Abstract
Leguminous plants have exclusive ability to form symbiotic relationship with soil bacteria of the genus Rhizobium. Symbiosis is a complex process that involves multiple molecular signaling activities, such as calcium fluxes, production of reactive oxygen species (ROS) and synthesis of nodulation genes. We analyzed the role of ROS in defense gene expression in Medicago truncatula during symbiosis and pathogenesis. Studies in Arabidopsis thaliana showed that the induction of pathogenesis-related (PR) genes during systemic acquired resistance (SAR) is regulated by NPR1 protein, which resides in the cytoplasm as an oligomer. After oxidative burst and return of reducing conditions, the NPR1 undergoes monomerization and becomes translocated to the nucleus, where it functions in PR genes induction. We show that ROS production is both stronger and longer during symbiotic interactions than during interactions with pathogenic, nonhost or common nonpathogenic soil bacteria. Moreover, root cells inoculated with Sinorhizobium meliloti accumulated ROS in the cytosol but not in vacuoles, as opposed to Pseudomonas putida inoculation or salt stress treatment. Furthermore, increased ROS accumulation by addition of H2O2 reduced the PR gene expression, while catalase had an opposite effect, establishing that the PR gene expression is opposite to the level of cytoplasmic ROS. In addition, we show that salicylic acid pretreatment significantly reduced ROS production in root cells during symbiotic interaction.
Keywords: PR genes, pathogenesis, reactive oxygen species, rhizobium, symbiosis
Introduction
Plants from the legume family possess the unique ability to symbiotically interact with rhizobacteria from the Rhizobium genus. This interaction provides reduced nitrogen to the plants that is necessary for biosynthesis of proteins and nucleic acids. In exchange, plants supply the bacteria with fixed carbon compounds, such as sugars. Interaction between the organisms is a multi-step process, which involves secretion and sensing of specific molecules by both parties. The symbiotic relationship begins by detection of plant flavonoids by Rhizobium, which triggers the production and exudation of bacterial lipochitooligosaccharides that act as nodulation (Nod) factors (NF) in the host. Perception of the NFs causes curling of root hairs that entraps the bacteria.1 The bacteria are allowed to penetrate into the root hairs and form an infection thread into the root cortex.2 Finally, the interaction gives rise to creation of a new organ that has suitable oxygen-free conditions for nitrogen fixation. These processes are part of complex signal transduction mechanism in the host that include: cytosolic calcium influx and spiking, generation of reactive oxygen species (ROS) and expression of nodulin genes, leading to root hair deformation and curling.
One of the hallmarks of plant interactions with microorganisms, both pathogenic, as well as symbiotic, is generation of ROS that cause the hypersensitive reaction (HR) or establishment of symbiosis.3,4 Moreover, we showed earlier by using pharmacological agents that ROS production is necessary for symbiosis establishment.1,5 ROS were shown to induce different responses in plant pathogen interaction, particularly when occurring in conjunction with salicylic acid (SA).6
In contrast to symbiotic interaction, when plants are attacked by pathogens they activate a variety of local defense responses, followed by induction of systemic acquired resistance (SAR) against further attacks.7 During SAR, plants produce high concentration of SA, both in the infected area and in other tissues that functions in the signaling of defense responses, such as induction of pathogenesis related (PR) genes.8,9 SA is sensed and transduced by Nonexpressor of PR1 Gene (NPR1), which is a redox sensitive protein that contains several ankyrin repeats and a BTB/POZ domain with a limited homology to IkB.10,11 The NPR1 protein exists as an oligomer in the cytoplasm in its uninduced form, but during pathogen attack, NPR1 becomes reduced to its monomeric form that is translocated to the nucleus, where it interacts with bZIP transcription factors of the TGA/OBF family and activates the transcription of PR genes.12
Analysis of SA levels during initial stages of symbiotic interactions showed that SA is reduced in the first 48 h post inoculation (h.p.i.) with S. meliloti,13 suggesting that the SA-dependent signaling pathway has a negative effect on symbiosis. Moreover, exogenous addition of SA reduced the number of curled hairs during inoculation with S. meliloti.14 Recently, we showed that cytoplasmic ROS inhibits NPR1 translocation to the nucleus in Arabidopsis thaliana.15 Moreover, plants expressing antisense CATALASE gene, as well as the CATALASE inhibitor 3-amino-1,2,4-triazole, or mutants of ASCORBATE PEROXIDASE, all of which had high levels of ROS, showed reduced expression of PR1 gene.15
Here, we show that during symbiosis there is an extended period of ROS accumulation, and that this continuous ROS production functions in maintaining an oxidative atmosphere in the cytoplasm, which prevents the NPR1 translocation to the nucleus.
