Abstract
Severe mental illnesses have been linked to white matter abnormalities, documented by postmortem studies. However, cause and effect have remained difficult to distinguish. CNP (2′,3′-cyclic nucleotide 3′-phosphodiesterase) is among the oligodendrocyte/myelin-associated genes most robustly reduced on mRNA and protein level in brains of schizophrenic, bipolar or major depressive patients. This suggests that CNP reduction might be critical for a more general disease process and not restricted to a single diagnostic category. We show here that reduced expression of CNP is the primary cause of a distinct behavioural phenotype, seen only upon aging as an additional ‘pro-inflammatory hit’. This phenotype is strikingly similar in Cnp heterozygous mice and patients with mental disease carrying the AA genotype at CNP SNP rs2070106. The characteristic features in both species with their partial CNP ‘loss-of-function’ genotype are best described as ‘catatonia-depression’ syndrome. As a consequence of perturbed CNP expression, mice show secondary low-grade inflammation/neurodegeneration. Analogously, in man, diffusion tensor imaging points to axonal loss in the frontal corpus callosum. To conclude, subtle white matter abnormalities inducing neurodegenerative changes can cause/amplify psychiatric diseases.
Keywords: anxiety, axonal degeneration, diffusion tensor imaging, low-grade inflammation, social withdrawal
INTRODUCTION
The CNP gene encodes the enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) which is present in non-compacted myelin areas such as the inner mesaxon, paranodal loops and Schmidt-Lantermann incisures (Braun et al, 2004; Yu et al, 1994), and accounts for about 4% of total central nervous system myelin proteins (Braun et al, 2004). CNP is expressed early in development of oligodendrocytes (Yu et al, 1994), increases with onset of myelination and remains detectable in these cells throughout life (Scherer et al, 1994). In vitro and in vivo studies demonstrated a regulatory function of CNP for process outgrowth in oligodendrocytes (Gravel et al, 1996; Lee et al, 2005; Yin et al, 1997), as well as an interaction with microtubules, cytoskeleton and RNA (Bifulco et al, 2002; De Angelis & Braun, 1996; Gravel et al, 2009; Lee et al, 2005).
Studies employing homozygous Cnp-null mutant mice revealed that Cnp is essential for axonal survival but not for myelin assembly (Lappe-Siefke et al, 2003). In fact, Cnp−/− mice show progressive axonal swellings and brain inflammation with first motor deficits occurring at 4 months that progress to severe hindlimb paralysis and death at 8–15 months (Lappe-Siefke et al, 2003). In contrast, Cnp+/− mice with a 50% reduced Cnp expression do not exhibit any signs of inflammation nor of abnormalities in neurological scoring or behaviour at least until the age of 12 months (Lappe-Siefke et al, 2003; Wieser et al, in preparation), indicating that lower Cnp levels can be fully compensated for.
Nevertheless, decreased CNP expression could have pathophysiological significance. CNP is among the oligodendrocyte/myelin-associated genes identified to be most robustly reduced both on mRNA and protein level in postmortem brains of schizophrenic, bipolar or major depressive patients (Aston et al, 2005; Mitkus et al, 2008; Tkachev et al, 2003). These findings suggest that CNP reduction might be critical in a more general disease process and that the potential role of this molecule is not restricted to a single diagnostic category but of global relevance for severe mental disorders.
Several genetic association studies have explored a potential impact of genetic variability in the CNP gene (chr17q21.2, 11Kb) on the overall risk for schizophrenia, with inconclusive results so far (Che et al, 2009; Peirce et al, 2006). Interestingly, however, a synonymous (Gly/Gly) single nucleotide polymorphism (SNP), localized in the fourth exon of the gene (rs2070106), influences CNP expression in the human cortex, especially in frontal areas, with the rarer A-allele showing lower expression in comparison to the G-allele (Iwamoto et al, 2008; Mitkus et al, 2008; Peirce et al, 2006).
Recent work indicates that in major psychiatric disorders like schizophrenia and depression, low-grade inflammation constitutes a crucial mechanism in the final common disease pathway (reviewed in Monji et al, 2009). Already the normal aging process is associated with slightly increased brain inflammation characterized by, for example, enhanced levels of pro-inflammatory cytokines, higher microglial numbers and increased reactivity with augmented expression of microglial surface markers (reviewed in, e.g. Miller & Streit, 2007; Sparkman & Johnson, 2008; Streit, 2006).
To address the pathophysiological relevance of reduced CNP expression, we chose CNP partial ‘loss-of-function’ genotypes with aging as an additional ‘pro-inflammatory hit’. We examined old Cnp+/− mice and schizophrenic patients with the AA versus GG genotype in the CNP SNP rs2070106. We report here the surprising association of CNP partial loss-of-function with a catatonia-depression syndrome both in mouse and man upon aging. Importantly, we provide evidence for late-onset low-grade inflammation in mice as a plausible pathophysiological mechanism. In patients carrying the low-expression genotype (AA), a comparable process might be reflected by axonal loss in the frontal corpus callosum as detectable by neuroimaging.
RESULTS
Brains of aging Cnp+/− mice are characterized by enhanced inflammation, astrogliosis and axonal degeneration
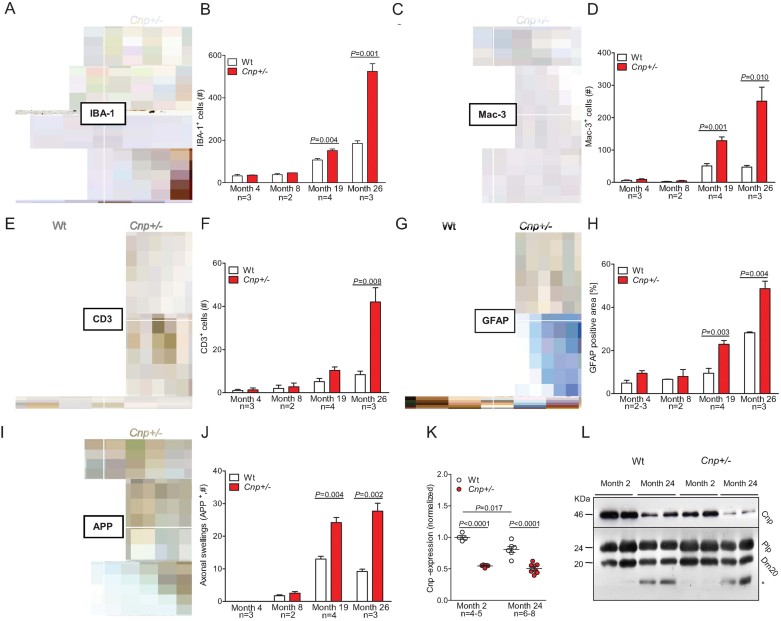
Immunohistochemical analysis of mouse brains from age 4 to 26 months revealed an age-related increase in the number of ionized calcium-binding adapter molecule 1 (IBA-1) and Mac-3 positive microglia, infiltrating T-lymphocytes (cluster of differentiation 3; CD3) and astrocytes (glial fibrillary acidic protein, GFAP) in corpus callosum, striatum and anterior commissure (month 4 vs. month 26: all p ≤ 0.01; for wild-type (Wt) as well as Cnp+/− mice). This increase was significantly more pronounced in old Cnp+/− as compared to Wt mice (Fig 1A–H). Axonal swellings (spheroids) as readout of neurodegeneration were determined in corpus callosum, striatum and anterior commissure using amyloid precursor protein (APP) immunoreactivity (Fig 1I/J). At the age of 4 months, no positive APP staining was detected. Thereafter, an age-dependent increase in axonal swellings became evident, again more prominent in Cnp+/− mice (Fig 1I/J). Determination of Cnp mRNA expression in brains of young versus old mice revealed a remarkable decrease upon aging in Wt mice, which, however, still maintained levels above those in Cnp+/− mice (Fig 1K). In both Wt and Cnp+/− mice, we found a corresponding age-dependent reduction of Cnp protein in purified myelin membranes, with the lowest overall level in aged Cnp+/− (Fig 1L). Proteolipid protein (Plp), a control protein for compact myelin, also decreased with age but independent of the Cnp genotype (Fig 1L). Taken together, old Cnp+/− mice show a more pronounced low-grade inflammatory phenotype with axonal degeneration compared to Wt mice.
