Abstract
OBJECTIVE
This study investigated the cost-effectiveness of treating mild gestational diabetes mellitus (GDM).
STUDY DESIGN
A decision analytic model was built to compare treating vs not treating mild GDM. The primary outcome was the incremental cost per quality-adjusted life year (QALY). All probabilities, costs, and benefits were derived from the literature. Base case, sensitivity analyses, and a Monte Carlo simulation were performed.
RESULTS
Treating mild GDM was more expensive, more effective, and cost-effective at $20,412 per QALY. Treatment remained cost-effective when the incremental cost to treat GDM was less than $3555 or if treatment met at least 49% of its reported efficacy at the baseline cost to treat of $1786.
CONCLUSION
Treating mild GDM is cost-effective in terms of improving maternal and neonatal outcomes including decreased rates of preeclampsia, cesarean sections, macrosomia, shoulder dystocia, permanent and transient brachial plexus injury, neonatal hypoglycemia, neonatal hyperbilirubinemia, and neonatal intensive care unit admissions.
Keywords: cost-effectiveness, decision analysis, gestational, diabetes mellitus
Gestational diabetes mellitus (GDM), defined as glucose intolerance that first occurs or is first identified in pregnancy, is thought to occur in 2–5% of all pregnancies and along with the rate of obesity is rising in frequency in the United States.1 It is associated with higher rates of preeclampsia, operative deliveries, macrosomia, shoulder dystocia, and birth injuries.2
Current recommendations from the American College of Obstetricians and Gynecologists are that all patients be screened during the second trimester of pregnancy, those that screen positive have a confirmatory test, and those that are diagnosed with GDM be treated first by medical nutrition therapy (diet), and if that fails, then with insulin.1 However, the US Preventative Services Task Force in their 2008 recommendation stated that there was insufficient evidence to support screening for GDM and that it was uncertain whether treatment led to improved health outcomes.3,4
Until recently the best evidence for treating GDM was from a multicenter study (Australian Carbohydrate Intolerance Study in Pregnant Women [ACHOIS]) that randomized women diagnosed with “glucose intolerance of pregnancy” by pre-1998 World Health Organization (WHO) criteria (fasting glucose <140 mg/dL and a 75 g, 2 hour oral glucose tolerance test between 140 and 198 mg/dL) to treatment vs no treatment. The results showed that treating these women with GDM with dietary advice, glucose monitoring, and insulin if necessary reduced serious perinatal morbidities, including neonatal death, shoulder dystocia, bone fracture, and nerve palsy.5
A more recent (2009) multicenter randomized controlled trial (RCT) investigated women with mild GDM, defined as a normal fasting glucose (<95 mg/dL) but 2 or more values exceeding the postprandial thresholds of the Carpenter-Coustan criteria after a 100 g, 3 hour oral glucose tolerance test, which is commonly used in the United States as opposed to the WHO criteria. Women were assigned to receive formal nutritional counseling, diet therapy, and insulin if required (treatment group) or usual prenatal care (control group). The results showed that treating mild GDM resulted in reduced risks of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders.6
In the setting of wide variations in the definition and management of GDM, cost-effectiveness analyses on the results of clinical trials are an integral component of public policy decision making. Understanding the cost-effectiveness of treating mild GDM is particularly important because a normal fasting glucose in the setting of abnormal postprandial glucose levels represents the margin of GDM care because its definition and clinical implications continue to evolve as a result of ongoing research efforts. To date, there has been no cost-effectiveness analysis on treating mild GDM based on the most recent clinical trial results.
Materials and Methods
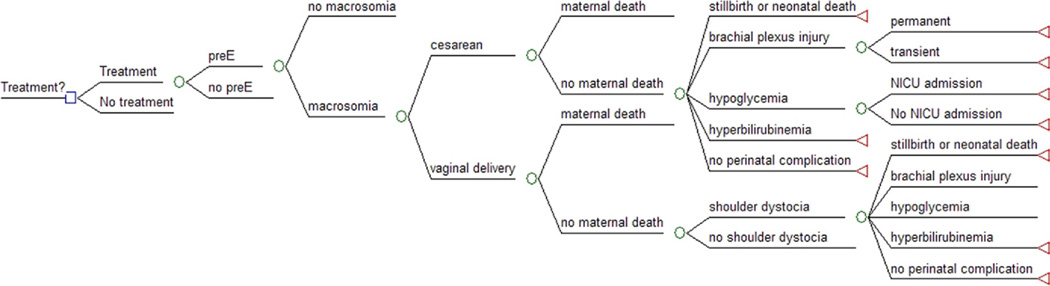
A decision-analytic model (Figure 1) from the societal perspective was created using TreeAge Pro (version 2009; Tree-Age, Williamstown, MA) to simulate a cohort of pregnant women diagnosed with mild GDM and divided into a treatment and no treatment arm. Because no human subjects were involved in creating this theoretical model, this study was exempt from institutional review board approval. Maternal outcomes in the model included preeclampsia, shoulder dystocia, cesarean vs vaginal delivery, and maternal death. Neonatal outcomes included macrosomia (>4000 g), permanent or transient brachial plexus injury, hypoglycemia, admission to a neonatal intensive care unit (NICU), hyperbilirubinemia, and neonatal death. All probabilities, costs, and utilities were derived from the literature. A cost-effectiveness threshold of $100,000 per quality-adjusted life year (QALY) was used.7
FIGURE 1. The decision analytic model comparing treating vs not treating mild GDM.
