Abstract
The protozoan parasite Trypanosoma cruzi, the etiologic agent of Chagas disease, is an obligate intracellular protozoan pathogen. Overlapping mechanisms ensure successful infection, yet the relationship between these cellular events and clinical disease remains obscure. This review explores the process of cell invasion from the perspective of cell surface interactions, intracellular signaling, modulation of the host cytoskeleton and endosomal compartment, and the intracellular innate immune response to infection.
Keywords: Trypanosoma cruzi, invasion, lysosome, microtubules, signaling
1. Introduction
The protozoan parasite Trypanosoma cruzi is the etiologic agent of Chagas disease, a disorder of poverty endemic to Central and South America. 10-16 million people are chronically infected with T. cruzi, with widely variable clinical sequelae, ranging from no disease (the majority) to an inflammatory cardiomyopathy and dilatation of the enteric viscera from denervation injury [1-3]. Chagas disease is emerging in North America in animals and humans, likely from the economic migration of infected individuals and extending range of the insect vector [4, 5]. The early diagnosis and treatment of Chagas disease remains a challenge for resource-poor nations, with the acute phase often passing undetected, and therapy during the chronic phase being largely supportive rather than curative [6, 7].
Typically, T. cruzi infection occurs when parasites excreted by the triatomine insect vector contaminate the bite wound or a mucous membrane. In non-endemic areas, transmission may occur congenitally, via blood transfusion or organ transplantation, or as a result of a laboratory accident [8]. Despite a century of scientific study, the relationship between the cell biology of the host-parasite relationship and the pathophysiology of Chagas disease remains incompletely understood. This review will explore the process of cell invasion with a focus on known cell surface interactions, review the evidence surrounding tissue tropism, explore the intracellular response to infection, and highlight several experimental unknowns and challenges.
T. cruzi has developed complex and redundant mechanisms to ensure successful cell invasion. We will examine the common features that underlie critical events involved in host cell infection by the parasite, with a focus on the trypomastigote. Considerable heterogeneity exists at each step of this process, and the specifics may vary with each unique combination of parasite strain, stage, and host cell. Therefore, the reader is cautioned not to apply a reductionistic viewpoint broadly. Further, the outcome of T. cruzi infection is highly heterogeneous across cell types. Aspects of cell invasion which vary across cell types include surface-surface interactions, enzymatic events, calcium-mediated signaling, trafficking of donor and host membranes, cytoskeletal contributions to parasite uptake and, finally, cytoplasmic entry via escape from the parasitophorous vacuole. Many excellent reviews from leaders in the field have been published on the mechanistic aspects underlying cell invasion [9-14].
2. Tissue Tropism
Infective metacyclic trypomastigotes inoculated into a wound generally infect local macrophages, fibroblasts, and other mesenchymal tissues at the site of primary infection, followed by hematogenous dissemination and stable infection of distant tissues [15] (Figure 1). Although the parasite is capable of infecting nearly any nucleated cells in vitro, a restricted tissue pool, involving cardiac and skeletal muscle and enteric nerves, develop apparent pathology [16]. It is tempting to conclude that the parasite has intrinsic tissue tropism and, indeed, this idea was initially established in classic work from Melo and Brener [17]. Chagas disease demonstrates geographically-restricted clinical profiles [18], lending support to the notion of strain-dependent tissue-specific tropism, and genetically distinct strains and clones can be isolated from patients with primary cardiac or gastrointestinal disease [19]. Further support for tissue tropism comes from the results of experimental infection employing two isolates of the parasite, in which one strain was found to preferentially localize to the heart, and the other to the gastrointestinal tract [20]. However, the rich genetic variation in parasite population clearly contributes to disease outcome [21], as does the host genetic background [22, 23]. A clear molecular or immunologic explanation for apparent tissue tropism is lacking and, at best, the hallmarks of clinical disease appear to result from a complex interplay between parasite and host genetic variation, inflammation, and immunity.
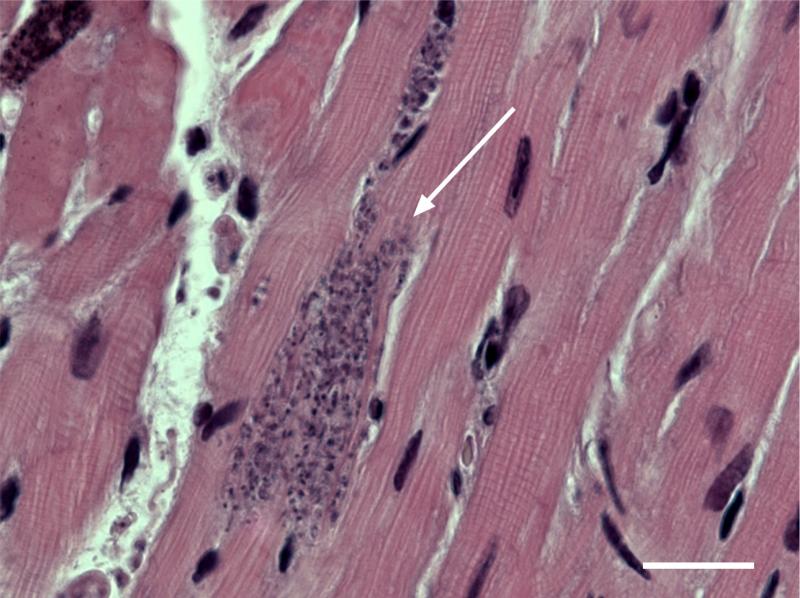
Figure 1.
Trypanosoma cruzi forms nests of intracellular parasites (white arrow) when it infects mammalian cells, especially cardiac and skeletal muscle. Shown here is an H&E stained section of a mouse heart demonstrating parasitosis of adjacent cardiac myocytes. Bar = 20 micrometers.
Interestingly, and somewhat surprisingly, given the extensive literature on interactions between host leukocytes and vascular endothelium, the method of parasite egress from the bloodstream into the tissues remains to be established. The parasite is capable of directly infecting the endothelium, and cardiac-specific studies of established Chagas heart disease demonstrate endothelial injury, inflammation, and microcirculatory compromise [24, 25]. The time-course for tissue dissemination is 7-10 days following inoculation [15], yet it is has not been established that parasites must first infect and lyse the vascular endothelium prior to spreading in the surrounding tissues. Alternatively, the parasite could engage in regulated transmigration/diapedesis, stimulate a cytotoxic or inflammatory injury resulting in breach of the permeability barrier, enter and exit the endothelial cell without establishing an infection, or escape via infected inflammatory cells acting as a Trojan horse. The rich complement of surface proteases suggest that enzymatic digestion between the endothelial cell and into the underlying connective tissues is likely a direct, parasite-driven processes. Indeed surface proteases, notably cruzipain [26, 27], play an important role in cellular infection, and almost certainly are fundamental to permit passage through the intact endothelium as well as the extracellular matrix. It has also been recognized that surface residue modifications through trans-sialidase also contribute to endothelial cell interactions [28]. Further studies are needed to specifically address this fundamental step in parasite dissemination through escape from the vascular compartment.
3. Cell Invasion
Interactions with host cells and the extracellular matrix occur through a large and diverse group of surface glycoproteins and proteases. Since the pioneering work of Dvorak and Hyde [29, 30], researchers have gained tremendous insight into the specific molecules involved during initial cell-cell interactions. Interestingly, many of the glycoproteins share the glycosylphosphatidylinositol (GPI) moiety. GPI-anchored proteins are first synthesized in the ER, conjugated to a GPI-anchor, and attached to the membrane as luminal facing proteins in the endoplasmic reticulum. In the Golgi, they undergo extensive sugar and side-chain modifications, and then fuse with the plasma membrane resulting in extracellular membrane-associated proteins [31, 32]. The structures and functions of these proteins are incredibly diverse, from adhesion, paracrine signaling, surface enzymes, and cell differentiation [33-35]. The GPI anchor confers several additional properties. First, enzymatic cleavage via glycosylphosphatidylinositol-specific phospholipase C (GPI-PLC) can release the head group, and is implicated in lipid and paracrine signaling, as well as signal termination [36, 37]. Additionally, GPI anchored proteins are thought to ubiquitously associate with, and in fact may help define, the lipid raft microdomain compartment [38] in other eukaryotic systems. Trypanosomes were recognized early as cells with abundant expression of GPI-anchored proteins [39], and these proteins form the classic VSG coat critical to immune evasion by T. brucei [40, 41]. Many GPI-anchored proteins of T. cruzi are involved in both the host response and macrophage infection, as reviewed in [42]. Given the differences in synthesis and side-chain modifications, the responsible enzymes are potential drug targets in the mammalian host.