Figure 1. Low-grade brain inflammation and axonal degeneration in aged Cnp +/− mice.
- Representative microscopic images of the corpus callosum from 4 months (upper panels) and 26 months (lower panels) old Wt and Cnp+/− mice, immunostained for IBA-1; scale bar 20 µm.
- Bar graph gives the age-dependent quantification of the total number of IBA-1 positive microglia in the corpus callosum of Wt and Cnp+/− mice. For all quantifications (B, D, F, H, J), n numbers indicated; mean ± s.e.m. presented; two-sided Student's t-test used.
- Representative microscopic images of the corpus callosum from 4 months (upper panels) and 26 months (lower panels) old Wt and Cnp+/− mice, immunostained for Mac-3; scale bar 20 µm.
- Bar graph gives the age-dependent quantification of the total number of Mac-3 positive microglia in the corpus callosum of Wt and Cnp+/− mice.
- Representative microscopic images of the corpus callosum from 4 months (upper panels) and 26 months (lower panels) old Wt and Cnp+/− mice, immunostained for CD3; black arrows exemplify respective positive cells; scale bar 20 µm.
- Bar graph gives the age-dependent quantification of the total number of CD3 positive T-lymphocytes in the corpus callosum, striatum and anterior commissure of Wt and Cnp+/− mice.
- Representative microscopic images of the corpus callosum from 4 months (upper panels) and 26 months (lower panels) old Wt and Cnp+/− mice, immunostained for GFAP; scale bar 20 µm.
- Densitometrical quantification of the GFAP positive area in the corpus callosum.
- Representative microscopic images of the striatum from 4 months (upper panels) and 26 months (lower panels) old Wt and Cnp+/− mice, immunostained for APP; black arrows exemplify respective positive cells; scale bar 20 µm.
- Bar graph gives the age-dependent quantification of the APP positive axonal swellings in the corpus callosum, striatum and anterior commissure of Wt and Cnp+/− mice.
- Cnp mRNA expression level of Wt and Cnp+/− mice at months 2 and 24, normalized to mean value of ATP synthase subunit beta (Atp5b) and acidic ribosomal phosphoprotein P0 (Rplp0) as housekeeper genes and to 2 months old Wt (1.0); mean ± s.e.m. presented; two-sided Student's t-test used.
- Cnp protein expression of Wt and Cnp+/− mice at months 2 and 24, compared to Plp as control protein of compact myelin; * low-size band detected in aged brain myelin with the Plp antibody directed against the C-terminus of PLP/DM20.
Aged Cnp+/− mice have a slightly elevated anxiety profile but normal motor activity, coordination and strength
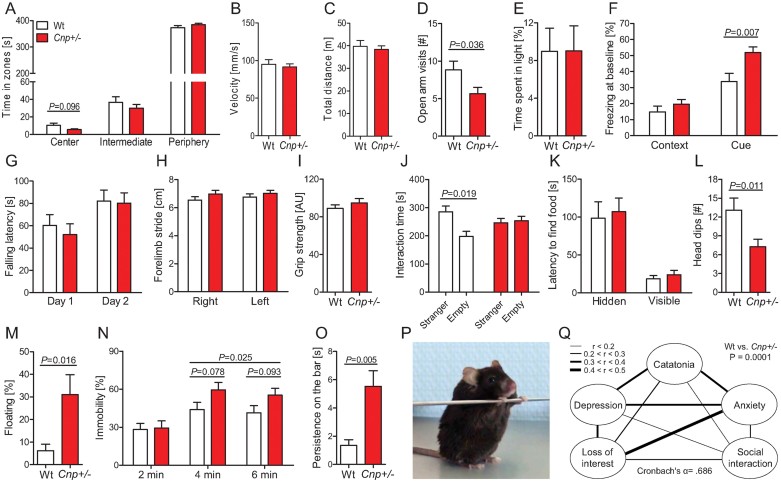
To test whether the pronounced histological changes upon aging are associated with any behavioural consequences, we investigated aged (24 months old) Cnp+/− and Wt mice employing a comprehensive test battery. In the open field test, a measure for general locomotor activity and anxiety, Cnp+/− mice tended to spend less time in the centre than Wt (p = 0.096; Fig 2A). Velocity and total distance travelled in the open field were comparable in both genotypes (Fig 2B and C), indicating normal activity. In the elevated plus maze, a classical anxiety test, open arm visits were reduced in Cnp+/− mice (p = 0.036; Fig 2D), whereas, the light/dark-box did not yield differences in the time spent in light (Fig 2E). Freezing behaviour is seen as another indicator of anxiety/fear in rodents. Cnp+/− mice showed higher percentage of freezing in the fear conditioning chamber already at baseline, that is before measurement of conditioned or cued memory (p = 0.007; Fig 2F), precluding the use of fear conditioning for memory assessment in these mice. Like basic motor activity, which proved to be normal, motor performance, coordination and motor learning, as evaluated in a 2-day rota-rod testing, were not different between genotypes (Fig 2G). Also, gait analysis detected no motor abnormalities or ataxia (see, e.g. Fig 2H depicts forelimb stride of left and right paw) and muscle strength, measured by the grip strength test, did not differ between genotypes (Fig 2I). To summarize, 24 months old Cnp+/− mice show normal overall motor performance and a mildly elevated anxiety profile in different anxiety-relevant tests compared to Wt mice.
Figure 2. Aged Cnp +/− mice show a phenotype composed of catatonia, depression, loss of interest, impaired social interaction and anxiety.
- A-C Open arm parameters.
- D. Elevated plus maze.
- E. Light/dark box paradigm.
- F. Baseline freezing in the context and cue memory task of fear conditioning.
- G. Rota-rod.
- H. Gait analysis.
- I. Grip strength.
- J. Sociability testing in the three-partite chamber.
- K. Buried-food finding test – latency to find hidden versus visible food pellets.
- L. Hole board.
- M. Floating rate in a 90 s swim trial.
- N. Tail suspension test.
- O. Bar test for catatonia.
- P. Typical posture of a catatonic Cnp+/− mouse during the bar test; see also videos of Supporting Information.
- Q. Behavioural composite score displayed as intercorrelation network of Z-transformed items. Line thickness indicates the degree of correlation between 2 respective items. The composite score differs between genotypes (p = 0.0001). For all behavioural experiments, 24 months old mice were used: Wt n = 9–11 and Cnp+/− n = 10–16; mean ± s.e.m. presented; two-sided or paired t-tests used where applicable.
