SUMMARY
Background
Adefovir and tenofovir are nucleotide analogues used as long-term therapy of chronic hepatitis B. Side effects are few, but prolonged and high-dose therapy has been associated with proximal renal tubular dysfunction (RTD).
Aim
To assess the incidence of RTD during long-term nucleotide therapy of chronic hepatitis B.
Methods
A total of 51 patients being treated at the Clinical Center, National Institutes of Health were studied. Diagnosis of RTD required de novo appearance of at least three of five features: hypophosphataemia, hypouricaemia, serum creatinine elevation, proteinuria or glucosuria.
Results
Among 51 patients treated for 1–10 (mean 7.4) years with adefovir (n = 42), tenofovir (n = 4) or adefovir followed by tenofovir (n = 5), 7 (14%) developed RTD. Time to onset ranged from 22 to 94 (mean 49) months with an estimated 10-year cumulative rate of 15%. All seven had low urinary percent maximal tubular reabsorption of phosphate (<82%). Patients with RTD were older (58 vs. 44 years; P = 0.01) and had lower baseline glomerular filtration rates (82 vs. 97 cc/min; P = 0.08) compared to those without; but did not differ in other features. Six patients with RTD were switched to entecavir, all subsequently had improvements in serum phosphate (2.0–3.0 mg/dL), creatinine (1.6–1.1 mg/dL), uric acid (2.7 –3.8 mg/dL) and proteinuria.
Conclusions
Renal tubular dysfunction develops in 15% of patients treated with adefovir or tenofovir for 2–9 years and is partially reversible with change to other antivirals. Monitoring for serum phosphate, creatinine and urinalysis is prudent during long-term adefovir and tenofovir therapy.
INTRODUCTION
The current preferred approach to therapy of chronic hepatitis B is long-term use of oral nucleoside or nucleotide analogues until evidence of a lasting remission of disease occurs, either loss of hepatitis B e antigen (HBeAg) or, more preferably, hepatitis B surface antigen (HBsAg).1–3 The ultimate efficacy and safety of this approach has not been adequately shown. The first generation of oral nucleos(t)ides with activity against hepatitis B virus (HBV) included lamivudine and adefovir dipivoxil, both of which led to rapid suppression of serum HBV DNA levels and improvements in both biochemical and histological features of disease,4–7 at least during the first 1 or 2 years of therapy. Long-term use of these agents, however, was associated with a high rate of antiviral resistance (28%–75%) and consequent treatment failures.8–10 The more recently developed, second generation of nucleos(t)ides with activity against HBV, such as tenofovir and entecavir, have been associated with better and more sustained responses 11, 12 and a low rate of antiviral resistance (<1%).13, 14 Long-term therapy with the nucleoside analogues in hepatitis B has also been well tolerated with few adverse events.
Prolonged therapy with the acyclic nucleotide analogues adefovir and tenofovir, however, is associated with rare instances of proximal renal tubular dysfunction (RTD) that typically arise only after months or years of treatment.15 Renal dysfunction is particularly common with higher doses of adefovir (30–60 mg daily). In contrast, tenofovir which is a close analogue of adefovir in a dose of 300 mg daily is rarely associated with this toxicity, at least during the first year or two of treatment.16 Recently, however, renal dysfunction has been reported in patients with human immunodeficiency virus (HIV) infection treated with tenofovir or chronic hepatitis B treated with adefovir for several years.17–22 This renal injury resembles Fanconi’s syndrome and presents with variable degrees of proximal renal tubular dysfunction, including hypophosphataemia, hypouricaemia, aminoaciduria and glucosuria. This hypophosphataemia can result in bone mineralisation defects, osteomalacia and fractures. The frequency of this syndrome has not been well defined, and few cases have been reported in patients without HIV infection. We have encountered several patients who have developed evidence of renal Fanconi’s syndrome while on long-term nucleotide therapy for chronic hepatitis B and report on its incidence, clinical features, underlying risk factors and potential resolution with cessation of the drug.
