Abstract
Xanthomonas oryzae pv. oryzae is a causal agent of bacterial leaf blight of rice. Recently, an efficient hrp-inducing medium, XOM2, was established for this bacterium. In this medium, more than 10 proteins were secreted from the wild-type strain of X. oryzae pv. oryzae. Many of these proteins disappeared or decreased in amount in culture on XOM2 when incubated with the strain that has a mutation in the hrp regulatory gene. Interestingly, the secretory protein profile of a mutant lacking a type III secretion system (TTSS), components of which are encoded by hrp genes, was similar to that of the wild-type strain except that a few proteins had disappeared. This finding suggests that many HrpXo-dependent secretory proteins are secreted via systems other than the TTSS. By isolating mutant strains lacking a type II secretion system, we examined this hypothesis. As expected, many of the HrpXo-dependent secretory proteins disappeared or decreased when the mutant was cultured in XOM2. By determining the N-terminal amino acid sequence, we identified one of the type II secretory proteins as a cysteine protease homolog, CysP2. Nucleotide sequence analysis revealed that cysP2 has an imperfect plant-inducible-promoter box, a consensus sequence which HrpXo regulons possess in the promoter region, and a deduced signal peptide sequence at the N terminus. By reverse transcription-PCR analysis and examination of the expression of CysP2 by using a plasmid harboring a cysP2::gus fusion gene, HrpXo-dependent expression of CysP2 was confirmed. Here, we reveal that the hrp regulatory gene hrpXo is also involved in the expression of not only hrp genes and type III secretory proteins but also some type II secretory proteins.
In general, plant-pathogenic bacteria possess hypersensitive response and pathogenicity (hrp) genes, which are clustered in their chromosomes. hrp genes encode a type III secretion system (TTSS) that delivers virulence and avirulence factors from the bacteria to plant cells and are required for pathogenesis in host plants and for triggering a hypersensitive response in nonhost plants (1, 38). Transcriptional regulation of hrp genes depends on environmental conditions. The expression of hrp genes is generally suppressed in complex media and induced in planta and under certain in vitro conditions (6, 24, 35, 39).
In xanthomonads, the hrp cluster comprises six hrp loci, hrpA to hrpF, which are all required for full pathogenicity, and their expression is regulated by two genes, hrpX and hrpG, which are located outside the hrp gene cluster region (7, 36). The HrpG protein belongs to the OmpR family of two-component regulatory systems and activates the expression of hrpA and hrpX (37). HrpX, an AraC-type transcriptional activator, has been reported to control the expression of the operons hrpB to hrpF, which contain the hrp genes encoding a component of TTSS (36). It has also been suggested that HrpX controls some effector proteins (5). Several genes that are regulated in a HrpX-dependent manner possess the consensus nucleotide sequence TTCGC(N15)TTCGC, which has been termed the plant-inducible-promoter (PIP) box (12).
Xanthomonas oryzae pv. oryzae is a causal agent of bacterial leaf blight of rice (28). Recently, an efficient hrp-inducing medium, XOM2, was established for the bacterium (31). Using this medium, we have identified Hpa1 as one of the HrpXo-regulated type III secretory proteins in X. oryzae pv. oryzae (14). Hpa1 is encoded by an hrp cluster with a PIP box, and its requirement for disease development in rice plants has been reported (14, 41). We also detected HrpXo-regulated secretory proteins other than HpaI, none of which have been identified (14). Some of these proteins might be involved in pathogenicity.
Other than hrp gene products, extracellular polysaccharide, extracellular enzymes, and toxins have been proposed as possible virulence factors in X. oryzae pv. oryzae (3, 4, 22, 40). Suvendra et al. (27) reported that mutants of X. oryzae pv. oryzae deficient in a type II secretion system also lack virulence.