Aged Cnp+/− mice show impaired social and exploratory behaviour
Social behaviour of aged Wt and Cnp+/− mice was tested in a three-partite chamber. This test measures the preference of a mouse for a chamber containing a small wire cage with a stranger mouse in comparison to a chamber with an empty wire cage. Aged Wt mice displayed the expected behaviour, that is spent significantly more time close to the cage with the stranger mouse compared to the empty wire cage (p = 0.019), whereas, Cnp+/− mice did not show preference. To control for altered olfaction as a potential confounder of social behaviour in mice, the buried-food-finding test was performed, confirming normal olfactory function in both groups (Fig 2K). In the hole board test, measuring exploratory behaviour of mice, old Cnp+/− mice had significantly less head dips (p = 0.011; Fig 2L), indicating loss of interest (in the absence of any signs of altered basic motor activity). To conclude, old Cnp+/− mice demonstrate several facets of a loss of interest in the outside world.
Aged Cnp+/− mice exhibit features of depression and catatonia
In the Morris water maze task, Cnp+/− mice displayed prominent floating behaviour, precluding analysis of this test for learning and memory. Analysis of the time mice spent floating within a swim trial of 90 s yielded threefold higher floating rates of Cnp+/− mice in comparison to Wt, which we interpret as a potential sign of depression (p = 0.016; Fig 2M). To further consolidate this hypothesis, we performed an established test to measure depression in rodents, the tail suspension test, which determines over 6 min the time mice spend immobile. Fractionated analysis revealed that Cnp+/− mice had a higher duration of immobility in the second and last third of the test period compared to Wt (p = 0.025; Fig 2N), consistent with the typical ‘give up’ behaviour of depressed individuals. A phenotype, thus far observed in mice only upon induction (e.g. body pinch or drug exposure; Amir, 1986; Chaperon & Thiebot, 1999) is catatonia/catalepsy, a state of immobility where mice persist in an externally imposed abnormal posture for a prolonged time period. Mice are put into a position where they have to grab a bar while standing with their hind paws on the floor (as illustrated in Fig 2P; for a striking example see videos of Supporting Information). Wt mice swiftly left this position, whereas, Cnp+/− mice persisted in this posture (p = 0.005; Fig 2O). Taken together, old Cnp+/− mice exhibit a catatonia-depression syndrome.
Creating a mouse behavioural composite, the ‘catatonia-depression score’
For translational purposes and confirmation of the internal consistency of our behavioural readouts in aged mice, we calculated intercorrelations between the observed behavioural sub-phenotypes catatonia, depression, loss of interest, impaired social interaction and anxiety as target variables. These variables, put together in a composite score, were internally consistent (Cronbach's α = .686; Fig 2Q). Operationalization of the score items is detailed in the Materials and Methods Section. Expectedly, the score was significantly higher in Cnp+/− (0.32 ± 0.44) than in Wt mice (−0.43 ± 0.41; p = 0.0001). Based on these findings, we wondered whether reduced expression of the CNP gene in aging human patients may have a similar influence on the phenotype.
Exploiting the GRAS data base for a phenotype-based genetic association study on the role of CNP genotypes in a ‘catatonia-depression syndrome’
To search for potential behavioural consequences of a previously described CNP loss-of-function genotype in humans (Iwamoto et al, 2008; Mitkus et al, 2008; Peirce et al, 2006), we conducted a phenotype-based genetic association study (PGAS) targeting the CNP SNP rs2070106 (A/G; Fig 3A) in >1000 schizophrenic patients of the Göttingen Research Association for Schizophrenia (GRAS) data collection (Begemann et al, 2010; Ribbe et al, 2010). As a first step, we performed a case–control analysis (schizophrenic patients vs. healthy controls) and found that this genetic marker does not contribute to an increased risk of schizophrenia in our population, as proven by the genotypic and the allelic chi-square comparison (p > 0.05; Table I of Supporting Information).
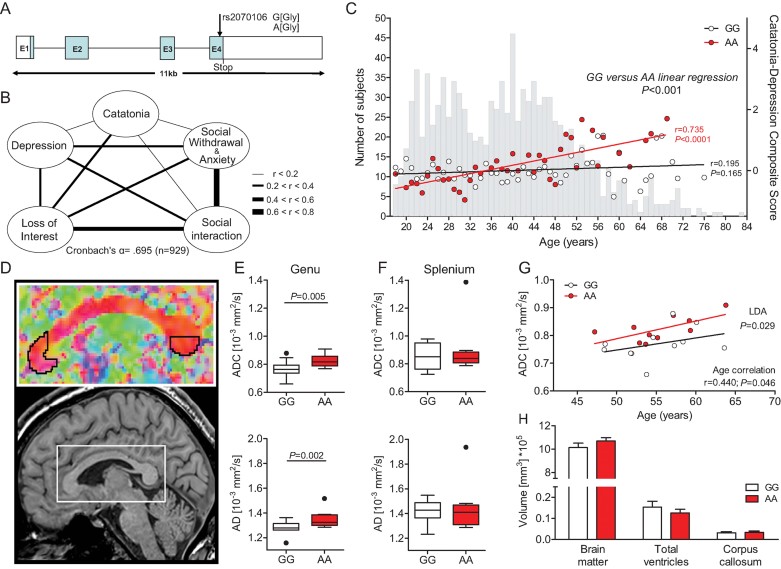
Figure 3. Age- and genotype-dependent association of the CNP rs2070106 SNP with a catatonia-depression syndrome in the GRAS sample of schizophrenic patients.
- A. Schematic view of the human CNP gene structure and location of the synonymous SNP rs2070106 (A/G).
- B. Intercorrelation network of all Z-transformed items of the catatonia-depression composite in the GRAS population. Line thickness indicates the degree of correlation between two respective items.
- C. Correlation of genotypes with the catatonia-depression composite score across age groups. Grey bars in the background display the age distribution of the total GRAS sample of schizophrenic patients (n = 1048). Red or white circles denote mean values of the composite score for the respective age group and genotype (red, AA; black, GG). Linear regression lines of the genotypes dissociate after the age of 40 years. Pearson product-moment correlation applied.
- D. Diffusion tensor imaging (DTI) study selecting the frontal (genu) and caudal (splenium) areas of the corpus callosum as regions of interest.
- E,F ADC and AD values plotted according to rs2070106 homozygosity status in genu (E, target region) and splenium (F, control region) of the corpus callosum in a subgroup of schizophrenic individuals >40 years of age (GG n = 11 and AA n = 10); results corrected for chlorpromazine equivalents (CPZ). Mean ± s.e.m. presented and ANCOVA applied.
- G. Correlation of ADC and age in AA and GG genotypes; linear discriminant analysis (LDA) with genotype as grouping variable and ADC and age as independent variables. Pearson product-moment correlation applied.
- H. Magnetic resonance imaging (MRI) volumetric comparison of brain matter, ventricular system and corpus callosum between genotypes. Mean ± s.e.m. presented; two-sided Student's t-test applied.
Next, a composite score including all variables represented in the mouse behaviour composite was created that also yielded good internal consistency with a Cronbach's α = .695 (Fig 3B). The operationalization of the score items is explained in the Materials and Methods Section. As illustrated in Fig 3C, the composite score shows a clear age and genotype (rs2070106) association: AA subjects develop a significantly higher score with increasing age as compared to GG carriers, with the dissociation of the regression lines starting at around the age of 40 years. We therefore set a cut-off of 40 years and focused on the older schizophrenic patients with our further PGAS analysis.
The characteristics of the GRAS patients with an age ≥40 years, separated by AA versus GG genotype of rs2070106, are presented in Table 1. These data demonstrate that both genotype groups are comparable with respect to basic sociodemographic and clinical/disease control variables but differ highly significantly in the composite score measuring the catatonia-depression syndrome. Interestingly, heterozygote individuals (GA) are very similar to GG subjects. They do not show an intermediate phenotype in the composite score (Table II of Supporting Information). Importantly, when screening all items of the composite separately, a significant age-associated genotype (GG vs. AA) effect, comparable to the mouse findings, becomes evident for all (Fig 1 of Supporting Information).
