Abstract
Vibrio cholerae switches between smooth and rugose colonial variants. The rugose variant produces more vibrio polysaccharides (VPSEl Tor) and forms well-developed biofilms. Both phenotypes depend on expression of vps biosynthesis genes. We identified a positive transcriptional regulator of vps gene expression, VpsT, which is homologous to response regulators of two-component regulatory systems. Disruption of vpsT in the rugose variant yields smooth colonies, prevents formation of mature biofilms, and decreases vps gene expression. The interaction between VpsT and VpsR, a previously identified positive regulator of vps genes, was also investigated.
Vibrio cholerae, the causative agent of the disease cholera, is a natural inhabitant of aquatic ecosystems. The pathogen causes periodic, seasonal cholera outbreaks in regions where the disease is endemic and can spread worldwide in pandemics (10). The ability of V. cholerae to cause epidemics is linked to its survival in aquatic habitats.
During its life cycle, V. cholerae undergoes phase variation which results in the generation of two morphologically different variants termed smooth and rugose (23). Compared to smooth variants, rugose variants have increased capacity to produce exopolysaccharide VPSEl Tor, which enables them to form well-developed biofilms and to better resist environmental stresses (14, 21-23, 27). Exopolysaccharide VPSEl Tor production depends on transcription of the vibrio polysaccharide synthesis (vps) genes (27). vps genes are clustered in two regions in the V. cholerae chromosome. One cluster harbors genes vpsA through vpsK, and the other one harbors genes vpsL through vpsQ. Rugose variants lacking vpsA or vpsL do not produce VPSEl Tor and exhibit a smooth colonial morphology (27). One positive regulator of vps genes, VpsR (25), and two negative regulators, HapR (5, 8, 28) and CytR (6), have been identified. In this communication, we report the identification of a second positive regulator of vps genes, designated VpsT, which is required for the formation of a corrugated colonial morphology, biofilm formation, and vps gene expression in the rugose variant. We further show that VpsT and VpsR positively autoregulate their own expression and also form a complex regulatory network by positively regulating each other's expression.
Identification of vpsT.
Whole-genome expression profiling of exponentially grown smooth and rugose variants revealed that expression of gene VCA0952 (TIGR annotation; now named vpsT) was fourfold elevated in the rugose variant compared to that in the smooth variant (F. H. Yildiz et al., submitted for publication). The vpsT gene product is 671 bp long and is predicted to encode a 224-amino-acid, 25.8-kDa protein that is similar to proteins that belong to the UhpA (FixJ) family of transcriptional response regulators (12, 15). VpsT is homologous (44% homology and 65% similarity) to the transcriptional regulators CsgD and AgfD from Escherichia coli and Salmonella enterica, respectively. CsgD and AgfD are required for the production of extracellular matrix components, cellulose and curli fimbriae, which are important for the development of wrinkled colonies and biofilm formation in these bacteria (3, 4, 16, 17).
VpsT affects colony morphology.
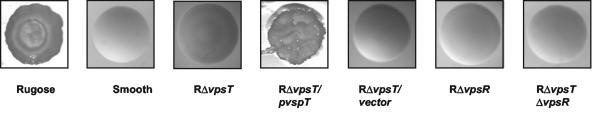
In order to determine the role of vpsT in maintaining the rugose colonial morphology and associated phenotypes, we deleted vpsT in the V. cholerae O1 El Tor rugose variant (FY_Vc_0004) (Table 2), designated the RΔvpsT mutant (FY_Vc_0005). Deletions were done according to the modified method of Horton (2, 7, 9), using the VCA0952 primer set listed in Table 1. The RΔvpsT mutant exhibited a smooth colonial morphology on Luria-Bertani (LB) agar plates (Fig. 1), indicating that the vpsT gene product is required for the formation of a rugose colonial morphology. To verify that the smooth colony phenotype of the RΔvpsT mutant was caused by the vpsT deletion, we amplified the wild-type copy of vpsT, including 559 bp upstream and 380 bp downstream, and cloned it into the low-copy-number plasmid pACYC177, generating pCC17. Introduction of pCC17 into the RΔvpsT mutant resulted in conversion of the smooth colonial morphology to the wild-type rugose colonial morphology (Fig. 1). We also found that vpsT cloned from either the smooth (pCC14) or the rugose (pCC16) variant complemented the RΔvpsT mutant, indicating that this gene is not physically altered during phase variation. Introduction of the cloning vector alone did not result in complementation. Deletion of the previously identified transcriptional regulator VpsR (using primer set VC0665) (Table 1) in the rugose variant also resulted in formation of a smooth colonial morphology (25). To determine any epistasis between the two positive transcriptional regulators, we generated a ΔvpsR ΔvpsT double mutant in the rugose variant and observed that the colonial morphologies of the single and double mutants did not differ in their characteristics (Fig. 1). Taken together, these results indicate that both VpsT and VpsR are involved in the formation of corrugated colonies.