In this study, we detected not only HrpXo-regulated type III secretory proteins but also some HrpXo-regulated type II secretory proteins in culture supernatant from the hrp-inducing medium XOM2. We identified HrpE1 and HrpF as HrpXo-regulated type III proteins and a protein homologous with cysteine protease as one of the HrpXo-regulated type II secretory proteins.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli DH5αMCR (Stratagene) was grown at 37°C in Luria-Bertani medium (25). X. oryzae pv. oryzae strains were usually grown at 28°C in nutrient broth-yeast extract (NBY) medium (32) or in an hrp-inducing medium, XOM2 (31). Xanthomonas axonopodis pv. citri was grown at 28°C in NBY medium. All media were supplemented with the following antibiotics at the indicated concentrations: rifampin, 20 μg/ml; ampicillin, 50 μg/ml; cycloheximide, 50 μg/ml; kanamycin, 25 μg/ml for X. oryzae pv. oryzae and 50 μg/ml for E. coli; spectinomycin, 25 μg/ml for X. oryzae pv. oryzae and 50 μg/ml for E. coli.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Characteristics | Reference |
|---|---|---|
| Bacterial strains | ||
| E. coli DH5αMCR | F−mcrAΔ(mrr-hsd RMS-mcrBC) recA φ80dlacZ ΔM15 | Stratagene |
| X. oryzae pv. oryzae | ||
| T7174R | Spontaneous mutant of T7174, Rfr, used as the wild-type strain in this study | 11, 34 |
| 74ΔHrpXo | Transposon insertion mutant of T7174R, hrpXo mutant, Rfr Kmr | 30 |
| 74ΔHrcV | Transposon insertion mutant of T7174R, hrcV mutant, Rfr Kmr | 13 |
| 74ΔXpsE | Transposon insertion mutant of T7174R, xpsE mutant, Rfr Kmr | This study |
| 74ΔXpsL | Transposon insertion mutant of T7174R, xpsL mutant, Rfr Kmr | This study |
| 74ΔXpsN | Transposon insertion mutant of T7174R, xpsN mutant, Rfr Kmr | This study |
| 74ΔCysP2 | Transposon insertion mutant of T7174R, cysP2 mutant, Rfr Kmr | This study |
| X. axonopodis pv. citri MAFF302104a | ||
| Plasmid | ||
| pBluescript II SK+ | Phagemid, pUC derivative, Amr | Stratagene |
| pUC119 | Plasmid, Amr | 33 |
| pBSGUS | gus gene in pBluescript II KS(+) | 31 |
| pHM1 | Broad-host-range vector with pUC19 polylinker, Spr | 17 |
| pHMCysP2PIP | Promoter region of cysP2 is inserted in pHM1 | This study |
| pHMCysP2GUS | cysP2::gus fusion gene is inserted in pHM1 | This study |
| pGLCysP | Cosmid clone from the genomic library of T7174R containing cysP2 | This study |
| pUCCysP2 | 5-kb EcoRI-HindIII fragment of pGLCysP is inserted in pUC119 | This study |
| pUCΔCysP2 | Transposon insertion in cysP2 of pUCCysP2 | This study |
MAFF, Collection of Ministry of Agriculture, Forestry, and Fisheries, Tsukuba, Japan.
Recombinant DNA techniques.
DNA manipulations were performed by standard procedures (25).
Sequence analysis.
A dye terminator cycle sequencing reaction was performed with a DNA sequencing kit (Applied Biosystems, Piscataway, N.J.) according to the manufacturer's instructions followed by electrophoresis and analysis with an autosequencer (model 373A; Applied Biosystems). Similarity searches were made by using the BLAST program (2). A potential signal peptide at the N terminus was predicted by PSORT (21).
Isolation of mutants lacking a type II secretion system of X. oryzae pv. oryzae.
An EZ::TN <KAN-2> transposome, a mixture of the transposon EZ::TN <KAN-2> and EZ::TN transposase (Epicentre, Madison, Wis.), was introduced directly into X. oryzae pv. oryzae strain T7174R by electroporation. Three strains out of 1,000 kanamycin-resistant clones were then selected for deficiencies in extracellular cellulase and xylanase activities, which are known to be secreted by the type II secretion system (10, 14, 15). Assays for these enzymatic activities were done according to the procedures described by Tsuchiya et al. (29) and Keen et al. (18), respectively. Sequence analysis of the regions flanking the transposon revealed that the transposon was inserted into homologs of xpsE, xpsL, and xpsN of Xanthomonas campestris pv. campestris (DDBJ accession no. AE012165), which are deduced to be genes encoding a component of the type II secretion system (26), and the mutant strains were named 74ΔXpsE, 74ΔXpsL, and 74ΔXpsN, respectively.
