Abstract
Mercury is neurotoxic and increasing evidence suggests that environmental exposure to mercury may contribute to neuropathologies including Alzheimer's disease and autism spectrum disorders. Mercury is known to disrupt immunocompetence in the periphery, however, little is known about the effects of mercury on neuroimmune signaling. Mercury-induced effects on central immune function are potentially very important given that mercury exposure and neuroinflammation both are implicated in certain neuropathologies (i.e., autism). Furthermore, mounting evidence points to the involvement of glial activation in autism. Therefore, we utilized an in vivo model to assess the effects of mercury exposure on neuroimmune signaling. In prairie voles, 10 week mercury exposure (60 ppm HgCl2 in drinking water) resulted in a male-specific increase in TNFα protein expression in the cerebellum and hippocampus. These findings are consistent with our previously reported male-specific mercury-induced deficits in social behavior and further support a role for heavy metals exposure in neuropathologies such as autism. Subsequent studies should further evaluate the mechanism of action and biological consequences of heavy metals exposure. Additionally, these observations highlight the potential of neuroimmune markers in male voles as biomarkers of environmental mercury toxicity.
Keywords: heavy metals, environmental toxins, voles, cytokines, chemokines, autism
Introduction
Environmental exposure to heavy metals is a significant risk to human health [12]. Mercury, for example, certainly is neurotoxic and accumulation of mercury in the brain is accompanied by abnormal neuronal function in several brain regions, including in the cerebellum and the hippocampus [5, 17, 54]. Increasing evidence suggests that environmental mercury exposure may contribute to neuropathologies such as Alzheimer's disease (AD) and the autism spectrum disorders (ASD) [20-22, 28, 38-40].
Among the mechanisms implicated in mercury-induced neurotoxicity are mitochondrial dysfunction and oxidative stress [33, 45]. However, sub-lethal exposure to mercury also disrupts immunocompetence [29, 30] suggesting that changes in neuroimmune function may provide a link between metals exposure and the development of disorders such as autism. Consistent with this idea, recent studies have shown that glial activation and neuroinflammation may contribute to the neuropathology of autism [3, 11, 35, 47, 53]. For example, levels of proinflammatory cytokines, including tumor necrosis factor alpha (TNFα), consistently are elevated in the central nervous system (CNS) of autism patients [9, 35, 53].
Unfortunately, relatively little is known about the effects of mercury exposure on neuroimmune function [18, 46, 56] and how such effects may contribute to the development of disorders such as autism. Prairie voles have been used extensively in studies of the physiological and neurochemical underpinnings of social behaviors [2, 26, 57]. These animals are highly social: individual prairie voles appear to actively seek contact with conspecifics, and, in fact, display evidence of significant stress when isolated [16]. We recently have been able to produce behaviors in voles that model two of the major characteristics of autism: prairie voles that ingest heavy metals in their drinking water subsequently exhibit sex-specific deficits in social behavior [13]. Male, but not female, prairie voles that receive metals treatment significantly reduce social contact when confronted with an unfamiliar individual, apparently via a dopamine-mediated mechanism. Importantly, the social avoidance is not displayed when a familiar sibling rather than a stranger is encountered. In the present investigation, we used the prairie vole model to examine the effects of chronic mercury exposure on neuroimmune signaling.