MATERIALS AND METHODS
Patients
All patients with chronic hepatitis B started on therapy with either adefovir dipivoxil or tenofovir disoproxil fumarate by the Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), between 2002 and 2007 were assessed for evidence of hypophosphataemia and Fanconi’s syndrome. The majority of patients had been enrolled in a prospective, randomised controlled study comparing adefovir mono-therapy (10 mg/day) to the combination of adefovir (10 mg/day) and lamivudine (100 mg/day) (Clinical Trial NCT00023309).23 The remaining patients were treated ‘off-of-protocol’ with tenofovir (300 mg/day) because they failed to meet enrolment criteria but warranted treatment. In all instances, patients underwent pre-treatment evaluation including liver biopsy. During therapy, patients were seen and had blood testing at 1–3 month intervals. Therapy was continued indefinitely and stopped only if there was loss of HBsAg sustained for at least 6 months. Patients who developed significant side effects or who failed to have a virological and biochemical response despite at least 1 year of treatment were switched to other therapies. All patients, however, continued to be followed. These patients were participants in clinical research protocols approved by the institute review board of NIDDK, and all gave written consent. Adefovir dipivoxil was kindly provided by Gilead Sciences (Foster City, CA, USA) under a Clinical Trial Agreement with the NIDDK and administered under an Investigational New Drug application (#66221) held by the senior author.
Serum and urine monitoring during therapy
On each outpatient clinic visit, blood was taken for complete blood counts and routine chemistries which included serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, direct and total bilirubin, albumin, total protein, creatinine, blood urea nitrogen, phosphate, calcium and uric acid. HBV DNA was tested at 1–3 month intervals and HBV serology (HBsAg, HBeAg, anti-HBe, anti-HBs) was done at least once yearly. Urinalysis was done at baseline and at least yearly. Starting in July 2010, a routine urinalysis and urine phosphate, creatinine and beta-2-microglobulin were tested at each clinic visit.
Definitions
The diagnosis of phosphate wasting and RTD was based upon retrospective review of blood and urine testing results and required sustained hypophosphataemia (#1) with at least two other manifestations of tubular dysfunction (#2–5), each defined below:
Sustained decrease in serum phosphate to below 2.5 mg/dL and by at least 0.5 mg/dL from baseline.
Sustained increase in serum creatinine to above 1.2 mg/dL and by at least 0.5 mg/dL from baseline.
Sustained decrease in serum uric acid to below 3.7 mg/dL and by at least 0.5 mg/dL from baseline.
De novo and sustained appearance of proteinuria (trace to 4 + on urinalysis).
De novo and sustained appearance of glucosuria in the absence of the diagnosis of diabetes mellitus.
Baseline values were defined using the average of at least three samples taken before starting adefovir or tenofovir therapy. A feature was defined as sustained if present on at least half of subsequent determinations while continuing on nucleotide therapy. In each instance, other causes of abnormalities (such as diabetes, drug-induced renal dysfunction or use of uricosuric medications) were sought. Glomerular filtration rate (GFR) was calculated based upon serum creatinine, race and age using the updated Modification-of-Diet- in-Renal-Disease (MDRD) equation [GFR = 175 × (Scr)−1.154 × (Age)−0.203 × (0.742 if female patient) × (1.212 if African American)].24 The tubular reabsorption of phosphate (TRP) was calculated according to the formula: TRP = (urine phosphate/serum phosphate) / (urine creatinine/serum creatinine) × 100%; normal values being >85%.25 Liver biopsies were scored based upon the histology activity index (HAI: range 0–18) and the Ishak fibrosis scoring system (range 0–6).26
Stored serum samples were tested for fibroblast growth factor-23 (FGF23), a bone-derived hormone that inhibits renal tubular phosphate transport and overexpression of which is the cause of several osteomalacic disorders.27 Normally, FGF23 levels rise in response to high serum phosphate levels and fall in response to low levels.28 FGF23 was assayed using an enzyme-linked immunoassay (Kainos, Tokyo, Japan) with a normal range of 10–50 pg/mL and a sensitivity of 3 pg/mL.29 In patients who developed RTD, yearly samples were chosen for FGF23 testing. A similar number of controls (treated patients who did not develop RTD) were also tested.
Statistical analysis
Two-sample t-tests were used to compare the means for continuous variables. Categorical variables were tested using Fisher’s exact test or chi-square test if there were more than three groups in comparison. A two-tailed P-value of <0.05 was considered statistically significant. Life-table analysis of time to first evidence of RTD was performed using Kaplan–Meier plots. All analyses were performed using SAS 9.1.3 and JMP 8.0 (SAS Inc., Cary, NC, USA).