Detection of secretory proteins of X. oryzae pv. oryzae in XOM2.
X. oryzae pv. oryzae strains were preincubated on NBY agar medium for 1 day and adjusted to an optical density at 600 nm of 2 with sterilized water. Forty microliters of the bacterial suspension was inoculated into 1 ml of XOM2 (pH 6.0). After 2 days of incubation (28°C, 180 rpm), bacteria were removed by centrifugation at 10,000 × g for 5 min and filtration, and the supernatant was precipitated on ice with 10% (vol/vol) trichloroacetic acid. After centrifugation at 16,000 × g for 30 min at 4°C, protein precipitates were washed twice with acetone and resuspended in 150 μl of Laemmli buffer (20). Protein samples were boiled for 3 min and separated by sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis (SDS-15% PAGE). Proteins were detected by silver staining with a Wako (Osaka, Japan) silver stain kit.
Amino acid sequence analysis.
For the analysis of N-terminal amino acid sequences of secretory proteins from 14 ml of XOM2 culture medium, proteins were separated on a large preparative SDS-15% PAGE gel or Tricine-SDS-17.5% PAGE gel and transferred to a polyvinylidene difluoride membrane (Immobilon-P [Millipore, Bedford, Mass.] or a 0.2 μm-pore-size Immun-Blot polyvinylidene difluoride membrane [Bio-Rad, Richmond, Va.], respectively). The membranes were stained with 0.025% Coomassie brilliant blue R-250, and the protein bands were excised. The N-terminal amino acid sequences of the proteins were determined with an Applied Biosystems model 492 Procise protein sequencing system. Homology searches were done with the NCBI BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/).
Reverse transcription (RT)-PCR.
Total RNA from bacteria cultured in XOM2 for 1 day was extracted with an RNeasy kit (QIAGEN, Valencia, Calif.). cDNA synthesis and PCR were conducted with RiverTra-Ace (Toyobo, Osaka, Japan) and KOD Dash (Toyobo), respectively.
Cloning of a DNA fragment containing cysP2.
pGLCysP, a cosmid clone from a genomic library of X. oryzae pv. oryzae T7174R containing cysP2, was selected by colony hybridization with the internal fragment of the X. axonopodis pv. citri cysteine protease gene (XAC2853; refer to GenBank accession no. AE011926) as a probe. The internal fragment of XAC2853 was amplified by PCR with genomic DNA of X. axonopodis pv. citri MAFF302104 used as a template and the primers 5′-ATGGGCCTGAAGCCTTCGTC-3′ and 5′-TCGGCGCCGATCACATCCTT-3′. An approximately 5-kb EcoRI-HindIII fragment from pGLCysP containing cysP2 was subcloned into pUC119 (33) to give pUCCysP2.
Construction of a plasmid harboring a cysP2::gus fusion gene.
A 459-bp fragment containing the 91-bp 5′ coding region and 368-bp noncoding region of cysP2 was amplified by PCR using pUCCysP2 as a template and the primers 5′-GAGGCGAATTCGAAAACGAATGTGACG-3′ and 5′-AGGCCCTTTCCGAGCTCTTCCGCCTGT-3′. The PCR product which was digested with EcoRI and SacI was cloned into a broad-host-range vector, pHM1 (17), to obtain pHMCysP2PIP. An approximately 1.8-kb SacI-KpnI fragment containing the gus gene from pBSGUS (31) was then inserted into pHMCysP2PIP, and the plasmid obtained was named pHMCysP2GUS. Plasmid pHMCysp2GUS was then introduced into X. oryzae pv. oryzae.
Assay of GUS activity.
β-Glucuronidase (GUS) activity was assayed as described previously (31).
Isolation of a cysP2 mutant of X. oryzae pv. oryzae T7174R.
The transposon EZ::TN <KAN-2> was then introduced into pUCCysP2 according to the manufacturer's instructions. Plasmid pUCΔCysP2, which has the transposon inserted in cysP2 at the +198-bp position (+1 represents A of the start codon ATG) was selected by Southern blot analysis and sequence analysis. The plasmid was introduced into strain T7174R by electroporation, and kanamycin-resistant clones were selected. Marker exchange mutagenesis was confirmed by genomic Southern blot analysis (data not shown), and one of the clones was named 74ΔCysP2.