Methods
We first wanted to test whether prairie voles display a typical central response to an immune challenge, in this case, systemic administration of bacterial lipopolysaccharide (LPS). Twenty male voles were injected with 3 mg/kg of LPS. Four animals were sacrificed immediately after LPS administration to provide a baseline value against which longer LPS exposures could be compared. Additional groups of 4 animals each were sacrificed at 6, 12, 24, and 48 hours after LPS administration. At each time point, brains were removed at termination and the cerebellum and hippocampus were quickly frozen and subsequently assayed for TNFα protein expression. Brain tissue was prepared using a modified version of the protocol described by Vargas et al, [53]. Briefly, tissue was sonicated on ice for 10 sec. in 0.5 ml buffer [50nM Tris-HCl, pH 7.4, 150nM NaCl, 0.02% sodium azide, 0.1% sodium dodecyl sulfate, 1.0% IGEPAL, 0.5% sodium deoxycholate, 10ug/mL aprotinin and 0.5mM phenylmethylsulfonyl fluoride. A standard dual-antibody solid phase immunoassay (ELISA Development Kits, Peprotech) was used for quantitation of TNFα protein in brain tissue homogenates, according to the manufacturer's instructions and as previously described [14]. Brain samples and standards (100 μl for each) were added to antibody-coated wells of 96 well plates. Next, 100 μl of antigen-specific biotinylated detection antibody was added to each well followed by a 2 h incubation at room temperature. Liquid contents then were aspirated and the wells were washed 3× with wash buffer. Avidin-peroxidase conjugate (100 μl) was added to each well and incubated for 30 min at room temperature. Wells again were aspirated and washed 3×, and 100 μl of ABTS liquid substrate solution (Sigma cat. # A3219) was added to each well followed by 25 min incubation at room temperature. Absorbance was measured at 450 nm (with wavelength correction set at 650 nm) on a BIO-TEK HT spectrophotometer. Total protein/well was determined using the bicinchoninic acid (BCA) protein assay as previously described [15] in order to normalize data when appropriate (pg chemokine protein/mg total tissue protein). One-way Analysis of Variance (ANOVA) was used to test for significant effects of time after LPS administration. Student-Neuman-Kuels (SNK) pair-wise comparisons were used to further probe significant (p < 0.05) main effects. Data are presented as means ± standard errors.
We then examined vole brain tissue after chronic exposure to low-dose mercury ingestion. Tissue for this experiment was collected at termination of subjects used in a previously reported study of the effects of chronic metals exposure on vole social behavior [13]. Details of animal husbandry are provided in that report and all experimental manipulations, animal handling procedures, and behavioral testing were approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee. Same-sex pairs were randomly assigned to treatment groups that received a dilute (60 ppm) mercuric chloride (HgCl2) solution as their sole drinking water source for 10 weeks. This concentration is near the lower end of the range of concentrations found during a survey of the toxicology literature. Control pairs received normal drinking water for the same period. Groups otherwise were treated identically. Fresh metals solutions were prepared weekly and solutions were replaced as part of normal cage maintenance, but drinking bottles were replaced if they became contaminated with bedding outside of the normal cage maintenance regimen. Since animals required multiple housing, the amounts of mercury ingested were assumed to be equal between the two individuals in each pair. Mercury exposure was followed by measurement of TNFα, and chemokine (CCL2 and CXCL10) levels in the cerebellum and hippocampus using ELISA as described above with kits specific for each analyte (Peprotech). Two-way ANOVA was used to assess group differences in the neurochemical measures with sex and treatment as factors. Student-Neuman-Kuels pair-wise comparisons were used to further probe significant (p < 0.05) main effects or interactions. Data are presented as means ± standard errors.