Results
Production of ROS in Medicago truncatula roots during pathogenic and symbiotic interactions
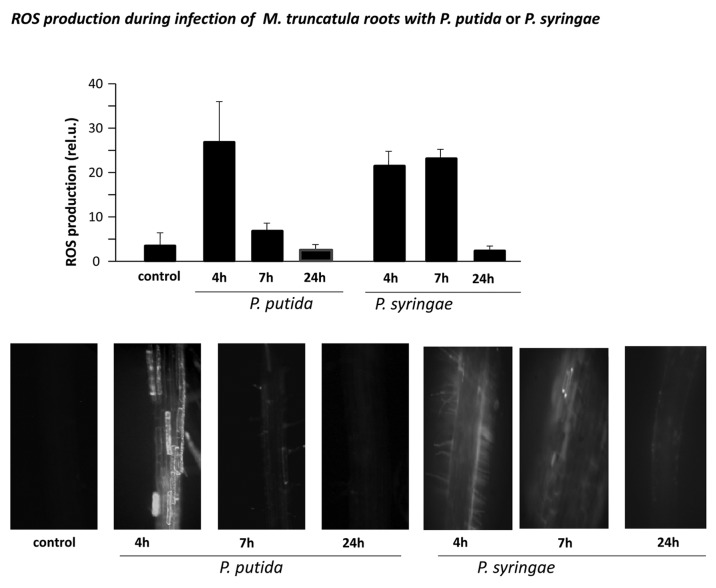
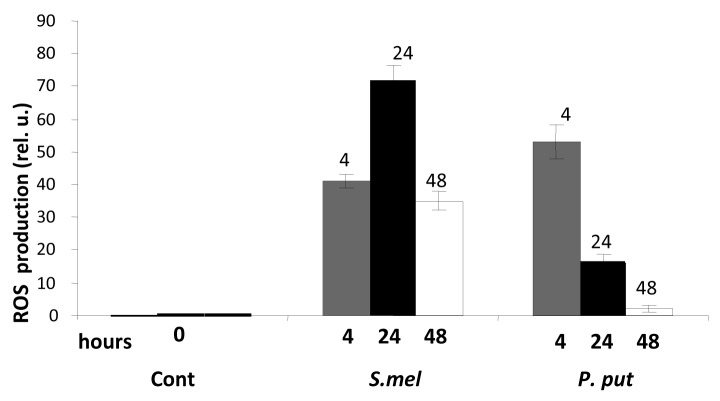
Generation of oxidative burst is a common plant response to pathogenic bacteria and fungi.3 To compare ROS production in legumes during different plant-microbe interactions we, we inoculated M. truncatula seedlings with incompatible bacteria, Pseudomonas syringae pv phaseolicula, nonpathogenic soil bacteria, P. putida and with compatible rhizobacteria Sinorhizobium meliloti. Peak oxidative burst was detected after inoculation of Pseudomonas syringae and P. putida after 4 h, which returned to the level observed in untreated (control) cells after 7 or 24 h later, respectively (Fig. 1). In contrast, inoculation of M. truncatula with S. meliloti resulted in gradual increase of ROS accumulation in the root cells, which peaked at 24 h after inoculation and remained high even after 48 h (Fig. 2).
Figure 1.