RESULTS
Patient characteristics
Between 2002 and 2007, 51 patients with chronic hepatitis B were started on therapy with adefovir (n = 47) or tenofovir (n = 4). Among those started on adefovir, 42 were participants in a prospective randomised controlled trial comparing adefovir monotherapy to the combination of adefovir and lamivudine (23), of whom five were eventually switched to tenofovir because of an inadequate virological or histological response. The total duration of therapy ranged from 1 to 10 years and averaged 7.4 years. The patients were mostly men (84%) and either Asian (47%) or non-Hispanic white (43%). Thirty-nine were initially HBeAg-positive and eight had cirrhosis before starting treatment. Eighteen patients (35%) had received lamivudine previously and 27 (53%) received lamivudine in combination with adefovir. All except three patients continued to be followed at the time of these analyses. One patient was lost to follow up after 2.5 years of adefovir therapy when he returned to his country of origin. Two other patients died of hepatocellular carcinoma, 5 and 6 years after starting nucleotide analogue therapy.
Patients with renal tubular dysfunction
Seven of 51 patients (14%) developed persistent hypophosphataemia with at least two other criteria for RTD during nucleotide analogue therapy (Table 1). All except one were men and the average age was 58 years. Hypophosphataemia arose after 22–95 (average 49) months of therapy and was accompanied by hypouricaemia and mild proteinuria in all seven. All patients also had increases in serum creatinine levels which reached criteria used in the diagnosis of RTD in four. No patient had glucosuria or severe proteinuria (>3+ on a fasting, spot urine sample). Overall, the abnormalities were mild and no patient reported symptoms of hypophosphataemia, renal or bone disease. Testing of urine and serum creatinine and phosphate concurrently, showed that all seven patients had urinary phosphate wasting with a low estimated TRP (<82%). Six patients with RTD were tested for urinary beta-2-microglobulin levels which were raised in five (peak levels 0.88–29.26 mg/dL, normal <0.30 mg/L), and the one patient with normal levels was first tested more than 6 months after stopping adefovir. In contrast, among 26 patients who were not diagnosed as having renal tubular dysfunction and were tested, urine beta-2-microglobulin levels were normal in all but one.
Table 1.
Clinical features of seven patients with renal tubular dysfunction
| Pt No. | Age | Sex | Results at the start of therapy | Therapy | Years on therapy | Results at diagnosis of RTD | New Medication | Last results recorded | Follow-up after switch (years) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phos | Uric acid | Cr | Urine protein | Urine glucose | Phos | Uric acid | Cr | Urine protein | Urine glucose | Phos | Uric acid | Cr | Urine protein | Urine glucose | |||||||
| 1 | 56 | M | 3.1 | 6.5 | 1.3 | None | None | Adv/Lam | 2.1 | 2.1 | 2.9 | 2.0 | 1+ | None | Entecavir | 3.0 | 5.1 | 1.3 | None | None | 6.3 |
| 2 | 40 | M | 3.1 | 4.5 | 0.9 | None | None | Adv/Lam | 4.9 | 1.7 | 2.5 | 1.4 | Trace | None | Entecavir | 2.1 | 2.7 | 0.9 | None | None | 1.0 |
| 3 | 62 | M | 4.0 | 3.6 | 1.0 | None | None | Ten | 3.9 | 2.3 | 2.4 | 1.8 | Trace | None | Entecavir | 3.1 | 3.2 | 1.2 | None | None | 1.0 |
| 4 | 66 | M | 3.2 | 5.8 | 1.1 | None | None | Adv → Ten* | 7.7 | 2.1 | 3.6 | 1.7 | 1+ | None | Entecavir | 2.7 | 3.7 | 1.4 | None | None | 1.0 |
| 5 | 76 | F | 3.5 | 5.3 | 0.6 | None | None | Adv/Lam | 3.4 | 1.9 | 1.5 | 1.3 | 2+ | None | Entecavir | 3.1 | 3.9 | 0.7 | None | None | 2.0 |
| 6 | 33 | M | 3.5 | 5.7 | 1.1 | None | None | Adv/Lam | 6.5 | 2.0 | 2.3 | 1.4 | Trace | None | Not switched | 2.7† | 2.7 | 1.4 | 1+ | None | |
| 7 | 71 | M | 3.7 | 5.3 | 0.8 | None | None | Adv/Lam | 8.4 | 2.0 | 1.8 | 1.6 | Trace | None | Entecavir | 3.7 | 4.2 | 1.2 | Trace | None | 1.0 |
Adv, adefovir; Cr, creatinine; Phos, phosphate; RTD, renal tubular dysfunction; Ten, tenofovir.