The mechanisms and route of cell invasion vary greatly with the host cell type, and the reader is cautioned against broad generalizations across cell types. Unlike some infectious agents that rely on uptake and escape from professional phagocytic cells, T. cruzi trypomastigotes are capable of directly invading both professional phagocytes and nonphagocytic cells. Among the professional phagocytes, tissue resident macrophages are critical targets for early infection [43], where they initiate both a robust innate immunity and the systemic anti-parasite inflammatory response through epitope processing and presentation. Professional phagocytes have long been recognized both as necessary cellular targets and as a defense mechanisms for the host. Macrophages form the backbone of the infection models exploring the professional phagocytes-parasite interaction. These cells successfully harbor infection [44] yet limit their own infection [45], likely through oxidative burst-dependent killing [46], thus serving as important parasite reservoirs. Trypomastigotes specifically induce their uptake by professional phagocytes by engaging both TLR2 [47] and TLR 9-dependent pathways. The cellular mechanisms of phagocytosis have been well studied, and several reviews are suggested [48-51].
For infection of non-phagocytic cells, at least two major pathways have been characterized. The first relies upon a calcium-mediated signaling at the surface for lysosomal trafficking to provide donor membranes for the vacuole in a manner dependent upon actin polymerization and microtubules [52-55], while the second is a plasma membrane-mediated invagination involving PI3 kinase signaling and independent of actin polymerization [56-59]. While these observations form a core understanding of cell invasion, significant diversity, complexity, and redundancy in the process have emerged over the past two decades.
The capacity for cell invasion is not restricted to metacyclic or cell-derived trypomastigotes. Both the dividing amastigotes [60] and insect stage epimastigotes [61] are fully capable of establishing infections, and amastigotes are increasingly recognized to share comparable infectivity to trypomastigotes. Amastigote cell entry may follow more stereotyped pattern of cell invasion than the relatively diverse patterns noted between trypomastigotes and their targets. Unlike trypomastigotes, amastigotes invade in a Ca2+-dependnet manner insensitive to PI3 inhibition but involving both cAMP signaling and Ca2+-release from parasite acidocalcisomes. This has been modeled in HeLa cells [62] and MDCK cells, where infection with G-strain amastigotes demonstrate a role for Rac1-mediated cell invasion [63]. While this review will largely focus on pathways established in trypomastigotes, do not discount the important role for infective amastigotes in propagating the local spread of infection within tissues of the parasitized host.
4. Surface Interactions
At the outset, parasites must survive, gain access to the cell surface, and form stable attachments to host cells prior to entry. A cadre of protease-resistant surface glycoproteins either attach to matrix components, bind cell surface receptors, or possess proteolytic activity against matrix components. Many of these surface molecules serve as adhesion anchors, some enabling matrix destruction or ligand cleavage, others help with immune evasion, and others initiate bidirectional signaling events in the parasite and host cell. Nearly 50 percent of the T. cruzi genome is dedicated to encoding these surface proteins, broadly divided into several families: the gp63 surface proteases, the gp85/trans-sialidase superfamily (TS), the mucins, and the mucin-associated surface proteins [9, 64, 65]. A few of the prominent surface glycoproteins and, if known, their ligands, together with selected (not comprehensive) references are shown (Table 1). A seminal example is the role of sialic acid in parasite virulence [66]. Surface mucins, a subset of the GPI-linked proteins, are modified by sialic acid scavenged from the host through the action of the trans-sialidase [67], as the parasite lacks the ability to produce these modifications directly. These sugar-modified residues, have been demonstrated to have critical roles in cell attachment, invasion, and replication [68]. Other surface sugar residues upon glycoproteins, notably mannose and galactose, also figure prominently in the interaction and infection of host cells [69-71]. Many of the surface glycoproteins impact invasion or serve as virulence factors, since deletion, disruption of their enzymatic activity, or blockade of the receptor-ligand interaction, usually reduces cell invasion in vitro and improves outcome of infection in vivo. The extent and diversity of these surface protein families cannot be overemphasized. Despite decades of study, a unified single invasion mechanism has not emerged. Rather, a series of redundant and overlapping mechanisms, varying with the parasite-host strain-strain combination, have been reported [9]. This diversity likely contributes to the co-evolutionary success of this parasite in the infection of vertebrate cells and tissues.
Table 1.
Surface glycoproteins of T. cruzi with extracellular matrix binding or proteolytic activity
| Protein | Host Target or Ligand | Biology | Reference(s) |
|---|---|---|---|
| gp82 | mucin, unknown surface ligand | binding, signaling | [138] |
| gp63 | fibronectin, laminin | ECM protease, binding | [139] |
| Penetrin (gp60) | heparan, heparan sulfate, collagen | binding | [139] |
| Tc-85/ gp85/TS | fibronectin, laminin, cytokeratin 18 | binding, retention | [140, 141] |
| gp35/50 | mucins | binding, signaling | [142] |
| gp90 | unknown | inhibitor of invasion, signaling | [143] |
| gp30 | unknown | binding | [79] |
| Mucins/Trans-sialidase | 2,3-sialyl containing host surface glycoproteins (galectin-3) | sialidase, secreted (SAPA) immunogen | [144, 145] |
| Mucin p45 | unknown | cardiac myocyte binding | [146] |
| gp83 | unknown | sialidase, Ca signaling | [147] |
| Cruzipain | bradykinin | cysteine proteases | [148, 149] |
| POP Tc80 serine protease | collagen I, IV, fibronectin | ECM protease | [150, 151] |
The importance of the plasma membrane lipid environment is rapidly gaining attention. Specialized regions, the lipid microdomains/rafts, coordinate and regulate signaling events through temporal-spatial organization of proteins. The kinetoplastids are no exception, and GPI-anchored proteins are known to cluster in lipid rafts in this family [72]. The host-parasite signaling event likely depends upon surface-surface events coordinated through lipid rafts, and indeed, cholesterol scavengers, which impair membrane fluidity and raft lateral reorganization, also impair cell invasion [73, 74].
5. Intracellular Signaling and Calcium
The end result of host-parasite surface interaction is triggering of bidirectional (host and parasite) signaling cascades which initiate the invasion event. After extracellular matrix proteolysis and surface binding through robust and redundant mechanisms, the parasite initiates a bidirectional calcium signaling cascade. This event can also be trigged in a cell-free system by isolated membrane components or parasite lysate [52, 75]. This calcium signaling is fundamental to the downstream signaling cascade, which ends with the parasite encased in an acidic parasitophorous vacuole [13, 76]. Some of the major signaling events downstream of these surface receptors in the host and parasite trypomastigotes are tabulated (Table 2).
Table 2.
Signaling properties of selected T. cruzi surface glycoproteins
| Protein | Biology | Reference |
|---|---|---|
| gp82 | parasite: Ca increase, PLC dependent tyrosine phosphorylation of Tc-p175 | [144, 152] |
| gp83 | parasite: Ca increase Host: MAPK signaling | [36, 153] |
| gp30 | parasite: Ca increase | [79] |
| gp35/50 | host and parasite cAMP and calcium increase | [154-156] |
| gp90 | phosphatase, ? downregulates gp82 signaling | [157] |
| cruzipain | bradykinin signaling, calcium increase, kinin generation | [86] |
| oligopeptidase B | cytosolic, cleaves a 120 kDa substrate, secreted, direct calcium release | [91, 158] |
The precise molecular mechanisms leading to host and parasite intracellular calcium release remain unknown. In the parasite, at least two pathways have been identified. In metacyclic trypomastigotes, the engagement of gp82 with an unknown ligand triggers a cascade in the parasite involving tyrosine phosphorylation of p175 [77], the serine protein kinase C, and IP3-medated release of ER calcium stores [78]. An alternative, overlapping pathway occurs upon gp30 activation [79]. Another major pathway, mediated by gp35/50 binding to an unknown ligand, induces calcium release from acidocalcisomes through adenylate cyclase and a rise in cAMP [11, 13], a pathway shared during amastigote invasion. The protein tyrosine phosphatase gp90 is a negative regulator of invasion [14, 80-82]. Additionally, TGF-β and integrin signaling on host cells have been implicated in the invasion process, as have toll-like receptors (TLR2 and 9) [83-85], and the nerve growth factor receptor TrkA has been identified to bind to a trans-sialidase [14]. Signaling in the host cell is even less well characterized. The generation of kinins by cruzipain results in bradykinin receptor (B2R)-mediated signaling through PLC and IP3-kinase to release ER-bound calcium, opposed by the actions of the kininases (angiotensin converting enzyme-ACE) [14, 86, 87]. Evidence suggests that the anti-inflammatory properties of ACE inhibition is useful to modulate cardiac inflammation in Chagas [87, 88], as it does in models of experimental autoimmune myocarditis [89]. Surface signaling through other bradykinin receptors (B1R) by the actions of kininase I, support invasion [90], and the action of oligopeptidase B on its substrate is thought to generate an agonist for host cell calcium release through adenylate cyclase and phospholipase C [91]. Additional receptors are proposed for ligand interactions with TS/Tc85, as well as additional substrates for cruzipain and chagasin, which interface with downstream signaling in both the lysosome-dependent and independent pathways. Scharfstein and Lima recently published a detailed review on the subject of the cysteine proteases, cruzipain, and protease inhibitors [92]. MAPK pathways have also been implicated in macrophages through gp83 signaling [36]. Alternative pathways for amastigote involving calcium release from acidocalcisomes in a PI3 insensitive manner have already been described above [62].
