Abstract
Problem
Hepatocyte Growth Factor (HGF) secretion facilitates epithelial cell growth and development in the female reproductive tract (FRT) and may contribute to pathological conditions such as cancer and endometriosis. We hypothesized that estradiol and Poly (I:C), a synthetic RNA mimic, may have a regulatory effect on HGF secretion by stromal fibroblasts from FRT tissues.
Method of Study
Following hysterectomies, normal tissue from the uterus, endocervix and ectocervix were dispersed into stromal cell fractions by enzymatic digestion and differential filtering. Stromal fibroblasts were cultured and treated with estradiol and/or Poly (I:C) and conditioned media were analyzed for HGF via ELISA.
Results
Treating uterine fibroblasts with estradiol or Poly (I:C) significantly increased HGF secretion. When uterine fibroblasts were co-treated with estradiol and Poly (I:C), the effect on HGF secretion was additive. In contrast, stromal fibroblasts from endo- and ecto-cervix were unresponsive to estradiol but were stimulated to secrete HGF by Poly (I:C).
Conclusions
HGF secretion is uniquely regulated in the uterus, but not in ecto- and endo-cervix, by estradiol. Moreover, potential viral pathogens further induce HGF. These findings have potential applications to understanding both hormonal regulation of normal tissue as well as the role of HGF in tumorogenesis, endometriosis and HIV infection.
Keywords: Estradiol, Poly (I:C), Hepatocyte Growth Factor, Stromal Fibroblasts, Female Reproductive Tract
INTRODUCTION
Hepatocyte Growth Factor (HGF) was discovered as a mitogen of hepatocytes more than 25 years ago1, 2. We now know that HGF is a pleiotropic agent produced by stromal cells that induces epithelial cell proliferation, motility, morphogenesis as well as angiogenesis in several organs2. HGF initiates its effects on epithelial cells through tyrosine phosphorylation of its receptor, c-Met. In addition to organ development, HGF has protective effects on cells and tissues through wound-healing as well as anti-apoptotic and anti-inflammatory actions.
Epithelial-stromal cell interactions are essential in facilitating steroid hormone-induced growth and development in the endometrium3, 4. Interestingly, estradiol-induced actions on epithelial cells may be indirect, and estradiol-induced HGF from underlying stromal cells may be the primary mediator of estradiol’s effects on the epithelial cells. Cooke et al used FRT tissues from estrogen receptor (ER) knockout mice to demonstrate that underlying estrogen receptor positive stromal cells regulate the differentiation of the adjacent epithelial cells3. These studies indicated that estradiol acts on stromal cells to release one or more paracrine factors that then modulate estradiol’s effects on epithelial cells for growth and differentiation. Many actions of HGF are mirrored by estradiol in the female reproductive tract (FRT). We have shown that some of estradiol’s effects on mouse uterine epithelial cells are mediated via HGF secreted by stromal cells in response to estradiol stimulation5. For example, HGF was identified as the key paracrine mediator of TNFα production by uterine epithelial cells since TNFα secretion was abrogated by the addition of blocking antibodies to either c-Met or HGF6. Sugawara et al7 also have identified HGF as a critical factor in estradiol-mediated regulation of uterine epithelial cells. We recently showed that estradiol treatment of human primary uterine stromal fibroblasts results in increased secretion of HGF8, again implying a relationship between estradiol and HGF. In addition to similarities between endogenous HGF and estradiol in normal FRT physiology, several investigators have reported a parallel of HGF and estradiol in pathologies of the FRT. In reproductive tissue cancers and endometriosis, relatively high estradiol concentrations are correlated with increased HGF levels9–17.
Uterine and Fallopian tube epithelial cells are known to express Toll-like Receptors (TLR) as does the lower FRT18. TLR are designed to recognize pathogen-associated molecular patterns (PAMP) that are characteristic of various pathogens, such as lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, and double-stranded RNA, associated with viral infection. Upon activation, TLR stimulate the secretion of cytokines and chemokines, as well as both extra- and intra-cellular anti-microbicidal agents, such as secretory leukocyte protease inhibitor (SLPI)19 and 2’, 5’ OAS20, respectively. Whereas the response to TLR agonists has been reported in immune cells and FRT epithelial cells, relatively little research has been reported with TLR stimulation of FRT stromal fibroblasts in the presence of estradiol.
The goal of this study was to determine if TLR stimulation of FRT stromal fibroblasts alters HGF secretion, which then may have effects on innate immune functions of FRT epithelial cells. We also wanted to determine whether the response, if any, was differentially modulated in the upper vs lower FRT (uterine, cervical, and ectocervical compartments). Finally, we wished to compare the magnitude of any TLR3-induced HGF increase to that induced by estradiol, as well as any modulation of HGF secretion of FRT stromal fibroblasts to the co-stimulation of FRT stromal fibroblasts with a TLR3 agonist and estradiol.
Materials and Methods
Human Uterus, Cervix, and Ectocervix tissues
FRT mucosal tissue was obtained following surgery from women who underwent hysterectomies at Dartmouth-Hitchcock Medical Center. Tissues used in this study, including uterine, cervical, and ectocervical tissues, were distal to the sites of pathology and were determined to be unaffected by disease upon inspection by a trained pathologist. Pathologists also determined the menstrual status, as well as the stage in the cycle of pre-menopausal donors. Tissues were transported from Pathology and procedures to prepare purified epithelial sheets and stromal cells began within 2 hours of surgery. Approval to use tissues was previously obtained from the IRB.