TABLE 2.
Plasmids and strains used in this study
| Plasmid or strain | Properties | Reference or source |
|---|---|---|
| Plasmid | ||
| pCC2 | pGP704sac-28ΔlacZ | This study |
| pCC9 | pGP704sac-28ΔvpsT | This study |
| pCC27 | pGP704sac-28ΔvpsR | This study |
| pGP704sac-28 | Laboratory collection | |
| pRS415 | 19 | |
| pCC11 | pRS415 vpsA promoter | This study |
| pCC12 | pRS415 vpsL promoter | This study |
| pCC25 | pRS415 vpsT promoter | This study |
| pCC10 | pRS415 vpsR promoter | This study |
| pCC14 | pCR 2.1-TOPO SvpsTa | This study |
| pCC16 | pCR 2.1-TOPO RvpsT | This study |
| pACYC177 | Fermentas | |
| pCC17 | pACYC177 RvpsT | This study |
| GFP | pv25.1 | 20 |
| Strain | ||
| FY_Vc_0001 | O1 El Tor, Inaba, smooth, Rifr | 26 |
| FY_Vc_0002 | O1 El Tor, Inaba, rugose, Rifr | 25 |
| FY_Vc_0003 | smoothΔlacZ | This study |
| FY_Vc_0004 | rugoseΔlacZ | This study |
| FY_Vc_0005 | RΔvpsT ΔlacZ | This study |
| FY_Vc_0006 | RΔvpsR ΔlacZ | This study |
| FY_Vc_0007 | RΔvpsR ΔvpsT ΔlacZ | This study |
S denotes the gene that originated from a smooth variant; R denotes the gene that originated from a rugose variant.
TABLE 1.
Primers used in this study
| Primer | Sequencea |
|---|---|
| VC2338_A | CATCACCCTAAGCAGCAG |
| VC2338_B | TTTACTCCTCGGCTTGCCACAATAAGCCAGAG AGC |
| VC2338_C | ATTGTGGCAAGCCGAGGAGTAAAGAAG |
| VC2338_D | GCCAGACCACCACGATGATAACCAATC |
| VCA0952_A | GTCCCATGGCGATATTGTGGGTGGACGC |
| VCA0952_B | TGTTAGGAGTCTCTTCGAGTTTTAGTTCAATA GGC |
| VCA0952_C | CTCGAAGAGACTCCTAACACATCAAGGCTAAC ATG |
| VCA0952_D | CTCGAGCTCGATACGCTATGAATAAATTGAGT GGTAAG |
| VCA0952_rev_prom | GCGCTATCTTTTGTTTACTTGACGC |
| VC0665_AII | CCGCCATGGGCTCCTGACCAATACTCACACTA TCCG |
| VC0665_BII | GTTGCACGCGCAGTCTCGACAAATTACGCATC GG |
| VC0665_C | TTCCGTATGGCGCGTGCAACCATGTATCGC |
| VC0665_D | CTGGAGCTCGCTCAAATCATTGCCCATTGCG |
| vpsL_prom_5′ | GGATCCTGAGTGAGTGACATATTGTTCTGTTT TTCCT |
| vpsL_prom_3′ | GGATCCTTAGTTAGTTAGTACTGAATCCATA CGGAAT |
| vpsA_prom_5′ | GGATCCTGAGTGAGTGATTTCTTAGCAAGGCG AAT |
| vpsA_prom_3′ | GGATCCTTAGTTAGTTACAGAGGTGCCATTTT GAT |
| vpsR_prom_5′ | GGATCCTGAGTGAGTGAACGATGCTGAAGAC CAAGAT |
| vpsR_prom_3′ | GGATCCTTAGTTAGTTAGTACTGAATCCATAC GGAAT |
| lacZrev4 | GCCAGTGAATCCGTAATCATGGTC |
Restriction sites are underlined and additional primer sequences that anneal with another primer sequence are shown in italics.
