Abstract
Phytophthora is the most devastating pathogen of dicot plants. There is a need for resistance sources with different modes of action to counteract the fast evolution of this pathogen. In order to better understand mechanisms of defense against P. infestans, we analyzed several clones of potato. Two of the genotypes tested, Sarpo Mira and SW93-1015, exhibited strong resistance against P. infestans in field trials, whole plant assays and detached leaf assays. The resistant genotypes developed different sizes of hypersensitive response (HR)-related lesions. HR lesions in SW93-1015 were restricted to very small areas, whereas those in Sarpo Mira were similar to those in Solanum demissum, the main source of classical resistance genes. SW93-1015 can be characterized as a cpr (constitutive expressor of PR genes) genotype without spontaneous microscopic or macroscopic HR lesions. This is indicated by constitutive hydrogen peroxide (H2O2) production and PR1 (pathogenesis-related protein 1) secretion. SW93-1015 is one of the first plants identified as having classical protein-based induced defense expressed constitutively without any obvious metabolic costs or spontaneous cell death lesions.
Keywords: Phytophthora infestans, PR proteins, cell death, constitutive defence, cpr, hypersensitive response, late blight, plant-pathogen interaction, potato, resistance
Introduction
Despite more than a century of resistance breeding, potato (Solanum tuberosum) is still severely hit by late blight, currently causing billion dollar losses annually.1 Phytophthora infestans, the oomycete causing late blight disease, can totally devastate a potato field in a matter of days. Frequent application of fungicides, sometimes on more than a weekly basis, is therefore required during severe epidemics. In parallel to overcoming plant defense mechanisms,2 many pesticides have become inefficient against the pathogen.3 Environmental hazards and toxicity to farmers have led to increased concerns regarding the use of pesticides. Lack of appropriate human protection standards and resources for frequent use of fungicides causes even greater health problems and economic losses for farmers in developing countries.1
Most plant pathogenic microorganisms actively penetrate the plant surface to access intracellular nutrients. Plants have therefore developed different mechanisms to counteract such pathogen attacks. Some of these defense mechanisms are preformed (constitutive), providing constant physical and chemical barriers to prevent pathogen infection, whereas others are induced after pathogen perception. Locally induced plant responses include production of reactive oxygen species (ROS), hypersensitive response (HR), callose deposition and production of pathogenesis-related (PR) proteins.4 Reactive oxygen species (ROS) have antimicrobial activity and play a role in cross-linking of cell wall proteins and as inducers of defense-related gene expression, and are often linked with early stages of HR.5,6 ROS generation prior to HR is reported to be elicited by P. infestans in potato7 and plays a pivotal role in disease resistance to P. infestans.8,9
The first interaction between the pathogen and the host occurs in the apoplast, where recognition and lysis of the pathogen occur in cases of successful defense. Proteases and hydrolases (such as cysteine proteases) secreted in the apoplast contribute to the defense against the pathogens, some of which are under diversifying selection in natural environments.10 Several of the apoplastic proteins are induced after pathogen attack and are therefore referred to as PR proteins. These PR proteins have been classified into 17 families (from 1 to 17) based on time of their discovery. Examples include the thaumatin-like PR5 protein family, which has been associated with activity against oomycete pathogens,11 peroxidases (PR9), which are involved in cell wall cross-linking or lignification, and glucanases, which degrade the cell wall and release glucosidal elicitors. PR1 is secreted in the apoplast and is a commonly used marker for defense activation in plants.12 In order to evade these host defenses, pathogen not only alters the extracellular proteome13 but also inhibits14 and neutralizes15 key proteases that are important for defense.
Many Arabidopsis lesion mimic mutants (mutants which produce spontaneous HR-like lesions without pathogen attack) with constitutively activated defense have been identified.16,17 There are also a few Arabidopsis mutants with constitutive defense without formation of spontaneous HR-like lesions, including constitutive PR producer (cpr1 and cpr6) mutants showing increased resistance to pathogens accompanied with normal HR.18,19 Other similar Arabidopsis mutants are the defense no death (dnd1 and dnd2) mutants that have enhanced resistance against the bacterial pathogen Pseudomonas syringae without producing any HR indicating that resistance against pathogens and HR can be uncoupled.20,21 However dnd1 and dnd2 mutants can display spontaneous lesions in high light and low humidity conditions.22 Moreover, the Arabidopsis mpk4 (MAP kinase 4) mutant constitutively expresses PR proteins without spontaneous lesions and has increased resistance to bacterial and oomycete pathogens.23 Arabidopsis cpr-type mutants are generally dwarfed and are believed to have high fitness cost. Interestingly, the recently discovered Arabidopsis mutant cdd1 (constitutive defense without defect in growth and development 1) shows enhanced resistance against a bacterial pathogen without apparent cost of resistance.24 Several of the Arabidopsis mutants have increased salicylic acid levels in uninfected plants compared with wild-type plants. In contrast, potato has high basal salicylic acid levels but this does not lead to constitutive defense activation.25
Modeling studies show that inducible defense is likely to be favored when: (1) pathogen attack varies in time or space; and/or (2) there is a relatively high cost of expressing the defense, provided that induced defense leads to a net benefit (benefit-cost).26 It has been postulated that the magnitude of fitness costs of a disease determine whether constitutive or inducible defense is favored, i.e., constitutive defense becomes the optimal choice when there are fast and severe infections by the pathogen.27,28 Another proposed model for optimal defense points at the usefulness of storing defense-related proteins rather than producing new proteins, and concludes that storage strategies can be more cost-favorable in the event of a severe attack.29 Both models predict that constitutive responses are favorable when there is higher probability of pathogen attack.