Enzymatic Digestion of Tissues
Uterine, cervical and ectocervical cells were dispersed into epithelial and stromal cell fractions by enzymatic digestion as described previously21. Briefly, tissues were minced under sterile conditions into 1 – 2 mm fragments and then digested at 37°C for 1 hour using a "PHC" enzyme mixture that contains final concentrations of 3.4 mg/ml pancreatin (Gibco Life Technologies, Rockville, MD), 0.1 mg/ml hyaluronidase (Worthington Biochemical Corporation, Freehold, NJ), 1.6 mg/ml collagenase (Worthington) and 2 mg/ml D-glucose (Sigma, St Louis, MO) in Hank's balanced salt solution (HBSS, Gibco, Invitrogen). This procedure was chosen to maximize digestion of the extracellular matrix, as verified by microscopy of hematoxylin and eosin stained frozen sections after digestion, while minimizing digestion of cell surface antigens as determined by flow cytometry. After incubating in this enzyme cocktail, uterine, cervical, and ectocervical cells were collected, washed with media consisting of DMEM/F-12 without phenol red (Gibco), 2 mM fresh L-glutamine, 25 mM HEPES, 50 ng/ml primocin (InvivoGen, San Diego, CA) and separated from tissue aggregates by sedimentation for 5 minutes. Medium containing predominantly single cells and small epithelial cell glands was removed and dispersed through a 250µm nylon mesh screen (Small Parts, Miami Lake, FL). To further separate epithelial and stromal cells, tissue fragments were re-suspended in complete media. Dispersed uterine cells were then pooled and separated on a 40 mm mesh screen (Small Parts) into fractions containing epithelial cell glands and a mixed population of sub-epithelium stromal cells. Stromal cells passed through this fine mesh whereas small epithelial cell glands did not. Stromal cells were centrifuged at 500×g for 5 minutes in a Beckman-Coulter (Fullerton, CA) centrifuge and resuspended in complete media. Stromal cells were cultured in T75 flasks (Falcon) in an atmosphere of 95% air and 5% CO2 at 37°C in an InCuSafe cell culture incubator (Sanyo Scientific, Wood Dale, IL).
Culture of Stromal Fibroblasts
Stromal fibroblasts were grown to confluence in T75 flasks, cultured in complete media consisting of DMEM/F-12 without phenol red (Gibco) supplemented with 10% heat inactivated fetal calf serum (Hyclone, Logan UT), 2 mM fresh L-glutamine, 25 mM HEPES, 50 ng/ml primocin (InvivoGen, San Diego, CA). Once stromal fibroblasts reached confluence, they were trypsinized with 0.05% trypsin EDTA (Invitrogen), counted for viability via trypan blue exclusion, then seeded into 24 well plates at a density of 5 × 105 cells/well (1 × 106 cells/ml) and cultured in complete media. Twenty four hours after seeding, wells were washed with HBSS and agitated to remove remaining red blood cells and other non-fibroblastic stromal cells. After four days of culture, all cells had the characteristic fibroblast morphology, and the culture was devoid of other cell types22. Media was replaced at 48-hour intervals. Once the cells reached confluence, they were cultured in media containing 10% stripped FBS and allowed to acclimate to this media for 48 hours prior to treatment with estradiol. Charcoal-dextran stripped FBS was substituted for defined FBS, because there are very low, yet measurable levels of estradiol (10−11 M) present in defined FBS (Hyclone).
Preparation of Estradiol
17β-estradiol (CalBiochem, San Diego, CA) was weighed and diluted in ethanol to a concentration of 10−3 M. 20µl of this stock solution was evaporated in a clean glass scintillation vial and resuspended in culture media to make a working stock solution of 10−5M; specific treatment concentrations were diluted from the working stock as needed. A control media was made using 20µl 100% ethanol evaporated in a clean glass scintillation vial and resuspended in culture media, parallel to the estradiol solution. Fresh steroid preparations were made for each experiment, and all treatment media needed for a specific culture/experiment was made at the time of estradiol preparation.
Treatment with Estradiol
Stromal fibroblasts were grown to confluence in 24 well plates, and then switched to media containing 10% stripped FBS. After 24 hours, the media were replaced and the fibroblasts were treated. Conditioned stromal medium (CSM) was collected at 48 hours, centrifuged and stored at −80° and assayed for HGF by ELISA. Quadruplicate wells of stromal fibroblasts were treated with estradiol at a dose of 10−8M, with a control treatment made with the ethanol control media. The secretion of HGF was tested over a period of eight days. This time course covered the collections that have yielded significant HGF secretion, while minimizing the risk of over-culturing the stromal fibroblasts and altering the primary phenotype. Unless otherwise indicated, stromal fibroblasts were treated with 10−8M estradiol. This is the standard estradiol treatment concentration used by our laboratory and is within the physiological range of estradiol concentration23. Since FRT cells are constantly exposed to estradiol throughout the menstrual cycle, in some cases estradiol was added for a second 48 hour treatment.
Cell Counts
To determine if estradiol treatment increased the number of uterine stromal fibroblasts, confluent cultures in 24 well plates treated with or without estradiol at a concentration of 10−8 M were washed with HBSS, trypsinized with 0.05% trypsin/EDTA; individual wells were collected in individual microfuge tubes and washed with culture media. Cells were assessed for viability via trypan blue dye exclusion and counted manually with a hemocytometer. Our results indicated that estradiol did not increase cell numbers of the stromal fibroblasts in any experiment (data not shown).
Treatment with TLR Agonists
For these experiments, cultures were treated with a panel of TLR agonists. The TLR-agonists used included 1 µg/ml LPS (TLR 4) (InVivogen),, 1 µg/ml Pam3cys-Ser-(Lys)4 (TLR2) (EMC Microcollections, Tubingen, Germany), 25 µg/ml Poly I:C (TLR3) (InVivogen), 100 ng/ml flagellin isolated from E. coli (TLR5) (Inotek Pharmaceuticals, Beverly, MA), 10 µg/ml Zymosan isolated from Saccharomyces cerevisiae (TLR2) (InVivogen), 10 µg/ml peptidoglycan isolated from S. aureus (TLR2) (InVivogen), 5 µg/ml Imiquimod (TLR7, 8) (InVivogen), and 108 heat-killed Listeria monocytogenes (HKLM, TLR2) (InVivogen). We have shown these doses to be effective in stimulation of human uterine epithelial cell TLR24. In further experiments, uterine stromal fibroblasts were treated with both estradiol at 10−8M and Poly (I:C) at 25 µg/ml. Treatment groups consisted of four wells per treatment. After 48 hours, conditioned stromal media (CSM) were collected, spun at 10,000×g to remove cellular debris, and stored at −80° C. In other studies, the effect of both estradiol and TLR treatment were assessed. Treatment groups included control, estradiol-treatment (10−8 M), TLR agonist treatment, and co-treatments of estradiol plus TLR agonist. Time-course experiments over six to eight days of treatment with estradiol and TLR agonists were performed. Supernatants from each well were collected at 48-hour intervals, centrifuged at 10,000× g in a microfuge (Eppendorf, Westbury, NY) to remove any cellular debris, and stored in a −80°C freezer (Revco Scientific, Asheville, NC) until assayed.