Results
Neuroimmune response to systemic LPS challenge
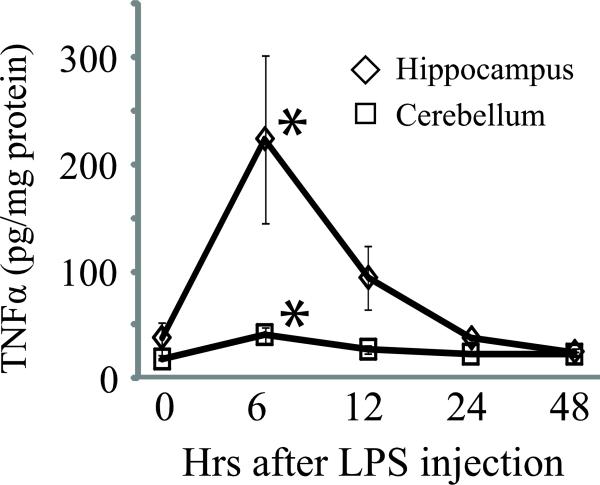
Neuroimmune responses in males after peripheral administration of LPS were compared at five time points after administration. Values at time 0 (for animals terminated immediately after LPS administration) served as the baseline against which values from subsequent time points were compared. During the experiment, there were no group differences in the animals’ ages (F4,15 = 0.29, p = 0.88) or body masses (F4,15 = 0.10, p = 0.98). There were significant effects of LPS administration on TNFα in both the cerebellum (F4,15 = 4.56, p < 0.02) and the hippocampus (F4,15 = 4.71, p < 0.02). In both brain regions, TNFα was significantly elevated over baseline at six hours after LPS administration (p < 0.01), but not at 12, 24, or 48 hours after treatment.
TNFα expression in vole brain after chronic mercury ingestion
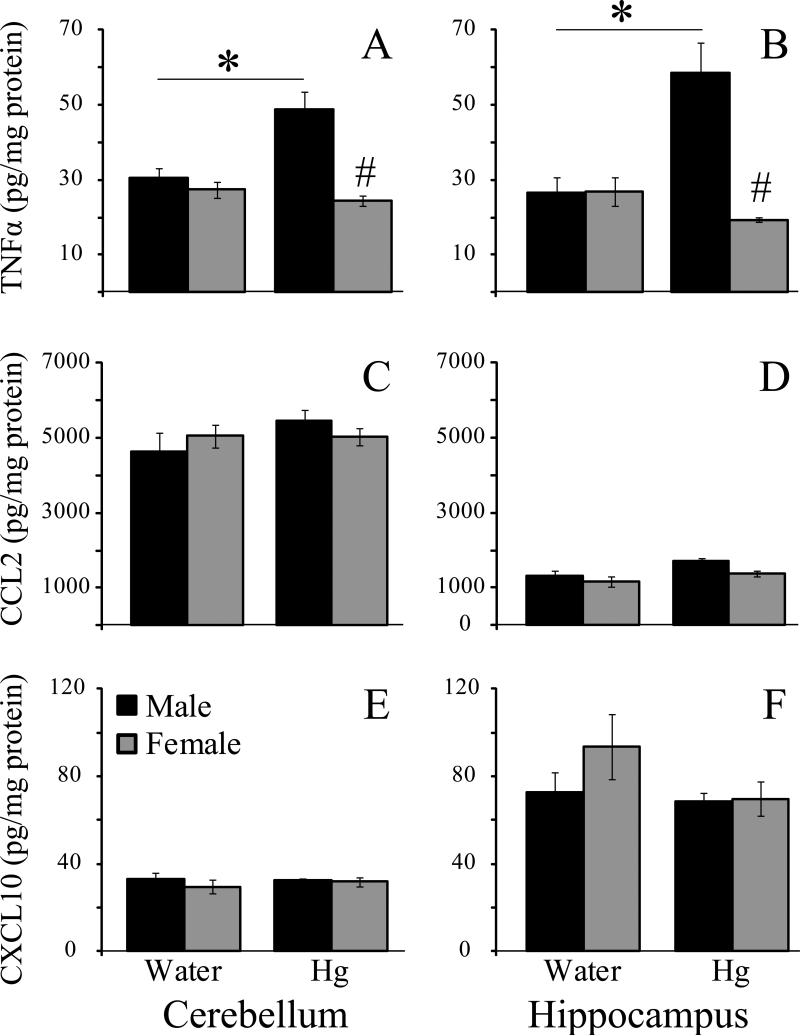
Two-way ANOVA for TNFα in the cerebellum revealed significant main effects of treatment (F1,56 = 4.13, p < 0.001), sex (F1,56 = 13.35, P < 0.05), and a significant interaction (F1,56 = 8.07, P < 0.01). SNK pair-wise comparisons established that cerebellum TNFα expression (Fig 1A) was greater in mercury-treated males than in water-treated males (p < 0.002) or in either group of females (both p-values < 0.001). No other group differences were found (all p-values > 0.49). In general, the same pattern was seen for TNFα in the hippocampus (Fig 1B). There was a significant effect of sex (F1,57 = 8.22 , p < 0.01) and a significant treatment × sex interaction (F1,57 = 8.44, p < 0.01), however, the main effect of treatment failed to reach statistical significance (F1,57 = 3.26, p = 0.076). Pair-wise comparisons again established mercury-treated males as having significantly greater TNFα expression than that for any other group (all p-values < 0.005). No other pair-wise comparisons reached statistical significance (all p-values > 0.46).