Accumulation of reactive oxygen species (ROS) in roots of M. truncatula seedlings, inoculated with P. putida or P. syringae. ROS production was assayed in roots of 4 d-old M. truncatula seedlings inoculated with common soil bacteria P. putida or with nonhost P. syringae pv phaseolicola after 4, 7 and 24 h post inoculation by epi-fluorescent microscopy. Above, ROS were quantified using ImagePro Plus software. Error bars indicate standard deviation of the mean (n = 12). Below, representativ pictures of ROS production in the roots at the indicated time periods. Bar = 60 μm.
Figure 2.
Redox changes in roots of M. truncatula seedlings, inoculated with S. meliloti or with P. putida. Roots of 4 d-old Medicago seedlings were inoculated with S. meliloti (S.mel) or P. putida (P.put). ROS production was assayed and quantified 4, 24 and 48 h after inoculation as described in Figure 1. Numbers above bars indicate h.p.i. Error bars indicate standard deviation of the mean (n = 8) from two separate assays.
Subcellular localization of ROS during biotic and abiotic stresses
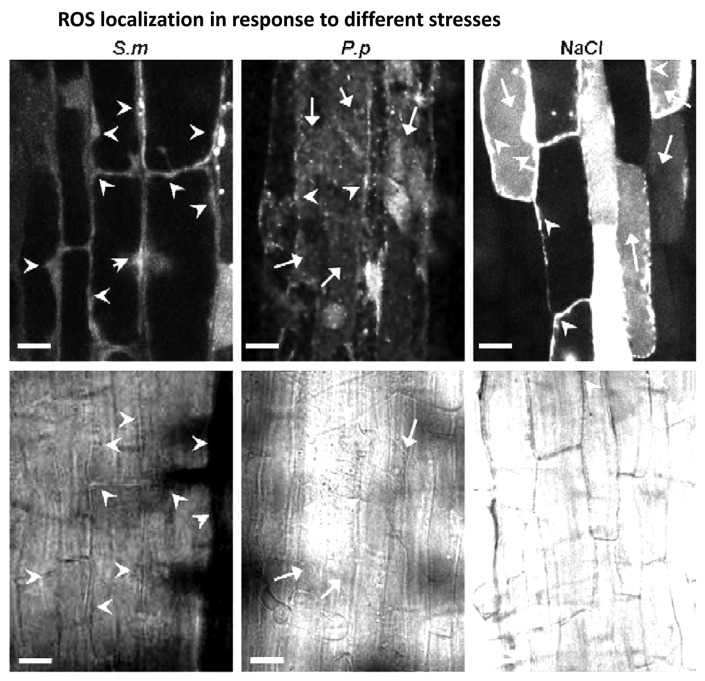
To analyze the distribution of ROS in the cytoplasm during different (biotic and abiotic) stresses, we inoculated the M. truncatula roots with P putida, S. meliloti or treated them with high salinity stress. The accumulation of ROS was analyzed by confocal microscopy. In roots inoculated with P. putida or with P. syringae (data not shown), ROS appeared scattered throughout the cytoplasm, including the vacuole (Fig. 3). Such ROS distribution, including the vacuolar localization, was also observed during the salt stress. However, in M. truncatula plants that were inoculated with S. meliloti the accumulation of ROS was observed exclusively in the cytosol and nuclei, with no ROS detected in the vacuoles.
Figure 3.
Subcellular localization of ROS as a response to stress. Production of ROS in roots of 4 d-old Medicago seedlings (upper panel) at 24 h.p.i. with S. meliloti, or at 4 h.p.i. with P. putida, or treated with 150 mM NaCl as described by (Leshem et al. 2007). Lower panel shows bright field images. Arrowheads point to cytoplasm and arrows indicate vacuoles. Bar = 20 μm. The assay was repeated tree times with very similar results.