Conventional breeding with introgression of resistance genes from wild potato relatives such as Solanum demissum into susceptible potato varieties was initially successful in conferring resistance against P. infestans. However, this narrow, race-specific resistance has been quickly overcome by the pathogen.30 Transgenic approaches to introduce resistance genes into susceptible cultivars have also been utilized. One example is the Katahdin potato containing the RB gene, which provides slightly broader resistance than the resistance genes from S. demissum.2 A transgenic strategy to stack resistance genes for more durable resistance has also been used,31 but field trials over several years are needed to test the durability of such gene combination against the rapidly evolving pathogen. The unusually large number of repeated sequences and highly mobile transposable elements in the P. infestans genome32 might explain its great ability to modify virulence capability to escape host defense mechanisms. Ideally, several different resistance mechanisms should be utilized in different spatiotemporal combinations.
In this study we examined defense mechanisms in two potato genotypes that we found to be highly resistant against Phytophthora in field experiments. In one genotype the typically induced defense system was constitutively active without any apparent metabolic cost.
Results
SW93-1015 and Sarpo Mira resistance to Phytophthora infestans in the field
Field trials were performed in southern Sweden using different potato cultivars and breeding clones from the Swedish national potato breeding program. Results from the 2007 trial, shown as relative Area under Disease Progression Curve (rAUDPC), indicated that SW93-1015 and Sarpo Mira were highly resistant to P. infestans (Table 1), while other cultivars showed only partial resistance compared with the susceptible cultivar Bintje. No spontaneous HR lesions were found in any of the genotypes tested. Similar results were obtained in field trials performed 2008 and 2009, indicating that SW93-1015 and Sarpo Mira are resistant to Phytophthora populations in Sweden.
Table 1. Infection rates of P. infestans in field trials measured as relative area under disease progression curve (rAUDPC). Means of four replications.
| Genotype | Mean relative AUDPC ±SD |
|---|---|
| Bintje |
0.60 ± 0.13 |
| Danva |
0.36 ± 0.06 |
| Matilda |
0.40 ± 0.03 |
| Superb |
0.39 ± 0.06 |
| Escort |
0.22 ± 0.13 |
| Robijn |
0.23 ± 0.05 |
| SW93-1015 |
0.0075 ± 0.0006 |
| Sarpo Mira | 0.0020 ± 0.0017 |
SW93-1015 and Sarpo Mira highly resistant to P. infestans under greenhouse conditions
Based on the field data (Table 1), we selected Sarpo Mira and SW93-1015 for further analysis. To confirm the high field resistance shown by Sarpo Mira and SW93-1015, we tested these genotypes for resistance against P. infestans in whole plant greenhouse resistance assays and in detached leaf assays. The susceptible cultivar Desiree was used as a control. SW93-1015 and Sarpo Mira were found to be fully resistant to P. infestans, whereas Desiree was susceptible (Fig. 1A and B). Similar results were observed in the detached leaf assays (Fig. 1C). These results were obtained in three independent experiments.
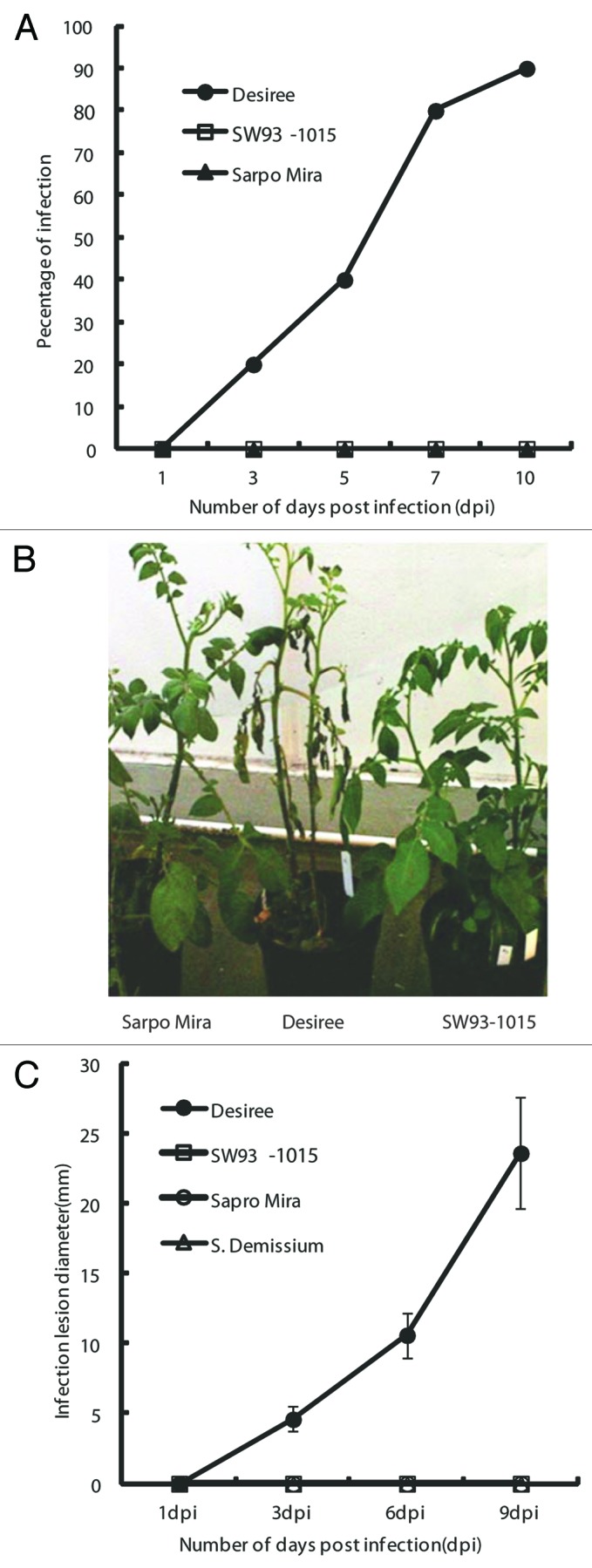
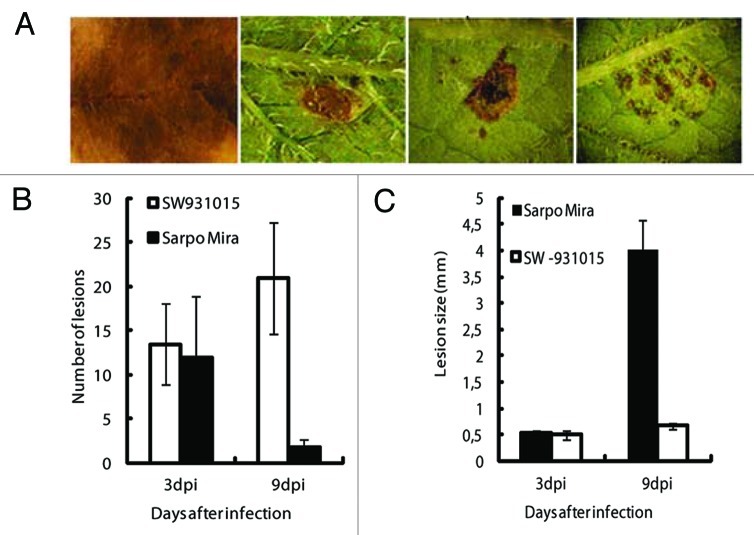