RESULTS
Detection of secretory proteins from a mutant strain lacking a type II secretion system or a TTSS.
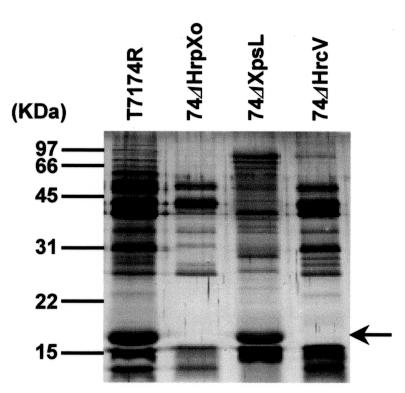
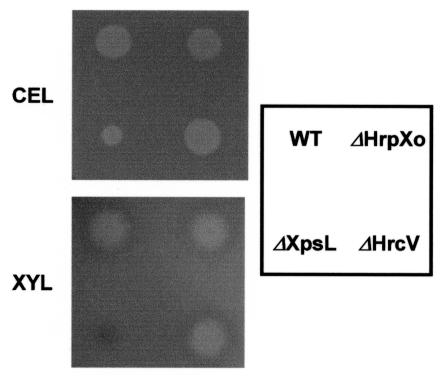
Secretory proteins from the wild-type strain X. oryzae pv. oryzae T7174R in the hrp-inducing medium XOM2 were compared with those from strain 74ΔHrpXo, in which an hrp-regulatory gene, hrpXo, is disrupted. Many of the proteins which were detected in the culture of T7174R disappeared or decreased in that of 74ΔHrpXo (Fig. 1). To clarify whether those proteins were secreted via a TTSS, secretory proteins in 74ΔHrcV, which has a transposon insertion in a conserved TTSS component gene and cannot secrete Hpa1, a type III secretory protein (13), were investigated. Interestingly, most of the proteins detected in the culture of T7174R were also detected in that of 74ΔHrcV, although a few proteins containing Hpa1 were not detected (Fig. 1). These results suggest that many of the secretory proteins from the wild-type strain detected in XOM2 are secreted not via a TTSS but via other systems. To examine the involvement of the type II system in the secretion of these proteins, mutants lacking this system were isolated (74ΔXpsE, 74ΔXpsL, and 74ΔXpsN; see Materials and Methods). A deficiency in secretion in these mutants was confirmed by a decrease of extracellular cellulase and xylanase activities (Fig. 2). The secretory proteins of 74ΔXpsL were compared with those of 74ΔHrcV, wild-type strain T7174R, and 74ΔHrpXo (Fig. 1). Several signals detected in strains T7174R and 74ΔHrcV had disappeared or weakened in 74ΔXpsL along with 74ΔHrpXo. Protein profiles from another two mutants lacking a type II secretion system (74ΔXpsE and 74ΔXpsN) were similar to the profile for 74ΔXpsL (data not shown). To test whether a mutation in hrpXo influences the construction of the type II secretion system, extracellular cellulase and xylanase activities of an hrpXo mutant were investigated (Fig. 2). While 74ΔXpsL showed low levels of these enzymatic activities, the hrpXo mutant 74ΔHrpXo showed both activities to the same extent as the wild-type strain did. These results suggest that HrpXo regulates not only the expression of type III secretory proteins but also that of some type II secretory proteins.
FIG. 1.
Comparison of secretory proteins from the wild type (T7174R), the hrpXo mutant (74ΔHrpXo), and mutants lacking a type II and type III secretion system (74ΔXpsL and 74ΔHrcV, respectively) in an hrp-inducing medium, XOM2. The proteins in the supernatants were separated by SDS-PAGE and detected by silver staining. An arrow indicates Hpa1.
FIG. 2.
Extracellular cellulase and xylanase activities in strains of X. oryzae pv. oryzae. Strains T7174R (WT), 74ΔHrpXo (ΔHrpXo), 74ΔXpsL (ΔXpsL), and 74ΔHrcV (ΔHrcV) were cultured on XOM2 agar plates containing carboxymethyl cellulose (upper panel) or RBB-xylan (4-O-methyl-d-glucurono-d-xylan-remazol brilliant blue R) (lower panel). The presence of a halo around a colony in T7174R, 74ΔHrpXo, or 74ΔHrcV indicates cellulase (CEL) and xylanase (XYL) proficiency. The halo was highly reduced when 74ΔXpsL was cultured with XOM2 agar containing carboxymethyl cellulose, and no halo was observed on XOM2 agar containing RBB-xylan. The same results as those for 74ΔXpsL were obtained for 74ΔXpsE and 74ΔXpsN.