Fig. 1.
TNFα expression in male vole brain was elevated in both the hippocampus and cerebellum 6 h after peripheral administration of 3 mg/kg LPS. * P < 0.05 vs. 0 hr.
CCL2 expression in vole brain after chronic mercury ingestion
Two-way ANOVA for CCL2 in the cerebellum (Fig 1C) did not reveal any significant main effects (sex: F1,36 = 0.0005, p = 0.98; treatment: F1,36 = 1.28, p = 0.27), or interaction (F1,36 = 1.41, p = 0.24). Similarly, there were no significant main effects of sex (F1,35 = 2.13, p = 0.15) or treatment (F1,35 = 3.20, p =0.08), and no interaction (F1,35 = 0.27, p = 0.61) for CCL2 in the hippocampus (Fig 1D).
CXCL10 expression in vole brain after chronic mercury ingestion
Two-way ANOVA for CCL2 in the cerebellum (Fig 1 E) and the hippocampus (Fig 1F) also did not reveal any significant main effects of sex (cerebellum: F1,87 = 1.16, p = 0.29; hippocampus: F1,23 = 0.88, p = 0.36) or treatment: (cerebellum: F1,87 = 0.19, p = 0.67; hippocampus: F1,23 = 1.45, p = 0.24), and there were no significant sex × treatment interactions (cerebellum: F1,87 = 0.69, p = 0.41; hippocampus: F1,23 = 0.70, p = 0.41).
Discussion
We examined neuroimmune responses in prairie voles as part of an on-going series of studies into the potential for heavy metals exposure being a risk factor in the development of autism. We found that the expression of at least some neuroimmune factors is altered after chronic metals exposure and that the effects are consistent with previously reported metals-induced changes in social behavior.
As a first step in this process we needed to establish that vole neuroimmune responses were similar to those in other species. This was found to be the case for TNFα, which was elevated in at least two brain regions by six hours after administration of an LPS challenge. For purposes of this study, the elevation of TNFα in a temporally appropriate timeframe suggests that vole neuroimmune responses are not fundamentally different from those of the other species.
There is substantial evidence that mercury affects function in peripheral immune cells, including, lymphocyte adhesion [50], mast cell activation [27] and cytokine expression in numerous cell types [19, 27, 29, 42]. In addition, there is mounting evidence suggesting that immune signaling is instrumental in the neuropathology of autism. For instance, glial activation and several markers of neuroinflammation are present in autism [11, 35, 47, 53]. Among the neuroinflammatory markers often associated with autism is TNFα [9, 35], but it remains unclear exactly how elevated TNFα contributes to the symptoms or development of ASD. However, the importance of TNFα likely extends beyond its classic, proinflammatory and pro-apoptotic roles [8, 48]. Indeed, TNFα is increasingly recognized as instrumental in complex physiologic processes including neuroprotection, ionic homeostasis and synaptic plasticity [8, 23, 48, 49]. For example, overexpression of TNFα in response to methamphetamine (METH) treatment appears to activate vesicular dopamine uptake, thereby attenuating the METH-induced increase in extracellular dopamine in the striatum [41]. The ability of TNFα to modulate dopamine metabolism is particularly intriguing in an autism context given the apparent role for central dopamine functioning in the switching from social to asocial behavior in voles [13] and other CNS processes [51]. Taken together, as postulated by Chertoff et al., [8], the detrimental (or beneficial) effects of TNFα likely involve numerous factors including the level and site of TNFα expression, as well as the time at which this cytokine is expressed relative to other stimuli.