Figure 1. Whole plant assay in the greenhouse performed on Desiree, SW93-1015 and Sarpo Mira after spraying with P. infestans sporangia (15,000/ml). (A) Graph showing scoring of P. infestans infection development, represented as percentage of plant area infected based on the Malcolmson 1–9 scale. Readings were taken after 1, 3, 5, 7 and 9 dpi in three independent experiments where the respective plant had the same score at a given time point in all three experiments. (B) Picture taken at 9 dpi after P. infestans treatment of Sarpo Mira, Desiree and SW93-1015 (from left to right). (C) Graph showing infection lesion diameter (mm) in SW93-1015, Sarpo Mira and S. demissum at 1, 3, 5, 7 and 9 dpi.
SW93-1015 and Sarpo Mira respond differently to phytophthora challenge
To determine whether the two genotypes have different mechanisms of resistance, we followed the resistance reaction carefully after spraying with P. infestans zoospores. No visible HR resistance lesions were observed on SW93-1015 in whole plant assays or in field trials. Sarpo Mira occasionally formed macroscopic resistance lesions on older leaves (data not shown). In detached leaf assays we compared these two genotypes with the susceptible cultivar Desiree and the resistant wild relative to potato S. demissum. Following inoculation, severe pathogen growth was observed at 9 dpi on Desiree, whereas S. demissum, Sarpo Mira and SW93-1015 did not develop any pathogen growth and instead developed HR lesions (Fig. 2A). In both Sarpo Mira and SW93-1015, small HR lesions appeared at 3 dpi. No significant difference in number and size of lesions between the two genotypes was observed at this time point (Fig. 2B and C). However, at a later time point (9 dpi), there was a notable difference in lesion size, with Sarpo Mira having developed larger HR lesions similar in size to those of S. demissum, while HR lesions in SW93-1015 were restricted to very small areas (Fig. 2A). Number of HR lesions increased in SW93-1015 at 9 dpi (Fig. 2B), whereas size had not increased significantly (Fig. 2C). In Sarpo Mira, lesions observed at 3 dpi increased in size and fused, thereby reducing the number of lesions (Fig. 2B) and making them significantly larger (Fig. 2C). Similar results were obtained in three independent experiments.
Figure 2. Detached leaf assay showing infection lesion or resistance reaction HR against P. infestans. (A) Desiree showing necrosis after infection, while S. demissum, Sarpo Mira and SW93-1015 displayed HR at 9 dpi. (B) Graph showing number of lesions formed per leaf on Sarpo Mira and SW93-1015 at 3 and 9 dpi. Error bars indicate standard deviation of the mean. (C) Graph showing mean size of lesions on Sarpo Mira and SW93-1015 at 3 and 9 dpi. Error bars indicate standard deviation of the mean.
Restricted microscopic HR in SW93-1015 in response to Phytophthora challenge
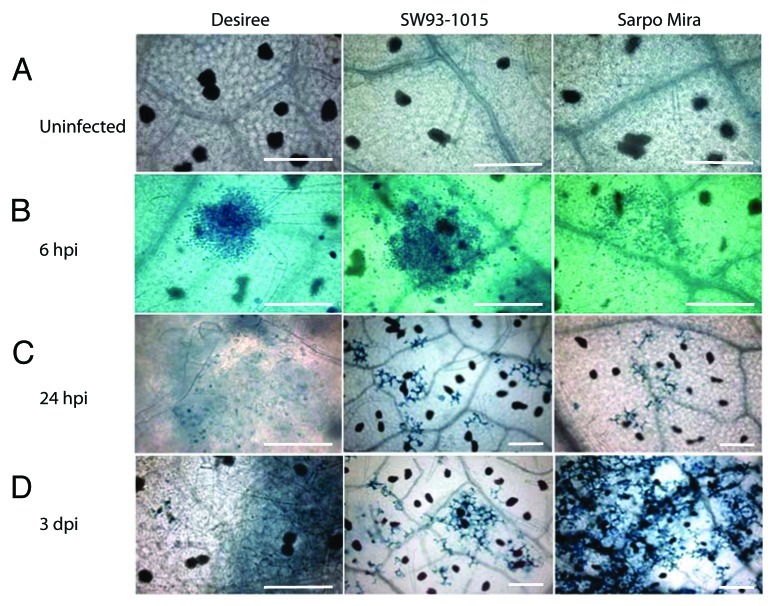
Microscopic observation with Trypan Blue staining of the two resistant genotypes revealed a similar pattern of HR as observed at macroscopic level (Fig. 3). Microscopic HR was not observed in uninfected plants, or in any of the three genotypes at 6 hpi (Fig. 3A and B). However, HR was visible at 24 hpi in both SW93-1015 and Sarpo Mira. At this stage HR was scattered and limited to few cells in both resistant genotypes, SW93-1015 and Sarpo Mira (Fig. 3C). In the susceptible genotype Desiree, Phytophthora germination was observed without HR (Fig. 3C). At 3 dpi, the scattered HR phenotype remained limited to a few cells in SW93-1015, in contrast to HR lesion size in Sarpo Mira, which had increased to a larger extent (Fig. 3D). In Desiree, Phytophthora germination observed at 24 hpi resulted in intense hyphal growth at 3 dpi (Fig. 3D). Similar results were obtained in three independent experiments. Thus, we did not detect spontaneous cell death in SW93-1015 or Sarpo Mira at either macroscopic or microscopic level.