Identification of an HrpXo-regulated secretory protein.
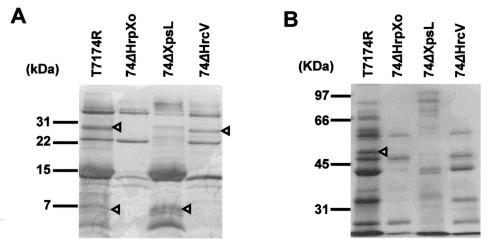
We identified one of the type II secretory proteins, whose molecular mass was about 30 kDa, which was not detected in 74ΔHrpXo nor in a type II secretion system-deficient mutant (Fig. 3A). The N-terminal sequence (EVHGKGLKPS) of the 30-kDa protein was almost identical to the internal sequence of X. axonopodis pv. citri cysteine protease (AVHGMGLKPS, amino acids [aa] 25 to 34; XAC2853 [refer to GenBank accession no. AE011926]) and that of X. campestris pv. campestris (AMHGMGLKPS, aa 25 to 34; XCC2693 [refer to GenBank accession no. AE012381]). The PSORT program predicted the presence of a signal peptide sequence at the N terminus (aa 1 to 24) of these proteins. These cysteine proteases are predicted to be secreted via the type II secretion system, and the N terminus of the mature form must start from aa 25.
FIG. 3.
HrpXo-regulated type II and type III secretory proteins identified in this study. Proteins in 14 ml of culture supernatant incubated with T7174R, 74ΔHrpXo, 74ΔXpsL, and 74ΔHrcV were separated on a Tricine-SDS-17.5% PAGE gel (A) and an SDS-7.5% PAGE gel (B) and transferred to polyvinylidene difluoride membranes. The membranes were stained with 0.025% Coomassie brilliant blue R-250. Triangles indicate the protein bands whose N-terminal amino acid sequences were determined.
We also identified three type III secretory proteins whose molecular masses were approximately 50, 7, and 6 kDa (Fig. 3). The N-terminal sequence (NDEFNPKDIKGS) of the 50-kDa protein was perfectly consistent with the internal sequence of HrpF of X. oryzae pv. oryzae. The predicted size of HrpF is 84.9 kDa, and the consistency of the sequence determined with HrpF started from aa 223. Therefore, the 50-kDa protein is likely to be a processed or degraded product of HrpF. The N-terminal sequences of the 7- and 6-kDa proteins were MEILPQISSL and SLNSRFQQGM, respectively, perfectly consistent with the start and internal (aa 9 to 18) sequences of HrpE1 of X. oryzae pv. oryzae, whose molecular mass is predicted to be 9.7 kDa. The smaller protein might be a degraded product.
Identification of the gene encoding the 30-kDa secretory protein.