Pretreatment of M. truncatula with salicylic acid reduced ROS production in plants inoculated with S. meliloti
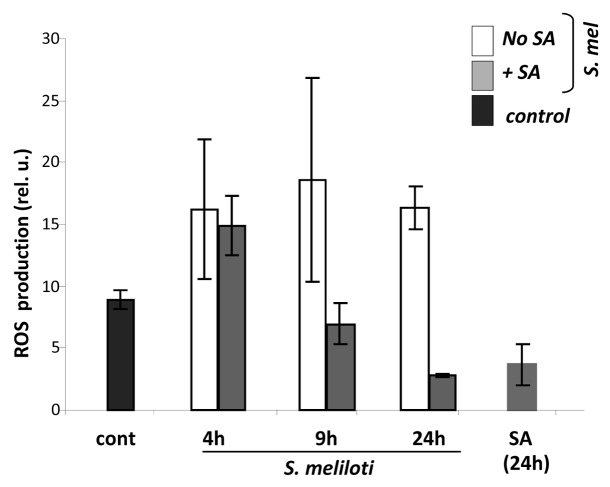
Next, we analyzed the effect of salicylic acid on the length of time of ROS production in M. truncatula roots inoculated with S. meliloti (see Fig. 2). SA and its biologically active analogs, such as benzo-(1,2,3)-thiadiazole-7-carbothioic S-methyl ester (BTH) have been shown to inhibit catalase and ascorbate peroxidase activities during pathogenic infection.10,16 The seedlings were pretreated with SA for 24 h prior to inoculation of S. meliloti, and ROS accumulation was measured after additional 4, 9 and 24 h. Pre-treatment of seedlings with SA significantly reduced the amount of ROS that accumulated after 9 h, and decreased it even further after 24 h (Fig. 4).
Figure 4.
Effect of Salicylic acid on accumulation of ROS in M. truncatula inoculated with S. meliloti. Roots of 4 d-old Medicago seedlings were pretreated with 0.5 mM SA for 2 h and then inoculated with S. meliloti. ROS production was assayed and quantified 24 h after inoculation using 2',7'-dichlorodihydrofluorescein diacetate dye as described in Methods. Error bars indicate standard deviation of the mean (n = 10).
Downregulation of pathogenesis related gene expression during symbiosis
Recent data in A. thaliana showed that the induction of pathogenesis-related genes is regulated by the NPR1protein, which undergoes nuclear translocation after infection.12,17 Analysis of genes that are regulated by the NPR1 protein in A. thaliana showed induction of several PR genes, including PR1 (At2g14610) and WAK1 (At1g21250).18 To monitor the NPR1-mediated gene expression in M. truncatula we analyzed transcription of the putative Medicago orthologs of PR1 and WAK1 (Wall-associated kinase, presently called Protein Kinase, TC135700) genes. The Medicago PR1 protein is listed in the public M. truncatula EST database as TC143335. Both proteins are highly conserved between A. thaliana and M. truncatula: the PR1 protein has 51% identity and 66% similarity with the A. thaliana (At2g14610) PR1 protein, while the WAK- PK shares 50% identity and 69% similarity with the WAK1 protein (Fig. S1).