This patient was on adefovir for 4 years before switching to tenofovir due to inadequate virological response. At the time of switching therapies, his phosphate, uric acid and creatinine were 2.9, 5.6 and 1.3, respectively, and had no proteinuria or glucosuria. RTD was diagnosed 3.7 years after switch to tenofovir.
On phosphate replacement.
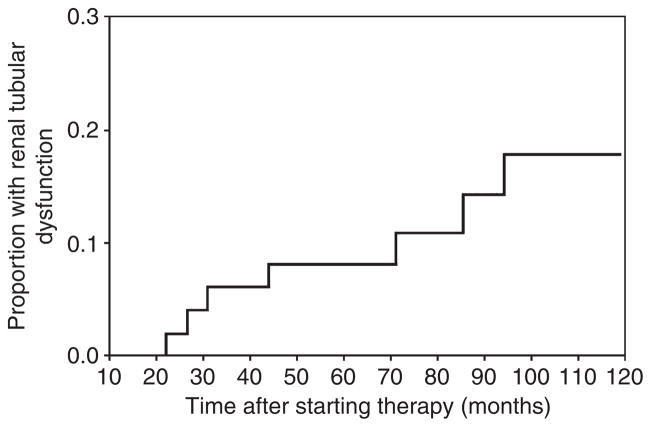
Life-table analysis of time to onset of RTD is shown in Figure 1. No patient developed renal abnormalities during the first 2 years of treatment. During the third year of therapy, however, 6% of patients developed RTD, and thereafter the average rate of onset was ~1% per year. The accumulative estimated proportion at 10 years was 15%. Using time-to-first-evidence of hypouricaemia, elevated creatinine or proteinuria did not change these estimates (data not shown).
Figure 1.
Life-table analysis of time to renal tubular dysfunction (RTD). Each vertical line in the graph represents a case of adefovir or tenofovir induced RTD. As depicted, the first case occurred at 22 months and the seventh case at 95 months. About 15% of patients developed RTD at the time of last recorded data for the entire 51 patients.
Comparisons of patients with and without RTD are shown in Table 2. Patients who developed hypophosphataemia were older (58 vs. 44 years; P = 0.01) and had slightly higher baseline serum creatinine levels (0.97 vs. 0.87 mg/dL: P = 0.18) and lower calculated GFR values (82 vs. 97 cc/min: P = 0.08). In other regards, patients with and without renal dysfunction were similar, differing minimally if at all in duration of therapy, presence of comorbidities such as diabetes or hypertension, distribution of gender or in average initial biochemical, histological or virological features of liver disease and HBV infection. Patients who developed RTD were not on any known nephrotoxic drugs concomitantly.
Table 2.
Comparison of baseline features in patients with and without renal tubular dysfunction (RTD)
| Features (at time of starting therapy) | With RTD (n = 7) | Without RTD (n = 44) | P-value |
|---|---|---|---|
| Sex (male) | 84% | 84% | 0.91 |
| Age (years)* | 58 (16) | 44 (12) | 0.01 |
| Race | |||
| White | 71% | 39% | 0.17† |
| Black | 14% | 9% | |
| Asian | 14% | 52% | |
| Diabetes (DM) | 14% | 7% | 0.49‡ |
| Hypertension (HTN) | 57% | 34% | 0.24‡ |
| Renal disease (RD) | 0% | 2% | 1.00‡ |
| DM or HTN or RD | 57% | 39% | 0.41‡ |
| ALT (U/L)* | 107 (60) | 155 (202) | 0.54 |
| AST (U/L)* | 107 (89) | 102 (130) | 0.92 |
| Alk P (U/L)* | 100 (52) | 75 (21) | 0.02 |
| Albumin (g/dL)* | 3.6 (0.4) | 3.8 (0.4) | 0.12 |
| Bilirubin (mg/dL)* | 1.3 (1.6) | 1.0 (0.5) | 0.29 |
| Creatinine (mg/dL)* | 0.97 (0.23) | 0.87 (0.18) | 0.18 |
| GFR (mL/min)* | 82 (13) | 97 (21) | 0.08 |
| Phosphate (mg/dL)* | 3.3 (0.6) | 3.4 (0.5) | 0.61 |
| Uric Acid (mg/dL)* | 5.2 (0.9) | 5.4 (0.9) | 0.72 |
| Proteinuria (trace or greater) | 0% | 7% | 1.00‡ |
| HBV DNA (log10 copies/mL)* | 7.97 (1.28) | 7.83 (1.54) | 0.85 |
| HBeAg | 86% | 75% | 1.00‡ |
| Liver histology | |||
| HAI score* | 7 (3) | 8 (3) | 0.36 |
| Ishak Fibrosis* | 3 (2) | 3 (2) | 0.77 |
| Cirrhosis | 0% | 20% | 0.57‡ |
| Concurrent lamivudine | 71% | 50% | 0.42‡ |
| Lamivudine resistance | 29% | 23% | 0.66‡ |
| Duration of therapy (years)* | 5.3 | 6.5 | 0.22 |
Alk P, alkaline phosphatase; GFR, glomerular filtration rate as estimated by MDRD equation; HAI, histology activity index (ranging from 0 to 18).