ELISA Assay for HGF
Culture supernatants were analyzed for HGF via a commercially available ELISA duoset (Quantikine; R&D system, Minneapolis, MN). This kit measures the biologically active form of HGF. The concentrations of HGF in the supernatants were measured from quadruplicate wells. The limit of sensitivity for this assay was 20 pg/ml. The plate was read on an Elisa reader (Dynex, Chantilly, VA). Standard curves and sample concentrations were determined using the Revelation software package called (Dynex).
Analysis and Statistics
The data for HGF secretion by the uterine, cervical, and ectocervical stromal fibroblasts are presented as the mean ± SEM. InSTAT software (GraphPad Software, San Diego, CA) was used to perform a one-way repeated-measures analysis of variance (ANOVA) for each individual donor. Thus, significance in a donor is relative to that donor’s control secretion. When ANOVA analysis indicated that significant differences existed among means, paired comparisons were made using the Tukey method to adjust p-values. A p-value of <0.05 was considered statistically significant.
RESULTS
Stromal fibroblasts from FRT tissues constitutively secrete HGF
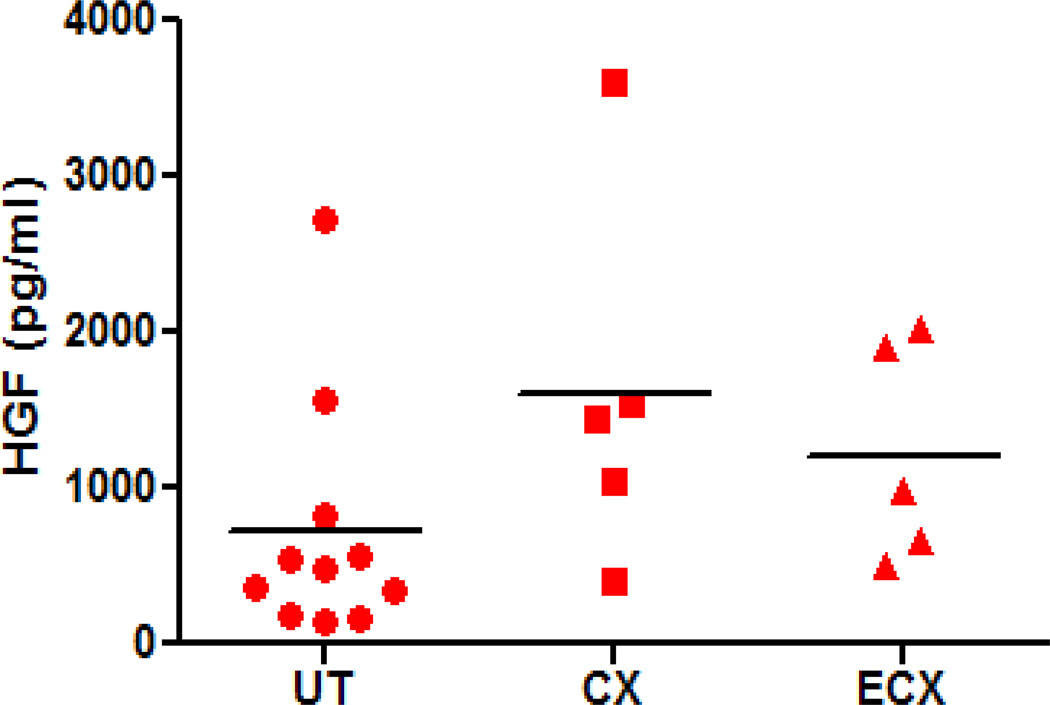
To determine if ectocervical, cervical and uterine stromal fibroblasts secrete HGF, fibroblasts from each tissue were isolated and grown in primary culture to confluence. Conditioned stromal media (CSM) were recovered from a minimum of 4 wells after 48 hours and individually assayed for HGF by ELISA. As shown in Figure 1, stromal fibroblasts from all three tissues constitutively secrete HGF, although levels vary among individual donors tested. Values were assessed after stromal fibroblasts grew to confluence in culture for 1–2 weeks with media changes every 48 hours, so the effect of endogenous hormones on HGF secretion would be eliminated. Also, since the stromal fibroblasts were cultured in media supplemented with stripped FBS, there should be no effect from exogenous steroid hormones. There was no significant difference in the mean values of HGF secretion from stromal fibroblasts derived from the uterus, endocervix and ectocervix. In other studies, we observed that constitutive HGF secretion was consistent over time when uterine stromal fibroblasts were cultured for 10 days during which 48 hour conditioned media were obtained and tested for HGF. There was no difference in the levels of secreted HGF assessed at 2, 4, 6, 8 and 10 days (data not shown).
Figure 1.
Constitutive secretion of HGF by human FRT stromal fibroblasts. Stromal fibroblasts were isolated and cultured to confluence in 24-well plates from uterine (n=11), endocervix (n=5) and ectocervix (n=5) tissue. Forty-eight hour conditioned stromal media were assayed for HGF by ELISA.