6. Host Membranes and the Parasite Vacuole
The classic model for parasite entry is based upon the rapid recruitment of lysosomes to the parasite attachment point [93] in a manner dependent upon microtubules and kinesin motors [94]. Host cell plasma membrane and lysosomes have been assumed to be the donor membranes necessary for vacuole formation, and inhibition of membrane fusion, vesicle trafficking, microtubule reorganization, molecular motors, or calcium/cAMP signaling impairs successful invasion. This vesicle-dependent pathway has been shown to be sensitive to wortmannin, a PI3 kinase inhibitor, known to involve G-protein coupled receptors, and depend upon synaptotagmin-VII [56, 95, 96]. The precise characterization and sources of these donor membranes have become more diverse with further investigation, including early and late endosomes [56, 97], involvement of dynamin and Rab5 [97, 98], and, recently, the autophagocytic pathway [99]. Localized alterations in calcium concentration are known signals for both microtubule-dependent lysosomal trafficking and fusion [100, 101]. More recently, this classic pathway was usurped by a dominant alternative, a direct invagination of the plasma membrane at the site of attachment in a wortmannin-insensitive and lysosome-independent [102] manner. However, lysosomal fusion is thought to remain fundamental for a productive infection to occur through vacuole acidification [102], and thus these diverse results represent further insight into the components of the maturing parasitophorous vacuole. While the precise molecular events that lead to successful invasion have yet to be elucidated, the overarching theme is one of parasite entry through surface-initiated signaling leading to a bi-directional rise in intracellular calcium, causing reorganization, trafficking, and fusion of selected donor membranes along the host cytoskeleton to the site of membrane attachment and invagination.
7. Host Cytoskeleton
The host cytoskeleton is critical for successful invasion. Host cells are encased in an actin corset parallel to the inner membrane. Calcium-mediated actin de-polymerization likely facilitates initial parasite entry and negatively impacts parasite retention [53, 56, 103]. The specific role of actin polymerization appears to depend on the specific cell type and parasite stage examined, with cytochalasin D treatment enhancing trypomastigote, yet impairing amastigote, invasion [104]. A host of actin-associated elements has been identified, including intermediate filaments, myosin-associated components, integrins, and extracellular matrix components, as noted in a recent review [105]. The Rho/Rac family of small GTPases is known to be a critical link between surface signaling and changes in the underlying cytoskeleton. However, evidence suggests that trypomastigotes do not rely on this family for productive infection. In contrast, the invasion mechanism employed by amastigotes does depend upon Rac1 signaling, again highlighting the diversity of cell invasion [63, 106]. Members of the Rab family of GTPases, necessary for endosomal compartment trafficking, are essential for infection [97]. The many components of the endosomal compartment (early, late, lysosomal, and autophagocytic) traffic along microtubules, which are necessary for infection. Evidence suggests that parasite entry itself may serve as a nucleation point for microtubule radiation from the parasitophorous vacuole membrane, further facilitating endosomal attraction initiated by calcium flux [107]. The relationship between apparent parasite microtubule nucleation and lysosomal attraction is unknown, nor is it understood if the parasite stimulates this organization, or if this represents part of the host response to invasion. It remains possible that the forming vacuole is somehow attractive for γ-tubulin but that the vacuole simply “sticks” to microtubules in the vicinity and that lysosome fusion is a relatively passive rather than an active process.
8. Cytoplasmic Entry and Parasite Differentiation
Now encased in the acidic parasitophorous vacuole (Figure 2), parasite protection is offered by surface trans-sialidases [108], which also serve to facilitate parasite maturation and release [109]. There is clear evidence that the parasite breaks down the vacuolar membrane to facilitate cytoplasmic entry [110]. The mechanism for vacuolar escape is known to be lysosome and pH dependent [111]. Early reports indicated that secretion of a porin-like/complement 9-related factor TcTOX [112], and later a lytic factor LYT1 [113, 114] were critical for this final step in cell invasion. While TcTOX has defined further molecular characterization, LYT1 null mutants [113] demonstrate markedly attenuated infectivity [115]. At the acidic pH of the vacuole achieved through lysosomal fusion, LYT1 and/or TcTOX, are expressed and assume conformations capable of promoting membrane lysis to permit cytoplasmic entry. The invasive trypomastigote thus functions as a loaded weapon, and, teleologically, has completed its task to achieve successful invasion.
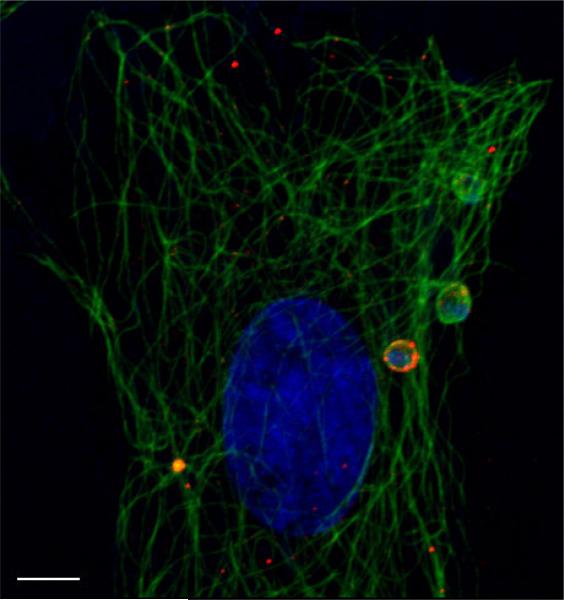
Figure 2.
T. cruzi interacts with the host cytoskeleton during cell invasion. Shown here are H9C2 rat cardiomyoblasts during invasion with Trypanosoma cruzi. Host and parasite DNA in blue (DAPI) host lysosomes in red, host α-tubulin in green. Bar = 5 micrometers
As a digenetic organism, T. cruzi follows a differentiation continuum from insect vector to mammalian host and back again. The epimastigote differentiates into the metacyclic trypomastigote in the insect hindgut and, once introduced into the host, differentiates into the replicative intracellular amastigote. These ultimately differentiate again into bloodform trypomastigotes, which lyse out of the cell. Both the amastigote and trypomastigote forms are capable of propagating the local and metastatic infection. Transient intermediate forms are thought to develop during the differentiation process from amastigotes to trypomastigotes and appear in the mammalian host with the general morphology of epimastigotes. [54, 116]. Of specific interest here are the factors regulating intracellular differentiation. Most notably is the acidic pH achieved in the vacuole. This environment initiates the differentiation program into the amastigote over a period of several (2-8) hours. In vitro the parasite spontaneously undergoes differentiation if placed in an acidic environment [117]. The replication of amastigotes also demonstrates an absolute requirement for L-proline [116], and the activity of phosphatases may be required for differentiation as well [118]. After a period of quiescence, the amastigotes reenter the cell cycle and undergo nine rounds of replication prior to further differentiation (or perhaps considered de-differentiation) into motile trypomastigotes [10, 59]. Interestingly, this process of invasion, infection, and replication will occur even in cells stripped of their nuclei, indicating that new host gene transcription is not necessary [119]. The trypomastigotes destroy the host cell by unclear mechanisms, although evidence does not support apoptotic cell death [120]. After cytolysis, the infection cycle begins again for new host cell targets or uptake by a naïve triatomine taking a bloodmeal.
The transcriptional events in the trypanosome that regulate these critical differentiation steps are poorly understood, and current dogma dictates that virtually all regulation occurs after transcription and trans-splicing. Thus message stability, transcription initiation, and post-transcriptional processing are critical events for the kinetoplastids, which generate polycistronic transcripts at a relatively constant rate, and control gene dosage largely through genomic copy amplification [121-123]. Not surprising, proteosome activity is known to be essential for degrading stage-specific proteins during the cytoskeletal remodeling that occurs during the transformation from trypomastigotes to amastigotes [124]. Several T. cruzi-specific proteases and other enzymes have been identified in the differentiation event [125, 126], but the upstream signals remain largely unknown. Notably, at the transcriptional level, evidence suggests down-regulation of RNA polymerases I and II occurs upon differentiation from proliferative to nonproliferative forms [127]. Additionally, stage-specific regulation of histone and ubiquitin genes has been reported [128, 129]. Overall, identifying and understanding changes in gene transcription, splicing, mRNA stability, and translational events governing differentiation and replication are incompletely understood and will benefit from additional study to develop specific therapies targeting against T. cruzi.