Table 1.
Sociodemographic variables, composite score (target variable) and clinical/disease control variables of the GRAS sample of schizophrenic patients ≥40 years with homozygosity in CNP SNP rs2070106 (A/G) and – for comparison – in the subset of patients selected for DTI
| GRAS sample ≥40 years | Pb (F/χ2) | DTI subsample | |||
|---|---|---|---|---|---|
| AA (n = 45) | GG (n = 235) | AA (n = 10) | GG (n = 11) | ||
| Sociodemographic variables | |||||
| Age, years, mean ± SD (range) | 51.04 ± 7.65 (40.44–69.93) | 50.16 ± 8.42 (44.03–79.49) | 0.515 | 52.07 ± 4.74 (44.08–60.30) | 49.50 ± 5.34 (40.66–58.71) |
| Gender, No. (%), male | 26 (57.8%) | 142 (60.4%) | 0.740 | 7 (70%) | 8 (72.7%) |
| Ethnicity, No. (%), Caucasian | 43 (95.6%) | 225 (95.7%) | 0.892 | 10 (100%) | 11 (100%) |
| Years of educationa, mean ± SD (range) | 12.27 ± 3.82 (0–21) | 12.32 ± 3.36 (0–27) | 0.933 | 12.45 ± 3.39 (9–19.5) | 14.14 ± 3.16 (8–19) |
| Target variable | |||||
| Catatonia-depression composite score, mean ± SD (range) | 0.38 ± 0.86 (−1.13–1.91) | 0.03 ± 0.74 (−1.20–2.44) | 0.009 | 0.72 ± 0.94 (−0.99–1.85) | 0.02 ± 0.76 (−0.97–0.97) |
| 0.006c | |||||
| Clinical/disease control variables | |||||
| Age at first episode, years, mean ± SD (range) | 29.77 ± 10.18 (15.26–55.61) | 30.71 ± 9.58 (14.73–57.35) | 0.533 | 30.80 ± 11.03 (19.35–49.61) | 30.80 ± 11.03 (22.10–41.89) |
| Duration of disease (1st episode), years, mean ± SD (range) | 21.02 ± 10.27 (0.16–47.35) | 19.39 ± 10.86 (0.04–58.23) | 0.359 | 21.27 ± 11.26 (0.16–39.13) | 19.26 ± 9.19 (3.30–34.18) |
| CPZ, mean ± SD (range) | 650.68 ± 515.35 (37.5–2295.0) | 805.76 ± 915.14 (0–7375.0) | 0.271 | 525.93 ± 276.29 (175–940) | 352.61 ± 338.62 (0–1200) |
| PANSS pos, mean ± SD (range) | 14.00 ± 6.81 (7–36) | 14.55 ± 6.63 (7–35) | 0.619 | 12.9 ± 6.01 (7–25) | 11.27 ± 3.66 (7–17) |
| PANSS neg, mean ± SD (range) | 19.79 ± 8.48 (7–38) | 18.86 ± 8.21 (7–46) | 0.503 | 21.1 ± 8.94 (7–35) | 17.55 ± 5.87 (7–27) |
| PANSS gen, mean ± SD (range) | 35.66 ± 14.17 (16–68) | 34.65 ± 12.54 (16–82) | 0.644 | 35.1 ± 11.20 (20–55) | 29.82 ± 9.97 (17–51) |
| PANSS total, mean ± SD (range) | 69.22 ± 27.27 (30–128) | 68.10 ± 24.70 (30–160) | 0.795 | 69.1 ± 23.64 (37–115) | 58.64 ± 16.35 (31–90) |
| GAF, mean ± SD (range) | 42.68 ± 20.22 (11–90) | 43.82 ± 17.56 (10–90) | 0.703 | 43.80 ± 14.21 (25–63) | 56.55 ± 17.41 (35–85) |
| CGI, mean ± SD (range) | 5.75 ± 1.35 (3–8) | 5.62 ± 1.13 (2–8) | 0.488 | 6.00 ± 0.94 (5–7) | 5.09 ± 1.14 (3–7) |
CPZ, chlorpromazine equivalents as measure of antipsychotic drug dose; PANSS, positive and negative syndrome scale (consisting of three parts: pos; positive symptoms; neg, negative symptoms; gen, general psychopathology); GAF, global assessment of functioning; CGI, clinical global impression (see Ribbe et al, 2010 for further details).
Due to missing data upon phenotyping, sample size varies between n = 242 and 280 in the sample of individuals with age equal to or above 40 years.
Rating according to graduation/certificate; patients currently in school or in educational training are excluded.
Statistical methods used: ANOVA or χ2-test.
Result after correction for CPZ.
CNP rs2070106 genotypes influence myelin/axon integrity in the frontal corpus callosum fibres, a candidate region of catatonia-depression
Based on clinical observation of the affected individuals – both mouse and man – and the scarce information in the literature on brain areas potentially involved in the catatonic phenomenon (Arora & Praharaj, 2007; Northoff et al, 2004), we hypothesized that aging AA individuals displaying the catatonia-depression syndrome, in contrast to GG subjects, should show differences in axonal integrity of frontal crossing fibres. To prove this hypothesis, a subset of older patients of both genotypes (GG n = 11; AA n = 10) from the GRAS sample was selected and matched according to age, gender and duration of disease (Table 1). These patients, living all over Germany, were re-invited to Göttingen for diffusion tensor imaging (DTI). Indeed, DTI identified higher axial diffusivity (AD) and a higher apparent diffusion coefficient (ADC) in the frontal part of the corpus callosum (genu) of AA subjects as compared to GG patients (p ≤ 0.005 for both values; Fig 3E), consistent with a more progressed axonal loss/degeneration. This effect was specific for the frontal commissural fibres and was not observed in the posterior corpus callosum taken as a control region (Fig 3F). ADC values in the genu were generally correlated with age but, despite the small number of imaged subjects, resulted in a significant difference between genotypes upon linear discriminant analysis (LDA; p < 0.05; Fig 3G). Importantly, there were no global brain volume differences detectable between GG and AA subjects that could have accounted for DTI results (p > 0.05 for all comparisons; Fig 3H).
DISCUSSION
We report here the unexpected finding that CNP loss-of-function genotypes are causative of a mental syndrome, consisting of catatonia, depression, mild anxiety/social withdrawal, impaired social interaction and reduced interest in the outside world, which is remarkably similar in mouse and man. In both species, age becomes an important cofactor, supporting the view that the underlying mechanism of this mental syndrome is a slowly progressive neurodegeneration, beginning in subcortical white matter, as described for the more rapid axonal loss in Cnp null mutant mice (Edgar et al, 2009; Lappe-Siefke et al, 2003). Importantly, the CNP loss-of-function genotype is causative of the here described behavioural syndrome but not of schizophrenia where it may only shape the aging phenotype.
In fact, the human part of this study has been obtained from a phenotypically extremely well characterized schizophrenic population (the ‘GRAS data collection’), which was accessible and where all assessed items of the catatonia-depression syndrome are potentially relevant for disease subphenotypes. If a similar database on patients with, for example major depression had been available, the study would have been extended to this population. We expect that in individuals suffering from other mental disorders and even to some (perhaps mild) degree in healthy subjects, the phenotypical consequence of the CNP rs2070106 AA genotype will be comparable. Along these lines, we show that many schizophrenic patients (and virtually all patients younger than 40 years) lack this syndrome. We would therefore like to stress again that this syndrome is independent of the diagnosis schizophrenia, which is also supported by the behavioural homology of the Cnp mouse model.