Estradiol enhances HGF secretion by uterine stromal fibroblasts
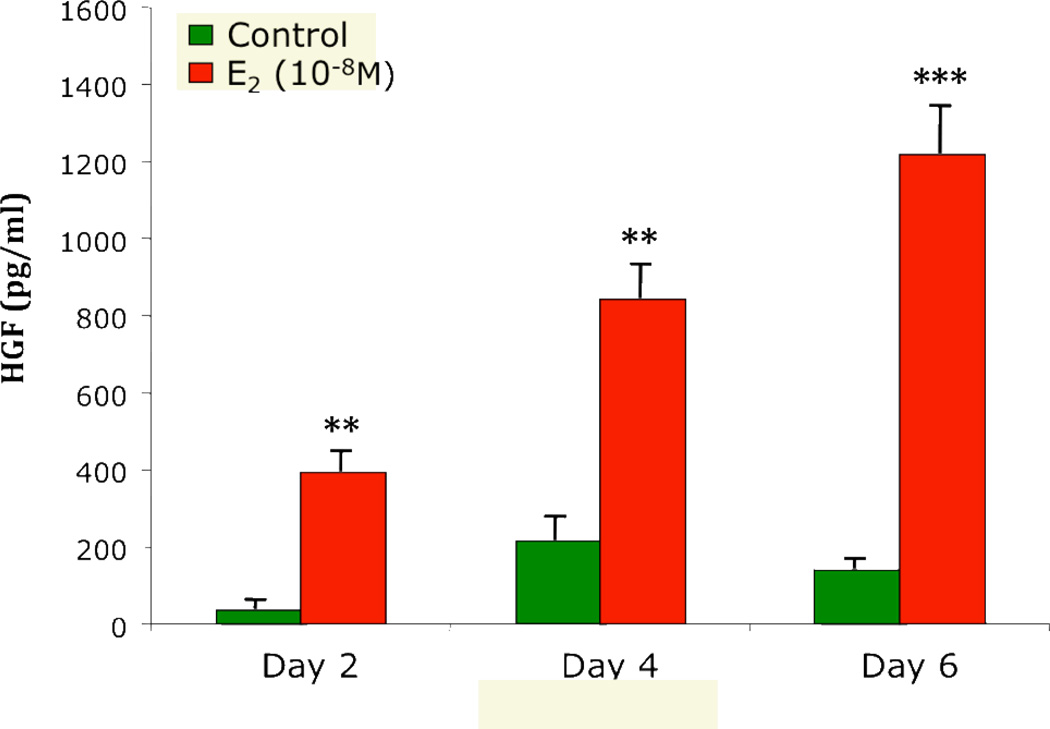
We have previously reported that estradiol stimulates uterine stromal fibroblasts to secrete HGF from 10 donor patients8. In order to better understand these results, we explored the effect of two different estradiol doses on HGF secretion. As seen in Figure 2, addition of estradiol for 48 hours more than doubled HGF secretion at concentrations of 10−8M and 10−7M, indicating that physiological non-saturating and saturating receptor doses elicit an HGF response. In other studies, to determine if uterine stromal fibroblasts can respond to repeated doses of estradiol, media containing 10−8M estradiol were completely removed from the cells after 48 hours and replaced with fresh media containing 10−8M estradiol. This was repeated after another 48 hours in culture. As shown in Figure 3, HGF induced by successive estradiol treatment at 2, 4 and 6 days showed a trend toward increasing HGF production, perhaps related to receptor induction and saturation or other mechanisms.
Figure 2.
Estradiol at two concentrations enhances HGF secretion by human uterine stromal fibroblasts. Confluent cultures of uterine stromal fibroblasts were treated with estradiol at 10−8 and 10−7M for 48 hours. Conditioned stromal media were assayed for HGF by ELISA. Similar results were obtained with cells derived from 3 patients. The mean +/− SEM are shown. ***, p<0.001, significantly different from control.
Figure 3.
Repeated addition of estradiol increases HGF secretion by human uterine stromal fibroblasts over time. The secretion of HGF, with or without 10−8M estradiol treatment, was assessed every 48 hours over a 6 day period from uterine stromal fibroblasts. At 48 hour intervals, the conditioned stromal fibroblast media were completely removed and replaced with either fresh media (Control) or fresh media containing 10−8M estradiol. At each time point, the CSM was assayed for HGF by ELISA. Similar results were obtained with uterine stromal fibroblasts from 4 patients. The mean +/− SEM are shown. **, p<0.01, ***, p<0.001, significantly different from control.
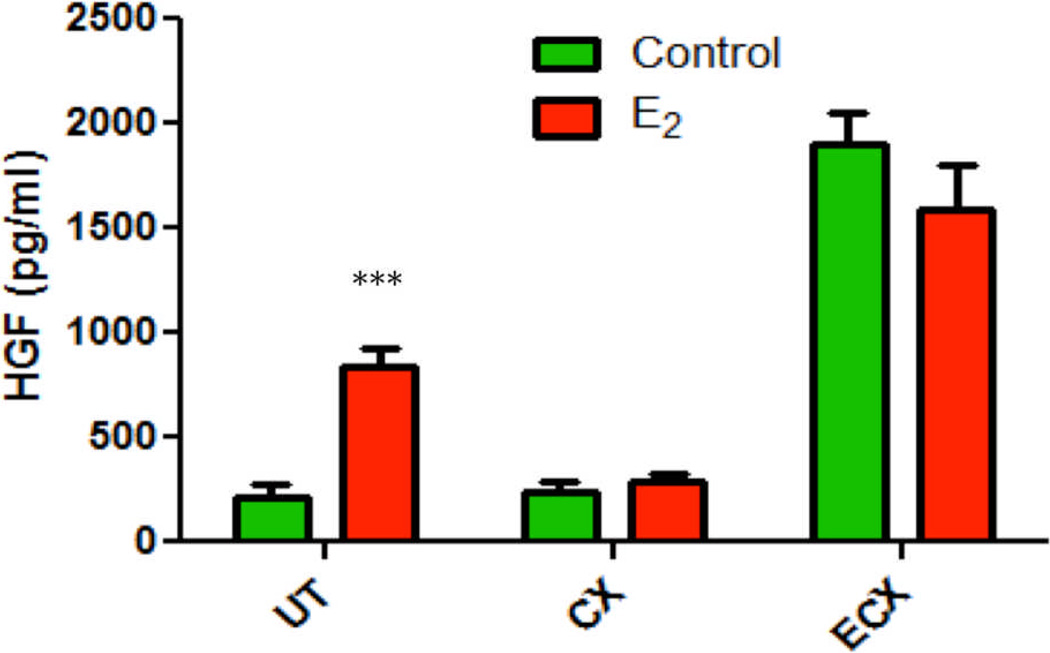
Since estradiol increased HGF secretion by uterine stromal fibroblasts, we determined if estradiol would have a similar effect on endo- and ecto-cervix cells. Figure 4 shows that estradiol increases HGF secretion from uterine stromal fibroblasts, but not from stromal fibroblasts derived from the endo- and ecto-cervix of the same patient. Similar results were obtained from 4 other patients.
Figure 4.
Estradiol stimulates HGF secretion from uterine but not endocervical and ectocervical epithelial cells. Confluent wells of stromal fibroblasts derived from uterine, endocervix and ectocervix tissue of one patient were treated with or without 10−8M estradiol for 48 hours. CSM was assessed for HGF. The mean +/− SEM are shown. ***, p<0.001, significantly different from control.