Figure 3. Microscopic analysis of hypersensitive response (HR). Trypan blue staining performed on Desiree, SW93-1015 and Sarpo Mira. (A) Trypan blue staining did not show any HR in uninfected leaves of the three genotypes. (B) Phytophthora zoospores at 6 hpi in Desiree (left), SW93-1015 (middle) and Sarpo Mira (right). (C) Phytophthora zoospore germination in Desiree (left), scattered HR in SW93-1015 (middle) and Sarpo Mira (right) indicated by blue coloration at 24 hpi. (D) Intense hyphal growth in Desiree (left), scattered HR in SW93-1015 (middle) and increased cell death in Sarpo Mira (right) at 3 dpi. Size bars represent 100 µm.
Constitutive H2O2 generation in SW93-1015
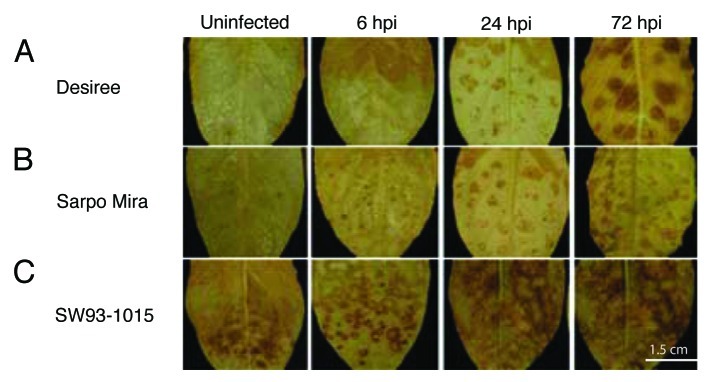
Reactive oxygen species are linked to early stages of HR.6 To determine whether H2O2 production was involved in resistance signaling and might constrain HR lesion size in SW93-1015, we performed DAB (3,3-diaminobenzedine) staining at different time points after inoculation with P. infestans zoospores (Fig. 4). Elevated levels of H2O2 were found in uninfected SW93-1015 leaves and at 6 h post inoculation compared with Desiree and Sarpo Mira where no H2O2 was detected at these time points (Fig. 4A and B). In Desiree and Sarpo Mira, H2O2 appeared at 24 hpi and increased at 72 hpi, while SW93-1015 maintained high levels of H2O2 at these time points (Fig. 4C and D). The elevated DAB staining in uninfected SW93-1015 leaves indicated that H2O2 generation was constitutive. Activation of H2O2 production in Desiree and Sarpo Mira indicated that the recognition exists in both genotypes, but Desiree was unable to prevent pathogen growth. Similar results were obtained in three independent experiments. This finding was confirmed using nitro blue tetrazolium (NBT) staining (data not shown).
Figure 4. 3,3-Diaminobenzidine (DAB) staining performed on Desiree, Sarpo Mira and SW93-1015 to detect H2O2 produced in detached leaves after P. infestans inoculation. H2O2 detected in (A) Desiree, (B) Sarpo Mira and (C) SW93-1015 on uninfected leaves at 6 hpi, 24 hpi and 72 hpi (from left to right). Size bar represents 15 mm.
SW93-1015 secretes PR1 protein constitutively in the apoplast
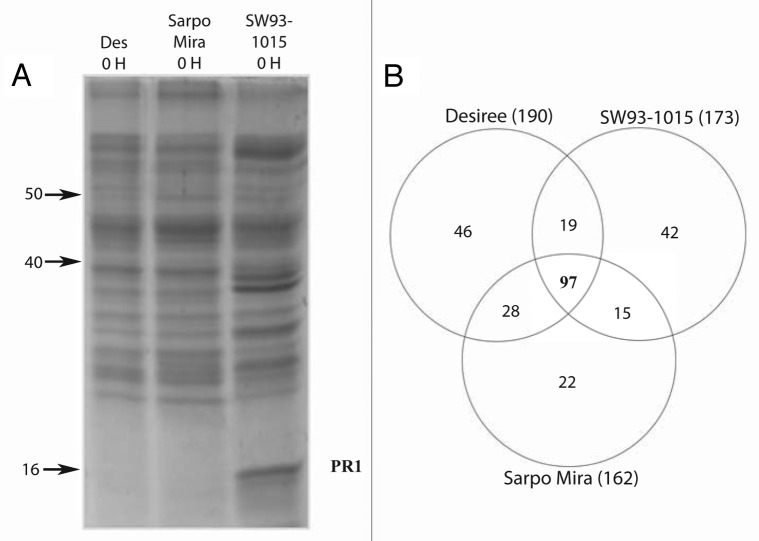
In order to further verify the constitutive activation of defense-related compounds in SW93-1015, apoplast liquid was isolated and analyzed for secreted proteins. Separation of samples from the three genotypes on SDS-polyacrylamide gels (Fig. 5A) showed higher basal level of PR1 in the apoplast of SW93-1015 than in Sarpo Mira and Desiree. PR1 was identified by mass spectrometry according to our earlier study.33 Constitutive PR1 expression in SW93-1015 apoplast was observed repeatedly in several independent experiments.
Figure 5. (A) SDS-PAGE showing apoplast proteins from untreated Desiree, SW93-1015 and Sarpo Mira. (B) Venn diagram showing number of proteins identified by mass spectrometry in the three genotypes, also showing number of proteins overlapping and unique for the genotypes.