Mean (standard deviation).
Chi-square test.
Fisher’s exact test; all other P-values are from t-tests.
Course of renal tubular dysfunction
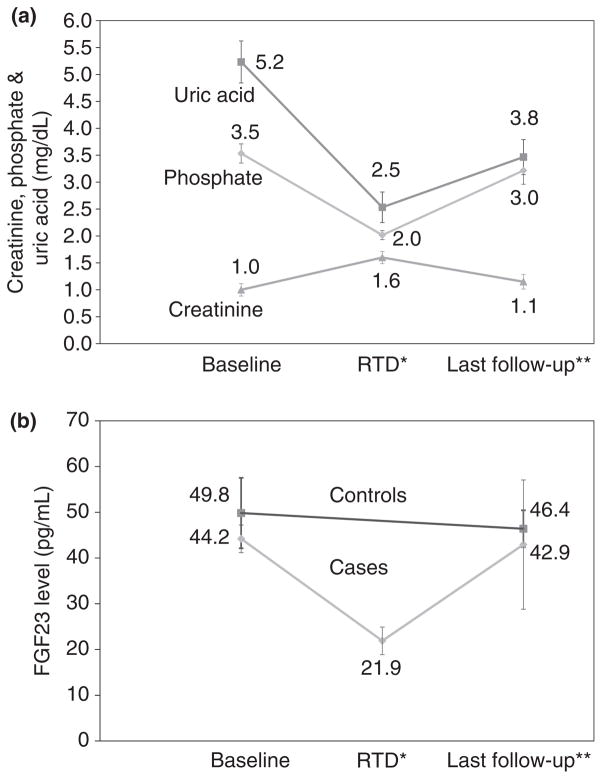
Six of the seven patients with RTD were switched from adefovir (n = 4) or tenofovir (n = 2) to entecavir therapy and followed for 1–6.3 (mean 2.0) years after switching. All subsequently had improvements in serum phosphate (mean = 2.0 rising to 3.0 mg/dL), creatinine (1.6–1.1 mg/dL), uric acid (2.7–3.8 mg/dL) and proteinuria (becoming undetectable in five). However, some values remained abnormal and most differed from pre-treatment levels (Table 1 and Figure 2a). In particular, uric acid levels were invariably lower after than before treatment and remained below the lower limit of normal in five patients. Serial determinations of TRP before and after switching to entecavir were available from four patients. Although TRP increased in all four patients (from mean of 71% to 82%), values remained low in three patients (77%, 81% and 81%), 1–6 years after switching therapies. Among 35 patients without RTD who underwent testing, the TRP on the last available specimen averaged 90% and ranged from 82% to 100%.
Figure 2.
Changes in serum markers of RTD with therapy. (a) Mean serum uric acid, phosphate and creatinine levels from six patients from before therapy, at the time of diagnosis of renal tubular dysfunction (RTD: *mean 48 months), and at the time of last follow-up after switching to entecavir (**mean 24 months later). Mean values improve but remain lower than pre-treatment values. (b) Mean serum FGF23 levels in 6 patients who developed RTD during adefovir or tenofovir therapy from before treatment (Baseline), at the time of diagnosis (RTD: *mean 48 months), and at the time of last follow-up after switching to entecavir (**mean 24 months later). FGF23 levels decreased during therapy and rose to baseline values after adefovir and tenofovir were stopped. Also shown are mean values of serum FGF23 in 7 control subjects who did not develop RTD from before therapy and at the time of last follow-up.