TLR agonist treatment of primary human uterine stromal fibroblasts
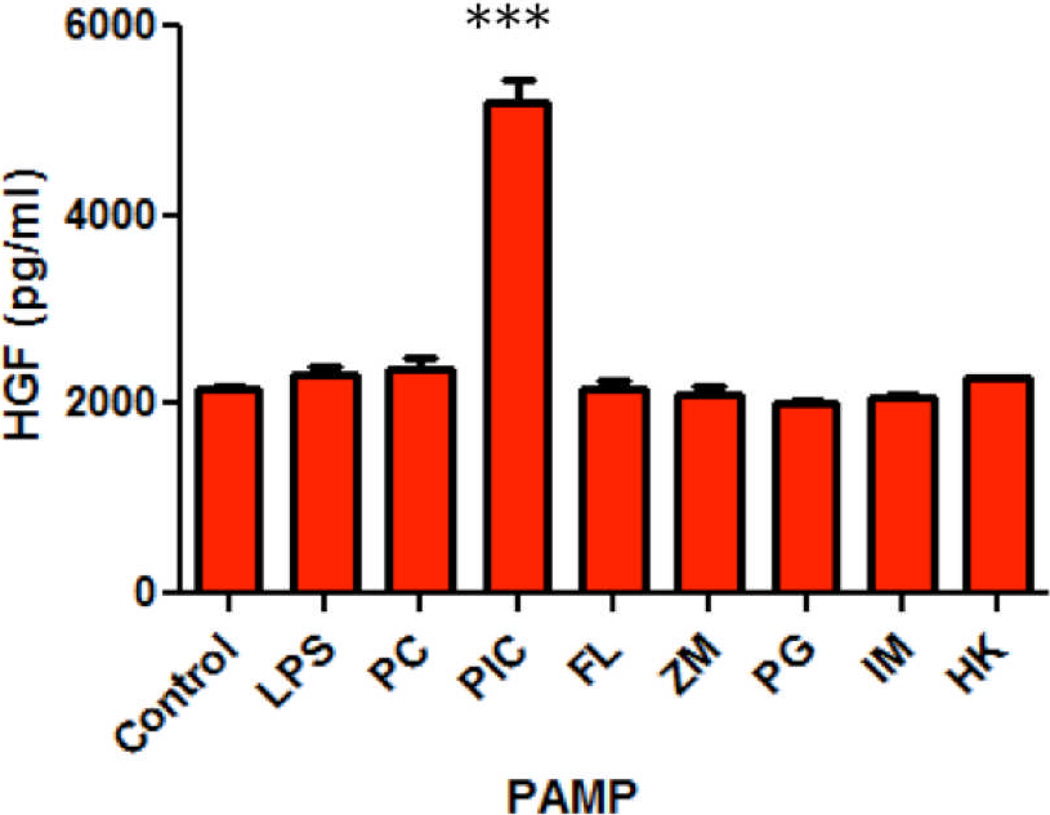
Since TLR agonists induce innate immune functions in epithelial cells, and HGF also regulates aspects of epithelial cell function, studies were undertaken to determine if treatment of uterine stromal fibroblasts with a panel of TLR agonists would alter the secretion of HGF. Uterine stromal fibroblasts were treated with a panel of specific TLR agonists, including LPS (TLR 4), Poly (I:C) (TLR 3), Pam3Cys (TLR 2), Flagellin (TLR 5), Zymosan (TLR 2), Peptidoglycan (TLR 2), Imiquimod (TLR 7,8)24, and heat-killed Listeria monocytoides (HKLM) (TLR 2)25. Most TLR agonists had no effect on HGF secretion (Figure 5). However, treatment of human uterine stromal fibroblasts with the TLR 3 agonist Poly (I:C), which mimics viral double-stranded viral RNA, at 25 µg/ml24, resulted in significant HGF secretion compared to control at 48 hours. Since Poly (I:C) increased HGF secretion from uterine stromal fibroblasts, we selected Poly (I:C) for further studies with FRT stromal fibroblasts.
Figure 5.
Treatment of uterine stromal fibroblasts with a panel of TLR agonists. Uterine stromal fibroblasts were treated with specific TLR agonists, including LPS, Pam3Cys (PC), Poly (I:C) (PIC), Flagellin (FL), Zymosan (ZM), Peptidoglycan (PG), Imiquimod (IM) or heat-killed Listeria monocytoides (HK) for 48 hours and assayed for HGF. Representative of experiments performed on uterine stromal fibroblasts from 3 patients. The mean +/− SEM are shown. ***, p<0.001, significantly different from control.