Not all branches are shown to facilitate display. Lines that do not terminate in a circle or a triangle indicate they are collapsed to facilitate display and are the same as branches that are already open.
GDM, gestational diabetes mellitus.
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
Probabilities
The baseline probabilities for preeclampsia, macrosomia, cesarean delivery, neonatal death, and NICU admission and how treatment affected these probabilities were derived directly from a 2009RCTon treating mild GDM (Table 1).6 Because the presence of macrosomia affects the probability of shoulder dystocia, brachial plexus injury, hypoglycemia, and hyperbilirubinemia, these probabilities were derived from a separate body of work that examined these factors independently in patients with GDM (Table 2).8,9 To estimate the effect of GDM treatment on shoulder dystocia, brachial plexus injury, hypoglycemia, and hyperbilirubinemia, their baseline probabilities were multiplied by the relative risk associated with treatment published in the 2009 RCT.6 Although treating mild GDM affected the probability of brachial plexus injury occurring, once it occurred, the probability that it would be permanent (vs transient)9 did not change because there were no data to support how treatment affected the severity of a brachial plexus injury. Probabilities for maternal death were derived from the literature.10
TABLE 1.
Probabilities, costs, and utilities used in the decision analytic model of treatment vs no treatment of mild GDM
| Parameter | Probabilities | Utilities | Costs | Reference | |
|---|---|---|---|---|---|
| GDM treatment | $1786 | 16 | |||
| Maternal outcomes | Without treatment | With treatment | |||
| Preeclampsia | 0.136 | 0.086 | $19,184 | 6,12 | |
| Cesarean delivery | 0.338 | 0.269 | 0.99 | $11,979 | 6,13,19,22 |
| Vaginal delivery | 1 | $7790 | 13,21 | ||
| Maternal death | |||||
| Cesarean | 0.000022 | 0.000022 | 0 | $100,000 | 10,17 |
| Vaginal | 0.000002 | 0.000002 | 0 | $100,000 | 10,17 |
| Shoulder dystocia | |||||
| With macrosomia | 0.105 | 0.03885 | 6,8 | ||
| Without macrosomia | 0.016 | 0.00592 | 6,8 | ||
| Neonatal outcomes | |||||
| Macrosomia | 0.143 | 0.059 | 6 | ||
| Brachial plexus injury | Please see Table 2 for the probabilities of brachial plexus injury | ||||
| Permanent | 0.067 | 0.067 | 0.6 | $15,699 | 6,9,18 |
| Transient | 0.99 | $1757 | 6,9,18 | ||
| Hypoglycemia | 1 | $2419 | 6,8,14 | ||
| With macrosomia | 0.053 | 0.05618 | |||
| Without macrosomia | 0.026 | 0.02756 | |||
| Hyperbilirubinemia | 1 | $2006 | 6,8,26 | ||
| With macrosomia | 0.132 | 0.0977 | |||
| Without macrosomia | 0.104 | 0.077 | |||
| NICU admission | 0.116 | 0.09 | 1 | $15,065 | 6,14,21 |
| Neonatal death | 0 | 0 | $82,361 | 6,15,21,22 | |
| Maternal perspective | 0.92 | ||||
| Neonatal perspective | 0 | ||||
GDM, gestational diabetes mellitus; NICU, neonatal intensive care unit.
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
TABLE 2.
| Variable | Macrosomia | |
|---|---|---|
| Yes | No | |
| Without treatment | ||
| Shoulder dystocia | ||
| Yes | 0.18000 | 0.09000 |
| No | 0.00790 | 0.00057 |
| With treatment | ||
| Shoulder dystocia | ||
| Yes | 0.08640 | 0.04320 |
| No | 0.00379 | 0.00027 |
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
Costs
All costs are in 2009 US dollars and inflated using the medical component of the consumer price index (Table 1).11–15 Of note, the cost of treating GDM was set at a baseline of $1786, which included pharmacotherapy, antenatal visits, ancillary diabetes-related visits, and antepartum fetal surveillance; it did not include the cost of screening, diagnosis, or antepartum admissions for GDM-related conditions nor broader indirect costs.16 For maternal mortality, because it is the most difficult to quantify with a point estimate because the values vary based on the circumstances of death, a baseline of $100,000 was used, and this figure was also subject to sensitivity analysis.17 For brachial plexus injury, published costs for management were $15,699 for permanent injury and $1757 for transient injury.18 All costs were subject to sensitivity analysis.