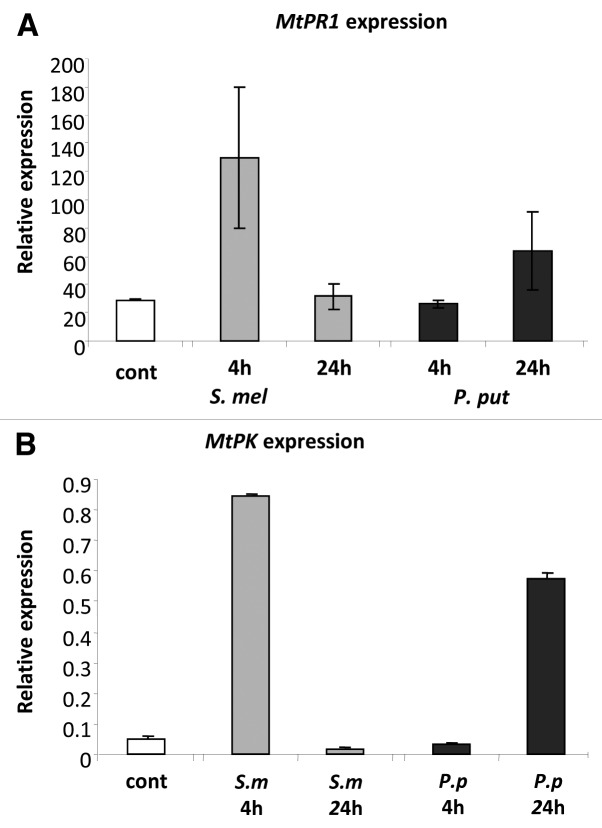
The expression of the NPR1-dependent genes was analyzed at the time points that corresponded to low and high levels of ROS (Fig. 2). Four hours post inoculation with P. putida, which corresponds to the peak time point of ROS accumulation with this bacteria, the expression of both genes was similar to their level in uninoculated plants (Fig. 5). The expression of the PR1 and WAK-PK genes in M. truncatula plants inoculated with P. putida increased 24 h.p.i., which corresponds to sharp reduction in the level of ROS (Fig. 1). These results are in agreement with the nuclear translocation of the NPR1 protein in A. thaliana that occurred 24 h.p.i. with bacteria or after SA treatment (Mou et al. 2003). Furthermore, the expression of both genes was high in the plants 4 h.p.i. with S. meliloti, but decreased at 24 h.p.i. (Fig. 5), which corresponded to the concomitant rise in the accumulation of ROS at 24 h.p.i with symbiotic bacteria (see Fig. 2 to compare the ROS levels at 4 and 24 h.p.i. with S. meliloti).
Figure 5.
Pathogenesis related gene expression in M. truncatula plants inoculated with symbiotic or pathogenic bacteria. Roots of 4 d-old Medicago seedlings were inoculated with S. meliloti or P. putida. RNA was Isolated and reverse transcribed after 4 and 24 h post inoculation. (A) Expression levels of M. truncatula Pathogenesis Related1 (MtPR1) gene. (B) Expression levels of M. truncatula protein kinase (MtPK). Error bars indicate standard deviation of the mean (n = 8) from two separate assays.
Downregulation of PR1 expression by ROS
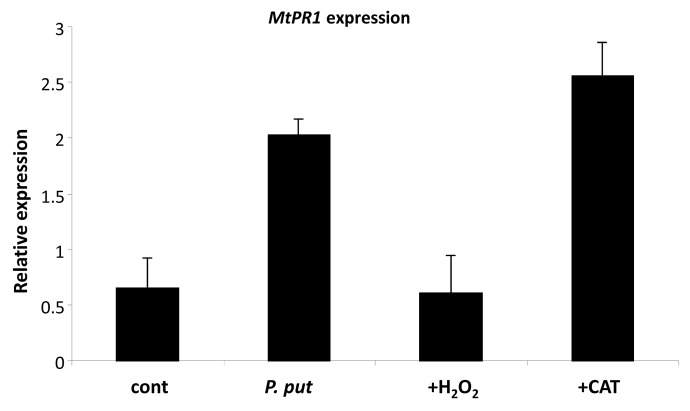
Finally, we analyzed the effect of cytoplasmic ROS on the translocation of NPR1 to the nucleus in plants that were infected with P. putida. To manipulate the production/ accumulation of ROS in the roots we either added H2O2, or increased the scavenging of ROS by catalase. The expression of the MtPR1 gene, which depends on the nuclear localization of NPR1 protein was tested at 24 h.p.i., when the level of ROS returned to normal (see Fig. 1). Pretreatment of roots with H2O2 significantly reduced the MtPR1 gene expression, resulting in transcription level that was similar to uninoculated (control). In contrast, addition of catalase to plants inoculated with P. putida increased the MtPR1 gene expression even higher (Fig. 6).
Figure 6.