HGF secretion following Poly (I:C) treatment of primary human ectocervical, cervical and uterine stromal fibroblasts
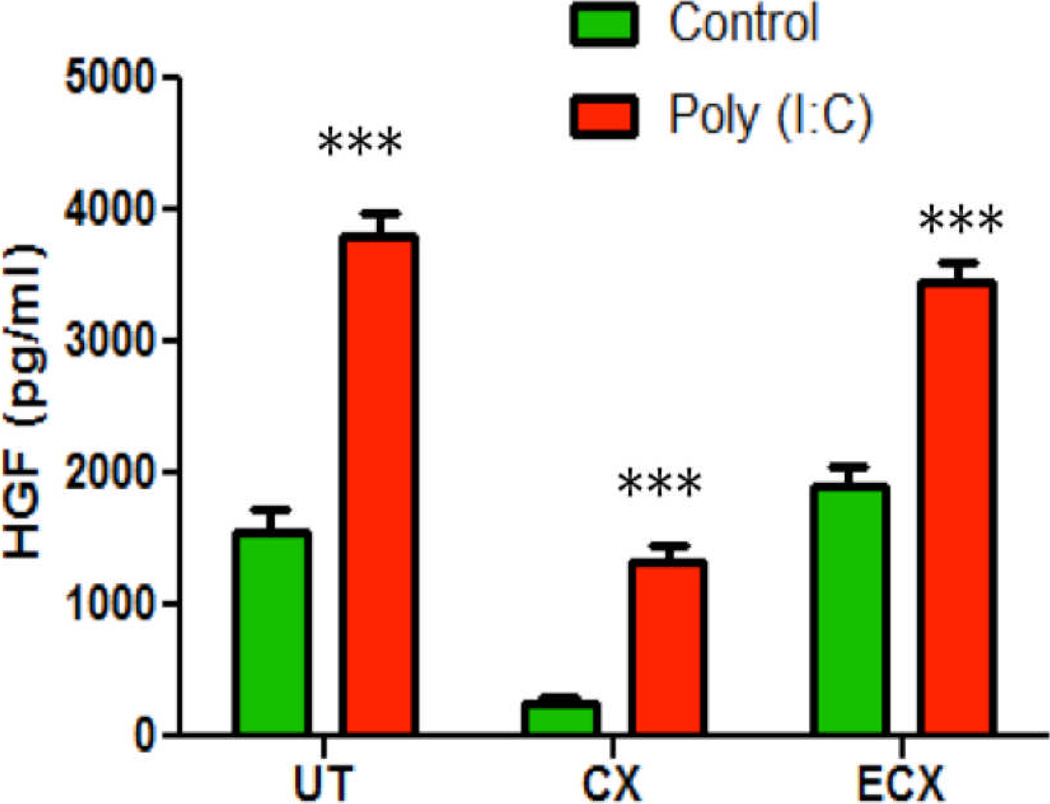
Figure 6 shows the HGF secretion from stromal fibroblasts isolated from 3 FRT tissues of one donor following treatment with Poly (I:C). The TLR3 ligand significantly increased HGF secretion in UT, CX and ECX. This is in contrast to the effects of estradiol treatment, which increased HGF secretion by uterine stromal fibroblasts only (see Figure 2). Of the 3 FRT tissues studied, stromal fibroblasts from the ectocervix and uterus secreted the most HGF, both constitutively and in response to Poly (I:C). In other studies, stromal fibroblasts from all 3 FRT tissues expressed mRNA for TLR3, with the ectocervix expressing approximately twice that of the cervix and uterus (data not shown). The stromal fibroblasts from only 3 of 5 donors of cervical tissue responded to Poly (I:C) with an increase in HGF secretion, while stromal fibroblasts from the ectocervix and the uterus of all donors produced HGF (5/5).
Figure 6.
Poly (I:C) stimulates HGF secretion from human uterine, cervical and ectocervical stromal fibroblasts. Confluent cultures of stromal fibroblasts from 3 FRT tissues were treated with 25µg/ml Poly (I:C) for 48 hours and CSM was assayed for HGF. Representative of experiments performed on uterine (UT, n=8), endocervix (CX, n=3) and ectocervix (ECX, n=5) stromal fibroblasts. The mean +/− SEM are shown. ***, p<0.001, significantly different from control.
Combined Poly (I:C) and estradiol treatment of uterine stromal fibroblasts
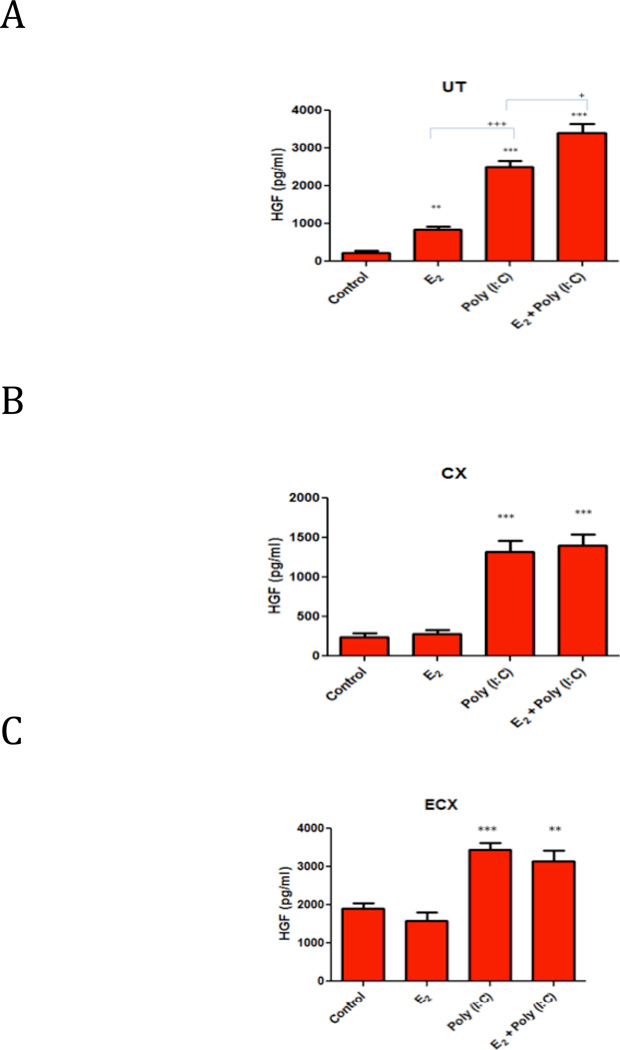
To compare estradiol-induced HGF secretion with that of Poly (I:C)-induced secretion, stromal fibroblasts from 3 FRT tissues were treated with 10−8M 17-β estradiol, a physiological dose of estradiol, or 25 µg/ml Poly (I:C) or both for 48 hours. As shown in Figures 7(A–C) and in previous Figures, both Poly (I:C) and estradiol individually enhanced HGF secretion only by uterine stromal fibroblasts. The Poly (I:C)-induced increase in HGF secretion from uterine stromal fibroblasts was significantly greater that that seen with estradiol. Co-treatment showed an additive HGF response compared with either estradiol or Poly (I:C) alone (Figure 7A). Since estradiol did not increase HGF secretion by either endo- or ecto-cervix stromal fibroblasts (Figures 4, 7(B), 7(C), co-treatment with estradiol and Poly (I:C) showed only an equivalent response to Poly (I:C) alone (Figures 7 (B) and (C)).
Figure 7.
Effect of estradiol and/or Poly (I:C) treatments on HGF secretion by (A) uterine, (B) endocervix and (C) ectocervix stromal fibroblasts. Confluent wells were treated with control media, estradiol (10−8M) and/or Poly (I:C) (25µg/ml) for 48 hours, and CSM was assayed for HGF. The mean +/− SEM are shown. **, p<0.01, ***, p<0.001, significantly different from control; +, p<0.05, significantly different comparing Poly (I:C) and co-treatment of Poly (I:C) and E2, +++, p<0.001, significantly different comparing E2 and Poly (I:C) treatments. Representative of 3 experiments.