SW93-1015 and Sarpo Mira have candidate resistance proteins in the apoplast
The secreted protein profile of the three potato genotypes was studied in more detail using mass spectrometry. More than 150 proteins from each of the three genotypes were identified (Fig. 5B). The most prominent proteins present in all three genotypes included multiple PR proteins from several families such as peroxidases (e.g., PR9), 1,3-β-glucanases, chitinases, NtPRp27-like protein (PR17), subtilase P69 proteinases (PR7) and 24K germin-like proteins (PR15), all of which were identified with at least six peptides. Some proteins could only be found in the resistant potato genotypes and not in Desiree. Among these were two PR5 proteins that were identified in both resistant genotypes. A β-D-xylosidase (Lexyl1) involved in degradation of xylan was only found in the secretome of Sarpo Mira, while Nectarin V was only found in SW93-1015. Nectarin V is a glucose oxidase that participates in hydrogen peroxide production.34 The identification of Nectarin V in leaves of uninfected SW93-1015 plants coincides with the high constitutive level of H2O2 in this genotype.
Phytophthora resistance in SW93-1015 specific to the shoot
Inoculation with P. infestans on tubers resulted in mycelium growth on SW93-1015 (2.3 on a scale where 0 = lack of mycelium, and 3 = very intense growth). Sarpo Mira showed very low to almost absent mycelium growth (average 0.1). Inoculation of susceptible Bintje tubers resulted in comparable mycelia growth to SW93-1015 (2.4) (Table 2). Sarpo Mira also had smaller necrotic symptom-related lesions in the tuber than SW93-1015. These data indicate that the resistance in SW93-1015 is specific against P. infestans shoot infections.
Table 2. Tuber resistance to P. infestans measured as mycelial growth and lesion sizes. Recordings have been made on seven tubers per genotype.
| Cultivar/line | Mean mycelium growth ±SD | Mean lesion size ±SD |
|---|---|---|
| SW93-1015 |
2.3 ± 0.48 |
4.7 ± 0.48 |
| Bintje |
2.4 ± 0.53 |
3.4 ± 0.78 |
| Asterix |
0.1 ± 0.37 |
6.0 ± 0.92 |
| Escort |
0.1 ± 0.37 |
6.4 ± 0.53 |
| Sarpo Mira |
0.1 ± 0.37 |
6.1 ± 0.89 |
| Robijn |
1.4 ± 0.53 |
4.7 ± 0.75 |
| Superb |
2.1 ± 0.37 |
5.0 ± 0.75 |
| Victoria | 1.9 ± 1.21 | 5.3 ± 0.48 |
Mycelium growth score: 0 (lack)–3 (intensive growth). Lesion size score: 1 (susceptible)–9 (most resistant).
Constitutive defense in SW93-1015 has no effect on growth and tuber yield
SW93-1015 did not show any reduction in growth of aboveground parts due to constitutive defense, which was comparable to growth of Sarpo Mira (Fig. 1B). Furthermore, SW93-1015 produced regular-sized tubers with a yield of 224 ± 51 g plant−1, which was not significantly different from Desiree (184 ± 17 g plant−1) and Sarpo Mira (180 ± 28 g plant−1) in optimal growing conditions. Thus, the constitutive expression of defense in SW93-1015 did not show any apparent cost.
Discussion
Bearing in mind the persistent problem of late blight in potato cultivation, several sources of resistance are needed. In our studies the potato genotypes SW93-1015 and Sarpo Mira proved highly resistant to P. infestans attack in repeated field trials, whole plant and detached leaf assays, in contrast to several other cultivars tested. We therefore analyzed these two genotypes in more detail to investigate the underlying source of resistance. We found clear differences between the two genotypes in HR pattern and in other defense reactions such as H2O2 and PR protein accumulation, which was constitutively activated in one of the genotypes.
A typical response in resistant plants to microbial attack is development of HR. In our greenhouse whole plant assays and field experiments, Sarpo Mira developed few macroscopic HR lesions. A similar pattern has been shown for example in transgenic Katahdin potato plants with the RB gene from S. bulbocastanum.2 Data from our detached leaf assays also indicated that the HR lesions in Sarpo Mira are similar in size to those formed in S. demissum. This means that resistance in Sarpo Mira might be mediated by classical R genes. Furthermore, tuber resistance was high in Sarpo Mira and no constitutive activation of immune response was found.
SW93-1015 showed an unusual reaction to Phytophthora challenge, since there were no visible HR lesions on the plants in the field trials and whole plant assays. Furthermore, no spontaneous cell death was observed in uninfected plants at the microscopic level and HR lesions were restricted to very small areas in the detached leaf assays. SW93-1015 can be classified as a cpr (constitutive PR) genotype in analogy to Arabidopsis cpr mutants without spontaneous cell death such as cpr1, cpr6 and mpk4.18,19,23 Compared with these mutants, SW93-1015 seems to be a weak cpr genotype with normal growth showing constitutive H2O2 production and PR protein accumulation in the apoplast, including PR1 and an H2O2-producing enzyme. The constitutively active immune system in this paranoid genotype without any apparent cost raises the question of whether such an immune system exists in cultivated or natural plant populations. Earlier described cpr genotypes in Arabidopsis generally showed reduced growth, indicating a metabolic cost for the constitutive defense. In addition to absence of spontaneous cell death, smaller or no HR lesions after the pathogen attack may lead to the hypothesis that a less active form corresponding to MPK4 or CNGC2 (DND1) is the cause of constitutive defense and reduced HR in SW93-1015.
The potato genotype SW93-1015 might be a source of a unique type of Phytophthora resistance compared with Sarpo Mira and S. demissum. Although SW93-1015 is a cpr genotype, it does not show any apparent growth defects and has the capability to maintain constitutively active defense at a low metabolic cost, at least in an agricultural system. This conclusion is supported by tuber yield and normal plant growth, which are comparable to those of other genotypes. The SW93-1015 clone is among the few examples of a classical induced immune system expressed constitutively without a major metabolic cost or manipulation of the cell death system. Models have predicted a benefit of constitutive defense in a situation of severe pathogen pressure29,35 or have shown that a combination of constitutive and induced defense is optimal.36 To avoid high metabolic costs, fine-tuning of the constitutive part could be necessary. SW93-1015, found in artificial selection, might be an example of such a balance between metabolic cost and constitutive immune system.