9. Host Response
Successful intracellular pathogens often co-opt the very cellular self-preservation mechanisms designed to thwart parasitism. T. cruzi has developed mechanisms of evading the immune responses and suppressing host apoptosis by modulating the expression of host cell surface receptors, secreted factors, and signaling molecules. The pathogenesis of Chagas disease, and the relative contributions of the parasite, inflammation, and autoimmunity remains a matter of much investigation and debate, and is beyond the scope of this review.
In addition to protective, anti-parasite immunity, individuals with Chagas disease develop aberrant, potentially deleterious immune responses. Notably, invasion with the parasite triggers a type I interferon response, known to be critical in during intracellular invasion from bacteria and virus (a foreign protein/DNA/RNA response), which may drive a local immune and autoimmune response [130]. An ancient cellular response to infection, termed the IFN-stimulatory DNA response (ISD), may be a key player in the local and adaptive immune response [131]. Both TLR2 and TLR9 mediated innate immune response from presentation of T. cruzi methylated CpG antigens [132] are involved (reviewed in [133]. Detailed reviews of the TLR-dependent and independent pathways are noteworthy [134], as well as the role for innate immunity clinical disease persistence [135]. In experimental models, dysregulation of several components of the type I interferon response, notably the members of the IFN regulatory factors (1-4) and their cognate binding proteins, drive spontaneous autoimmunity [136]. Stetson et al. found that deletion of the negative regulator Trex1, which serves to downregulate the ISD response, resulting in spontaneous cardiac autoimmunity [137]. It is intriguing to surmise that the cardiac autoimmunity observed in Chagas was the product of an imbalanced IFN response or failure to reset the inflammatory response even after parasite clearance. Further study will be necessary to understand the involvement of the ISD response as a driver of both adaptive immunity and the resultant cardiac autoimmunity.
10. Perspective
Understanding the cellular interaction between parasite and host and the host immune response are fundamental to treating parasitosis and preventing progression to Chagas disease. Decades of research have revealed an incredibly rich surface proteome that confers some degree of tissue specificity while retaining broad plasticity for cellular invasion. Pathways characterizing the upstream and downstream signaling events mediating cell invasion are partially understood at best, and many critical steps lack ligand-receptor pairing. With the developments in advanced proteomic and lipidomic analysis, the time is right to dissect the surface interactions between host and parasite. Our understanding of parasite differentiation, from trypomastigote to amastigote and back, remains incomplete, and detailed investigation into parasite transcriptional and transcriptional regulation underlying cell differentiation is likely to yield important insights. Finally, the emerging role of innate cellular immunity both in facilitating effective cell invasion and differentiation, and perhaps in affording resistance to overwhelming infection, is just now beginning to be investigated.
Acknowledgments
CLE and DME were supported in part by grants from the United States Public Health Service.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coura JR, Dias JC. Epidemiology, control and surveillance of Chagas disease--100 years after its discovery. Mem Inst Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- 2.Coura JR. Chagas disease: what is known and what is needed--a background article. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):113–122. doi: 10.1590/s0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- 3.Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz. 2009;104(Suppl 1):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- 4.Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. Prognostic impact of Chagas disease in the United States. Am Heart J. 2009;157:22–29. doi: 10.1016/j.ahj.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Schmunis GA. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102(Suppl 1):75–85. doi: 10.1590/s0074-02762007005000093. [DOI] [PubMed] [Google Scholar]
- 6.Rassi A, Jr., Dias JC, Marin-Neto JA, Rassi A. Challenges and opportunities for primary, secondary, and tertiary prevention of Chagas’ disease. Heart. 2009;95:524–534. doi: 10.1136/hrt.2008.159624. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Molina JA, Perez-Ayala A, Moreno S, Fernandez-Gonzalez MC, Zamora J, Lopez-Velez R. Use of benznidazole to treat chronic Chagas’ disease: a systematic review with a meta-analysis. J Antimicrob Chemother. 2009;64:1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez FR, Guedes PM, Gazzinelli RT, Silva JS. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 9.Alves MJ, Colli W. Role of the gp85/trans-sialidase superfamily of glycoproteins in the interaction of Trypanosoma cruzi with host structures. Subcell Biochem. 2008;47:58–69. doi: 10.1007/978-0-387-78267-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Alves MJ, Colli W. Trypanosoma cruzi: adhesion to the host cell and intracellular survival. IUBMB Life. 2007;59:274–279. doi: 10.1080/15216540701200084. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida N, Cortez M. Trypanosoma cruzi: parasite and host cell signaling during the invasion process. Subcell Biochem. 2008;47:82–91. doi: 10.1007/978-0-387-78267-6_6. [DOI] [PubMed] [Google Scholar]
- 12.Mott GA, Burleigh BA. The role of host cell lysosomes in Trypanosoma cruzi invasion. Subcell Biochem. 2008;47:165–173. doi: 10.1007/978-0-387-78267-6_13. [DOI] [PubMed] [Google Scholar]
- 13.Mortara RA, Andreoli WK, Taniwaki NN, Fernandes AB, Silva CV, Fernandes MC, L'Abbate C, Silva S. Mammalian cell invasion and intracellular trafficking by Trypanosoma cruzi infective forms. An Acad Bras Cienc. 2005;77:77–94. doi: 10.1590/s0001-37652005000100006. [DOI] [PubMed] [Google Scholar]
- 14.Villalta F, Scharfstein J, Ashton AW, Tyler KM, Guan F, Mukherjee S, Lima MF, Alvarez S, Weiss LM, Huang H, Machado FS, Tanowitz HB. Perspectives on the Trypanosoma cruzi-host cell receptor interactions. Parasitol Res. 2009;104:1251–1260. doi: 10.1007/s00436-009-1383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteon VM, Furuzawa-Carballeda J, Alejandre-Aguilar R, Aranda-Fraustro A, Rosales-Encina JL, Reyes PA. American trypanosomosis: in situ and generalized features of parasitism and inflammation kinetics in a murine model. Exp Parasitol. 1996;83:267–274. doi: 10.1006/expr.1996.0074. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. The Journal of Infectious Diseases. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 17.Melo RC, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64:475–482. [PubMed] [Google Scholar]
- 18.Macedo AM, Machado CR, Oliveira RP, Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- 19.Vago AR, Andrade LO, Leite AA, d'Avila Reis D, Macedo AM, Adad SJ, Tostes S, Jr., Moreira MC, Filho GB, Pena SD. Genetic characterization of Trypanosoma cruzi directly from tissues of patients with chronic Chagas disease: differential distribution of genetic types into diverse organs. Am J Pathol. 2000;156:1805–1809. doi: 10.1016/s0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade LO, Machado CR, Chiari E, Pena SD, Macedo AM. Differential tissue distribution of diverse clones of Trypanosoma cruzi in infected mice. Mol Biochem Parasitol. 1999;100:163–172. doi: 10.1016/s0166-6851(99)90035-x. [DOI] [PubMed] [Google Scholar]
- 21.Manoel-Caetano Fda S, Silva AE. Implications of genetic variability of Trypanosoma cruzi for the pathogenesis of Chagas disease. Cad Saude Publica. 2007;23:2263–2274. doi: 10.1590/s0102-311x2007001000002. [DOI] [PubMed] [Google Scholar]