Several studies have suggested that schizophrenia and affective disorders are on a continuum of liability. Genetic linkage and association studies have proposed common disease loci for both disorders (Berrettini, 2000; O'Donovan et al, 2008). Family studies document that first-degree relatives of bipolar patients have a threefold higher risk for schizophrenia compared with first-degree relatives of healthy controls (Sham et al, 1994; Valles et al, 2000). Psychopathological syndromes, as the catatonia-depression syndrome shown here, shared by subgroups of both patient populations, would also be compatible with this overlap. Indeed, catatonia has been found to be highly prevalent in elderly patients with major depression (Starkstein et al, 1996). It will be interesting to determine whether depressed individuals that exhibit catatonic signs are also preferentially carriers of the CNP rs2070106 AA genotype.
To our knowledge, no spontaneous catalepsy in mice has as yet been reported, in contrast to pinch- or drug-induced catalepsy/catatonia (for review see, e.g. Amir, 1986; Chaperon & Thiebot, 1999). The here observed Cnp+/− associated catalepsy/catatonia represents, therefore, the first clearly defined genetic catatonia model. Catatonia as a prominent phenotype has been extensively described by Karl Kahlbaum in 1874 (Kahlbaum, 1874) and entered the Diagnostic and Statistic Manual of Mental Disorders (APA, 2000) from its first edition in 1952 on, where it appears until now in connection with mood disorders, schizophrenia, and general medical conditions (Heckers et al, 2010). Nevertheless, reports on potential brain areas involved in this phenomenon in man are still scarce and point to frontal regions, based on, for example pronounced catatonia in a case with butterfly glioma of the frontal corpus callosum (Arora and Praharaj, 2007) or on a functional magnetic resonance imaging (MRI) study in akinetic catatonic patients during negative emotional stimulation (Northoff et al, 2004). We hypothesized that genotype-dependent axonal degeneration should be detectable in the frontal commissural fibres of the corpus callosum. These considerations were supported by the fact that the catatonia presented here in the context of a syndrome is characterized by several features of a primarily executive control (frontal lobe) deficiency in the absence of any ‘classical’ motor dysfunction. Indeed, we could localize axonal degeneration, determined by an increased axonal diffusivity in DTI, selectively to the genu corporis callosi.
The CNP rs2070106 AA genotype leads to reduced expression of CNP (Mitkus et al, 2008; Peirce et al, 2006), constituting ‘partial loss-of-function’. Since there is an appreciable degree of linkage disequilibrium across the CNP gene (http://www.hapmap.org), the effects seen with the synonymous SNP rs2070106 might well be due to the influence of another genetic variant in close vicinity (e.g. in the 3′-untranslated region (3′-UTR) of the CNP gene). Alternatively, according to previous studies, synonymous SNPs may modify translational timing due to differential codon usage (Kimchi-Sarfaty et al, 2007) or inactivate an exonic splicing silencer that compensates for other genetic variations in exonic splicing enhancers (Nielsen et al, 2007).
We demonstrated increased numbers of inflammatory cells, gliosis and axonal degeneration in old Cnp+/− mice suggesting an important role of low-grade inflammation in the described syndrome. Even though brain sections of human patients with the respective CNP genotypes were not available in the present study, the axonal degeneration detected by DTI is an intriguing observation that might point to the hypothesis of a comparable disease mechanism in mouse and man. Mechanistic details on the subcellular functions of CNP in myelinating oligodendrocytes have been reported (Gravel et al, 2009) and are under further investigation. The secondary neuroinflammation is a well-known cause of nitric oxide-mediated axonal stress and neurodegeneration (for review see Amor et al, 2010; Smith & Lassmann, 2002). We note that a diverse group of inherited myelinopathies in the nervous system of mice can trigger the recruitment of microglia/macrophages and T-cells (Ip et al, 2006; Kassmann et al, 2007; Martini & Toyka, 2004), demonstrating that low-grade inflammation is a rather unspecific response of myelinating glial cells to cellular stress, possibly related to perturbed lipid metabolism (Dumser et al, 2007). Interestingly, low-grade inflammation has been found to be associated with behavioural consequences in mouse studies (Bercik et al, 2010) and hypothesized to play a role in mental diseases (Gardner & Boles, 2011; Monji et al, 2009; Muller & Schwarz, 2008; Schnieder & Dwork, 2011). Respective first clinical trials employing antiinflammatory strategies in bipolar disease and schizophrenia yielded positive signals (Laan et al, 2010; Muller et al, 2010). Having information available on a predisposing genotype, individualized preventive and therapeutic approaches may be possible in the future.
To conclude, the major finding of the present study is the proof-of-principle that subtle changes of subcortical white matter can be the cause, rather than merely the consequence, of a complex neuropsychiatric syndrome. This distinction is extremely difficult in human patients with a psychiatric disease of unknown etiology, specifically when pharmacologically treated and only diagnosed (by MRI) with minor abnormalities of white matter tracts (Davis et al, 2003). Our analysis was possible by building on genetic variants of the cell type-specific CNP gene that lead to a partial loss-of-function genotype in both mouse and man. Importantly, Cnp heterozygosity (in mice) and moderately reduced CNP expression levels (in humans) are well tolerated until an advanced age. At that point, however, haplo-insufficiency causes a striking phenotype in mice and shapes the phenotype of a complex psychiatric disease in humans. Although we have no ultimate proof that moderately reduced CNP levels in any individual (diseased or healthy) suffice to trigger a catatonia-depression syndrome upon aging, they clearly add to other genetic factors (here in patients diagnosed with schizophrenia) such that the catatonia-depression syndrome can be well defined and emerges as remarkably similar to the isolated behavioural phenotype of aged Cnp heterozygous mice. This amazing similarity of the behavioural phenotype in two different species emphasizes the relevance of glial dysfunction in psychiatric disorders, and supports the exploration of therapeutic strategies to target the associated low-grade neuroinflammation.
MATERIALS AND METHODS
Human studies
Healthy subjects
Blood donors (n = 1045; Begemann et al, 2010) were recruited for the case-control study. Ethnicity (Caucasian 97.8%; other ethnicities 2%; unknown 0.2%) was comparable to the patient population (Caucasian 95.5%; other ethnicities 1.8%; unknown 2.7%).
Schizophrenic patients
The GRAS data collection was approved by Ethics Committees of the Georg-August-University of Göttingen and participating centres, and comprises at present 1048 patients with Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV; APA, 2000) diagnosis of schizophrenia (81.7%) or schizoaffective disorder (18.3%), examined between 2005–2010 in 23 centres all over Germany (for details see Ribbe et al, 2010). Interviews, testing and ratings were conducted by an invariable team of trained examiners using the ‘GRAS Manual’ (Begemann et al, 2010; Ribbe et al, 2010).