Utilities
All utilities were derived from the literature and included utilities from the maternal and neonatal perspective (Table 1). The utility of a vaginal delivery was assumed to be 1, and the utility of a cesarean delivery was set to a baseline of 0.99 based on previously published research on women’s preference in mode of delivery.19–21 Maternal death by definition was set to a utility of 0. The utility of a neonatal death from the maternal perspective was set at a baseline of 0.92, which is the published maternal utility of a miscarriage and thus applied over the maternal lifetime.21,22 The utility of a neonatal death from the neonatal perspective was by definition 0 and applied over the neonatal lifetime. The neonatal utility of a transient brachial plexus injury was set to a baseline of 0.99 based on brachial plexus injury that resolves within 2 months18; for a permanent brachial plexus injury, a conservative value of 0.6 was used based on a published value for mild to moderate injury, compared with a utility of 0.45 for a severe injury.18 Both utilities for brachial plexus injury were applied over the neonatal lifetime. Because there are no published utilities on short-term neonatal consequences such as hypoglycemia, NICU admission, or hyperbilirubinemia, these utilities were set to a conservative baseline value of 1.21 Utilities were calculated over the course of maternal life expectancy (56.1 years) and neonatal life expectancy (77.2 years)23 at a discount rate of 3%.7 All utilities were subject to sensitivity analysis.
Analysis
Initial analysis compared treating vs not treating mild GDM using baseline values to estimate differences in maternal and neonatal outcomes, total costs and QALYs for each strategy, and the incremental cost-effectiveness ratio. Next, deterministic sensitivity analyses were performed on all probabilities, costs, and utilities to better understand the variables that had the most influence on the outcomes and to test the robustness of the model.
An additional variable was introduced in the sensitivity analysis, which was a measure of the efficacy of treatment. Its purpose was to account for variations in practice that can result in a range of outcomes. This variable was defined as 0 equal to treatment being ineffective, or no difference between the treatment and no treatment values, and increased linearly to a value of 1 meaning perfect efficacy, or 100% attainment of the expected difference in outcomes between the treatment and no-treatment groups.
Finally, a Monte Carlo simulation was created to simultaneously vary all of the models inputs across a distribution of values for a theoretical cohort of 10,000 random women with GDM. Because of its inherent randomness, Monte Carlo simulations can be used to extrapolate real-world outcomes from a stochastic model by changing multiple variables simultaneously. One simulation represents a woman undergoing the decision to treat or not treat mild GDM, and her probabilities and costs are randomly chosen from a prespecified distribution; this simulation is repeated with a different set of randomly chosen values and the aggregate represents a theoretical cohort of random women.21 Based on these simulations, an acceptability curve can be constructed that shows the probability of achieving cost-effectiveness. To adopt the deterministic model to a stochastic one for the Monte Carlo simulation, costs were modeled using a gamma distribution and probabilities were modeled using a beta distribution.
Results
Maternal and neonatal outcomes associated with treatment of a theoretical cohort of 100,000 women were estimated (Table 3). Treatment decreased all maternal outcomes, including shoulder dystocia (1902 without treatment vs 575 with treatment), cesarean delivery (33,800 without treatment vs 26,900 with treatment), and preeclampsia (13,600 without treatment vs 8600 with treatment). Furthermore, all neonatal outcomes were also decreased, including macrosomia (14,300 without treatment vs 5900 with treatment), permanent brachial plexus injury (28 without treatment vs 5 with treatment), transient brachial plexus injury (386 without treatment vs 74 with treatment), hypoglycemia (2986 without treatment vs 2925 with treatment), and hyperbilirubinemia (10,800 without treatment vs 7818 with treatment).
TABLE 3.
Maternal and neonatal outcomes in treating vs not treating mild GDM per 100,000 women
| Outcome | Without treatment | With treatment |
|---|---|---|
| Maternal outcomes | ||
| Preeclampsia | 13,600 | 8600 |
| Cesarean delivery | 33,800 | 26,900 |
| Shoulder dystocia | 1902 | 575 |
| Neonatal outcomes | ||
| Macrosomia | 14,300 | 5900 |
| Permanent brachial plexus injury | 28 | 5 |
| Transient brachial plexus injury | 386 | 74 |
| Hypoglycemia | 2986 | 2925 |
| Hyperbilirubinemia | 10,800 | 7818 |
GDM, gestational diabetes mellitus.