Three patients with renal tubular dysfunction developed elevations in serum alkaline phosphatase levels after the development of hypophosphataemia (peak levels 155, 174 and 172 U/L: normal <116 U/L). Concurrent ALT, AST and gammaglutamyltranspeptidase values were normal, suggesting that alkaline phosphatase elevations were due to bone disease and possibly subclinical osteomalacia. These elevations also returned to baseline levels after switching to entecavir.
Serum HBV DNA levels were undetectable (<20 IU/mL) in all six patients at the time that they were switched to entecavir. In follow-up, three patients became HBV DNA positive at low levels (<104 copies/mL). All three patients were HBeAg-positive at the time of the switch and had a history of lamivudine resistance documented before initial treatment with adefovir. Nevertheless, serum aminotransferase levels remained unchanged and were within the normal range in two patients.
Several other patients in the total cohort had one or more abnormality in creatinine, phosphate, uric acid or urinalysis during the years of nucleotide analogue therapy but did not meet the criteria used to define RTD. One patient developed elevations in serum creatinine without hypophosphataemia or hypouricaemia which was attributed to diabetic nephropathy. A second patient was found to have marked but intermittent glucosuria without a change in serum creatinine, phosphate or uric acid which did not change upon switching to entecavir. A final patient had intermittent hypophosphataemia, but had similar transient decreases before treatment and had no change in serum creatinine, uric acid or proteinuria.
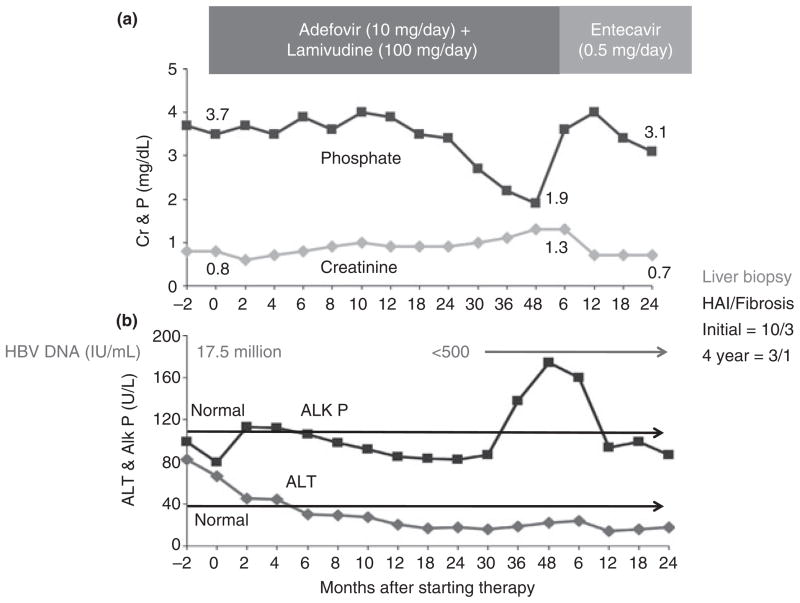
The clinical course of a representative patient is shown in Figure 3. She was a 76 year old woman with HBeAg-negative chronic hepatitis B who had an excellent clinical and virological response to the combination of adefovir and lamivudine. However, during year 3 of treatment, serum phosphate and uric acid levels fell and creatinine rose. At 48 months, she had proteinuria (953 mg/24 h), hypouricaemia (1.5 mg/dL), hypophosphataemia (1.9 mg/dL rising to 3.1 mg/dL on phosphate replacement therapy) and mild renal dysfunction (creatinine 1.3 mg/dL). Serum alkaline phosphatase had also risen during the third year of treatment. Once she was switched to entecavir, all these abnormalities resolved.
Figure 3.
Patient with adefovir-induced RTD. Panel A shows that serial serum creatinine (Cr) levels rose and phosphate (P) levels began to decline after 24 months of adefovir therapy but returned to normal levels after switching to entecavir. Panel B shows a concomitant rise in alkaline phosphatase (Alk P) which fell back to baseline levels within 12 months of switching to entecavir. Also shown are serial levels of ALT which fell and remained normal on antiviral therapy as did HBV DNA levels. Results of liver histology are given in the right corner based upon biopsies done before treatment and after 4 years. The histology activity index (HAI) decreased from 10 to 3 and the Ishak fibrosis score from 3 to 1.