- 22.Marinho CR, Bucci DZ, Dagli ML, Bastos KR, Grisotto MG, Sardinha LR, Baptista CR, Goncalves CP, Lima MR, Alvarez JM. Pathology affects different organs in two mouse strains chronically infected by a Trypanosoma cruzi clone: a model for genetic studies of Chagas’ disease. Infect Immun. 2004;72:2350–2357. doi: 10.1128/IAI.72.4.2350-2357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitas JM, Andrade LO, Pires SF, Lima R, Chiari E, Santos RR, Soares M, Machado CR, Franco GR, Pena SD, Macedo AM. The MHC gene region of murine hosts influences the differential tissue tropism of infecting Trypanosoma cruzi strains. PLoS One. 2009;4:e5113. doi: 10.1371/journal.pone.0005113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Factor SM, Cho S, Wittner M, Tanowitz H. Abnormalities of the coronary microcirculation in acute murine Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- 25.Tanowitz HB, Kaul DK, Chen B, Morris SA, Factor SM, Weiss LM, Wittner M. Compromised microcirculation in acute murine Trypanosoma cruzi infection. J Parasitol. 1996;82:124–130. [PubMed] [Google Scholar]
- 26.Stoka V, Nycander M, Lenarcic B, Labriola C, Cazzulo JJ, Bjork I, Turk V. Inhibition of cruzipain, the major cysteine proteinase of the protozoan parasite, Trypanosoma cruzi, by proteinase inhibitors of the cystatin superfamily. FEBS Letters. 1995;370:101–104. doi: 10.1016/0014-5793(95)00798-e. [DOI] [PubMed] [Google Scholar]
- 27.McGrath ME, Eakin AE, Engel JC, McKerrow JH, Craik CS, Fletterick RJ. The crystal structure of cruzain: a therapeutic target for Chagas’ disease. Journal of Molecular Biology. 1995;247:251–259. doi: 10.1006/jmbi.1994.0137. [DOI] [PubMed] [Google Scholar]
- 28.Dias WB, Fajardo FD, Graca-Souza AV, Freire-de-Lima L, Vieira F, Girard MF, Bouteille B, Previato JO, Mendonca-Previato L, Todeschini AR. Endothelial cell signalling induced by trans-sialidase from Trypanosoma cruzi. Cell Microbiol. 2008;10:88–99. doi: 10.1111/j.1462-5822.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak JA, Hyde TP. Trypanosoma cruzi: Interaction with vertebrate cells in vitro. I. Individual interactions at the cellular and subcellular levels. Experimental Parasitology. 1973;34:268–283. doi: 10.1016/0014-4894(73)90087-8. [DOI] [PubMed] [Google Scholar]
- 30.Hyde TP, Dvorak JA. Trypanosoma cruzi: interaction with vertebrate cells in vitro. 2. Quantitative analysis of the penetration phase. Exp Parasitol. 1973;34:284–294. doi: 10.1016/0014-4894(73)90088-x. [DOI] [PubMed] [Google Scholar]
- 31.Brown D, Waneck GL. Glycosyl-phosphatidylinositol-anchored membrane proteins. J Am Soc Nephrol. 1992;3:895–906. doi: 10.1681/ASN.V34895. [DOI] [PubMed] [Google Scholar]
- 32.Tiede A, Bastisch I, Schubert J, Orlean P, Schmidt RE. Biosynthesis of glycosylphosphatidylinositols in mammals and unicellular microbes. Biol Chem. 1999;380:503–523. doi: 10.1515/BC.1999.066. [DOI] [PubMed] [Google Scholar]
- 33.DosReis GA, Pecanha LM, Bellio M, Previato JO, Mendonca-Previato L. Glycoinositol phospholipids from Trypanosoma cruzi transmit signals to the cells of the host immune system through both ceramide and glycan chains. Microbes & Infection. 2002;4:1007–1013. doi: 10.1016/s1286-4579(02)01616-7. [DOI] [PubMed] [Google Scholar]
- 34.Fujita M, Jigami Y. Lipid remodeling of GPI-anchored proteins and its function. Biochim Biophys Acta. 2008;1780:410–420. doi: 10.1016/j.bbagen.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Zacks MA, Garg N. Recent developments in the molecular, biochemical and functional characterization of GPI8 and the GPI-anchoring mechanism. Mol Membr Biol. 2006;23:209–225. doi: 10.1080/09687860600601494. [DOI] [PubMed] [Google Scholar]
- 36.Villalta F, Zhang Y, Bibb KE, Burns JM, Jr., Lima MF. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through the MAP kinase pathway. Biochem Biophys Res Commun. 1998;249:247–252. doi: 10.1006/bbrc.1998.9127. [DOI] [PubMed] [Google Scholar]
- 37.Gaulton GN, Pratt JC. Glycosylated phosphatidylinositol molecules as second messengers. Semin Immunol. 1994;6:97–104. doi: 10.1006/smim.1994.1014. [DOI] [PubMed] [Google Scholar]
- 38.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field MC, Medina-Acosta E, Cross GA. Characterization of a glycosylphosphatidylinositol membrane protein anchor precursor in Leishmania mexicana. Mol. Biochem. Parasitol. 1991;48:227–229. doi: 10.1016/0166-6851(91)90118-p. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson MA, Brimacombe JS, Cottaz S, Field RA, Guther LS, Homans SW, McConville MJ, Mehlert A, Milne KG, Ralton JE, et al. Glycosyl-phosphatidylinositol molecules of the parasite and the host. Parasitology. 1994;108(Suppl):S45–54. doi: 10.1017/s0031182000075715. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson MA, Cross GMA. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J. Biol. Chem. 1984;259:3011–3015. [PubMed] [Google Scholar]
- 42.Ropert C, Ferreira LR, Campos MA, Procopio DO, Travassos LR, Ferguson MA, Reis LF, Teixeira MM, Almeida IC, Gazzinelli RT. Macrophage signaling by glycosylphosphatidylinositol-anchored mucin-like glycoproteins derived from Trypanosoma cruzi trypomastigotes. Microbes & Infection. 2002;4:1015–1025. doi: 10.1016/s1286-4579(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Fernandez MA, Fernandez MA, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur. J. Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 44.Tanowitz H, Wittner M, Kress Y, Bloom B. Studies of in vitro infection by Trypanosoma cruzi. I. Ultrastructural studies on the invasion of macrophages and L-cells. American Journal of Tropical Medicine and Hygiene. 1975;24:25–33. doi: 10.4269/ajtmh.1975.24.25. [DOI] [PubMed] [Google Scholar]
- 45.McCabe RE, Remington JS, Araujo FG. Mechanisms of invasion and replication of the intracellular stage in Trypanosoma cruzi. Infection and Immunity. 1984;46:372–376. doi: 10.1128/iai.46.2.372-376.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bogdan C, Rollinghoff M. How do protozoan parasites survive inside macrophages? Parasitol Today. 1999;15:22–28. doi: 10.1016/s0169-4758(98)01362-3. [DOI] [PubMed] [Google Scholar]
- 47.Maganto-Garcia E, Punzon C, Terhorst C, Fresno M. Rab5 activation by Toll-like receptor 2 is required for Trypanosoma cruzi internalization and replication in macrophages. Traffic. 2008;9:1299–1315. doi: 10.1111/j.1600-0854.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mauel J. In vitro induction of intracellular killing of parasitic protozoa by macrophages. Immunobiology. 1982;161:392–400. doi: 10.1016/S0171-2985(82)80097-1. [DOI] [PubMed] [Google Scholar]
- 49.Thorne KJ, Blackwell JM. Cell-mediated killing of protozoa. Advances in Parasitology. 1983;22:43–151. doi: 10.1016/s0065-308x(08)60461-3. [DOI] [PubMed] [Google Scholar]
- 50.De Araujo-Jorge TC, Barbosa HS, Meirelles MN. Trypanosoma cruzi recognition by macrophages and muscle cells: perspectives after a 15-year study. Memorias do Instituto Oswaldo Cruz. 1992;87(Suppl 5):43–56. doi: 10.1590/s0074-02761992000900006. [DOI] [PubMed] [Google Scholar]
- 51.Kuhn RE. Macrophages in experimental Chagas’ disease. Immunology Series. 1994;60:495–502. [PubMed] [Google Scholar]
- 52.Tardieux I, Nathanson MH, Andrews NW. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic-free Ca2+ transients. J. Exp. Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tardieux I, Webster P, Ravesloot J, Boron W, Lunn JA, Heuser JE, Andrews NW. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- 54.Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int. J. Parasitol. 2001;31:472–481. doi: 10.1016/s0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 55.Schenkman S, Robbins ES, Nussenzweig V. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect. Immun. 1991;59:645–654. doi: 10.1128/iai.59.2.645-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woolsey AM, Sunwoo L, Petersen CA, Brachmann SM, Cantley LC, Burleigh BA. Novel PI 3-kinase-dependent mechanisms of trypanosome invasion and vacuole maturation. J. Cell Sci. 2003;116:3611–3622. doi: 10.1242/jcs.00666. [DOI] [PubMed] [Google Scholar]
- 57.Burleigh BA. Host cell signaling and Trypanosoma cruzi invasion: do all roads lead to lysosomes? Sci STKE. 20052005:e36. doi: 10.1126/stke.2932005pe36. [DOI] [PubMed] [Google Scholar]
- 58.De Souza W. Basic cell biology of Trypanosoma cruzi. Current Pharmaceutical Design. 2002;8:269–285. doi: 10.2174/1381612023396276. [DOI] [PubMed] [Google Scholar]