SW93-1015 has been used as a parent in crosses performed in the potato breeding program at Svalöf Weibull AB and later at the Swedish University of Agricultural Sciences. However, some of the drawbacks with SW93-1015 are relatively low tuber resistance, inferior taste, susceptibility to potato leaf roll virus and greening of tubers due to a tendency for aboveground tuberization, reducing its value in classic breeding programs.
Our apoplast analysis revealed that PR5 protein was present in both resistant genotypes but not in Desiree. PR5-like proteins have been shown to inhibit germination and growth of P. infestans,37,38 while leaves of transgenic potato plants overexpressing a PR5 gene exhibited delayed development of disease symptoms after inoculation with P. infestans.38 Nectarin V was only detected in SW93-1015 in the present study. Nectarin V is a flavin-containing berberine bridge enzyme (BBE)-like protein that has glucose oxidase activity.34 Transgenic potato expressing H2O2-generating glucose oxidase shows increased resistance against P. infestans.39 Both Nectarin V and PR5 transcripts are known to be upregulated in P. infestans-infected leaves.40
Our finding of a genotype with constitutive active defense in conventional breeding material without a notable loss of fitness creates a basis for a new molecular strategy to combat the Phytophthora disease problem. This kind of variation might be present in other genotypes and our current screening of wild populations of potato can add to our understanding of defense mechanisms existing in nature.
Materials and Methods
Field trials
In the field trials 10 tubers/genotype were planted in two rows beside each other, with 5 tubers/row. The plant spacing was 30 cm and the row spacing 75 cm. The trial consisted of three repeats in randomized blocks. Every 5th row was planted with Bintje to achieve an even infection pressure over the trial. Field trials relied on spontaneous infection and the first symptoms of late blight occurred on July 7, 2007. From that date, the trial was scored at of 3–4 d intervals until July 24 and one-week intervals after that until August 21st. Scoring was done according to the Eucablight protocol and given as percentage of foliage affected. Relative Area Under Disease Progression Curve (rAUDPC) was calculated as previously described.41 In 2008, late blight infections were rare and appeared late in the season. Because of this, only three assessments were performed (August 5th and 27th; September 2nd) and a simplified scale was used. In 2009, late blight assessments were performed on four occasions (July 28th; August 3rd, 11th and 21st). Scoring in that year was according to a 6-point scale (0 = without any infection, 5 = very severe infection).
Whole plant assays
Potato plants were grown in the greenhouse at 20°C with 16 h of light for 4–5 weeks with approximately 70% relative humidity. Plants were then moved to a humid chamber where they were kept in 100% humidity. After 8 h, plants were sprayed with a suspension containing 15,000 sporangia/ml until inoculum saturated the leaf surfaces. Relative humidity was maintained at 100% for 2 d after infection and then adjusted to 90% for the rest of the experiment. Scoring was performed according to a previously described classification system,42 in three independent experiments, where 1 = > 90% infection and 8 = ≤ 10% infection); no infection was given a score of 9. Tuber yield was analyzed from 12 plants of each genotype and a Student's t-test was performed.
Phytophthora detached leaf assay
For all disease testing we used P. infestans strain SE03058 (virulence 1, 3, 4, 7, 10, 11) obtained from Björn Andersson, Department of Forest Mycology and Pathology, SLU, Sweden. Cultures were maintained on rye agar medium according to standard protocols. For obtaining infectious sporangia, P. infestans was grown on detached leaves of the susceptible potato cultivar Desiree.43 Sporangia were kept at 4°C for 2 h before infecting the leaves to release zoospores. Eight to 10 fully expanded leaves from 4–5 week old plants were excised and kept in humid chambers for two hours before inoculation with a 20 µl drop containing 15,000 sporangia/ml. The inoculated leaves were kept in a climate chamber at 15°C with the following light regime: 16 h light and 8 h dark. Number of HR lesions and diameter were measured 3 dpi and 9 dpi.
Decapitated tuber assay
Tuber resistance was evaluated using the method of inoculation of decapitated tubers.44 Slightly decapitated tubers were drop-inoculated with the inoculums comprising 20,000 zoospores/ml and were kept in dark at 18°C for 10 d following inoculation. Aerial mycelia growth on decapitated surfaces was graded on a scale 0–3, where 0 = absence of mycelium and 3 = most intensive growth. Lesion size on longitudinally cut tubers was scored using 1–9 scale, where 9 = the most resistant.
Detection of H2O2
In order to detect H2O2 production in plant leaves, we used a modified version of a previously described DAB staining protocol,45 whereby 1 mg ml−1 3,3-diaminobenzedin (DAB; Sigma-Aldrich) dissolved in water and adjusted to pH 3.6 with concentrated HCl was used. The second fully expanded leaves were cut with a razor blade and put in a detached leaf assay box where optimal light and humidity conditions were maintained.43 Ten 20 µl drops of P. infestans inoculum (15,000 sporangia/ml) were deposited on the abaxial surface of the leaves. Six and 24 h post inoculation (hpi), the leaves were immersed in DAB solution and incubated overnight in darkness along with non-inoculated control leaves. The reaction was stopped by clearing the leaves with boiling 96% ethanol for 30 min. The experiment was repeated three times, with three replicates for each genotype.
Microscopy
In order to observe HR and Phytophthora structures, leaves from plants grown in the climate chamber were inoculated with P. infestans sporangia in detached leaf assays according to the procedure described above. Leaf discs cut around the inoculation drop from three leaves for each time point per genotype were cut using a cork borer and subjected to Trypan blue staining.46 This experiment was repeated three times, with three replicates per experiment.