FIG. 1.
VpsT is required for the rugose colonial morphology. Colonial morphology of the smooth and rugose variants and the indicated deletion mutants formed on LB plates after 2 days of incubation at 30°C is shown.
VpsT affects biofilm formation.
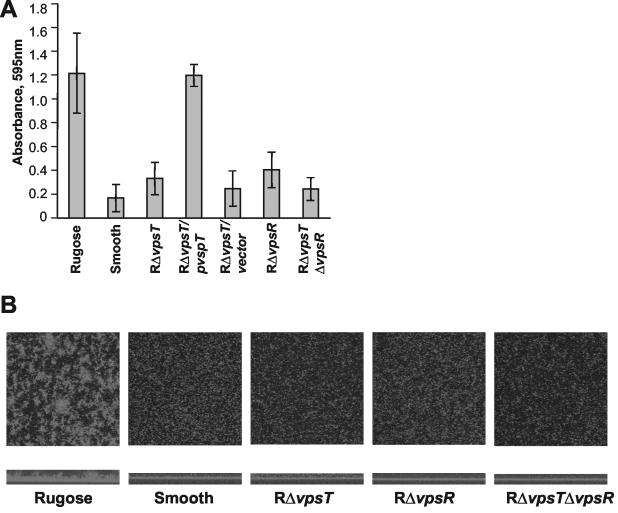
In the rugose variant, formation of corrugated colonies and well-developed biofilms depend on VPSEl Tor production. To determine whether VpsT influences biofilm formation, we compared quantitative and qualitative differences in biofilms of the RΔvpsT mutant to those of the rugose and smooth variants. For quantitative analysis, biofilms were formed on polyvinyl microtiter plates. After 8 h of growth in LB medium at 30°C under static conditions, biofilms were quantified by crystal violet staining (1). The results presented in Fig. 2A show that under the conditions tested the rugose variant formed six times more biofilm than the smooth variant. The biofilm-forming capacity of the RΔvpsT mutant was reduced to the level of that of the smooth variant. Complementation of the RΔvpsT mutant by pCC17 restored biofilm formation to wild-type levels. The cloning vector alone did not result in complementation. The biofilm formation of the RΔvpsR mutant and of the RΔvpsR ΔvpsT double mutant was similar to that of the RΔvpsT mutant and also to that of the smooth variant (Fig. 2A). The growth rates of the strains were similar (data not shown), indicating that the differences in biofilm formation were not due to different growth rates.
FIG. 2.
Biofilm formation of vpsT and vpsR mutants. (A) Biofilm biomass measured with crystal violet-staining assay. The figure shows the average and standard deviation of three independent measurements for each mutant and for the wild-type parents. (B) Confocal scanning laser microscopic images of biofilms formed on borosilicate cover glass chambers. Top panels, horizontal projections; bottom panels, vertical projections.