- 59.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 60.Mortara RA, Procopio DO, Barros HC, Verbisck NV, Andreoli WK, Silva RB, da Silva S. Features of host cell invasion by different infective forms of Trypanosoma cruzi. Memorias do Instituto Oswaldo Cruz. 1999;94(Suppl 1):135–137. doi: 10.1590/s0074-02761999000700014. [DOI] [PubMed] [Google Scholar]
- 61.Florencio-Martinez L, Marquez-Duenas C, Ballesteros-Rodea G, Martinez-Calvillo S, Manning-Cela R. Cellular analysis of host cell infection by different developmental stages of Trypanosoma cruzi. Experimental Parasitology. 2010 doi: 10.1016/j.exppara.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Fernandes AB, Neira I, Ferreira AT, Mortara RA. Cell invasion by Trypanosoma cruzi amastigotes of distinct infectivities: studies on signaling pathways. Parasitology Research. 2006;100:59–68. doi: 10.1007/s00436-006-0236-6. [DOI] [PubMed] [Google Scholar]
- 63.Fernandes AB, Mortara RA. Invasion of MDCK epithelial cells with altered expression of Rho GTPases by Trypanosoma cruzi amastigotes and metacyclic trypomastigotes of strains from the two major phylogenetic lineages. Microbes Infect. 2004;6:460–467. doi: 10.1016/j.micinf.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Kulkarni MM, Olson CL, Engman DM, McGwire BS. Trypanosoma cruzi GP63 proteins undergo stage-specific differential posttranslational modification and are important for host cell infection. Infection and Immunity. 2009;77:2193–2200. doi: 10.1128/IAI.01542-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, Aggarwal G, Tran AN, Ghedin E, Worthey EA, Delcher AL, Blandin G, Westenberger SJ, Caler E, Cerqueira GC, Branche C, Haas B, Anupama A, Arner E, Aslund L, Attipoe P, Bontempi E, Bringaud F, Burton P, Cadag E, Campbell DA, Carrington M, Crabtree J, Darban H, da Silveira JF, de Jong P, Edwards K, Englund PT, Fazelina G, Feldblyum T, Ferella M, Frasch AC, Gull K, Horn D, Hou L, Huang Y, Kindlund E, Klingbeil M, Kluge S, Koo H, Lacerda D, Levin MJ, Lorenzi H, Louie T, Machado CR, McCulloch R, McKenna A, Mizuno Y, Mottram JC, Nelson S, Ochaya S, Osoegawa K, Pai G, Parsons M, Pentony M, Pettersson U, Pop M, Ramirez JL, Rinta J, Robertson L, Salzberg SL, Sanchez DO, Seyler A, Sharma R, Shetty J, Simpson AJ, Sisk E, Tammi MT, Tarleton R, Teixeira S, Van Aken S, Vogt C, Ward PN, Wickstead B, Wortman J, White O, Fraser CM, Stuart KD, Andersson B. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 66.de Titto EH, Araujo FG. Mechanism of cell invasion by Trypanosoma cruzi: importance of sialidase activity. Acta Tropica. 1987;44:273–282. [PubMed] [Google Scholar]
- 67.Jacobs T, Erdmann H, Fleischer B. Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi. Eur J Cell Biol. 2010;89:113–116. doi: 10.1016/j.ejcb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Muia RP, Yu H, Prescher JA, Hellman U, Chen X, Bertozzi CR, Campetella O. Identification of glycoproteins targeted by Trypanosoma cruzi trans-sialidase, a virulence factor that disturbs lymphocyte glycosylation. Glycobiology. 2010 doi: 10.1093/glycob/cwq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snary D. Receptors and recognition mechanisms of Trypanosoma cruzi. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1985;79:587–590. doi: 10.1016/0035-9203(85)90163-4. [DOI] [PubMed] [Google Scholar]
- 70.Villalta F, Kierszenbaum F. Host cell invasion by Trypanosoma cruzi: role of cell surface galactose residues. Biochemical and Biophysical Research Communications. 1984;119:228–235. doi: 10.1016/0006-291x(84)91642-5. [DOI] [PubMed] [Google Scholar]
- 71.Villalta F, Kierszenbaum F. Role of cell surface mannose residues in host cell invasion by Trypanosoma cruzi. Biochimica et Biophysica Acta. 1983;736:39–44. doi: 10.1016/0005-2736(83)90167-0. [DOI] [PubMed] [Google Scholar]
- 72.Denny PW, Field MC, Smith DF. GPI-anchored proteins and glycoconjugates segregate into lipid rafts in Kinetoplastida. FEBS Lett. 2001;491:148–153. doi: 10.1016/s0014-5793(01)02172-x. [DOI] [PubMed] [Google Scholar]
- 73.Fernandes MC, Cortez M, Geraldo Yoneyama KA, Straus AH, Yoshida N, Mortara RA. Novel strategy in Trypanosoma cruzi cell invasion: implication of cholesterol and host cell microdomains. Int J Parasitol. 2007;37:1431–1441. doi: 10.1016/j.ijpara.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 74.Barrias ES, Dutra JM, De Souza W, Carvalho TM. Participation of macrophage membrane rafts in Trypanosoma cruzi invasion process. Biochem Biophys Res Commun. 2007;363:828–834. doi: 10.1016/j.bbrc.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez A, Rioult MG, Ora A, Andrews NW. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burleigh BA, Andrews NW. Signaling and host cell invasion by Trypanosoma cruzi. Curr Opin Microbiol. 1998;1:461–465. doi: 10.1016/s1369-5274(98)80066-0. [DOI] [PubMed] [Google Scholar]
- 77.Favoreto S, Jr., Dorta ML, Yoshida N. Trypanosoma cruzi 175-kDa protein tyrosine phosphorylation is associated with host cell invasion. Exp Parasitol. 1998;89:188–194. doi: 10.1006/expr.1998.4285. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida N, Favoreto S, Jr., Ferreira AT, Manque PM. Signal transduction induced in Trypanosoma cruzi metacyclic trypomastigotes during the invasion of mammalian cells. Braz J Med Biol Res. 2000;33:269–278. doi: 10.1590/s0100-879x2000000300003. [DOI] [PubMed] [Google Scholar]
- 79.Cortez M, Neira I, Ferreira D, Luquetti AO, Rassi A, Atayde VD, Yoshida N. Infection by Trypanosoma cruzi metacyclic forms deficient in gp82 but expressing a related surface molecule, gp30. Infect Immun. 2003;71:6184–6191. doi: 10.1128/IAI.71.11.6184-6191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Todorov AG, Einicker-Lamas M, de Castro SL, Oliveira MM, Guilherme A. Activation of host cell phosphatidylinositol 3-kinases by Trypanosoma cruzi infection. J Biol Chem. 2000;275:32182–32186. doi: 10.1074/jbc.M909440199. [DOI] [PubMed] [Google Scholar]
- 81.Vieira M, Dutra JM, Carvalho TM, Cunha-e-Silva NL, Souto-Padron T, Souza W. Cellular signaling during the macrophage invasion by Trypanosoma cruzi. Histochem Cell Biol. 2002;118:491–500. doi: 10.1007/s00418-002-0477-0. [DOI] [PubMed] [Google Scholar]
- 82.Zhong L, Lu HG, Moreno SNJ, Docampo R. Tyrosine phosphate hydrolysis of host proteins by Trypanosoma cruzi is linked to cell invasion. FEMS Microbiology Letters. 1998;161:15–20. doi: 10.1111/j.1574-6968.1998.tb12923.x. [DOI] [PubMed] [Google Scholar]
- 83.Campos MA, Gazzinelli RT. Trypanosoma cruzi and its components as exogenous mediators of inflammation recognized through Toll-like receptors. Mediators Inflamm. 2004;13:139–143. doi: 10.1080/09511920410001713565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ming M, Ewen ME, Pereira MEA. Trypanosome invasion of mammalian cells requires activation of the TGF beta signalling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- 85.Fernandez MA, Munoz-Fernandez MA, Fresno M. Involvement of beta 1 integrins in the binding and entry of Trypanosoma cruzi into human macrophages. Eur J Immunol. 1993;23:552–557. doi: 10.1002/eji.1830230238. [DOI] [PubMed] [Google Scholar]
- 86.Scharfstein J, Schmitz V, Morandi V, Capella MM, Lima AP, Morrot A, Juliano L, Muller-Esterl W. Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors. J. Exp. Med. 2000;192:1289–1300. doi: 10.1084/jem.192.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scharfstein J, Monteiro AC, Schmitz V, Svensjo E. Angiotensin-converting enzyme limits inflammation elicited by Trypanosoma cruzi cysteine proteases: a peripheral mechanism regulating adaptive immunity via the innate kinin pathway. Biol Chem. 2008;389:1015–1024. doi: 10.1515/BC.2008.126. [DOI] [PubMed] [Google Scholar]
- 88.Rassi A, Jr., Rassi A, Little WC. Chagas’ heart disease. Clinical Cardiology. 2000;23:883–889. doi: 10.1002/clc.4960231205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daniels MD, Hyland KV, Engman DM. Treatment of experimental myocarditis via modulation of the renin-angiotensin system. Current Pharmaceutical Design. 2007;13:1299–1305. doi: 10.2174/138161207780618812. [DOI] [PubMed] [Google Scholar]