Fibroblast growth factor-23 levels
Serum levels of FGF23 were measured on selected stored serum specimens from the seven patients who developed RTD and 7 controls. Overall, FGF23 levels correlated positively with serum phosphate levels (r = 0.053, P = 0.03: data not shown). Importantly, FGF23 levels decreased in all six patients who developed hypophosphataemia and were switched to entecavir, mean (±s.d.) values falling from 44.2 (±7.4) before treatment to 21.9 (±7.4) pg/mL at the time of diagnosis and rising back to 42.9 (±31.6) pg/mL at the time of the last follow-up sample (Figure 2b). On the other hand, FGF23 levels did not change in the controls, averaging 49.8 (±18.9) before treatment vs. 46.4 (±9.9) pg/mL at the time of the last sample.
DISCUSSION
This analysis of 51 patients with chronic hepatitis B and multiple published reports have shown that long-term therapy with adefovir or tenofovir (which are similar acyclic nucleotide analogues) can be associated with proximal renal tubular dysfunction resulting in significant hypophosphataemia, renal insufficiency and osteomalacia.17–22 The risk factors for this complication and its frequency are only partially understood. In this study, risk factors appeared to be age and pre-existing renal function, factors that also have been identified in studies of patients with HIV infection.17, 18 An important difference between this and previous studies, was that all subjects being treated with these agents were evaluated for this complication and therapy was stopped early, before symptomatic hypophosphataemia, renal failure or osteomalacia appeared. Although the diagnosis was made based upon serum and urine test results alone, the criteria used were strict and required a significant and sustained change from baseline values (as shown in Figure 3). In addition, the patients in this study had chronic hepatitis B without HIV infection, and most were asymptomatic and fully functional and active. Thus, even a mild degree of renal tubular dysfunction was considered unacceptable in view of the availability of other medications for this condition and its relatively good prognosis.
The cause of the low phosphate levels was clearly renal phosphate wasting. Phosphate loss was demonstrated by measuring the maximal TRP on a fasting urine specimen.25 All seven patients with persistent hypophosphataemia had a lower than normal per cent TRP. Furthermore, serum phosphate levels and TRP improved when therapy was switched to entecavir. Finally, measurement of FGF23 levels on stored serum specimens demonstrated a decrease with the development of hypophosphataemia, a normal response of this bone-derived hormone to hypophosphataemia due to renal loss.27–29
The strength of this study was that it included all patients treated with nucleotide analogues and monitoring was rigorous and follow-up excellent. The shortcomings of the study were its limited size, the lack of pre-treatment assessment of urinary phosphate transport and the lack of bone and renal biopsies to assess osteomalacia and structural renal tubular damage. Dual energy X-ray absorptiometry (DEXA) scans were done prospectively on the two patients who received tenofovir but not in those receiving adefovir. Although reliable in detecting osteoporosis, DEXA scanning is unreliable in osteomalacia in which there is increased bone volume.30 Finally, the majority of patients in this study were receiving both adefovir and lamivudine and the combination of nucleoside analogues may have predisposed to this renal complication.
Studies in HIV positive patients with severe phosphate wasting during tenofovir therapy have shown variable degrees of renal tubular damage with fibrosis and inflammation suggesting that the injury may not be reversible.20 In this study, the phosphate wasting was shown to be only partially reversible; however, the nucleotide analogues were stopped before severe hypophosphataemia, renal damage and osteomalacia occurred.
Both adefovir and tenofovir appear to cause RTD in a proportion of patients. For this reason, routine monitoring of serum creatinine and phosphate levels with routine urinalysis before treatment and at yearly intervals is prudent in managing patients in whom long-term therapy is planned. If abnormalities arise, confirmation of the diagnosis should be done using fasting urine and serum creatinine and phosphate levels to calculate the TRP as well as measurements of the degree of proteinuria and its characterisation using urinary amino acid or beta-2-microglobulin levels. Switching to another agent, such as entecavir, is appropriate if the diagnosis is made.
In the light of our data, adefovir and tenofovir should be used with caution in patients with evidence of pre-existing renal tubular dysfunction and in those taking concomitant nephrotoxic agents. Caution should be exercised in the elderly and in those with reduced GFR as well. However, it should be kept in mind that creatinine-based GFR estimates are not completely accurate in cirrhotics.31
Patients taking nucleotide analogues long-term should also receive recommendations about bone health, insuring adequate calcium and vitamin D intake. Phosphate replacement should be initiated if hypophosphataemia arises. Importantly, studies of the pathogenesis of renal tubular dysfunction due to adefovir and tenofovir are needed if there is any hope of avoiding this complication. Furthermore, other approaches at prevention such as using lower doses of tenofovir or altering doses based upon drug levels are needed.