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
The baseline cost-effectiveness analysis results are that treating GDM is more expensive at $12,623 vs $12,167 for no treatment but more effective shown by higher QALYs at 56.891002 vs 56.868753 for no treatment (Table 4). The incremental cost per QALY is cost-effective (below the cost-effectiveness threshold of $100,000/QALY) at $20,412/QALY.
TABLE 4.
Cost-effectiveness analysis of treating vs not treating mild GDM
| Variable | Costs | QALYs | $/QALY |
|---|---|---|---|
| Treatment | $12,623 | 56.891002 | $20,412 |
| No treatment | $12,167 | 56.868753 | Baseline |
| Treatment is more expensive | Treatment has higher QALYs | ||
| Cost threshold in which treatment is more expensive vs no treatment: $1330 | |||
| Cost threshold in which treatment is no longer cost-effective: $3555 | |||
GDM, gestational diabetes mellitus; QALY, quality-adjusted life year.
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
Sensitivity analysis
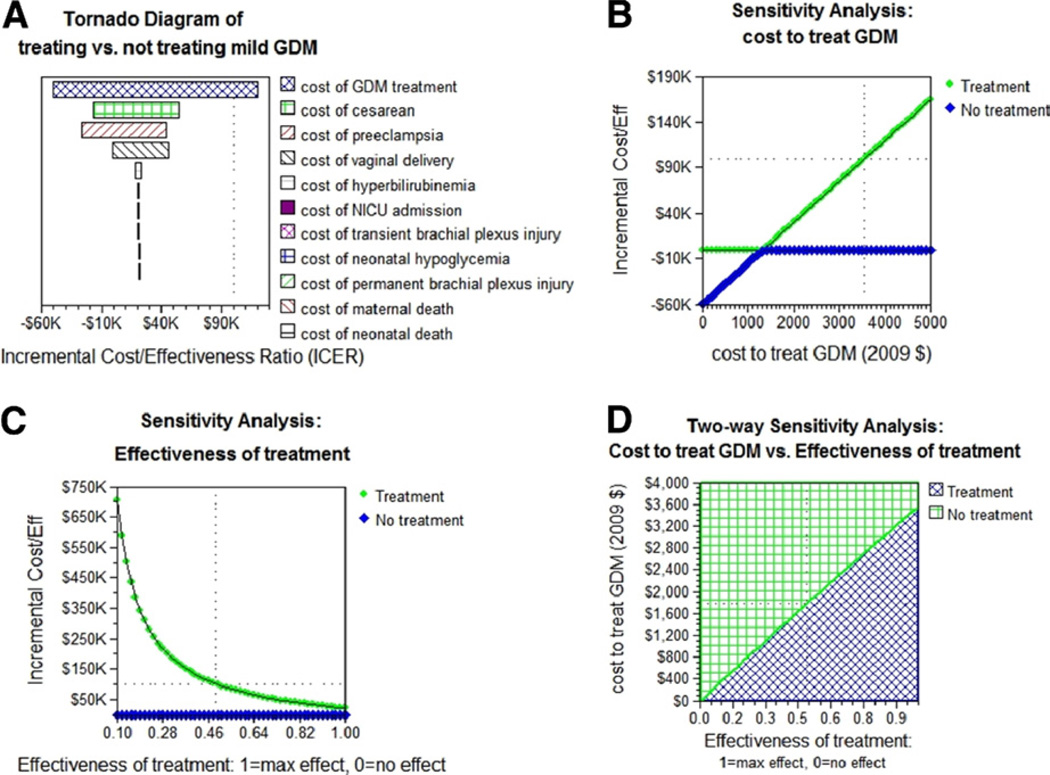
Univariate sensitivity analyses were conducted on all probabilities, costs, and utilities (Figure 2). The model remained robust across reasonable ranges for all of the variables. The only cost that made treatment no longer cost-effective (>$100,000/QALY) was the incremental cost of GDM treatment (Figure 2, A). Treating mild GDM was dominant (less expensive and more effective vs no treatment) until the incremental cost to treat GDM reached $1330. Above $1330, treating was more expensive but more effective vs no treatment and was still cost-effective (<$100,000/QALY) as long as the cost to treat GDM was less than $3555 (Figure 2, B).
FIGURE 2. Sensitivity analyses.