- 90.Todorov AG, Andrade D, Pesquero JB, Araujo Rde C, Bader M, Stewart J, Gera L, Muller-Esterl W, Morandi V, Goldenberg RC, Neto HC, Scharfstein J. Trypanosoma cruzi induces edematogenic responses in mice and invades cardiomyocytes and endothelial cells in vitro by activating distinct kinin receptor (B1/B2) subtypes. Faseb J. 2003;17:73–75. doi: 10.1096/fj.02-0477fje. [DOI] [PubMed] [Google Scholar]
- 91.Caler EV, Vaena de Avalos S, Haynes PA, Andrews NW, Burleigh BA. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharfstein J, Lima AP. Roles of naturally occurring protease inhibitors in the modulation of host cell signaling and cellular invasion by Trypanosoma cruzi. Subcell Biochem. 2008;47:140–154. doi: 10.1007/978-0-387-78267-6_11. [DOI] [PubMed] [Google Scholar]
- 93.Andrews NW. Lysosome recruitment during host cell invasion by Trypanosoma cruzi. Trends Cell Biol. 1995;5:133–137. doi: 10.1016/s0962-8924(00)88965-5. [DOI] [PubMed] [Google Scholar]
- 94.Rodriquez A, Samoff E, Rioult MG, Chung A, Andrews NW. Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. Journal of Cell Biology. 1996;134:349–362. doi: 10.1083/jcb.134.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leite MF, Moyer MS, Andrews NW. Expression of the mammalian calcium signaling response to Trypanosoma cruzi in Xenopus laevis oocytes. Mol Biochem Parasitol. 1998;92:1–13. doi: 10.1016/s0166-6851(97)00211-9. [DOI] [PubMed] [Google Scholar]
- 96.Caler EV, Chakrabarti S, Fowler KT, Rao S, Andrews NW. The Exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J Exp Med. 2001;193:1097–1104. doi: 10.1084/jem.193.9.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilkowsky SE, Barbieri MA, Stahl PD, Isola EL. Regulation of Trypanosoma cruzi invasion of nonphagocytic cells by the endocytically active GTPases dynamin, Rab5, and Rab7. Biochem Biophys Res Commun. 2002;291:516–521. doi: 10.1006/bbrc.2002.6474. [DOI] [PubMed] [Google Scholar]
- 98.Barrias ES, Reignault LC, De Souza W, Carvalho TM. Dynasore, a dynamin inhibitor, inhibits Trypanosoma cruzi entry into peritoneal macrophages. PLoS One. 2010;5:e7764. doi: 10.1371/journal.pone.0007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romano PS, Arboit MA, Vazquez CL, Colombo MI. The autophagic pathway is a key component in the lysosomal dependent entry of Trypanosoma cruzi into the host cell. Autophagy. 2009;5:6–18. doi: 10.4161/auto.5.1.7160. [DOI] [PubMed] [Google Scholar]
- 100.Luzio JP, Bright NA, Pryor PR. The role of calcium and other ions in sorting and delivery in the late endocytic pathway. Biochem Soc Trans. 2007;35:1088–1091. doi: 10.1042/BST0351088. [DOI] [PubMed] [Google Scholar]
- 101.Luzio JP, Poupon V, Lindsay MR, Mullock BM, Piper RC, Pryor PR. Membrane dynamics and the biogenesis of lysosomes. Mol Membr Biol. 2003;20:141–154. doi: 10.1080/0968768031000089546. [DOI] [PubMed] [Google Scholar]
- 102.Andrade LO, Andrews NW. Lysosomal fusion is essential for the retention of Trypanosoma cruzi inside host cells. J Exp Med. 2004;200:1135–1143. doi: 10.1084/jem.20041408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Woolsey AM, Burleigh BA. Host cell actin polymerization is required for cellular retention of Trypanosoma cruzi and early association with endosomal/lysosomal compartments. Cell. Microbiol. 2004;6:829–838. doi: 10.1111/j.1462-5822.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 104.Procopio DO, da Silva S, Cunningham CC, Mortara RA. Trypanosoma cruzi: effect of protein kinase inhibitors and cytoskeletal protein organization and expression on host cell invasion by amastigotes and metacyclic trypomastigotes. Exp Parasitol. 1998;90:1–13. doi: 10.1006/expr.1998.4314. [DOI] [PubMed] [Google Scholar]
- 105.Mortara RA, Andreoli WK, Fernandes MC, da Silva CV, Fernandes AB, L'Abbate C, da Silva S. Host cell actin remodeling in response to Trypanosoma cruzi: trypomastigote versus amastigote entry. Subcell Biochem. 2008;47:101–109. doi: 10.1007/978-0-387-78267-6_8. [DOI] [PubMed] [Google Scholar]
- 106.Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tyler KM, Luxton GW, Applewhite DA, Murphy SC, Engman DM. Responsive microtubule dynamics promote cell invasion by Trypanosoma cruzi. Cellular Microbiology. 2005;7:1579–1591. doi: 10.1111/j.1462-5822.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 108.Hall BF, Webster P, Ma AK, Joiner KA, Andrews NW. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J. Exp. Med. 1992;176:313–325. doi: 10.1084/jem.176.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rubin-de-Celis SS, Uemura H, Yoshida N, Schenkman S. Expression of trypomastigote trans-sialidase in metacyclic forms of Trypanosoma cruzi increases parasite escape from its parasitophorous vacuole. Cell Microbiol. 2006;8:1888–1898. doi: 10.1111/j.1462-5822.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 110.de Carvalho TM, de Souza W. Early events related with the behaviour of Trypanosoma cruzi within an endocytic vacuole in mouse peritoneal macrophages. Cell Structure & Function. 1989;14:383–392. doi: 10.1247/csf.14.383. [DOI] [PubMed] [Google Scholar]
- 111.Andrews NW. From lysosomes into the cytosol: the intracellular pathway of Trypanosoma cruzi. Braz J Med Biol Res. 1994;27:471–475. [PubMed] [Google Scholar]
- 112.Andrews NW, Abrams CK, Slatin SL, Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990;61:1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- 113.Manning-Cela R, Cortes A, Gonzalez-Rey E, Van Voorhis WC, Swindle J, Gonzalez A. LYT1 protein is required for efficient in vitro infection by Trypanosoma cruzi. Infect Immun. 2001;69:3916–3923. doi: 10.1128/IAI.69.6.3916-3923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manning-Cela R, Gonzalez A, Swindle J. Alternative splicing of LYT1 transcripts in Trypanosoma cruzi. Infection and Immunity. 2002;70:4726–4728. doi: 10.1128/IAI.70.8.4726-4728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zago MP, Barrio AB, Cardozo RM, Duffy T, Schijman AG, Basombrio MA. Impairment of infectivity and immunoprotective effect of a LYT1 null mutant of Trypanosoma cruzi. Infection and Immunity. 2008;76:443–451. doi: 10.1128/IAI.00400-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tonelli RR, Silber AM, Almeida-de-Faria M, Hirata IY, Colli W, Alves MJ. L-proline is essential for the intracellular differentiation of Trypanosoma cruzi. Cell Microbiol. 2004;6:733–741. doi: 10.1111/j.1462-5822.2004.00397.x. [DOI] [PubMed] [Google Scholar]
- 117.Tomlinson S, Vandekerckhove F, Frevert U, Nussenzweig V. The induction of Trypanosoma cruzi trypomastigote to amastigote transformation by low pH. Parasitology. 1995;110:547–554. doi: 10.1017/s0031182000065264. [DOI] [PubMed] [Google Scholar]
- 118.Grellier P, Blum J, Santana J, Bylen E, Mouray E, Sinou V, Teixeira AR, Schrevel J. Involvement of calyculin A-sensitive phosphatase(s) in the differentiation of Trypanosoma cruzi trypomastigotes to amastigotes. Mol Biochem Parasitol. 1999;98:239–252. doi: 10.1016/s0166-6851(98)00172-8. [DOI] [PubMed] [Google Scholar]
- 119.Coimbra VC, Yamamoto D, Khusal KG, Atayde VD, Fernandes MC, Mortara RA, Yoshida N, Alves MJ, Rabinovitch M. Enucleated L929 cells support invasion, differentiation, and multiplication of Trypanosoma cruzi parasites. Infect Immun. 2007;75:3700–3706. doi: 10.1128/IAI.00194-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clark RK, Kuhn RE. Trypanosoma cruzi does not induce apoptosis in murine fibroblasts. Parasitology. 1999;118(Pt 2):167–175. doi: 10.1017/s0031182098003631. [DOI] [PubMed] [Google Scholar]
- 121.Teixeira SM, daRocha WD. Control of gene expression and genetic manipulation in the Trypanosomatidae. Genet Mol Res. 2003;2:148–158. [PubMed] [Google Scholar]
- 122.Williams GT. Control of differentiation in Trypanosoma cruzi. Current Topics in Microbiology & Immunology. 1985;117:1–22. doi: 10.1007/978-3-642-70538-0_1. [DOI] [PubMed] [Google Scholar]
- 123.Teixeira SM. Control of gene expression in Trypanosomatidae. Braz J Med Biol Res. 1998;31:1503–1516. doi: 10.1590/s0100-879x1998001200001. [DOI] [PubMed] [Google Scholar]
- 124.Gonzalez J, Ramalho-Pinto FJ, Frevert U, Ghiso J, Tomlinson S, Scharfstein J, Corey EJ, Nussenzweig V. Proteasome activity is required for the stage-specific transformation of a protozoan parasite. J Exp Med. 1996;184:1909–1918. doi: 10.1084/jem.184.5.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meirelles MN, Juliano L, Carmona E, Silva SG, Costa EM, Murta AC, Scharfstein J. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol. Biochem. Parasitol. 1992;52:175–184. doi: 10.1016/0166-6851(92)90050-t. [DOI] [PubMed] [Google Scholar]