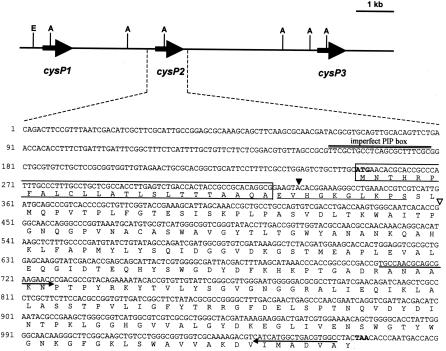
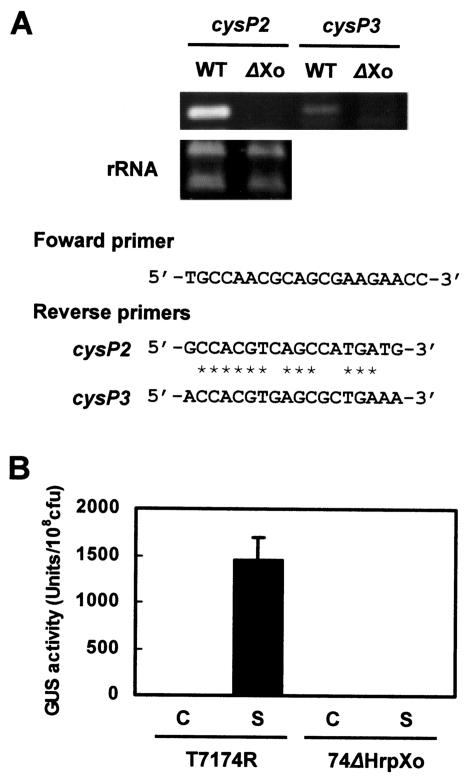
Sequence analysis of pGLCysP, which is a cosmid clone from the genomic library for X. oryzae pv. oryzae T7174R and contains the region hybridized with the cysteine protease gene from X. axonopodis pv. citri, revealed that three homologs are tandemly located in an approximately 10-kb genomic region (Fig. 4). The homology among the homologs (cysP1, cysP2, and cysP3) was 95 to 97%, and all encoded 271 amino acid residues with a predicted molecular mass of 29.1 to 29.5 kDa. These homologs possessed a deduced signal sequence at the N terminus. The amino acids of the homologs at positions 25 to 34 were EVHAKGLKPS for CysP1 and EVHGKGLKPS for CysP2 and CysP3, indicating that the 30-kDa protein was CysP2 or CysP3. To determine which of these was the 30-kDa secretory protein, strains T7174R and 74ΔHrpXo were cultivated in the hrp-inducing medium XOM2, and the transcription of cysP2 and cysP3 in each strain was analyzed by RT-PCR. We used specific primers for RT to distinguish transcriptional products of cysP2 and cysP3 (Fig. 5A). A specific DNA fragment corresponding to the internal sequence of cysP2 was amplified from T7174R, whereas it was not amplified from 74ΔHrpXo (Fig. 5A). Although a specific fragment derived from cysP3 mRNA that was dependent on the presence of HrpXo was also detected, the signal intensity of cysP3 was much lower than that of cysP2. These results suggest that the 30-kDa protein is CysP2 and that transcription of cysP2 is regulated by HrpXo.
FIG. 4.
Gene map of a region containing three copies of a cysteine protease homolog and the nucleotide sequence of cysP2 and the promoter region. The deduced amino acid sequence of CysP2 is given in the one-letter code below the nucleotide sequence. Restriction enzymes are abbreviated as E (EcoRI) and A (ApaI) on the gene map. The amino acid sequence determined in this study is underlined. The putative start codon and the termination codon are in boldface type. An imperfect PIP box, TTCGC(N12)TTCGC, is double underlined. Boxed sequences represent the deduced signal peptide of CysP2. An open triangle represents the transposon insertion site in 74ΔCysP2. A closed triangle indicates the position at which the gus gene was fused in pHMCysP2GUS. Arrows show the primers used for RT-PCR.
FIG. 5.
(A) Transcriptional regulation of the cysP2 and cysP3 genes by HrpXo. RT-PCR was performed to analyze the expression of cysP2 and cysP3. To distinguish the mRNA of cysP2 from that of cysP3, specific primers were used for RT. The same reverse primers were used for PCR as for RT, and a common forward primer was used for the amplification of the internal sequence of cysP2 or cysP3 (351 bp in common). Primer sets are shown. PCR products (upper gel) and rRNA (lower gel) were separated by agarose gel electrophoresis and stained with ethidium bromide. Asterisks indicate sequences common to the cysP2 and cysP3 reverse primers. (B) HrpXo-dependent expression of CysP2. Strains T7174R and 74ΔHrpXo transformed with an empty vector pHM1 (C) or with pHMCysP2GUS (S) were incubated in XOM2 for 1 day, and GUS activity was measured. Similar results were obtained from three independent experiments.
HrpXo-dependent expression of a cysP2::gus fusion gene.
To confirm HrpXo-dependent expression of CysP2, we constructed pHMCysP2GUS, which expresses a cysP2::gus fusion gene, and introduced it into X. oryzae pv. oryzae T7174R and 74ΔHrpXo. Each transformant was cultured in XOM2, and GUS activities were measured after a 1-day incubation. The transformant T7174R(pHMCysP2GUS) showed remarkable GUS activity, while T7174R transformed with the vector plasmid pHM1 and 74ΔHrpXo(pHMCysP2GUS) showed no activity (Fig. 5B). These results support the idea that HrpXo regulates the expression of CysP2.