Secretome sample preparation
Fully expanded middle leaves were treated with 1% Tween 20 by shaking mildly for 10 sec and dried briefly on blotting paper. They were then placed in Petri dishes, covered with a buffer with 150 mM sodium phosphate and 50 mM sodium chloride and placed in a vacuum chamber for 10 min to infiltrate with buffer. The leaves were then very briefly dried on blotting paper and centrifuged at 3,000 rpm for 3 min at 4°C in 15 ml Falcon tubes containing a metal ring to separate leaves from the secretome at the bottom of the tube and a protease inhibitor cocktail. This protocol was modified from previously published methods.47 Apoplast liquid was aliquoted into 1.5 ml tubes and stored at -80°C.
SDS-PAGE separation and identification of apoplastic proteins
Fpr proteomics analysis 120 µl of the secretome sample was precipitated using a standard methanol procedure, dissolved in 2x SDS-PAGE buffer containing DTT and separated for 1 cm with SDS-PAGE. After staining by Coomassie, the gel lane was cut into three pieces and each piece subjected to in-gel tryptic digestion. In brief, the gel pieces were de-stained and washed and, after dithiothreitol reduction and iodoacetamide alkylation, the proteins were digested with trypsin (modified sequencing grade; Promega) overnight at 37°C. The gel pieces were shaken vigorously at room temperature for 15 min, and the eluted peptides were subsequently analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using an LTQ-Orbitrap XL mass spectrometer (Thermo) with collision-induced fragmentation in the linear ion trap using top 7 data-dependent acquisition. Raw data files were converted to Mascot Generic Files using Proteowizard.48 Protein identification was performed in the Proteios Software Environment using Mascot and X!Tandem as described previously,49 in a locally assembled database consisting of UniProt proteins from Solanum, Nicotiana and potato protein predictions from ftp.plantbiology.msu.edu, extended with an equal number of random proteins to assess false discovery rates.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Lena Carlsson and Mia Mogren for expertly growing plants, David Norin for culturing and maintaining Phytophthora and Per Vestergren for arranging and maintaining the climate chamber for our whole plant infections. This work was funded by grants from FORMAS and the Swedish Foundation for Strategic Research (SSF).
Glossary
Abbreviations:
- ROS
reactive oxygen species
- HR
hypersensitive response
- PR
pathogenesis-related
- SAR
systemic acquired resistance
- rAUPDC
relative area under disease progression curve
- DAB
3,3- Diaminobenzidine
- cpr
constitutive expressor of PR proteins
- hpi
hours post infection
- dpi
days post infection
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19149
References
- 1.Haverkort A, Struik P, Visser R, Jacobsen E. Applied Biotechnology to Combat Late Blight in Potato Caused by Phytophthora Infestans. Potato Res. 2009;52:249–64. doi: 10.1007/s11540-009-9136-3. [DOI] [Google Scholar]
- 2.Song J, Bradeen JM, Naess SK, Raasch JA, Wielgus SM, Haberlach GT, et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc Natl Acad Sci U S A. 2003;100:9128–33. doi: 10.1073/pnas.1533501100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith JM, Grant BR, Zhang QS. Role of Phosphatidic-Acid during Differentiation of Phytophthora-Palmivora Zoospores. J Gen Microbiol. 1992;138:451–9. [Google Scholar]
- 4.Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol. 2010;13:459–65. doi: 10.1016/j.pbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence - a broad perspective. Physiol Mol Plant Pathol. 1997;51:347–66. doi: 10.1006/pmpp.1997.0129. [DOI] [Google Scholar]
- 6.Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–75. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 7.Doke N. Involvement of Superoxide Anion Generation in the Hypersensitive Response of Potato-Tuber Tissues to Infection with an Incompatible Race of Phytophthora-Infestans and to the Hyphal Wall Components. Physiol Plant Pathol. 1983;23:345–57. doi: 10.1016/0048-4059(83)90019-X. [DOI] [Google Scholar]
- 8.Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, et al. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15:706–18. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka H, Asai S, Yoshioka M, Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells. 2009;28:321–9. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaschani F, Shabab M, Bozkurt T, Shindo T, Schornack S, Gu C, et al. An effector-targeted protease contributes to defense against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiol. 2010;154:1794–804. doi: 10.1104/pp.110.158030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–62. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 12.Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- 13.Kaffarnik FA, Jones AM, Rathjen JP, Peck SC. Effector proteins of the bacterial pathogen Pseudomonas syringae alter the extracellular proteome of the host plant, Arabidopsis thaliana. Mol Cell Proteomics. 2009;8:145–56. doi: 10.1074/mcp.M800043-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Song J, Win J, Tian M, Schornack S, Kaschani F, Ilyas M, et al. Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defense protease Rcr3. Proc Natl Acad Sci U S A. 2009;106:1654–9. doi: 10.1073/pnas.0809201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozkurt TO, Schornack S, Win J, Shindo T, Ilyas M, Oliva R, et al. Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc Natl Acad Sci U S A. 2011;108:20832–7. doi: 10.1073/pnas.1112708109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeder W, Yoshioka K. Lesion mimic mutants: A classical, yet still fundamental approach to study programmed cell death. Plant Signal Behav. 2008;3:764–7. doi: 10.4161/psb.3.10.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorrain S, Vailleau F, Balagué C, Roby D. Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci. 2003;8:263–71. doi: 10.1016/S1360-1385(03)00108-0. [DOI] [PubMed] [Google Scholar]