- 126.Harth G, Andrews N, Mills AA, Engel JC, Smith R, McKerrow JH. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- 127.Elias MC, Marques-Porto R, Freymuller E, Schenkman S. Transcription rate modulation through the Trypanosoma cruzi life cycle occurs in parallel with changes in nuclear organisation. Mol Biochem Parasitol. 2001;112:79–90. doi: 10.1016/s0166-6851(00)00349-2. [DOI] [PubMed] [Google Scholar]
- 128.Manning-Cela R, Jaishankar S, Swindle J. Life-cycle and growth-phase-dependent regulation of the ubiquitin genes of Trypanosoma cruzi. Arch Med Res. 2006;37:593–601. doi: 10.1016/j.arcmed.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 129.Recinos RF, Kirchhoff LV, Donelson JE. Cell cycle expression of histone genes in Trypanosoma cruzi. Mol Biochem Parasitol. 2001;113:215–222. doi: 10.1016/s0166-6851(01)00214-6. [DOI] [PubMed] [Google Scholar]
- 130.Chessler AD, Ferreira LR, Chang TH, Fitzgerald KA, Burleigh BA. A novel IFN regulatory factor 3-dependent pathway activated by trypanosomes triggers IFN-beta in macrophages and fibroblasts. J Immunol. 2008;181:7917–7924. doi: 10.4049/jimmunol.181.11.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chessler AD, Unnikrishnan M, Bei AK, Daily JP, Burleigh BA. Trypanosoma cruzi triggers an early type I IFN response in vivo at the site of intradermal infection. J Immunol. 2009;182:2288–2296. doi: 10.4049/jimmunol.0800621. [DOI] [PubMed] [Google Scholar]
- 132.Bartholomeu DC, Ropert C, Melo MB, Parroche P, Junqueira CF, Teixeira SM, Sirois C, Kasperkovitz P, Knetter CF, Lien E, Latz E, Golenbock DT, Gazzinelli RT. Recruitment and endo-lysosomal activation of TLR9 in dendritic cells infected with Trypanosoma cruzi. Journal of Immunology. 2008;181:1333–1344. doi: 10.4049/jimmunol.181.2.1333. [DOI] [PubMed] [Google Scholar]
- 133.Tarleton RL. Immune system recognition of Trypanosoma cruzi. Current Opinion in Immunology. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 134.Kayama H, Takeda K. The innate immune response to Trypanosoma cruzi infection. Microbes Infect. 2010 doi: 10.1016/j.micinf.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 135.Sathler-Avelar R, Vitelli-Avelar DM, Teixeira-Carvalho A, Martins-Filho OA. Innate immunity and regulatory T-cells in human Chagas disease: what must be understood? Memorias do Instituto Oswaldo Cruz. 2009;104(Suppl 1):246–251. doi: 10.1590/s0074-02762009000900031. [DOI] [PubMed] [Google Scholar]
- 136.Taki S. Type I interferons and autoimmunity: lessons from the clinic and from IRF-2-deficient mice. Cytokine Growth Factor Rev. 2002;13:379–391. doi: 10.1016/s1359-6101(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 137.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neira I, Silva FA, Cortez M, Yoshida N. Involvement of Trypanosoma cruzi metacyclic trypomastigote surface molecule gp82 in adhesion to gastric mucin and invasion of epithelial cells. Infect Immun. 2003;71:557–561. doi: 10.1128/IAI.71.1.557-561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ortega-Barria E, Pereira ME. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991;67:411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- 140.Colli W. Trans-sialidase: a unique enzyme activity discovered in the protozoan Trypanosoma cruzi. FASEB J. 1993;7:1257–1264. doi: 10.1096/fasebj.7.13.8405811. [DOI] [PubMed] [Google Scholar]
- 141.Katzin AM, Colli W. Lectin receptors in Trypanosoma cruzi. An N-acetyl-D-glucosamine-containing surface glycoprotein specific for the trypomastigote stage. Biochim Biophys Acta. 1983;727:403–411. doi: 10.1016/0005-2736(83)90425-x. [DOI] [PubMed] [Google Scholar]
- 142.Ruiz RC, Favoreto S, Jr., Dorta ML, Oshiro ME, Ferreira AT, Manque PM, Yoshida N. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signalling activity. Biochemical Journal. 1998;330:505–511. doi: 10.1042/bj3300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoshida N, Blanco SA, Araguth MF, Russo M, Gonzalez J. The stage-specific 90-kilodalton surface antigen of metacyclic trypomastigotes of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1990;39:39–46. doi: 10.1016/0166-6851(90)90006-8. [DOI] [PubMed] [Google Scholar]
- 144.Yoshida N. Molecular basis of mammalian cell invasion by Trypanosoma cruzi. An Acad Bras Cienc. 2006;78:87–111. doi: 10.1590/s0001-37652006000100010. [DOI] [PubMed] [Google Scholar]
- 145.Moody TN, Ochieng J, Villalta F. Novel mechanism that Trypanosoma cruzi uses to adhere to the extracellular matrix mediated by human galectin-3. FEBS Lett. 2000;470:305–308. doi: 10.1016/s0014-5793(00)01347-8. [DOI] [PubMed] [Google Scholar]
- 146.Turner CW, Lima MF, Villalta F. Trypanosoma cruzi uses a 45-kDa mucin for adhesion to mammalian cells. Biochem Biophys Res Commun. 2002;290:29–34. doi: 10.1006/bbrc.2001.6189. [DOI] [PubMed] [Google Scholar]
- 147.Villalta F, Smith CM, Ruiz-Ruano A, Lima MF. A ligand that Trypanosoma cruzi uses to bind to mammalian cells to initiate infection. FEBS Lett. 2001;505:383–388. doi: 10.1016/s0014-5793(01)02853-8. [DOI] [PubMed] [Google Scholar]
- 148.Schenkman S, Diaz C, Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Experimental Parasitology. 1991;72:76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- 149.Souto-Padron T, Campetella OE, Cazzulo JJ, de Souza W. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. Journal of Cell Science. 1990;96:485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- 150.Murta AC, Persechini PM, Padron T, de Souza W, Guimaraes JA, Scharfstein J. Structural and functional identification of GP57/51 antigen of Trypanosoma cruzi as a cysteine proteinase. Molecular & Biochemical Parasitology. 1990;43:27–38. doi: 10.1016/0166-6851(90)90127-8. [DOI] [PubMed] [Google Scholar]
- 151.Santana JM, Grellier P, Schrevel J, Teixeira AR. A Trypanosoma cruzi-secreted 80 kDa proteinase with specificity for human collagen types I and IV. Biochem J. 1997;325(Pt 1):129–137. doi: 10.1042/bj3250129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ramirez MI, Ruiz Rde C, Araya JE, Da Silveira JF, Yoshida N. Involvement of the stage-specific 82-kilodalton adhesion molecule of Trypanosoma cruzi metacyclic trypomastigotes in host cell invasion. Infect Immun. 1993;61:3636–3641. doi: 10.1128/iai.61.9.3636-3641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Villalta F, Zhang Y, Bibb KE, Pratap S, Burns JM, Jr., Lima MF. Signal transduction in human macrophages by gp83 ligand of Trypanosoma cruzi: trypomastigote gp83 ligand up-regulates trypanosome entry through protein kinase C activation. Mol Cell Biol Res Commun. 1999;2:64–70. doi: 10.1006/mcbr.1999.0150. [DOI] [PubMed] [Google Scholar]
- 154.Dorta ML, Ferreira AT, Oshiro ME, Yoshida N. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol Biochem Parasitol. 1995;73:285–289. doi: 10.1016/0166-6851(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 155.Yoshida N, Mortara RA, Araguth MF, Gonzalez JC, Russo M. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect. Immun. 1989;57:1663–1667. doi: 10.1128/iai.57.6.1663-1667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neira I, Ferreira AT, Yoshida N. Activation of distinct signal transduction pathways in Trypanosoma cruzi isolates with differential capacity to invade host cells. Int J Parasitol. 2002;32:405–414. doi: 10.1016/s0020-7519(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 157.Manque PM, Neira I, Atayde VD, Cordero E, Ferreira AT, da Silveira JF, Ramirez M, Yoshida N. Cell adhesion and Ca2+ signaling activity in stably transfected Trypanosoma cruzi epimastigotes expressing the metacyclic stage-specific surface molecule gp82. Infect Immun. 2003;71:1561–1565. doi: 10.1128/IAI.71.3.1561-1565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Burleigh BA, Caler EV, Webster P, Andrews NW. A cytosolic serine endopeptidase from Trypanosoma cruzi is required for the generation of Ca2+ signaling in mammalian cells. Journal of Cell Biology. 1997;136:609–620. doi: 10.1083/jcb.136.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]