Effect of ROS on pathogenesis mediated PR1 gene expression. Roots of 4 d-old Medicago seedlings were exposed to exogenous H2O2 or Catalase or left intact. Two hours later plants were infected with P. putida. RNA was Isolated and reverse transcribed 24 h post infection. Error bars indicate standard deviation of the mean (n = 6) from three separate assays.
Discussion
The plant rhizosphere constitutes a rich biological system that contains many beneficial, as well as pathogenic microorganisms. The interactions between the different microorganisms and the plant roots constitute an important research area for plant development and stress physiology. However, the genetic, biochemical and regulatory components that have been identified reveal little difference between the pathogenic and symbiotic organisms, making it hard to understand which pathways govern the pathogenic vs. symbiotic interactions.19 For example, infection by either the pathogenic or symbiotic bacteria induces the oxidative burst in the host, and in both cases there is induction of PR gene expression. The major differences seem to be of quantitative nature, like the levels of ROS production.4,20
Our results show that inoculation of M. truncatula seedlings inoculated with pathogenic bacteria caused an oxidative burst 4 h.p.i. At that time-point expression of the pathogenesis-related genes was low; their transcription beginning only 24 h.p.i., when the ROS concentration was reduced (Mou, et al. 2003 and Fig. 5). In contrast, high levels of ROS were detected in M. truncatula seedlings during interaction with S. meliloti, which peaked at 24 h.p.i and remained high for 48 h (Fig. 2). Importantly, these later time-point coincided with the low expression of the pathogenesis-related genes (Fig. 5). These results are in line with infection of Arabidopsis thaliana seedlings with the pathogenic bacteria Pseudomonas syringae, which caused an oxidative burst that decreased and returned to control level 3–4 h later.3,21
Another important difference was observed in ROS localization: while ROS induced by nonsymbiotic bacteria are dispersed throughout the whole cytoplasm,22 the symbionts caused ROS production exclusively in the cytosol and nucleus. These differences are important in view of the nature of the NPR1 protein, which is a redox-sensitive protein that in its uninduced state is present in the cytosol as an oligomer.23 However, after return of reducing conditions that follow the oxidative burst the NPR1 protein undergoes reduction of the S-S bonds that hold the subunits together, which results in decomposition into monomers that are translocated to the nucleus, where they induce PR gene expression.12,24 Hence, accumulation of ROS in the cytosol is supporting the multimeric state of the NPR1 protein, keeping it away from the nucleus. This suggestion is also supported by ROS manipulations that increased the oxidative or the reductive states of the cells by addition of H2O2 or catalase, respectively, to the medium (Fig. 6).
Controversial reports on the levels of ROS production during symbiotic interactions have been described. For example, elevated levels of ROS were recorded in Medicago sativa during the rhizobial infection,25 or after treatment with purified Nod factor.5 On the other hand, other researchers detected a transient decrease in ROS production in M. truncatula roots treated with purified NOD factor.26 Decreased production of 22 and salicylic acid was also reported in Pisum sativum L. upon inoculation of S. meliloti.27
Here, we show that the level of Pathogenesis-related (PR) gene expression is opposite to the level of cytoplasmic ROS. We also show that salicylic acid, which is an inducer of the systemic acquired resistance (SAR) by the NPR1-dependent pathway, decreased ROS production during symbiotic interaction with S. meliloti, resulting in the levels of ROS that were similar to pathogenesis (see Fig. 4). It is notable that SA treatment was shown to reduce the oxidative burst in leaves in Cucurbita pepo plants infected with yellow mosaic virus.28 In summary, our results suggest that ROS function at the crossroad between pathogenesis and symbiosis by influencing the NPR1 location within the cell, thus regulating its activity and PR genes' expression.
Materials and Methods
Biological material and plant treatment
Seeds of M. truncatula were scarified for 5 min by exposure to concentrated sulfuric acid and then germinated and grown on a buffered nodulation medium (BNM) as described in (Ehrhardt et al., 1992). S. meliloti strain Rm1021, a streptomycin-resistant derivative of wild-type field isolate, was grown with shaking in yeast mannitol broth (YMB) at 28°C or yeast mannitol supplemented with 15 g/l of agar (YMA). Bacteria were inoculated on the roots in zone 1 at a concentration of 107 cells. P. putida that was a generous gift of Eduard Jurkevitch, Faculty of Agriculture, The Hebrew University of Jerusalem were grown in LB at 28°C.