A, Tornado diagram showing how varying the costs affects the incremental cost-effectiveness ratio (ICER). Only increasing the incremental cost of treating GDM above $3555 can make treating GDM not cost-effective (ICER >$100,000/QALY). B, Univariate sensitivity analysis on the incremental cost to treat mild GDM. At a cost of $1330 to treat, treating mild GDM becomes more expensive than not treating but still cost-effective. Treating mild GDM was no longer cost-effective if the cost to treat was greater than $3555. C, Univariate analysis on the efficacy of treatment. 1, 100% efficacy; 0, no difference between treatment and no treatment. At the baseline cost of $1786 to treat GDM, treating mild GDM is cost-effective as long as treatment meets at least 49% of its expected results. D, Two-way sensitivity analysis on the cost to treat GDM vs efficacy of treatment. The blue-hatch region represents where treating GDM is cost-effective (below the cost-effectiveness threshold of $100,000/QALY). At 100% efficacy, the cost to treat can be as high as $3555 and be cost-effective (baseline case). As the treatment efficacy decreases, the cost at which treating GDM no longer becomes cost-effective decreases. At the baseline cost of $1786 to treat GDM, treatment can be as low as 49% and still be cost-effective.
GDM, gestational diabetes mellitus; QALY, quality-adjusted life year.
Ohno. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am J Obstet Gynecol 2011.
Univariate sensitivity analysis on the efficacy of treatment showed that at the baseline cost to treat GDM of $1786, treating was cost-effective as long as it was at least 49% efficacious. Because the efficacy of treatment also affected the cost-effectiveness threshold (the lower the efficacy, the lower the cost-effectiveness threshold in which it was no longer cost-effective to treat GDM), 2-way sensitivity analysis was also performed on treatment efficacy vs incremental cost to treat GDM (Figure 2, D). At 100% treatment efficacy, the cost to treat GDM could be as high as $3555 and still be cost-effective; this represents the baseline cost-effectiveness threshold. As the treatment efficacy decreased (Figure 2, D, horizontal axis), the upper limit on the cost to treat GDM that was still cost-effective decreased linearly.
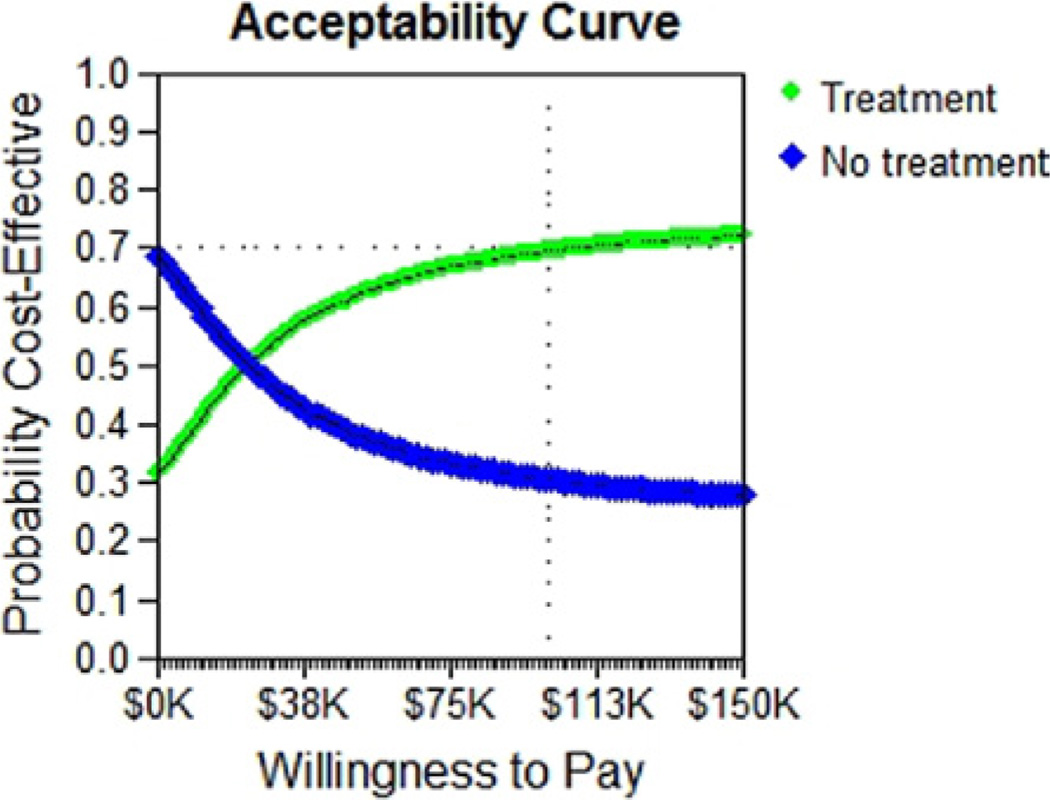
Monte Carlo analysis
A Monte Carlo analysis was performed to simulate the outcome of 10,000 random women with mild GDM. Based on these simulations, an acceptability curve showed that at a cost-effectiveness threshold of $100,000/QALY, there was a 70% probability that treating mild GDM would be cost-effective (Figure 3).
FIGURE 3. Monte Carlo simulation of 10,000 random women.