Acknowledgments
The authors thank the many physicians who cared for the 51 patients described in this report, including Drs. Edward Doo, Theo Heller, Yaron Rotman, Brian Borg, Jordan Feld, Rohit Loomba, Apurva Modi, Pothuraju Nagabhyru, Christopher Koh, Mazen Nourredin, Adil Abdulla and Souvik Sarkar; and the nursing staff of the Liver Diseases Branch Outpatient Clinic.
Declaration of funding interests: This study was supported by the Intramural Divisions of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Dental and Craniofacial Research, and National Cancer Institute of the National Institutes of Health.
Footnotes
Declaration of personal interests: None.
References
- 1.Ganem D, Prince AM. Hepatitis B virus infection - natural history and clinical consequences. N Engl J Med. 2004;350:1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health consensus development conference statement: management of hepatitis B. Hepatology. 2009;49:S4–12. doi: 10.1002/hep.22946. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 4.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–63. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 5.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 6.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–16. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 7.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–7. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 8.Chang TT, Lai CL, Chien RN, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276–82. doi: 10.1111/j.1440-1746.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 9.Manolakopoulos S, Bethanis S, Koutsounas S, et al. Long-term therapy with adefovir dipivoxil in hepatitis B e antigen-negative patients developing resistance to lamivudine. Aliment Pharmacol Ther. 2008;27:266–73. doi: 10.1111/j.1365-2036.2007.03567.x. [DOI] [PubMed] [Google Scholar]
- 10.Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology. 2009;49:S174–84. doi: 10.1002/hep.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–55. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 12.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–20. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 13.Snow-Lampart A, Chappell B, Curtis M, et al. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology. 2011;53:763–73. doi: 10.1002/hep.24078. [DOI] [PubMed] [Google Scholar]
- 14.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–14. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed April 4, 2012];Hepsera (adefovir dipivoxil) tablet [Gilead Sciences]. Product label. Available at: http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=a60adac4-57c2-4089-aefc-1a77a48676fc.
- 16.Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–43. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Peyriere H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. 2004;35:269–73. doi: 10.1097/00126334-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Quimby D, Brito MO. Fanconi syndrome associated with use of tenofovir in HIV-infected patients: a case report and review of the literature. AIDS Read. 2005;15:357–64. [PubMed] [Google Scholar]
- 19.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. doi: 10.1155/2011/354908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girgis CM, Wong T, Ngu MC, et al. Hypophosphataemic osteomalacia in patients on adefovir dipivoxil. J Clin Gastroenterol. 2011;45:468–73. doi: 10.1097/MCG.0b013e3181e12ed3. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22:99–103. doi: 10.1089/apc.2007.0052. [DOI] [PubMed] [Google Scholar]
- 22.Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–80. doi: 10.1053/j.ajkd.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Ghany MG, Feld JJ, Heller T, et al. Adefovir dipivoxil alone versus the combination of adefovir and lamivudine for chronic hepatitis B. Aliment Pharmacol Ther. 2012;35:1027–35. doi: 10.1111/j.1365-2036.2012.05059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Green T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Bijvoet OL, Morgan DB, Fourman P. The assessment of phosphate reabsorption. Clin Chim Acta. 1969;26:15–24. doi: 10.1016/0009-8981(69)90280-0. [DOI] [PubMed] [Google Scholar]
- 26.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 27.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Winer K, Econs MJ, et al. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–92. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 29.Imel EA, Peacock M, Pitukcheewanont P, et al. Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–61. doi: 10.1210/jc.2005-2105. [DOI] [PubMed] [Google Scholar]
- 30.Marie PJ, Glorieux FH. Bone Histomorphometry in asymptomatic adults with hereditary hypophosphatemic Vitamin D-resistant osteomalacia. Metab Bone Dis Relat Res. 1982;4:249–53. doi: 10.1016/0221-8747(82)90035-2. [DOI] [PubMed] [Google Scholar]
- 31.Caregaro L, Menon F, Angeli P, et al. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201–5. [PubMed] [Google Scholar]