ROS production in plants
ROS production was analyzed using 10 μM 2',7'-dichlorodihydrofluorescein diacetate and quantified by the ImagePro Plus analysis package (Media Cybernetics) as described by (Peleg-Grossman et al., 2007).1 Roots were photographed with Nikon Coolpix 4500 camera attached to Olympus IX70 microscope. The fluorescent light pass settings used narrow-band cube (Omega Optical Inc., Brattelboro). The pixels of mean density were collected from representative images for statistical analysis (n = 12). Confocal microscopy: roots were viewed using a MRC-1024 confocal microscope (Bio-Rad) equipped with 40X oil immersion objective (N.A. 1.3). Time series sections were collected once every 30 sec.
Pharmacological treatments
Three-day-old M. truncatula seedlings were treated as following: either transferred to liquid BNM containing 0.5 mM SA for 24 h; transferred to agar BNM containing H2O2 for 2 h; or transferred to agar BNM containing Catalase was from bovine liver (C40, Sigma-Aldrich) for 2 h. All treatments were followed by inoculation as described in the legends.
RT-PCR assay
Total RNA was extracted from roots before and after treatment. Roots were frozen in liquid nitrogen; RNA was extracted with Tri Reagent (Molecular Research Center, Inc.) and transcribed into cDNA using oligo dT as a primer with SuperScript II reverse transcriptase (Invitrogen). cDNA was amplified by quantitative PCR using a Rotor Gene 2000 thermocycler (Corbett Research), and the following primers: PR1 (TC143335): forward cacaactcttgtgttgatggaga, reverse tccatagtaattcccaggaggat; PK (TC135700): forward ttggttaatgaagcaaggaaaga, reverse tgttcatcacttggtgattcttg; EF1a gene was used for normalization: forward GTGGTGGTTTTGTTACAAGAATTG, reverse AAACGGTTCTAGTGAAACTTATCA.
The quantification and calibration curves were run simultaneously with experimental samples, and Ct calculations were performed by the Rotor-Gene 5.0 software (Corbett Research).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19217
References
- 1.Peleg-Grossman S, Volpin H, Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J Exp Bot. 2007;58:1637–49. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- 2.Lhuissier FGP, De Ruijter NCA, Sieberer BJ, Esseling JJ, Emons AMC. Time course of cell biological events evoked in legume root hairs by Rhizobium Nod factors: State of the art. Ann Bot (Lond) 2001;87:289–302. doi: 10.1006/anbo.2000.1333. [DOI] [Google Scholar]
- 3.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–93. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 4.Pauly N, Pucciariello C, Mandon K, Innocenti G, Jamet A, Baudouin E, et al. Reactive oxygen and nitrogen species and glutathione: key players in the legume-Rhizobium symbiosis. J Exp Bot. 2006;57:1769–76. doi: 10.1093/jxb/erj184. [DOI] [PubMed] [Google Scholar]
- 5.Ramu SK, Peng HM, Cook DR. Nod factor induction of reactive oxygen species production is correlated with expression of the early nodulin gene rip1 in Medicago truncatula. Mol Plant Microbe Interact. 2002;15:522–8. doi: 10.1094/MPMI.2002.15.6.522. [DOI] [PubMed] [Google Scholar]
- 6.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Shah J. Plants under attack: systemic signals in defence. Curr Opin Plant Biol. 2009;12:459–64. doi: 10.1016/j.pbi.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Vlot AC, Dempsey DA, Klessig DF. Salicylic Acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 9.Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. Plant Cell. 1996;8:1809–19. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mou Z, Fan W, Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–44. doi: 10.1016/S0092-8674(03)00429-X. [DOI] [PubMed] [Google Scholar]