Monte Carlo simulation of 10,000 random women shows there is a 70% probability that treatment is cost-effective at a threshold of $100,000/quality-adjusted life year.
Ohno. Treating mild gestational diabetes mellitus: a costeffectiveness analysis. Am J Obstet Gynecol 2011.
Comment
This decision analytic model showed that treating GDM was cost-effective as long as the cost to treat GDM was less than $3555 or at the baseline cost to treat GDM of $1786, when the efficacy of treatment met at least 49% of its expected goal. Above a cost of $3555 or below to treat GDM, an efficacy of 49% at baseline costs ($1786), treating GDM was no longer cost-effective in terms of reducing maternal and neonatal adverse outcomes, including preeclampsia, shoulder dystocia, maternal death, macrosomia, permanent and transient brachial plexus injury, neonatal hypoglycemia, neonatal hyperbilirubinemia, and NICU admissions.
Macrosomia, shoulder dystocia, and brachial plexus injury are among the well-described adverse maternal and neonatal outcomes associated with GDM.1 Another perspective on the benefits of treating mild GDM is a number-needed-to-treat (NNT) analysis to calculate the number of women who would need to be treated to decrease the incidence of a complication by 1. NNT analysis of this model showed an NNT of 12 for macrosomia, an NNT of 75 for shoulder dystocia, and an NNT of 320 for transient brachial plexus injury. Furthermore, only 14 women would need to be treated to reduce 1 case of a cesarean section.
The cost-effectiveness results are similar to a cost-consequence analysis of the ACHOIS trial, performed in Australia, which showed that treating mild GDM was more expensive vs routine care but cost-effective at $2186/QALY, based on an incremental cost to treat GDM of $247/patient (converted from 2002 Australian dollars to 2009 US dollars).24 Just as the costs to treat GDM in the United States are an order of magnitude higher than in Australia ($1786 in the United States vs $247 in Australia), the cost per QALY is also similarly higher ($20,412 in the United States vs $2186 in Australia).
Several costs were not included in this model because the clinical trials did not measure the outcomes associated with those costs or the data were not available. First, although antenatal admissions can add to the overall cost of managing women with GDM, they were not included in the costs because this outcome was not measured, and it was unclear how treating GDM would affect this parameter. For example, the cost-consequence analysis of the ACHOIS trial showed that the difference in antenatal admissions between the treatment and routine-care groups was not significant (health services use for antenatal inpatient admissions: 135 for treatment vs 133 for routine-care, adjusted treatment effect 1.11 (95% confidence interval, 0.91–1.36; P = .31).24 Thus, including antenatal admissions is unlikely to significantly affect the cost-effectiveness analysis. Similarly, emergency room visits were not taken into account because again the trials did not measure this outcome, and it is unclear how treating GDM would affect this factor.
Second, indirect costs from GDM such as time off from work were not included because these data were not available. Again, the cost-consequence analysis of the ACHOIS trial surveyed 108 of the 1000 women who participated in the RCT to determine the mean charges to women and their family from randomization to birth. These charges included paid child care, travel to/from appointments, food substitution, time off from work for both the mother and partner, and blood glucose monitoring equipment and consumables. There was no difference between the intervention and routine care group (Australian dollars 2002: $367 for the intervention group vs $302 for the routine-care group, P = .34). If the cost of the blood glucose monitoring equipment and consumables was removed because they were already accounted for in the cost to treat GDM, then the difference was even smaller ($314 for the intervention group vs $285 for the routine-care group because some in the routine-care group were diagnosed with GDM after randomization).24
Third, although the costs of the various complications associated with GDM were included in this model, the indirect costs associated with these complications, for example, the economic losses from a child with a permanent brachial plexus injury, were not included in this analysis. These costs are likely to be higher in the nontreatment group; thus, this analysis errs on the conservative side on this point.
One limitation of this model is the lack of recent data on the incremental cost to treat GDM (costs above routine care costs) in the United States. Although there are more recent studies that report this cost in other countries that range from $247 to $458,24,25 the most recent study in the United States is from a review published in 2000 in which the incremental costs ranged from $1786 to $3352.16 This analysis showed, however, that as long as the cost to treat GDM was below $3555, a value above the upper range of these cost estimates, treating GDM was cost-effective. More recent data are likely to show that the cost to treat GDM is lower than these 2000 estimates because GDM has become more prevalent and treatment practices more standardized, leading to more efficiently administered perinatal clinics that could absorb the additional costs over a larger patient population, thus lowering the per-patient cost.
In addition, the costs associated with various GDM outcomes represented in this analysis are taken from a wide range of published studies. These studies use varying methodologies to determine said costs and as such may not be valid when combined into a unifying analysis. However, univariate sensitivity analyses were performed over a wide range on all costs, and only the cost to treat GDM crossed the cost-effectiveness threshold.