To our knowledge, this is the first report on the effects of mercury exposure on cytokine/chemokine expression in the brain: chronic exposure to low levels of inorganic mercury enhanced TNFα expression in both the cerebellum and hippocampus. Of particular interest is that TNFα levels were elevated after mercury exposure in male voles only. This finding is important for two reasons. First, this is another example of sexual dimorphism in a response to an immune challenge (see also Klein et al., [31, 32]). Second, the brain tissue used in this study was from animals whose social behavior previously had been carefully characterized. In that study, we reported that prairie voles’ preference for social proximity is quite sensitive to exposure to environmental toxins. After 10 weeks of chronic, low-level exposure to metals (mercury or cadmium) in their drinking water, these normally social animals began to avoid strangers and displayed social withdrawal quite similar to the regressive aspects of autism that are seen in human autistic patients. Further, the metals-induced changes in social behavior occurred only in male voles [13], which parallels the well-established higher incidence of autism in males. Thus, these results are consistent with a number of studies in other animal models linking mercury in various forms to the neuropathology of autism [24, 25, 34, 44].
Chemokines also can be elevated in the CNS of those with autism: CXCL10 is elevated in the cerebrospinal fluid (CSF) and CCL2 is elevated in the CSF and brain (particularly the cerebellum) of autistic individuals [53]. Chemokines are instrumental in physiological and pathological process in the brain. Within the brain, CCL2 expression is predominately in the astrocytes [53]. CCL2 is expressed constitutively in astrocytes and neurons and is transcriptionally induced by proinflammatory cytokines, particularly in astrocytes [4, 7, 43]. Both CXCL10 and CCL2 are well characterized with respect to their roles in the recruitment of inflammatory cells to sites of insult [6]. Increasing data also indicate that these chemokines have neuromodulatory [1, 4, 37], neuroprotective [36, 55] and neurotoxic actions [52]. CCL2 reportedly has more of a neuromodulatory role, particularly on dopaminergic neurons, whereas, CXCL10 appears to be more of a neurotoxin. Thus, dysregulation of these cytokines/chemokines can impact multiple aspects of neurophysiology and neuropathology. We did not observe any mercury effect on chemokines in the specific brain regions we examined. However, this does not preclude changes in chemokine expression in other brain regions after mercury exposure. Further, we examined chemokine expression only at a single time-point after 10 weeks of chronic mercury exposure. It is possible that chemokine expression in these brain regions is transient, or that the length of exposure was not of sufficient duration to elicit a chemokine response. Interestingly, and of potential importance, is that our initial in vitro studies showed that chronic mercury exposure inhibited TNFα-induced chemokine (CCL2 and CXCL10) expression in human astroglial cells (data not shown). Further studies assessing the temporal effects of mercury are therefore warranted, as is the assessment of chemokine expression in other brain regions.
Together, these data demonstrate that neuroimmune signaling is altered by mercury exposure in vivo and that further study is needed to identify the mechanism of action and biologic consequences. Gaining a better understanding of mercury's effects on TNFα, CCL2, and CXCL10 is important given the physiological and pathological importance of these mediators. Furthermore, these observations in voles are particularly important given the potential for these neuroimmune factors to serve as biomarkers of mercury toxicity. Voles already are recognized as potentially useful sentinel animals for monitoring environmental levels of various toxins such as metals [10]. The present results suggest that inflammatory signaling in the vole brain, especially that in males, may be a useful biomarker of mercury contamination in the environment.
Highlights.