Protein secretion in a cysP2 mutant, 74ΔCysp2.
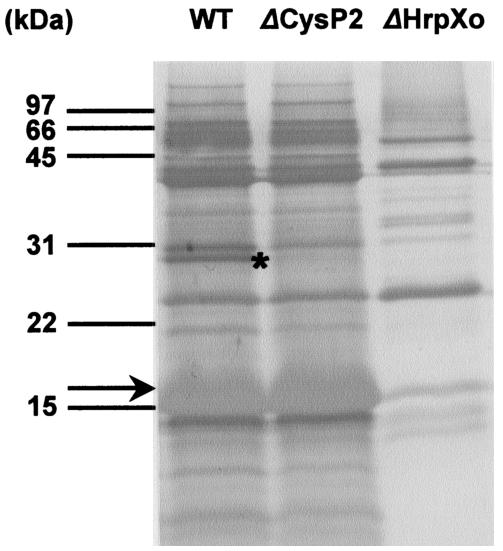
To clarify that the 30-kDa secretory protein which was not detected in mutants deficient in HrpXo and in the type II secretion system is CysP2, we generated the mutant 74ΔCysP2, in which an EZ::TN transposon was inserted in cysP2 (Fig. 4), and analyzed it for secretory protein by culturing it in XOM2. The mutant was incubated in XOM2 for 2 days, and secretory proteins were compared with those from T7174R. The 30-kDa protein was specifically missing from the culture supernatant of 74ΔCysP2 (Fig. 6). These results indicate that HrpXo regulates the expression of not only the components of a TTSS and type III secretory proteins but also some type II secretory proteins.
FIG. 6.
Detection of secretory proteins in 74ΔCysP2. Proteins in 14 ml of culture supernatant incubated with T7174R (WT), 74ΔCysP2 (ΔCysP2), and 74ΔHrpXo (ΔHrpXo) were separated on a Tricine-SDS-17.5% PAGE gel and transferred to the polyvinylidene difluoride membrane. The membrane was stained with 0.025% Coomassie brilliant blue R-250. The asterisk and arrow indicate CysP2 and Hpa1, respectively.
DISCUSSION
hrpX of xanthomonads has been thought to be a regulatory gene of other hrp genes which encode the components of a TTSS and effector proteins secreted via the TTSS (23, 36). For X. oryzae pv. oryzae, we have demonstrated that Hpa1, a harpin-like protein (19) whose expression is regulated by HrpXo, is secreted via a TTSS using an efficient hrp-inducing medium, XOM2 (14). However, there had been no reports of other secretory proteins. In this report, we newly identified HrpE1 and HrpF as HrpXo-regulated type III secretory proteins. Moreover, we indicated that HrpXo also regulates the expression of some type II secretory proteins and identified one of them as a cysteine protease homolog (CysP2).
By comparing the secretory proteins in culture incubated with wild-type strain T7174R and an hrcV mutant which lacks a TTSS, it was found that there were not many HrpXo-regulated type III secretory proteins in XOM2. By using mutants lacking a type II secretion system, many of the HrpXo-dependent proteins secreted from the wild-type strain were suggested to be secreted via such a system. In the culture of mutants deficient in a type II secretion system, more large-molecular-size proteins were detected than in that of the wild-type strain. It is likely that there are some products digested by proteases which are secreted via a type II secretion system in the culture of strains possessing this secretion system. On the other hand, in type II secretion system-deficient mutants, such protein digestion might not occur, and as a result, only large intact proteins might be detected. However, it is unlikely that all of the proteins detected in the culture of strains with a type II secretion system are products of digestion by proteases because the amounts of proteins detected were greater in those strains than in mutant strains lacking the secretion system. In fact, the size of the 30-kDa protein that we identified as a homolog of a cysteine protease from X. axonopodis pv. citri and X. campestris pv. campestris almost corresponded to that deduced from the nucleotide sequence.