Catatonia-depression composite
The score consists of five phenotype domains: Depression was operationalized by items 3 (guilt feelings) and 6 (depression) of general psychopathology subscale of Positive and Negative Syndrome Scale (PANSS) (Kay et al, 1987). Catatonia was based on catatonic signs subscale of the Cambridge Neurological Inventory (gait mannerism, gegenhalten, mitgehen, imposed posture, abrupt or exaggerated spontaneous movements, iterative movements, automatic obedience and echopraxia; Chen et al, 1995). Deficits in social interaction were built on items 1 (blunted affect) and 3 (poor rapport) of PANSS negative subscale, combined with item 44 (never feeling close to another person) of Brief Symptom Inventory (Derogatis & Melisaratos, 1983). Social withdrawal/anxiety was assessed by item 4 (social withdrawal) of PANSS negative subscale and item 12 (suddenly scared for no reason) of Brief Symptom Inventory. Loss of interest in the outside world was estimated by item 7 (self-centred attitude) of PANSS negative and item 15 (preoccupation) of general subscale. Phenotype domains were Z-standardized to be normally distributed with expectation zero and variance one. Higher values indicate worse outcome. Composite calculation was based on subjects without missing data (n = 929). Correlations of the five target phenotypes were assessed using Pearson product–moment correlation and internal consistency was determined using Cronbach's α.
Genotyping
Genotyping of SNP rs2070106 was performed using SimpleProbes (TIB Molbiol, Berlin, Germany) on LightCycler480 (Roche Diagnostics, Basel, Switzerland).
MRI/DTI
For MRI/DTI analyses, a subset of patients ≥40 years of both genotypes (GG n = 11; AA n = 10) from the GRAS sample was selected and matched according to age, gender and duration of disease. Studies were conducted at 3T (Tim Trio, Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. DTI was performed at 2 mm isotropic resolution using diffusion-weighted single-shot stimulated echo acquisition mode (STEAM) sequences (Hofer et al, 2010; Karaus and Frahm, 2009) combining 6/8 partial Fourier encoding and parallel imaging. Protocol comprised 24-independent diffusion gradient directions and b-values of 0 and 900 smm−2. A total of 55 transverse sections (2 mm thickness) covered brain parts dorsal and ventral to the corpus callosum. To increase signal-to-noise ratio, acquisition was repeated three times (17 min). Anatomic images were based on T1-weighted 3D fast low angle shot (FLASH) MRI sequence (repetition time TR = 11 ms, echo time TE = 4.9 ms, flip angle 15°).
DTI regions of interest (ROI)
Before calculation of diffusion tensor, diffusion-weighted MRI data sets were interpolated to 1 mm isotropic resolution and smoothed with a 3D Gaussian filter (half width 1 mm). Individual ROIs were manually defined on colour-coded maps of the main diffusion direction without thresholding. ROIs for the corpus callosum were placed in the midsagittal plane as well as in two directly neighbouring parasagittal sections covering central portions of genu and most posterior part of splenium (Hofer & Frahm, 2006). Mean values of fractional anisotropy (FA), ADC, AD and radial diffusivity (RD) were calculated.
MRI volumetry
Analyses were performed with an automatic brain segmentation tool for surface-based cortical thickness (http://surfer.nmr.mgh.harvard.edu). T1-weighted images underwent corrections for intensity inhomogeneity, skull strip and registration into Talairach space followed by segmentation into grey matter, white matter and various brain areas. Regional differences of cortical thickness between patient groups were investigated using Qdec (FreeSurfer for multiple comparisons and voxel-based morphometry). Statistics relied on p ≤ 0.05 (false discovery rate corrected for multiple comparisons). Visualization employed an inflated pial surface model.
Mouse studies
Mouse mutants
Experiments were carried out according to animal policies of the German Federal State of Niedersachsen. Cnp+/− mice were genotyped with primers Cnp-E3s, 5′-GCCTTCAAACTGTCCATCTC-3′; Cnp-E3as, 5′-CCCAGCCCTTTTATTACCAC-3′ and puro3, 5′-CATAGCCTGAAGAACGAGA-3′.
Immunostaining
Mice were anesthetized with Avertin (Sigma–Aldrich, Taufkirchen, Germany) and perfused through the left ventricle with 15 ml of Hank's balanced salt solution (Lonza, Basel, Switzerland), followed by 50 ml of 4% paraformaldehyde in phosphate buffered saline (PBS). Brains were harvested and postfixed in 4% paraformaldehyde overnight at 4°C and then embedded in Paraplast (Surgipath Paraplast; Leica, Wetzlar, Germany). Microtome sections of 5 µm (Microm HM400, Walldorf, Germany) were prepared. For diaminobenzidine (DAB)-based immunostaining of paraffin sections, Dako-LSAB2 system or Vectastain Elite ABC kit (Vector laboratories, Burlingame, CA, USA) were used according to manufacturer's instructions. Primary antibodies were directed against APP (1:750, Chemicon (Millipore) Billerica, MA, USA), CD3 (1:150, Serotec, Oxford, UK), GFAP (1:200, Novocastra (Leica) Newcastle Upon Tyne, UK), IBA-1 (1:1000, Wako, Neuss, Germany) and Mac-3 (1:400, BD Pharmingen, Franklin Lakes, NJ, USA).
Quantitative real time polymerase chain reaction (qRT-PCR)
qRT-PCR was performed using SYBR green master mix (Applied Biosystems, Foster City, CA, USA) and 7500 Fast Real-Time PCR System (Applied Biosystems). Specific qRT-PCR primers were designed by Roche Universal ProbeLibrary Assay Design Center (Cnp, forward 5′-TAACCCTCCCTTAGCCCCTG-3′, reverse 5′-GTCCCTAGCATGTGGCAGCT-3′; for normalization: Atp5b forward 5′-GGATCTGCTGGCCCCATAC-3′, reverse 5′-CTTTCCAACGCCAGCACCT-3′, Rplp0 forward 5′-GATGCCCAGGGAAGACAG-3′, reverse 5′-ACAATGAAGCATTTTGGATAATCA-3′). Data were analysed with Microsoft Excel 2010.
Western blot
Myelin purified from protein lysates was performed according to (Norton & Poduslo, 1973). For Western blotting, proteins were size-separated in 12% sodium dodecyl sulphate (SDS)–polyacrylamide gels (0.2 µg/µl), blotted onto polyvinylidene difluoride membranes (Hybond P; GE Healthcare, München, Germany), blocked with 5% milk powder in Tris-buffered saline (TBS) and Tris-buffered saline + Triton X-100 (TBST; 150 mM NaCl, 10 mM Tris/HCl, pH 7.4; 0.1% Tween20), and incubated with primary antibodies (CNPase, 1:5000, Sigma, Saint Louis, MO, USA; Plp (A431), 1:5000; Jung et al, 1996), overnight at 4°C. Blots were washed with TBS/0.05% Tween20, incubated with appropriate secondary horseradish peroxidase-conjugated antibodies (Dianova, Hamburg, Germany), washed with TBS/0.05% Tween20 and developed by enhanced chemiluminescence (Pierce, Rockford, IL, USA).
Morphometry
Digitized overlapping light microscopic images (20× if not otherwise stated), fused to a continuous image of a complete corpus callosum (bregma 0.74 mm) by using Photoshop CS5 and ImageJ software were analysed for absolute numbers of IBA-1 and Mac-3 positive cells. To quantify GFAP positive areas, a plug-in for the ImageJ software for semi-automated analysis was implemented (http://www1.em.mpg.de/wieser). APP positive axonal spheroids (analysed at 40× magnification) and CD3 positive T-cells are expressed as total numbers quantified in corpus callosum, anterior commissure and striatum. For all stainings, two sections per mouse were quantified.
