceptible and resistant wheat cultivars by Russian wheat aphid infestation. Plant Cell Rep. 1998;18:132–7. doi: 10.1007/s002990050545. [DOI] [Google Scholar]
- 47.Smith CM, Liu X, Wang LJ, Liu X, Chen MS, Starkey S, et al. Aphid feeding activates expression of a transcriptome of oxylipin-based defense signals in wheat involved in resistance to herbivory. J Chem Ecol. 2010;36:260–76. doi: 10.1007/s10886-010-9756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller HL, Neese PA, Ketring DL, Dillwith JW. Involvement of ethylene in aphid infestation of barley. J Plant Growth Regul. 1994;13:167–71. doi: 10.1007/BF00226033. [DOI] [Google Scholar]
- 49.van de Ven WTG, LeVesque CS, Perring TM, Walling LL. Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell. 2000;12:1409–23. doi: 10.1105/tpc.12.8.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Audenaert K, De Meyer GB, Höfte MM. Abscisic acid determines basal susceptibility of tomato to Botrytis cinerea and suppresses salicylic acid-dependent signaling mechanisms. Plant Physiol. 2002;128:491–501. doi: 10.1104/pp.010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones RL. Gibberellic acid-enhanced release of β-1,3- glucanase from barley aleurone cells. Plant Physiol. 1971;47:412–6. doi: 10.1104/pp.47.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–99. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 53.Pitzschke A, Forzani C, Hirt H. Reactive oxygen species signaling in plants. Antioxid Redox Signal. 2006;8:1757–64. doi: 10.1089/ars.2006.8.1757. [DOI] [PubMed] [Google Scholar]
- 54.Thompson GA, Goggin FL. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J Exp Bot. 2006;57:755–66. doi: 10.1093/jxb/erj135. [DOI] [PubMed] [Google Scholar]
- 55.Mohase L, Van Der Westhuizen AJ. Salicylic acid is involved in resistance responses in the Russian wheat aphid-wheat interaction. J Plant Physiol. 2002;159:585–90. doi: 10.1078/0176-1617-0633. [DOI] [PubMed] [Google Scholar]
- 56.Botha A-M, Swanevelder ZH, Lapitan NLV. Transcript profiling of wheat genes expressed during feeding by two different biotypes of Diuraphis noxia. Environ Entomol. 2010;39:1206–31. doi: 10.1603/EN09248. [DOI] [PubMed] [Google Scholar]
- 57.Abeles F. Ethylene in Plant Biology. New York, NY: Academic Press, 1992. [Google Scholar]
- 58.O’Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–7. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Zhu L, He G. Differential gene expression in response to brown planthopper feeding in rice. J Plant Physiol. 2004;161:53–62. doi: 10.1078/0176-1617-01179. [DOI] [PubMed] [Google Scholar]
- 60.Addicott FT, Carns HR. History and introduction. In: Addicott FT, ed. Abscisic Acid. New York, NY: Praeger, 1983:1-21. [Google Scholar]
- 61.Zeevaart JAD, Creelman RA. Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:439–73. doi: 10.1146/annurev.pp.39.060188.002255. [DOI] [Google Scholar]
- 62.Pierce M, Raschke K. Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta. 1980;148:174–82. doi: 10.1007/BF00386419. [DOI] [PubMed] [Google Scholar]
- 63.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 64.Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, ed. Plant Hormones. Dordrecht, The Netherlands: Kluwer, 1995:509-30. [Google Scholar]
- 65.Kawano T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003;21:829–37. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- 66.Quirino BF, Normanly J, Amasino RM. Diverse range of gene activity during Arabidopsis thaliana leaf senescence includes pathogen-independent induction of defense-related genes. Plant Mol Biol. 1999;40:267–78. doi: 10.1023/A:1006199932265. [DOI] [PubMed] [Google Scholar]
- 67.Suttle JC. Effect of ethylene treatment on polar IAA transport, net IAA uptake and specific binding of N-1-naphthylphthalamic acid in tissues and microsomes isolated from etiolated pea epicotyls. Plant Physiol. 1988;88:795–9. doi: 10.1104/pp.88.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Havlová M, Dobrev PI, Motyka V, Storchová H, Libus J, Dobrá J, et al. The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ. 2008;31:341–53. doi: 10.1111/j.1365-3040.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 69.Botha A-M, Swanevelder ZH, Schultz T, Van Eck L, Lapitan NLV. Deciphering defense strategies that are elucidated in wheat containing different Dn resistance genes. In: Proceeding of the 11th International Wheat Genetics Symposium, August 24-29, 2008. Brisbane, 2008:1-3. [Google Scholar]
- 70.Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol Biol. 2009;69:473–88. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 71.Ellis C, Karafyllidis I, Turner JG. Constitutive activation of jasmonate signaling in an Arabidopsis mutant correlates with enhanced resistance to Erysiphe cichoracearum, Pseudomonas syringae, and Myzus persicae. Mol Plant Microbe Interact. 2002;15:1025–30. doi: 10.1094/MPMI.2002.15.10.1025. [DOI] [PubMed] [Google Scholar]
- 72.Ellis C, Turner JG. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. Plant Cell. 2001;13:1025–33. doi: 10.1105/tpc.13.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leon-Reyes A, Du Y, Koornneef A, Proietti S, Körbes AP, Memelink J, et al. Ethylene signaling renders the jasmonate response of Arabidopsis insensitive to future suppression by salicylic Acid. Mol Plant Microbe Interact. 2010;23:187–97. doi: 10.1094/MPMI-23-2-0187. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Jones AD, Howe GA. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006;580:2540–6. doi: 10.1016/j.febslet.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 75.Paponov IA, Paponov M, Teale W, Menges M, Chakrabortee S, Murray JA, et al. Comprehensive transcriptome analysis of auxin responses in Arabidopsis. Mol Plant. 2008;1:321–37. doi: 10.1093/mp/ssm021. [DOI] [PubMed] [Google Scholar]
- 76.Leyser O. Dynamic integration of auxin transport and signalling. Curr Biol. 2006;16:R424–33. doi: 10.1016/j.cub.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 77.Thilmony R, Underwood W, He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;46:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- 78.O’Donnell PJ, Schmelz EA, Moussatche P, Lund ST, Jones JB, Klee HJ. Susceptible to intolerance—a range of hormonal actions in a susceptible Arabidopsis pathogen response. Plant J. 2003;33:245–57. doi: 10.1046/j.1365-313X.2003.01619.x. [DOI] [PubMed] [Google Scholar]
- 79.Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, et al. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science. 2006;312:436–9. doi: 10.1126/science.1126088. [DOI] [PubMed] [Google Scholar]
- 80.Chen Z, Agnew JL, Cohen JD, He P, Shan L, Sheen J, et al. Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc Natl Acad Sci U S A. 2007;104:20131–6. doi: 10.1073/pnas.0704901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Close TJ, Wanamaker SI, Caldo RA, Turner SM, Ashlock DA, Dickerson JA, et al. A new resource for cereal genomics: 22K barley GeneChip comes of age. Plant Physiol. 2004;134:960–8. doi: 10.1104/pp.103.034462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 84.SAS Institute Inc. SAS/STAT Software version 9.1. Cary, NC: SAS Institute, 2001. [Google Scholar]