- 18.Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong XI. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell. 1994;6:1845–57. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke JD, Liu YD, Klessig DF, Dong XN. Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell. 1998;10:557–69. doi: 10.1105/tpc.10.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu IC, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci U S A. 1998;95:7819–24. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu I, Fengler KA, Clough SJ, Bent AF. Identification of Arabidopsis mutants exhibiting an altered hypersensitive response in gene-for-gene disease resistance. Mol Plant Microbe Interact. 2000;13:277–86. doi: 10.1094/MPMI.2000.13.3.277. [DOI] [PubMed] [Google Scholar]
- 22.Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Jr., Bent AF. The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci U S A. 2000;97:9323–8. doi: 10.1073/pnas.150005697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–20. doi: 10.1016/S0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 24.Swain S, Roy S, Shah J, Van Wees S, Pieterse CM, Nandi AK. Arabidopsis thaliana cdd1 mutant uncouples the constitutive activation of salicylic acid signalling from growth defects. Mol Plant Pathol. 2011;12:855–65. doi: 10.1111/j.1364-3703.2011.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu D, Liu Y, Fan B, Klessig DF, Chen Z. Is the High Basal Level of Salicylic Acid Important for Disease Resistance in Potato? Plant Physiol. 1997;115:343–9. doi: 10.1104/pp.115.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvell CD. The ecology and evolution of inducible defenses. Q Rev Biol. 1990;65:323–40. doi: 10.1086/416841. [DOI] [PubMed] [Google Scholar]
- 27.Clark CW, Harvell CD. Inducible Defenses and the Allocation of Resources - a Minimal Model. Am Nat. 1992;139:521–39. doi: 10.1086/285342. [DOI] [Google Scholar]
- 28.Frank SA. A Model of Inducible Defense. Evolution. 1993;47:325–7. doi: 10.2307/2410142. [DOI] [PubMed] [Google Scholar]
- 29.Shudo E, Iwasa Y. Optimal defense strategy: storage vs. new production. J Theor Biol. 2002;219:309–23. doi: 10.1006/jtbi.2002.3126. [DOI] [PubMed] [Google Scholar]
- 30.Fry WE, Goodwin SB. Resurgence of the Irish potato famine fungus. Bioscience. 1997;47:363–71. doi: 10.2307/1313151. [DOI] [Google Scholar]
- 31.Zhu S, Li Y, Vossen JH, Visser RG, Jacobsen E. Functional stacking of three resistance genes against Phytophthora infestans in potato. Transgenic Res. 2012;21:89–99;. doi: 10.1007/s11248-011-9510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas BJ, Kamoun S, Zody MC, Jiang RH, Handsaker RE, Cano LM, et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature. 2009;461:393–8. doi: 10.1038/nature08358. [DOI] [PubMed] [Google Scholar]
- 33.Lenman M, Sörensson C, Andreasson E. Enrichment of phosphoproteins and phosphopeptide derivatization identify universal stress proteins in elicitor-treated Arabidopsis. Mol Plant Microbe Interact. 2008;21:1275–84. doi: 10.1094/MPMI-21-10-1275. [DOI] [PubMed] [Google Scholar]
- 34.Carter CJ, Thornburg RW. Tobacco nectarin V is a flavin-containing berberine bridge enzyme-like protein with glucose oxidase activity. Plant Physiol. 2004;134:460–9. doi: 10.1104/pp.103.027482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shudo E, Iwasa Y. Inducible defense against pathogens and parasites: optimal choice among multiple options. J Theor Biol. 2001;209:233–47. doi: 10.1006/jtbi.2000.2259. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton R, Siva-Jothy M, Boots M. Two arms are better than one: parasite variation leads to combined inducible and constitutive innate immune responses. Proc Biol Sci. 2008;275:937–45. doi: 10.1098/rspb.2007.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJM, Cornelissen BJC. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3:619–28. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu D, Raghothama KG, Hasegawa PM, Bressan RA. Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci U S A. 1994;91:1888–92. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu G, Shortt BJ, Lawrence EB, Levine EB, Fitzsimmons KC, Shah DM. Disease resistance conferred by expression of a gene encoding H2O2-generating glucose oxidase in transgenic potato plants. Plant Cell. 1995;7:1357–68. doi: 10.1105/tpc.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian ZD, Liu J, Wang BL, Xie CH. Screening and expression analysis of Phytophthora infestans induced genes in potato leaves with horizontal resistance. Plant Cell Rep. 2006;25:1094–103. doi: 10.1007/s00299-006-0169-7. [DOI] [PubMed] [Google Scholar]
- 41.Liljeroth E, Bengtsson T, Wiik L, Andreasson E. Induced resistance in potato to Phytphthora infestans-effects of BABA in greenhouse and field tests with different potato varieties. Eur J Plant Pathol. 2010;127:171–83. doi: 10.1007/s10658-010-9582-4. [DOI] [Google Scholar]
- 42.Cruickshank G, Stewart HE, Wastie RL. An Illustrated Assessment Key for Foliage Blight of Potatoes. Potato Res. 1982;25:213–4. doi: 10.1007/BF02359807. [DOI] [Google Scholar]
- 43.Vleeshouwers VGAA, van Dooijeweert W, Keizer LCP, Sijpkes L, Govers F, Colon LT. A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. Eur J Plant Pathol. 1999;105:241–50. doi: 10.1023/A:1008710700363. [DOI] [Google Scholar]
- 44.Zoteyeva NM, Zimnoch-Guzowska E. A new method for evaluation of potato tubers resistance to Phytophthora infestans. Mikol Fitopatol. 2004;38:89–93. [Google Scholar]
- 45.ThordalChristensen H Zhang ZG, Wei YD, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–94. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 46.Heath MC. Haustorial Sheath Formation in Cowpea Leaves Immune to Rust Infection. Phytopathology. 1971;61:383. doi: 10.1094/Phyto-61-383. [DOI] [Google Scholar]
- 47.Kalde M, Nühse TS, Findlay K, Peck SC. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc Natl Acad Sci U S A. 2007;104:11850–5. doi: 10.1073/pnas.0701083104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24:2534–6. doi: 10.1093/bioinformatics/btn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Häkkinen J, Vincic G, Månsson O, Wårell K, Levander F. The proteios software environment: an extensible multiuser platform for management and analysis of proteomics data. J Proteome Res. 2009;8:3037–43. doi: 10.1021/pr900189c. [DOI] [PubMed] [Google Scholar]