Behavioural testing
Tests were performed as described in detail previously, using the following order: Elevated plus maze (Radyushkin et al, 2010), open field (Radyushkin et al, 2010), light/dark box (Finn et al, 2003), rota-rod (Radyushkin et al, 2010), gait analysis (Brooks & Dunnett, 2009), grip strength (Radyushkin et al, 2010), hole board (Radyushkin et al, 2009), sociability (Moy et al, 2004), buried-food-finding test for olfaction (Radyushkin et al, 2009), floating behaviour (analysis of swimming/floating during a 90s trial in the Morris water maze pool; Morris, 1984; Stone & Lin, 2011), tail suspension test (Cryan et al, 2005), bar test (Kuschinsky & Hornykiewicz, 1972; see Fig 2P and videos of Supporting Information) and fear conditioning (Radyushkin et al, 2009).
The paper explained
PROBLEM:
Myelin and white matter abnormalities have been documented in neuropsychiatric diseases such as schizophrenia, major depression and bipolar disorder. However, their significance for disease mechanisms, pathogenesis or phenotypes is still obscure. A considerable number of postmortem studies found reduced expression of several myelin genes, including 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP), in the brains of individuals with severe mental disease. In the present translational approach, we report for the first time phenotypical consequences of moderate CNP ‘loss-of-function’ genotypes, that is genetic variants leading to decreased CNP expression, both in man (single nucleotide polymorphism rs2070106) and mouse (heterozygous Cnp null mutant mice).
RESULTS:
We show that reduced CNP expression causes a distinct behavioural abnormality, seen only upon aging as an additional ‘pro-inflammatory hit’. This phenotype is strikingly similar between Cnp heterozygous mice and patients with mental disease, carrying the AA genotype at CNP SNP rs2070106. The characteristic features in both species are best described as a ‘catatonia-depression’ syndrome and include bizarre posturing, depression, anxiety, loss of interest in the outside world and social withdrawal. As a consequence of perturbed CNP expression, mice show secondary low-grade inflammation and degeneration of nerve fibres. Analogously, in man, diffusion tensor imaging points to axonal loss in the frontal corpus callosum.
IMPACT:
Our genetic data demonstrate that subtle white matter abnormalities can be the cause of a psychiatric syndrome. To our knowledge, CNP is the first gene identified to be associated with catatonia, and aged heterozygous null mutant mice are the first animal model of spontaneous catatonia. Moderately reduced CNP expression contributes to a distinct phenotype, which is not restricted to a single diagnostic category but could explain features of catatonia-depression in different mental disorders and possibly – to a milder degree – even in aging healthy individuals. This knowledge will help defining subgroups of (aging) subjects who may profit from novel, more specific therapeutic approaches including anti-inflammatory strategies.
Mouse score
For the catatonia-depression score, five phenotype domains were created: (I) Depression was operationalized by floating time and tail suspension (delta time of immobility in the last 2 min minus first 2 min), (II) catatonia by time on bar, (III) deficit in social interaction by delta time spent with stranger versus empty compartment, (IV) anxiety by open field-duration in centre, elevated plus maze – open arm visits, and fear conditioning – freezing at baseline in cue task and (V) loss of interest by hole board – number of head dips. Composite score calculation was done in analogy to the human score and based on mice with not more than two variables missing (n = 27).
Statistical analysis
Statistical analyses were performed using SPSS for Windows version 17.0 (https://www.spss.com/de) and Prism5 (GraphPad Software, San Diego, CS, USA). Exact procedures are indicated in the respective sections.
Note: All experiments/analyses in both men and mice were performed by persons unaware of genotypes (‘blinded’).
Acknowledgments
We are indebted to all patients for their participation in the GRAS (Göttingen Research Association for Schizophrenia) study and all collaborating GRAS centres for their support. We are grateful to all colleagues who contributed to the GRAS data collection. This work was supported by the Max Planck Society, the DFG-Research Center for Molecular Physiology of the Brain (CMPB) and the Bernstein Center for Computational Neuroscience (BCCN) (Grant 01GQ0431). K.A.N. acknowledges grant support from ERA-Net Neuron (Grant 01EW1102) and is recipient of an ERC Advanced Grant (Axoglia).
Supporting Information is available at EMBO Molecular Medicine online.
The authors declare that they have no conflict of interest.
Author contributions
NH, SG, SP, KAN and HE developed study concept and design; NH performed all behavioural analysis of Cnp+/− mice; SP and AK carried out human genetic analyses and performed the human association study; UCG and GLW performed the histological analyses of Cnp+/− mice under supervision of SG; DTI study with human subjects was performed by SH and analysed under the supervision of SB and JF; Administrative, technical and material support was provided by MB, AR and AG in different aspects of the study; MB and AK coordinated and supervised the recruitment of subjects for the DTI study; SHH, SB and JF gave input to data analysis, interpretation, and manuscript preparation; NH, SG, SP, AK, KAN and HE wrote the manuscript; KAN and HE had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementaary material
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
References
- Amir S. Catalepsy induced by body pinch: relation to stress-induced analgesia. Ann N Y Acad Sci. 1986;467:226–237. doi: 10.1111/j.1749-6632.1986.tb14631.x. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington: American Psychiatric Association; 2000. [Google Scholar]
- Arora M, Praharaj SK. Butterfly glioma of corpus callosum presenting as catatonia. World J Biol Psychiatry. 2007;8:54–55. doi: 10.1080/15622970600960116. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, Friedrichs H, Radyushkin KA, El-Kordi A, Benseler F, et al. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch Gen Psychiatry. 2010;67:879–888. doi: 10.1001/archgenpsychiatry.2010.107. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. e2101. [DOI] [PubMed] [Google Scholar]
- Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Stingo S, Wolff J. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc Natl Acad Sci USA. 2002;99:1807–1812. doi: 10.1073/pnas.042678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun PE, Lee J, Gravel M. 2′,3′-cyclic nucleotide 3′-phosphodiesterase: structure, biology, and function. In: Lazzarini RA, editor. Myelin Biology and Disorders. San Diego: Elsevier Academic Press; 2004. pp. 499–522. [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user's guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Crit Rev Neurobiol. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Che R, Tang W, Zhang J, Wei Z, Zhang Z, Huang K, Zhao X, Gao J, Zhou G, Huang P, et al. No relationship between 2′,3′-cyclic nucleotide 3′-phosphodiesterase and schizophrenia in the Chinese Han population: an expression study and meta-analysis. BMC Med Genet. 2009;10:31. doi: 10.1186/1471-2350-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EY, Shapleske J, Luque R, McKenna PJ, Hodges JR, Calloway SP, Hymas NF, Dening TR, Berrios GE. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res. 1995;56:183–204. doi: 10.1016/0165-1781(95)02535-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- De Angelis DA, Braun PE. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase binds to actin-based cytoskeletal elements in an isoprenylation-independent manner. J Neurochem. 1996;67:943–951. doi: 10.1046/j.1471-4159.1996.67030943.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- Dumser M, Bauer J, Lassmann H, Berger J, Forss-Petter S. Lack of adrenoleukodystrophy protein enhances oligodendrocyte disturbance and microglia activation in mice with combined Abcd1/Mag deficiency. Acta Neuropathol. 2007;114:573–586. doi: 10.1007/s00401-007-0288-4. [DOI] [PubMed] [Google Scholar]