To compare the biofilm morphologies of the wild-type variants and the mutants, we introduced plasmid pV25, harboring a gene constitutively expressing the green fluorescent protein (20), into each strain. Biofilms were formed on a borosilicate cover glass in LB medium at 30°C under static conditions and analyzed after 12 h by confocal scanning laser microscopy. Horizontal projected views (Fig. 2B) show that the rugose variant formed biofilms with distinct islands, whereas the smooth variant mainly attached as evenly spread-out single cells. Vertical views of the same biofilms show that the rugose biofilm (approximately 60 μm) was about five times thicker than the smooth biofilm and displayed distinctive structures. Surface colonization of the RΔvpsR and RΔvpsT mutants and the RΔvpsR ΔvpsT double mutant was similar to that of the smooth variant. Together these results suggest that VpsR and VpsT both contribute to biofilm formation in the rugose variant.
VpsT induces vps gene expression.
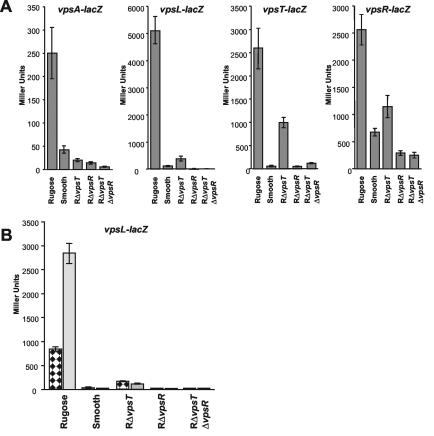
The RΔvpsT mutant is affected in its capacity to form rugose colony morphology and biofilms, which both depend on vps gene expression (6, 25). As VpsT is homologous to response regulators of two-component signal transduction systems, we examined whether VpsT affects vps gene expression. For the expression analysis, we chose vpsA (VC0917) and vpsL (VC0934), which are the first genes of the two vps operons (27), respectively. We constructed transcriptional fusions of the upstream regulatory sequences of vpsA (574 bp upstream) and vpsL (565 bp upstream) to the β-galactosidase (lacZ) gene. To this end, vpsAp and vpsLp were amplified and cloned upstream of promoterless lacZ in vector pRS415, yielding plasmids pCC11 and pCC12, respectively. Both plasmids and the parent vector (pRS415) (19) were introduced into the smooth, rugose RΔvpsT and RΔvpsR mutants and the RΔvpsR ΔvpsT double mutant. Transcription was measured by determining β-galactosidase activity (13) of cultures grown to mid-exponential phase (optical density at 600 nm, 0.3 to 0.4) in LB medium at 30°C by shaking. Figure 3A shows that vpsA transcription was five times higher in the rugose variant than in the smooth variant. Deletion of the vpsT gene in the rugose variant resulted in a significant reduction of vpsA gene transcription. Deletion of vpsR resulted in a similar decrease in vpsA gene transcription. The RΔvpsR ΔvpsT double mutant had low vpsA gene expression, similar to that for the individual deletion mutants. Strains transformed with vector pRS415 had no β-galactosidase activity (data not shown). vpsL transcription was forty times higher in the rugose variant than in the smooth variant. Transcription of vpsL was markedly (13-fold) decreased in the RΔvpsT mutant. In contrast, in the RΔvpsR mutant and the RΔvpsR ΔvpsT double mutant, vpsL transcription was below the detection level. The results indicate that VpsT and VpsR are both required for maximal transcription of the vpsA and vpsL promoters in the rugose variant during the logarithmic growth phase. Transcription of vpsL was higher than that of vpsA in all tested strains. The relative effects of VpsT were similar on vpsA and vpsL transcription. VpsR deletion, on the other hand, had a stronger effect on vpsL transcription than on vpsA transcription. The results suggest that the regulation of these two genes is different in the rugose variant. Interestingly, there is a computationally identified VpsR binding site upstream of vpsL but not upstream of vpsA (Yildiz et al., submitted). It remains to be determined if the actions of VpsT and VpsR are indeed mediated by the direct binding to the vpsL and vpsA promoter regions or through other regulatory proteins. Complex regulation of exopolysaccharide biosynthesis genes is a common phenomenon. In the alginate biosynthesis pathway of Pseudomonas aeruginosa, response regulators AlgB and AlgR are both required as positive regulators of algD, the first gene of the alginate biosynthetic operon (11, 24). Furthermore, activation of the eps operon, which harbors genes required for exopolysaccharide I production in Ralstonia solanacearum, is mediated by two response regulators that are themselves under the control of a complex regulatory network (18).