Peripheral LPS induces increased TNFα in vole brain
Chronic mercury induces increased TNFα in vole brain
Mercury induced TNFα in vole brain is male specific
Mercury induced TNFα in brain parallels previously reported social deficits in males
Fig 2.
Neuroimmune factor responses in vole brain after chronic mercury exposure. Ten weeks of chronic exposure to mercury in drinking water altered TNFα expression in a sex-specific fashion in the cerebellum and hippocampus of prairie voles (A, B). In both brain regions, exposure to mercury increased TNFα in male voles but not in females. This mercury exposure paradigm did not alter the expression of CCL2 (C, D) or CXCL10 (E, F). * - significantly greater than water-treated voles of either sex. # - significantly different from within-treatment opposite sex animals.
Acknowledgements
This project was supported in part by OSU-CHS Intramural funding (RLD, JTC), NIH Grant NS062664 (RLD) and NIH Grant HD48462 (JTC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2006;7:E865–870. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 3.Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 4.Banisadr G, Gosselin RD, Mechighel P, Rostene W, Kitabgi P, Melik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J Comp Neurol. 2005;492:178–192. doi: 10.1002/cne.20729. [DOI] [PubMed] [Google Scholar]
- 5.Basu N, Scheuhammer AM, Rouvinen-Watt K, Grochowina N, Evans RD, O'Brien M, Chan HM. Decreased N-methyl-D-aspartic acid (NMDA) receptor levels are associated with mercury exposure in wild and captive mink. Neurotoxicology. 2007;28:587–593. doi: 10.1016/j.neuro.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Brown KA. Factors modifying the migration of lymphocytes across the blood-brain barrier. Int Immunopharmacol. 2001;1:2043–2062. doi: 10.1016/s1567-5769(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 8.Chertoff M, Di Paolo N, Schoeneberg A, Depino A, Ferrari C, Wurst W, Pfizenmaier K, Eisel U, Pitossi F. Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor alpha in the nigrostriatal dopaminergic circuit of adult mice. Exp Neurol. 2011;227:237–251. doi: 10.1016/j.expneurol.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol. 2007;36:361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Cobb GP, Moore AW, Rummel KT, Adair BM, McMurry ST, Hooper MJ. Mercury and methylmercury accumulation and excretion in prairie voles (Microtus ochrogaster) receiving chronic doses of methylmercury. Arch Environ Contam Toxicol. 2007;52:441–449. doi: 10.1007/s00244-006-0006-6. [DOI] [PubMed] [Google Scholar]
- 11.Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–341. doi: 10.1016/s0074-7742(05)71013-8. [DOI] [PubMed] [Google Scholar]
- 12.Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol Appl Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Curtis JT, Hood AN, Chen Y, Cobb GP, Wallace DR. Chronic metals ingestion by prairie voles produces sex-specific deficits in social behavior: an animal model of autism. Behav Brain Res. 2010;213:42–49. doi: 10.1016/j.bbr.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RL, Buck DJ, Saffarian N, Stevens CW. The opioid antagonist, beta-funaltrexamine, inhibits chemokine expression in human astroglial cells. J Neuroimmunol. 2007;186:141–149. doi: 10.1016/j.jneuroim.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:1404–1411. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- 16.DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faro LR, do Nascimento JL, San Jose JM, Alfonso M, Duran R. Intrastriatal administration of methylmercury increases in vivo dopamine release. Neurochem Res. 2000;25:225–229. doi: 10.1023/a:1007571403413. [DOI] [PubMed] [Google Scholar]