DISCUSSION
Sex hormones play a key role in regulating immunity in the FRT26–31, and we have previously shown that estradiol increases HGF secretion by human uterine stromal fibroblasts8. Our results extend our prior observation to demonstrate that stimulation of primary human uterine stromal fibroblasts with the TLR 3 agonist Poly (I:C), the synthetic double stranded analog for viral RNA, significantly increases HGF secretion. The Poly (I:C)-induced HGF increase is more extensive in uterine stromal fibroblasts than that induced by estradiol; co-treatment of uterine stromal cells results in an additive effect in terms of HGF secretion. The additive response to combined estradiol and Poly (I:C) treatment in uterine stromal fibroblasts may reflect the cyclical relationship between estradiol levels and uterine morphology, as well as suggest that estradiol and Poly (I:C) increases HGF by different mechanisms. Poly (I:C) treatment of ECX stromal fibroblasts also results in increased HGF secretion, though combined estradiol and Poly (I:C) treatment does not differ from Poly (I:C) treatment alone. Poly (I:C) treatment of CX stromal fibroblasts resulted in variable HGF secretion; three of five donors increased HGF secretion while the other two were unresponsive. Co-treatment of these cells with Poly (I:C) and estradiol also had no effect on HGF secretion in cells from these two patients.
Published reports have identified individual types of TLR on fibroblasts, though studies measuring a full panel of TLR-specific RNA and protein expression are lacking on stromal fibroblasts. There are few published reports concerning TLR expression in stromal fibroblasts of the FRT. Human Fallopian tube stromal fibroblasts have been reported to express TLR 432. TLR 4 mRNA message and protein expression have been reported in human uterine stromal fibroblasts33. Hirata et al demonstrated that uterine stromal cells expressed TLR 4 mRNA via RT-PCR and in situ hybridization, as well as secreted IL-8 in response to LPS stimulation34. The stromal cells in that study were cultured to confluence for only two or three days and characterized only as CD14-positive; therefore, it is not clear what percentage of the stromal cells were fibroblasts and what leukocytes might be present. Uterine stromal fibroblasts were not LPS-responsive in our experiments, which may suggest TLR 4 inactivity or absence; perhaps the lack of an LPS-binding protein in our stripped FBS media prevented a response to LPS. We found that uterine and ectocervical stromal fibroblasts significantly increased HGF secretion in response to Poly (I:C) treatment, indicating that TLR 3 is both present and functional in uterine and ectocervical stromal fibroblasts. However, cervical stromal fibroblasts had variable responses to Poly (I:C) (3/5). This may be explained by patient variability, but conclusions cannot be drawn without a larger pool of donors. This finding does suggest the presence of functional TLR 3 in FRT stromal fibroblasts, at least from some patients.
To the best of our knowledge, this study is the first to demonstrate TLR 3 mediated modulation of HGF in the human FRT. HGF has modulatory effects on migration, mitosis, and morphogenesis of epithelial cells (reviewed in2). HGF may also be involved with the innate immune response to HIV. HGF has been shown to increase epithelial cell expression of the receptor CXCR4, an important cofactor in HIV infection eceptor35, 36, in human breast cancer cell lines37. Increased HGF secretion has been associated with oncogenic-type HPV and HIV co-infection in the cervix38.
It is also possible that HGF may link some viruses to development of cancer by the following mechanism: exposure of stromal fibroblasts to oncogenic viruses may increase HGF secretion, leading to metastatic cancer characterized by overexpression of epithelial c-Met or other epithelial receptors involved in viral infection. For example, a receptor (CXCR4) for a different factor, stromal derived factor-1 (SDF-1), is expressed on epithelial cells of the FRT39, 40. Importantly, HGF has been demonstrated to regulate human cervical carcinoma, a pathology of viral origin, in conjunction with SDF-115. Also, replication of SV40 in human mesothelial cells induces HGF/c-Met receptor activation41. Significantly, these studies link HGF with viral infection.
The results of these studies demonstrate a tissue-specific Poly (I:C) response; uterine and ectocervical stromal fibroblasts increase HGF secretion when treated with Poly (I:C), though cervical stromal fibroblasts from two of five did not. TLR 3 mRNA is expressed in uterine, cervical and ectocervical stromal fibroblasts from at least one donor; the fact that all three tissues responded with an increase in HGF secretion to Poly (I:C) indicates that the TLR 3 is functional in stromal fibroblasts from this donor.
Our studies suggest that a combination of estradiol secretion and viral infection on uterine stromal fibroblasts may enhance HGF production. These results suggest the potential that HGF may induce uterine epithelial cells to spread to other tissues and possibly enhance, under certain conditions, the development of endometrial cancer and endometriosis. Therapies to control HGF secretion and/or c-Met expression by stromal fibroblasts due to hormonal or viral causes have been suggested to control these serious diseases42.
Acknowledgments
This work was funded by National Institutes of Health grants AI-51877, AI-13541 and AI-071761 (CRW).