- 11.Després C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, et al. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. Plant Cell. 2003;15:2181–91. doi: 10.1105/tpc.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochon A, Boyle P, Wignes T, Fobert PR, Després C. The coactivator function of Arabidopsis NPR1 requires the core of its BTB/POZ domain and the oxidation of C-terminal cysteines. Plant Cell. 2006;18:3670–85. doi: 10.1105/tpc.106.046953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Abarca F, Herrera-Cervera JA, Bueno P, Sanjuan J, Bisseling T, Olivares J. Involvement of salicylic acid in the establishment of the Rhizobium meliloti—Alfalfa symbiosis. Mol Plant Microbe Interact. 1998;11:153–5. doi: 10.1094/MPMI.1998.11.2.153. [DOI] [Google Scholar]
- 14.Peleg-Grossman S, Golani Y, Kaye Y, Melamed-Book N, Levine A. NPR1 protein regulates pathogenic and symbiotic interactions between Rhizobium and legumes and non-legumes. PLoS One. 2009;4:e8399. doi: 10.1371/journal.pone.0008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peleg-Grossman S, Cohen G, Levine A. Cytoplasmic H2O2 prevents translocation of NPR1 to the nucleus and inhibits the induction of PR genes in Arabidopsis. Plant Signal Behav. 2010;5:1–6. doi: 10.4161/psb.5.11.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen ZX, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262:1883–6. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 17.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 18.Blanco F, Garretón V, Frey N, Dominguez C, Pérez-Acle T, Van der Straeten D, et al. Identification of NPR1-dependent and independent genes early induced by salicylic acid treatment in Arabidopsis. Plant Mol Biol. 2005;59:927–44. doi: 10.1007/s11103-005-2227-x. [DOI] [PubMed] [Google Scholar]
- 19.Hentschel U, Steinert M, Hacker J. Common molecular mechanisms of symbiosis and pathogenesis. Trends Microbiol. 2000;8:226–31. doi: 10.1016/S0966-842X(00)01758-3. [DOI] [PubMed] [Google Scholar]
- 20.Santos R, Hérouart D, Sigaud S, Touati D, Puppo A. Oxidative burst in alfalfa-Sinorhizobium meliloti symbiotic interaction. Mol Plant Microbe Interact. 2001;14:86–9. doi: 10.1094/MPMI.2001.14.1.86. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–84. doi: 10.1016/S0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- 22.Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R. Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol. 2007;143:1817–26. doi: 10.1104/pp.106.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laloi C, Apel K, Danon A. Reactive oxygen signalling: the latest news. Curr Opin Plant Biol. 2004;7:323–8. doi: 10.1016/j.pbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Dong XN. NPR1, all things considered. Curr Opin Plant Biol. 2004;7:547–52. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Rubio MC, James EK, Clemente MR, Bucciarelli B, Fedorova M, Vance CP, et al. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol Plant Microbe Interact. 2004;17:1294–305. doi: 10.1094/MPMI.2004.17.12.1294. [DOI] [PubMed] [Google Scholar]
- 26.Lohar DP, Haridas S, Gantt JS, VandenBosch KA. A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol. 2007;173:39–49. doi: 10.1111/j.1469-8137.2006.01901.x. [DOI] [PubMed] [Google Scholar]
- 27.Glyan’ko AK, Makarova LE, Vasil’eva GG, Mironova NV. Possible involvement of hydrogen peroxide and salicylic acid in the legume-rhizobium symbiosis. Biol Bull. 2005;32:245–9. doi: 10.1007/s10525-005-0096-0. [DOI] [PubMed] [Google Scholar]
- 28.Radwan DEM, Ali Fayez K, Younis Mahmoud S, Hamad A, Lu G. Salicylic acid alleviates growth inhibition and oxidative stress caused by zucchini yellow mosaic virus infection in Cucurbita pepo leaves. Physiol Mol Plant Pathol. 2006;69:172–81. doi: 10.1016/j.pmpp.2007.04.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.