- 18.Franco JL, Braga Hde C, Nunes AK, Ribas CM, Stringari J, Silva AP, Garcia Pomblum SC, Moro AM, Bohrer D, Santos AR, Dafre AL, Farina M. Lactational exposure to inorganic mercury: evidence of neurotoxic effects. Neurotoxicol Teratol. 2007;29:360–367. doi: 10.1016/j.ntt.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Gardner RM, Nyland JF, Evans SL, Wang SB, Doyle KM, Crainiceanu CM, Silbergeld EK. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009;117:1932–1938. doi: 10.1289/ehp.0900855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrecht M, Austin DW. The plausibility of a role for mercury in the etiology of autism: a cellular perspective. Toxicol Environ Chem. 2011;93:1251–1273. doi: 10.1080/02772248.2011.580588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geier DA, Kern JK, Garver CR, Adams JB, Audhya T, Nataf R, Geier MR. Biomarkers of environmental toxicity and susceptibility in autism. J Neurol Sci. 2009;280:101–108. doi: 10.1016/j.jns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Geier DA, Kern JK, Geier MR. The biological basis of autism spectrum disorders: Understanding causation and treatment by clinical geneticists. Acta Neurobiol Exp (Wars) 2010;70:209–226. doi: 10.55782/ane-2010-1792. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves J, Baptista S, Martins T, Milhazes N, Borges F, Ribeiro CF, Malva JO, Silva AP. Methamphetamine-induced neuroinflammation and neuronal dysfunction in the mice hippocampus: preventive effect of indomethacin. Eur J Neurosci. 2010;31:315–326. doi: 10.1111/j.1460-9568.2009.07059.x. [DOI] [PubMed] [Google Scholar]
- 24.Hewitson L, Lopresti BJ, Stott C, Mason NS, Tomko J. Influence of pediatric vaccines on amygdala growth and opioid ligand binding in rhesus macaque infants: A pilot study. Acta Neurobiol Exp (Wars) 70:147–164. doi: 10.55782/ane-2010-1787. [DOI] [PubMed] [Google Scholar]
- 25.Hornig M, Chian D, Lipkin WI. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry. 2004;9:833–845. doi: 10.1038/sj.mp.4001529. [DOI] [PubMed] [Google Scholar]
- 26.Insel TR, Wang ZX, Ferris CF. Patterns of brain vasopressin receptor distribution associated with social organization in microtine rodents. J Neurosci. 1994;14:5381–5392. doi: 10.1523/JNEUROSCI.14-09-05381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, Theoharides TC. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern JK, Jones AM. Evidence of toxicity, oxidative stress, and neuronal insult in autism. J Toxicol Environ Health B Crit Rev. 2006;9:485–499. doi: 10.1080/10937400600882079. [DOI] [PubMed] [Google Scholar]
- 29.Kim SH, Johnson VJ, Sharma RP. Mercury inhibits nitric oxide production but activates proinflammatory cytokine expression in murine macrophage: differential modulation of NF-kappaB and p38 MAPK signaling pathways. Nitric Oxide. 2002;7:67–74. doi: 10.1016/s1089-8603(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 30.Kim SH, Johnson VJ, Sharma RP. Oral exposure to inorganic mercury alters T lymphocyte phenotypes and cytokine expression in BALB/c mice. Arch Toxicol. 2003;77:613–620. doi: 10.1007/s00204-003-0497-0. [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Bird BH, Glass GE. Sex differences in immune responses and viral shedding following Seoul virus infection in Norway rats. Am J Trop Med Hyg. 2001;65:57–63. doi: 10.4269/ajtmh.2001.65.57. [DOI] [PubMed] [Google Scholar]
- 32.Klein SL, Cernetich A, Hilmer S, Hoffman EP, Scott AL, Glass GE. Differential expression of immunoregulatory genes in male and female Norway rats following infection with Seoul virus. J Med Virol. 2004;74:180–190. doi: 10.1002/jmv.20163. [DOI] [PubMed] [Google Scholar]
- 33.Konigsberg M, Lopez-Diazguerrero NE, Bucio L, Gutierrez-Ruiz MC. Uncoupling effect of mercuric chloride on mitochondria isolated from an hepatic cell line. J Appl Toxicol. 2001;21:323–329. doi: 10.1002/jat.763. [DOI] [PubMed] [Google Scholar]