REFERENCES
- 1.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology: life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86:588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse. Biol Reprod. 1998;59:470–475. doi: 10.1095/biolreprod59.3.470. [DOI] [PubMed] [Google Scholar]
- 4.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 5.Grant-Tschudy KS, Wira CR. Effect of oestradiol on mouse uterine epithelial cell tumour necrosis factor-alpha release is mediated through uterine stromal cells. Immunology. 2005;115:99–107. doi: 10.1111/j.1365-2567.2005.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Hepatocyte growth factor stimulated proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod. 1997;57:936–942. doi: 10.1095/biolreprod57.4.936. [DOI] [PubMed] [Google Scholar]
- 8.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukaya T, Sugawara J, Yoshida H, Murakami T, Yajima A. Intercellular adhesion molecule-1 and hepatocyte growth factor in human endometriosis: original investigation and a review of literature. Gynecol Obstet Invest. 1999;47(Suppl 1):11–16. doi: 10.1159/000052854. discussion 16–17. [DOI] [PubMed] [Google Scholar]
- 10.Haslam SZ, Woodward TL. Host microenvironment in breast cancer development: epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland. Breast Cancer Res. 2003;5:208–215. doi: 10.1186/bcr615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Hiraki K, Sekine I, Matsuyama T, Ishimaru T. Interleukin-6- and tumour necrosis factor alpha-mediated expression of hepatocyte growth factor by stromal cells and its involvement in the growth of endometriosis. Hum Reprod. 2005;20:2715–2723. doi: 10.1093/humrep/dei156. [DOI] [PubMed] [Google Scholar]
- 12.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Immunoexpression of hepatocyte growth factor and c-Met receptor in the eutopic endometrium predicts the activity of ectopic endometrium. Fertil Steril. 2003;79:173–181. doi: 10.1016/s0015-0282(02)04535-1. [DOI] [PubMed] [Google Scholar]
- 13.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril. 2004;81:652–661. doi: 10.1016/j.fertnstert.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, Ishimaru T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20:2004–2013. doi: 10.1093/humrep/deh897. [DOI] [PubMed] [Google Scholar]
- 15.Majka M, Drukala J, Lesko E, Wysoczynski M, Jenson AB, Ratajczak MZ. SDF-1 alone and in co-operation with HGF regulates biology of human cervical carcinoma cells. Folia Histochem Cytobiol. 2006;44:155–164. [PubMed] [Google Scholar]
- 16.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–483. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 17.Moghul A, Lin L, Beedle A, Kanbour-Shakir A, DeFrances MC, Liu Y, Zarnegar R. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: evidence for rapid decay of the 8 kb c-MET transcript. Oncogene. 1994;9:2045–2052. [PubMed] [Google Scholar]
- 18.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract, antiviral response of uterine epithelial cells to the TLR3 agonist poly(I: C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 19.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 20.Schroder HC, Kelve M, Muller WE. The 2-5A system and HIV infection. Prog Mol Subcell Biol. 1994;14:176–197. doi: 10.1007/978-3-642-78549-8_10. [DOI] [PubMed] [Google Scholar]
- 21.Fahey JV, Humphrey SL, Stern JE, Wira CR. Secretory component production by polarized epithelial cells from the human female reproductive tract. Immunol Invest. 1998;27:167–180. doi: 10.3109/08820139809089454. [DOI] [PubMed] [Google Scholar]
- 22.Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 23.McNatty KP, Baird DT, Bolton A, Chambers P, Corker CS, McLean H. Concentration of oestrogens and androgens in human ovarian venous plasma and follicular fluid throughout the menstrual cycle. J Endocrinol. 1976;71:77–85. doi: 10.1677/joe.0.0710077. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 26.Fahey JV, Schaefer TM, Wira CR. Sex hormone modulation of human uterine epithelial cell immune responses. Intgr Comp Biol. 2006;46:1082–1087. doi: 10.1093/icb/icl036. [DOI] [PubMed] [Google Scholar]
- 27.Wira CR, Fahey JV. The innate immune system: gatekeeper to the female reproductive tract. Immunology. 2004;111:13–15. doi: 10.1111/j.1365-2567.2003.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wira CRFJ, White HD, Givan A, Howell A. The mucosal immune system in the human female reproductive tract. Influence of stage in the menstrual cycle and menopause on mucosal immunity in the uterus. Boca Raton: CRC Press; 2002. [Google Scholar]
- 29.Wira CR, Richardson J, Prabhala R. Endocrine regulation of mucosal immunity: Effect of sex hormones and cytokines on the afferent and efferent arms of the immune system in the female reproductive tract. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Handbook of Mucosal Immunology. New York: Academic Press; 1994. pp. 705–718. [Google Scholar]
- 30.Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells: role of TGFbeta as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143:2872–2879. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- 31.Wira CRKC, Richardson JM. Role of sex hormones and cytokines in regulating the mucosal immune system in the female reproductive tract. New York: Academic Press; 2004. [Google Scholar]
- 32.Itoh H, Nasu K, Nishida M, Matsumoto H, Yuge A, Narahara H. Human oviductal stromal fibroblasts, but not oviductal epithelial cells, express Toll-like receptor 4: the site-specific mucosal immunity of the human fallopian tube against bacterial infection. Am J Reprod Immunol. 2006;56:91–101. doi: 10.1111/j.1600-0897.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Hirata M, Sato T, Tsumagari M, Shimada A, Nakano H, Hashizume K, Ito A. Differential regulation of the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases by cytokines and growth factors in bovine endometrial stromal cells and trophoblast cell line BT-1 in vitro. Biol Reprod. 2003;68:1276–1281. doi: 10.1095/biolreprod.102.006452. [DOI] [PubMed] [Google Scholar]
- 34.Hirata T, Osuga Y, Hirota Y, Koga K, Yoshino O, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Evidence for the presence of toll-like receptor 4 system in the human endometrium. J Clin Endocrinol Metab. 2005;90:548–556. doi: 10.1210/jc.2004-0241. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 37.Matteucci E, Locati M, Desiderio MA. Hepatocyte growth factor enhances CXCR4 expression favoring breast cancer cell invasiveness. Exp Cell Res. 2005;310:176–185. doi: 10.1016/j.yexcr.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Walker F, Kermorgant S, Darai E, Madelenat P, Cremieux AC, Henin D, Lehy T. Hepatocyte growth factor and c-Met in cervical intraepithelial neoplasia: overexpression of proteins associated with oncogenic human papillomavirus and human immunodeficiency virus. Clin Cancer Res. 2003;9:273–284. [PubMed] [Google Scholar]
- 39.Yeaman GR, Asin S, Weldon S, Demian DJ, Collins JE, Gonzalez JL, Wira CR, Fanger MW, Howell AL. Chemokine receptor expression in the human ectocervix: implications for infection by the human immunodeficiency virus-type I. Immunology. 2004;113:524–533. doi: 10.1111/j.1365-2567.2004.01990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeaman GR, Howell AL, Weldon S, Demian DJ, Collins JE, O'Connell DM, Asin SN, Wira CR, Fanger MW. Human immunodeficiency virus receptor and coreceptor expression on human uterine epithelial cells: regulation of expression during the menstrual cycle and implications for human immunodeficiency virus infection. Immunology. 2003;109:137–146. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cacciotti P, Libener R, Betta P, Martini F, Porta C, Procopio A, Strizzi L, Penengo L, Tognon M, Mutti L, Gaudino G. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci U S A. 2001;98:12032–12037. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naran S, Zhang X, Hughes SJ. Inhibition of HGF/MET as therapy for malignancy. Expert Opin Ther Targets. 2009;13:569–581. doi: 10.1517/14728220902853917. [DOI] [PubMed] [Google Scholar]