Another limitation is that the probabilities of the effect of GDM treatment on maternal and neonatal outcomes come from 1 trial. However, this was a well-powered, multicenter, RCT.6 Furthermore, the results of this trial were very similar to the results ACHOIS trial.5 Because the threshold analysis showed the model to be robust over a wide range of probabilities, costs, and utilities, this illustrates that there is a wide margin in which treatment is cost-effective as long as it decreases maternal and/or neonatal outcomes.
Finally, a decision analytic model has its inherent limits in simulating reality. For example, some neonatal outcomes are modeled as discrete, mutually exclusive outcomes; in other words, in this model a neonate cannot have both hypoglycemia and hyperbilirubinemia, even though both outcomes can occur in reality. Adding permutations for every possible combination of outcomes quickly makes decision analytic models unwieldy. However variables that were known to have strong associations, such as the relationship between macrosomia, shoulder dystocia, and brachial plexus injury, were taken into account in this model. For example, a neonate could have shoulder dystocia both in the presence and absence of macrosomia; similarly, shoulder dystocia and macrosomia were both factors that affected the probability brachial plexus injury. Moreover, we were unable to model the long-term downstream effects on the offspring from treatment of GDM. Within increasing evidence from the field of fetal programming, it does appear that there are effects from maternal diet and hyperglycemia that may lead to obesity and type 2 diabetes mellitus in the offspring. However, the incorporation of these results would only have made our findings more robust.
Whereas treating mild GDM is more expensive than not treating, it is also more effective with cost-effectiveness within usual cost-effective thresholds. Because the costs of treating GDM are expected to decrease with increased efficiency in management, technology advances, and lower cost of supplies and pharmaceuticals, it is likely to be both less expensive and more effective in the future. These results in combination with the results of the Hyperglycemia and Adverse Pregnancy Outcome study, which showed a continuous relationship between maternal glucose levels below what is considered overt GDM and various outcomes including birthweight above the 90th percentile and primary cesarean delivery,27 suggest that lowering the thresholds for diagnosing GDM may be cost-effective. Such policy efforts are currently underway,28 and more clinical research is warranted to clearly identify specific cut-off values.
Based on this analysis, the expected value of perfect information29 of such research is expected to be high because there is a small difference in cost-effectiveness between treating and not treating, there is some uncertainty about cost-effective estimates, the consequences of GDM can be serious, and the disease is increasing in prevalence.30
Acknowledgments
Y.W.C. is supported by the University of California, San Francisco, Women’s Reproductive Health Research Career Development Award funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grant no. K12 HD001262).
Footnotes
Presented at the 31st Annual Meeting of the Society for Maternal-Fetal Medicine, San Francisco, CA, Feb. 7–12, 2011.
REFERENCES
- 1.American College of Obstetricians and Gynecologists Committee on Practice Bulletins Obstetrics. Clinical management guidelines for obstetrician-gynecologists. ACOG practice bulletin, no. 30, September 2001 (replaces technical bulletin no. 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 2.Cheng YW, Block-Kurbisch I, Caughey AB. Carpenter-Coustan criteria compared with the National Diabetes Data Group thresholds for gestational diabetes mellitus. Obstet Gynecol. 2009;114:326–332. doi: 10.1097/AOG.0b013e3181ae8d85. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Screening for gestational diabetes mellitus: US Preventative Services Task Force Recommendation Statement. Ann Intern Med. 2008;148:759–765. doi: 10.7326/0003-4819-148-10-200805200-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hillier TA, Vesco KK, Pedula KL, Bell TL, Whitlock EP, Pettitt DJ. Screening for gestational diabetes mellitus: a systematic review for the US Preventative Services Task Force. Ann Intern Med. 2008;148:766–775. doi: 10.7326/0003-4819-148-10-200805200-00009. [DOI] [PubMed] [Google Scholar]
- 5.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 6.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey AB. Cost-effectiveness analysis of prenatal diagnosis: methodological issues and concerns. Gynecol Obstet Invest. 2005;60:11–18. doi: 10.1159/000083480. [DOI] [PubMed] [Google Scholar]