FIG. 3.
vps gene expression in logarithmic, planktonic, and biofilm growth states. (A) Expression of vpsA, vpsL, vpsT, and vpsR in logarithmically grown wild-type variants and mutants. (B) vpsL transcription in planktonic cells (stippled bars) and in biofilm cells (grey bars) in the indicated mutants. Error bars indicate the standard deviation. Note the different y axis scales in all panels.
To determine a possible interaction between the two positive regulators, we analyzed vpsT and vpsR transcription in the described strains (Fig. 3). To this end, we constructed vpsRp-lacZ and vpsTp-lacZ transcriptional fusions by amplifying the upstream regulatory sequences of vpsT (primers VCA0952_C and rev_prom) and vpsR (primers vpsR_prom_5′ and vpsR_prom_3′) and cloning them into pRS415. Transcription of vpsT and vpsR was determined during exponential growth (optical density at 600 nm, 0.3 to 0.4) in LB medium at 30°C by measuring β-galactosidase activity. The results revealed that vpsT transcription was 45 times higher in the rugose variant than in the smooth variant, confirming the trend of the initial microarray experiment (Yildiz et al., submitted). Furthermore, vpsT transcription was 2.5-fold lower in the RΔvpsT mutant than in the rugose variant, indicating that VpsT positively regulates its own expression. Deletion of vpsR from the rugose variant caused a 50-fold reduction in vpsT expression. vpsT expression in the RΔvpsR RΔvpsT double mutant was similar to that in the RΔvpsR mutant.
We also determined vpsR transcription in wild-type phase variants and the mutant strains. Figure 3 shows that vpsR transcription was fourfold higher in the rugose variant than in the smooth variant. The experiments also showed that, compared to that for the rugose variant, vpsR transcription was decreased twofold in the RΔvpsT mutant and ninefold in the RΔvpsR mutant and in the RΔvpsR ΔvpsT double mutant. The results indicate that VpsT and VpsR positively regulate vpsR expression and that VpsR had a more dramatic effect on its own expression.
Next we examined whether vpsA and vpsL transcription differ in planktonic and biofilm cells. For these measurements, overnight-grown cultures were diluted in LB medium, inoculated into polystyrene petri plates, and incubated at 30°C under static conditions for 12 h. β-Galactosidase activities of planktonic and attached bacteria were compared for each of the strains. In the rugose variant, vpsL transcription was three times higher in the biofilm cells than in the planktonic cells (Fig. 3B). This result is similar to measurements in the V. cholerae O139 strain (6). The smooth variant formed less-developed biofilms. Significantly, biofilm cells of the smooth variant did not have an increased vpsL transcription compared to that of planktonic cells. vpsL transcription in smooth planktonic and biofilm cells was 30- and 100-fold lower, respectively, than expression in the corresponding rugose cells. When vpsT was deleted from the rugose variant, the biofilm growth-dependent vpsL induction did not occur. In addition, vpsL transcription in the RΔvpsT mutant planktonic and biofilm cells was 5- and 25-fold lower than that for the respective rugose variant cells.
Deletion of vpsR in the rugose variant or in the double mutant prevented vpsL transcription altogether, similar to the results obtained from logarithmically grown cells. vpsA transcription in the rugose variant was increased 25% in the biofilm cells compared to that in the planktonic cells (data not shown). In contrast, this induction was not observed in the other strains.
In summary, we have identified VpsT, a positive regulator of vps gene expression. VpsT and the previously identified regulator VpsR (25) are both necessary for maximal vps transcription in the rugose variant. VpsT and VpsR both influence vpsA and vpsL expression and positively regulate their own and each other's expression.
VpsR and VpsT are homologous to response regulators of two-component regulatory systems. Response regulators usually act together with a sensor histidine kinase. Sensor histidine kinase(s) that regulates expression of vps genes and in turn the development of the rugose colonial morphology of V. cholerae O1 El Tor has not been identified thus far and is under investigation.