- Edgar JM, McLaughlin M, Werner HB, McCulloch MC, Barrie JA, Brown A, Faichney AB, Snaidero N, Nave KA, Griffiths IR. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia. 2009;57:1815–1824. doi: 10.1002/glia.20893. [DOI] [PubMed] [Google Scholar]
- Finn DA, Rutledge-Gorman MT, Crabbe JC. Genetic animal models of anxiety. Neurogenetics. 2003;4:109–135. doi: 10.1007/s10048-003-0143-2. [DOI] [PubMed] [Google Scholar]
- Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Gravel M, Peterson J, Yong VW, Kottis V, Trapp B, Braun PE. Overexpression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase in transgenic mice alters oligodendrocyte development and produces aberrant myelination. Mol Cell Neurosci. 1996;7:453–466. doi: 10.1006/mcne.1996.0033. [DOI] [PubMed] [Google Scholar]
- Gravel M, Robert F, Kottis V, Gallouzi IE, Pelletier J, Braun PE. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: a novel RNA-binding protein that inhibits protein synthesis. J Neurosci Res. 2009;87:1069–1079. doi: 10.1002/jnr.21939. [DOI] [PubMed] [Google Scholar]
- Heckers S, Tandon R, Bustillo J. Catatonia in the DSM–shall we move or not. Schizophr Bull. 2010;36:205–207. doi: 10.1093/schbul/sbp136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hofer S, Karaus A, Frahm J. Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Front Neuroanat. 2010;4:15. doi: 10.3389/fnana.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip CW, Kroner A, Bendszus M, Leder C, Kobsar I, Fischer S, Wiendl H, Nave KA, Martini R. Immune cells contribute to myelin degeneration and axonopathic changes in mice overexpressing proteolipid protein in oligodendrocytes. J Neurosci. 2006;26:8206–8216. doi: 10.1523/JNEUROSCI.1921-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Ueda J, Bundo M, Nakano Y, Kato T. Effect of a functional single nucleotide polymorphism in the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene on the expression of oligodendrocyte-related genes in schizophrenia. Psychiatry Clin Neurosci. 2008;62:103–108. doi: 10.1111/j.1440-1819.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- Jung M, Sommer I, Schachner M, Nave KA. Monoclonal antibody O10 defines a conformationally sensitive cell-surface epitope of proteolipid protein (PLP): evidence that PLP misfolding underlies dysmyelination in mutant mice. J Neurosci. 1996;16:7920–7929. doi: 10.1523/JNEUROSCI.16-24-07920.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlbaum K. Berlin: Hirschwald; 1874. Die Katatonie oder das Spannungsirresein. [PubMed] [Google Scholar]
- Karaus AH, Frahm J. Separation of Fiber Tracts within the Human Cingulum Bundle using Single-Shot STEAM DTI. Open Med Imaging J. 2009;3:21–27. [Google Scholar]
- Kassmann CM, Lappe-Siefke C, Baes M, Brugger B, Mildner A, Werner HB, Natt O, Michaelis T, Prinz M, Frahm J, et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat Genet. 2007;39:969–976. doi: 10.1038/ng2070. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Kuschinsky K, Hornykiewicz O. Morphine catalepsy in the rat: relation to striatal dopamine metabolism. Eur J Pharmacol. 1972;19:119–122. doi: 10.1016/0014-2999(72)90086-6. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003;33:366–374. doi: 10.1038/ng1095. [DOI] [PubMed] [Google Scholar]
- Lee J, Gravel M, Zhang R, Thibault P, Braun PE. Process outgrowth in oligodendrocytes is mediated by CNP, a novel microtubule assembly myelin protein. J Cell Biol. 2005;170:661–673. doi: 10.1083/jcb.200411047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Toyka KV. Immune-mediated components of hereditary demyelinating neuropathies: lessons from animal models and patients. Lancet Neurol. 2004;3:457–465. doi: 10.1016/S1474-4422(04)00822-1. [DOI] [PubMed] [Google Scholar]
- Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- Mitkus SN, Hyde TM, Vakkalanka R, Kolachana B, Weinberger DR, Kleinman JE, Lipska BK. Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res. 2008;98:129–138. doi: 10.1016/j.schres.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci. 2008;258:97–106. doi: 10.1007/s00406-008-2012-3. [DOI] [PubMed] [Google Scholar]
- Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, Moller HJ, Klauss V, Schwarz MJ, Riedel M. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Nielsen KB, Sorensen S, Cartegni L, Corydon TJ, Doktor TK, Schroeder LD, Reinert LS, Elpeleg O, Krainer AR, Gregersen N, et al. Seemingly neutral polymorphic variants may confer immunity to splicing-inactivating mutations: a synonymous SNP in exon 5 of MCAD protects from deleterious mutations in a flanking exonic splicing enhancer. Am J Hum Genet. 2007;80:416–432. doi: 10.1086/511992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Kotter R, Baumgart F, Danos P, Boeker H, Kaulisch T, Schlagenhauf F, Walter H, Heinzel A, Witzel T, et al. Orbitofrontal cortical dysfunction in akinetic catatonia: a functional magnetic resonance imaging study during negative emotional stimulation. Schizophr Bull. 2004;30:405–427. doi: 10.1093/oxfordjournals.schbul.a007088. [DOI] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Peirce TR, Bray NJ, Williams NM, Norton N, Moskvina V, Preece A, Haroutunian V, Buxbaum JD, Owen MJ, O'Donovan MC. Convergent evidence for 2′,3′-cyclic nucleotide 3′-phosphodiesterase as a possible susceptibility gene for schizophrenia. Arch Gen Psychiatry. 2006;63:18–24. doi: 10.1001/archpsyc.63.1.18. [DOI] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, Winter D, Frahm J, Fischer J, Brose N, et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Radyushkin K, El-Kordi A, Boretius S, Castaneda S, Ronnenberg A, Reim K, Bickeboller H, Frahm J, Brose N, Ehrenreich H. Complexin2 null mutation requires a ‘second hit’ for induction of phenotypic changes relevant to schizophrenia. Genes Brain Behav. 2010;9:592–602. doi: 10.1111/j.1601-183X.2010.00590.x. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Friedrichs H, Begemann M, Grube S, Papiol S, Kastner A, Gerchen MF, Ackermann V, Tarami A, Treitz A, et al. The cross-sectional GRAS sample: a comprehensive phenotypical data collection of schizophrenic patients. BMC Psychiatry. 2010;10:91. doi: 10.1186/1471-244X-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Differential regulation of the 2′,3′-cyclic nucleotide 3′-phosphodiesterase gene during oligodendrocyte development. Neuron. 1994;12:1363–1375. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
- Schnieder TP, Dwork AJ. Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry. 2011;69:134–139. doi: 10.1016/j.biopsych.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham PC, Jones P, Russell A, Gilvarry K, Bebbington P, Lewis S, Toone B, Murray R. Age at onset, sex, and familial psychiatric morbidity in schizophrenia. Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1994;165:466–473. doi: 10.1192/bjp.165.4.466. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–241. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–330. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Petracca G, Teson A, Chemerinski E, Merello M, Migliorelli R, Leiguarda R. Catatonia in depression: prevalence, clinical correlates, and validation of a scale. J Neurol Neurosurg Psychiatry. 1996;60:326–332. doi: 10.1136/jnnp.60.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EA, Lin Y. Open-space forced swim model of depression for mice. Curr Protoc Neurosci. 2011;9:36. doi: 10.1002/0471142301.ns0936s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date. Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P, Fananas L. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000;42:83–90. doi: 10.1016/s0920-9964(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Yin X, Peterson J, Gravel M, Braun PE, Trapp BD. CNP overexpression induces aberrant oligodendrocyte membranes and inhibits MBP accumulation and myelin compaction. J Neurosci Res. 1997;50:238–247. doi: 10.1002/(SICI)1097-4547(19971015)50:2<238::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yu WP, Collarini EJ, Pringle NP, Richardson WD. Embryonic expression of myelin genes: evidence for a focal source of oligodendrocyte precursors in the ventricular zone of the neural tube. Neuron. 1994;12:1353–1362. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.