- 8.Esakoff TF, Cheng YW, Sparks TN, et al. The association between birthweight 4000 g or greater and perinatal outcomes in patients with and without gestational diabetes mellitus. Am J Obstet Gynecol. 2009;200:672.e1–672.e4. doi: 10.1016/j.ajog.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Rouse DJ, Owen J. Prophylactic cesarean delivery for fetal macrosomia diagnosed by means of ultrasonography—a Faustian bargain? Am J Obstet Gynecol. 1999;181:332–338. doi: 10.1016/s0002-9378(99)70557-0. [DOI] [PubMed] [Google Scholar]
- 10.Clark SL, Belfort MA, Dildy GA, et al. Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol. 2008;199:36.e1–36.e5. doi: 10.1016/j.ajog.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Inflation calculator. [Accessed Feb. 1, 2011]; Available at: http://www.halfhill.com/inflation.html. [Google Scholar]
- 12.Meads CA, Cnossen JS, Meher S, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2008;12:iii–iv. 1–270. doi: 10.3310/hta12060. [DOI] [PubMed] [Google Scholar]
- 13.Bost BW. Cesarean delivery on demand: what will it cost? Am J Obstet Gynecol. 2003;188:1418–1423. doi: 10.1067/mob.2003.455. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd A, Townsend C, Munro V, Twena N, Nielsen S, Holman A. Cost-effectiveness of insulin as part compared to human insulin in pregnant women with type 1 diabetes in the UK. Curr Med Res Opin. 2009;25:599–605. doi: 10.1185/03007990802668208. [DOI] [PubMed] [Google Scholar]
- 15.Phibbs CA, Schmidt SK. Estimates of the cost and length of stay changes that can be attributed to one-week increases in gestational age for premature infants. Early Hum Dev. 2006;82:85–95. doi: 10.1016/j.earlhumdev.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzmiller JL. Cost analysis of diagnosis and treatment of gestational diabetes mellitus. Clin Obstet Gynecol. 2000;43:140–153. doi: 10.1097/00003081-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Grobman WA, Peaceman AM, Socol ML. Cost-effectiveness of elective cesarean delivery after one prior low transverse cesarean. Obstet Gynecol. 2000;95:745–751. doi: 10.1016/s0029-7844(00)00783-3. [DOI] [PubMed] [Google Scholar]
- 18.Culligan PJ, Myers JA, Goldberg RP, Blackwell L, Gohmann SF, Abell TD. Elective cesarean section to prevent anal incontinence and brachial plexus injuries associated with macrosomia—a decision analysis. Int Urogynecol J. 2005;16:19–28. doi: 10.1007/s00192-004-1203-3. [DOI] [PubMed] [Google Scholar]
- 19.Angeja A, Washington A, Vargas J, Gomez R, Rojas I, Caughey A. Chilean women’s preferences regarding the mode of delivery: which do they prefer and why? BJOG. 2006;113:1253–1258. doi: 10.1111/j.1471-0528.2006.01069.x. [DOI] [PubMed] [Google Scholar]
- 20.Caughey AB, Angeja A, Vargas J, Gomez R, Washington AE. Quantitative evaluation of preference for mode of delivery in pregnant Chilean women. Med Decis Making. 2003;23:634. [Google Scholar]
- 21.Kaimal AJ, Little SE, Odibo AO, et al. Cost-effectiveness of elective induction of labor at 41 weeks in nulliparous women. Am J Obstet Gynecol. 2010;204:137.e1–137.e9. doi: 10.1016/j.ajog.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kuppermann M, Nease RF, Learman LA, Gates E, Blumberg B, Washington AE. Procedure-related miscarriages and Down syndrome—affected births: implications for prenatal testing based on women’s preferences. Obstet Gynecol. 2000;96:511–516. doi: 10.1016/s0029-7844(00)00969-8. [DOI] [PubMed] [Google Scholar]
- 23.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. National Center for Health Statistics. Vital Health Stat Series no. 14. 2009;vol. 57 [PubMed] [Google Scholar]
- 24.Moss JR, Crowther CA, Hiller JE, Willson KJ, Robinson JS. Costs and consequences of treatment for mild gestational diabetes mellitus— evaluation from the ACHOIS randomized trial. BMC Pregnancy Childbirth. 2007;7:27–34. doi: 10.1186/1471-2393-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiCianni G, Volpe L, Casadidio I, et al. Universal screening and intensive metabolic management of gestational diabetes: cost-effectiveness in Italy. Acta Diabetol. 2002;39:69–73. doi: 10.1007/s005920200016. [DOI] [PubMed] [Google Scholar]
- 26.Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917–924. doi: 10.1542/peds.2004-0899. [DOI] [PubMed] [Google Scholar]
- 27.HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 28.Coustan DR, Lowe LP, Metzger BE, et al. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: paving the way for new diagnostic criteria for gestational diabetes mellitus. Am J Obstet Gynecol. 2010;202:654.e1–654.e6. doi: 10.1016/j.ajog.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felli JC, Hazen GB. Sensitivity analysis and the expected value of perfect information. Med Decis Making. 1998;18:95–109. doi: 10.1177/0272989X9801800117. [DOI] [PubMed] [Google Scholar]
- 30.Briggs A, Claxton K, Schulpher M. Decision modelling for health economic evaluation. Oxford, United Kingdom: Oxford University Press; 2006. [Accessed April 26, 2011]. Available at: http://healthcare-economist.com/tag/evpi/ [Google Scholar]