- 34.R.F. Laurente J, Avalos B, Chiquinta J, Ponce B, Avendano R, Maya L. Neurotoxic effects of thimerosal at vaccines doses on the encephalon and development in 7 days-old hamsters. An Fac Med Lima. 2007;68:222–237. [Google Scholar]
- 35.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci. 2009;29:263–267. doi: 10.1523/JNEUROSCI.4926-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melik-Parsadaniantz S, Rostene W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198:62–68. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 38.Monnet-Tschudi F, Zurich MG, Boschat C, Corbaz A, Honegger P. Involvement of environmental mercury and lead in the etiology of neurodegenerative diseases. Rev Environ Health. 2006;21:105–117. doi: 10.1515/reveh.2006.21.2.105. [DOI] [PubMed] [Google Scholar]
- 39.Mutter J, Curth A, Naumann J, Deth R, Walach H. Does inorganic mercury play a role in Alzheimer's disease? A systematic review and an integrated molecular mechanism. J Alzheimers Dis. 2010;22:357–374. doi: 10.3233/JAD-2010-100705. [DOI] [PubMed] [Google Scholar]
- 40.Mutter J, Naumann J, Schneider R, Walach H, Haley B. Mercury and autism: accelerating evidence? Neuro Endocrinol Lett. 2005;26:439–446. [PubMed] [Google Scholar]
- 41.Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noda M, Wataha JC, Lockwood PE, Volkmann KR, Kaga M, Sano H. Sublethal, 2-week exposures of dental material components alter TNF-alpha secretion of THP-1 monocytes. Dent Mater. 2003;19:101–105. doi: 10.1016/s0109-5641(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 43.Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- 44.Olczak M, Duszczyk M, Mierzejewski P, Meyza K, Majewska MD. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav Brain Res. 223:107–118. doi: 10.1016/j.bbr.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Pamphlett R, Slater M, Thomas S. Oxidative damage to nucleic acids in motor neurons containing mercury. J Neurol Sci. 1998;159:121–126. doi: 10.1016/s0022-510x(98)00161-0. [DOI] [PubMed] [Google Scholar]
- 46.Papp A, Nagymajtenyi L, Vezer T. Subchronic mercury treatment of rats in different phases of ontogenesis: functional effects on the central and peripheral nervous system. Food Chem Toxicol. 2005;43:77–85. doi: 10.1016/j.fct.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 48.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–983. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- 50.Roos A, Neeft M, Engelen L, Schilder-Tol EJ, Kunzendorf U, Weening JJ, Aten J. The immune dysregulatory compound mercuric chloride induces integrin-mediated T-lymphocyte adhesion. Immunology. 2001;102:31–38. doi: 10.1046/j.1365-2567.2001.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Exp Brain Res. 1997;117:428–436. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- 52.Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S. CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci. 2006;23:957–964. doi: 10.1111/j.1460-9568.2006.04631.x. [DOI] [PubMed] [Google Scholar]
- 53.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 54.Vezer T, Papp A, Kurunczi A, Parducz A, Naray M, Naggymajtenyi L. Behavioral and neurotoxic effects seen during and after subchronic exposure of rats to organic mercury. Environmental Toxicology and Pharmacology. 2005;19:785–796. doi: 10.1016/j.etap.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasutake A, Marumoto M, Yoshida M. Neurotoxic action of inorganic mercury injected in the intraventricular space of mouse cerebrum. J Toxicol Sci. 35:767–771. doi: 10.2131/jts.35.767. [DOI] [PubMed] [Google Scholar]
- 57.Young LJ, Pitkow LJ, Ferguson JN. Neuropeptides and social behavior: animal models relevant to autism. Mol Psychiatry. 2002;7(Suppl 2):S38–39. doi: 10.1038/sj.mp.4001175. [DOI] [PubMed] [Google Scholar]