V. cholerae occupies different niches during its life cycle and is likely to be exposed to fluctuating environmental conditions (26). Two-component signal transduction systems are involved in sensing and responding to environmental stimuli. Future work will focus on the identification of environmental signals sensed by signal transduction systems involving VpsR and VpsT and on the importance of the processes regulated by these two regulators in the adaptation responses of the pathogen.
Acknowledgments
This work was supported by grants from UC TSR&TP, the Ellison Medical Foundation, and NIH (5R01AI055987-02). F.H.Y. is a new scholar in the Ellison Medical Foundation Global Infectious Diseases Program.
We thank Uyen Tram and William Sullivan for help with the confocal microscopy and Sofie Salama and Karen Ottemann for critical reading of the manuscript.
REFERENCES
- 1.Christensen, G. D., L. Baldassarri, and W. A. Simpson. 1995. Methods for studying microbial colonization of plastics. Methods Enzymol. 253:477-500. [DOI] [PubMed] [Google Scholar]
- 2.Fullner, K. J., and J. J. Mekalanos. 1999. Genetic characterization of a new type IV-A pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect. Immun. 67:1393-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstel, U., and U. Romling. 2001. Oxygen tension and nutrient starvation are major signals that regulate agfD promoter activity and expression of the multicellular morphotype in Salmonella typhimurium. Environ. Microbiol. 3:638-648. [DOI] [PubMed] [Google Scholar]
- 4.Hammar, M., A. Arnqvist, Z. Bian, A. Olsen, and S. Normark. 1995. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 18:661-670. [DOI] [PubMed] [Google Scholar]
- 5.Hammer, B. K., and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-104. [DOI] [PubMed] [Google Scholar]
- 6.Haugo, A. J., and P. I. Watnick. 2002. Vibrio cholerae CytR is a repressor of biofilm development. Mol. Microbiol. 45:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton, R. M., S. N. Ho, J. K. Pullen, H. D. Hunt, Z. Cai, and L. R. Pease. 1993. Gene splicing by overlap extension. Methods Enzymol. 217:270-279. [DOI] [PubMed] [Google Scholar]
- 8.Jobling, M. G., and R. K. Holmes. 1997. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Mol. Microbiol. 26:1023-1034. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre, B., P. Formstecher, and P. Lefebvre. 1995. Improvement of the gene splicing overlap (SOE) method. BioTechniques 19:186-188. [PubMed] [Google Scholar]
- 10.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, S., U. Selvaraj, D. E. Ohman, R. Quarless, D. J. Hassett, and D. J. Wozniak. 1998. Phosphorylation-independent activity of the response regulators AlgB and AlgR in promoting alginate biosynthesis in mucoid Pseudomonas aeruginosa. J. Bacteriol. 180:956-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maris, A. E., M. R. Sawaya, M. Kaczor-Grzeskowiak, M. R. Jarvis, S. M. Bearson, M. L. Kopka, I. Schroder, R. P. Gunsalus, and R. E. Dickerson. 2002. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 9:771-778. [DOI] [PubMed] [Google Scholar]
- 13.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 14.Mizunoe, Y., S. N. Wai, A. Takade, and S. I. Yoshida. 1999. Isolation and characterization of rugose form of Vibrio cholerae O139 strain MO10. Infect. Immun. 67:958-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 16.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romling, U., Z. Bian, M. Hammar, W. D. Sierralta, and S. Normark. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180:722-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schell, M. A. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu. Rev. Phytopathol. 38:263-292. [DOI] [PubMed] [Google Scholar]
- 19.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 20.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 21.Wai, S. N., Y. Mizunoe, A. Takade, S. I. Kawabata, and S. I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, B. P. 1938. The rugose variant of Vibrios. J. Pathol. 46:1-6. [Google Scholar]
- 24.Wozniak, D. J., and D. E. Ohman. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. J. Bacteriol. 176:6007-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildiz, F. H., N. A. Dolganov, and G. K. Schoolnik. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPSETr-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]