N-terminal amino acid sequence analysis of the 30-kDa protein, which was detected in culture supernatants of T7174R and 74ΔHrcV but not in those of 74ΔHrpXo and 74ΔXpsL, revealed that this protein is a homolog of cysteine protease. We found that at least three copies of this cysteine protease homolog are present in the genomic DNA of X. oryzae pv. oryzae and that these copies (products of cysP1, cysP2, and cysP3) are tandemly located in an approximately 10-kb region. Detailed nucleotide sequence analysis of cysP genes revealed that they are highly homologous (95 to 97%) and that their deduced products have a signal peptide at the N terminus. The amino acid sequence that we determined starts just after the most likely cleavage site by signal peptidase (Fig. 4 [CysP2] and data not shown [CysP1 and CysP3]), suggesting that the 30-kDa protein is secreted via the type II system.
Among three cysteine protease homologs, we considered CysP2 to be the most probable candidate for the 30-kDa secretory protein. The reasons we considered CysP2 are (i) the amino acid sequence was not completely consistent with the corresponding sequence of CysP1 and (ii) the transcriptional level was much higher in cysP2 than in cysP3 according to RT-PCR with specific primers. Actually, the mutant that had a transposon insertion in cysP2 did not secrete the 30-kDa protein.
Nucleotide sequence analysis of cysP genes revealed that all of them, not only cysP2 but also cysP1 and cysP3, have an imperfect PIP box, TTCGC(N12)TTCGC, upstream of each open reading frame (Fig. 4 [cysP2] and data not shown [cysP1 and cysP3]). The PIP box, a consensus sequence consisting of TTCGC(N15)TTCGC, is reported to be located upstream of HrpX regulons such as hrp genes and some avirulence genes and is required for the transcription of the regulons in xanthomonads (12). We show HrpXo-dependent transcription of cysP2 and cysP3 in Fig. 5A. By using a plasmid harboring a cysP1::gus fusion gene, HrpXo-dependent expression was also observed, although the GUS activity was extremely weak (data not shown). These results imply the importance of the imperfect PIP boxes. However, we do not have any experimental evidence that the imperfect PIP boxes upstream of cysP genes function as cis elements for the transcription activator. There might be some unknown sequence recognized by HrpXo or other regulatory genes that mediate between HrpXo and each cysP gene. Anyway, although the secretion of CysP1 and CysP3 via a type II secretion system was unclear, at least for CysP2, we obtained the first evidence that HrpXo regulates the expression of a type II secretory protein. Besides CysP2, we detected some proteins secreted from the wild type and the TTSS mutant but not from the HrpXo mutant and the type II secretion system-deficient mutant. Some of these genes must also be HrpXo regulons secreted via the type II secretion system.
The genomic sequences of X. axonopodis pv. citri and X. campestris pv. campestris have now been completely determined (9). By detecting the PIP box or a sequence similar to it, da Silva et al. (9) have provided candidates for hrpX regulons. The cysteine protease of X. campestris pv. campestris (XCC2693), which is homologous to CysP2, is one of the candidates for HrpX regulons (9). Like CysP2, some of the candidates might be regulated in their transcription by HrpXo. The candidates of the regulons contain hrp gene products and effector proteins which are secreted via a TTSS. They also include some proteins with amino-terminal type II signal peptide sequences and are, therefore, likely to be secreted via a type II secretion system. On the other hand, genes that are unlikely encoding components of the secretion system or secretory proteins are contained in the candidates, suggesting that HrpXo is some sort of a global regulatory factor.
In this study, we also identified two HrpXo-dependent type III secretory proteins. The function of HrpE1 remains unclear, and HrpF is suggested to play a role at the bacterium-plant interface as part of a bacterial translocon which mediates effector protein delivery across the host cell membrane in X. campestris pv. vesicatoria (8, 16). Both proteins are required for pathogenicity on host plants and hypersensitive-response induction on nonhost plants. Many effector proteins have been shown to be secreted via a TTSS both in animals and in plant-pathogenic bacteria. There have been few reports regarding type III secretory effector proteins from X. oryzae pv. oryzae (14). By comparing secretory protein profiles between the wild-type and the type III-defective strains, effector proteins from the bacterium could be detected and identified.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research B (no. 13460024) and C (no. 14560043) from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Alfano, J. R., and A. Collmer. 1997. The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179:5655-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Angadi, C. V. 1978. Extra-cellular slime of Xanthomonas oryzae in bacterial leaf blight of rice. Phytopathol. Z. 93:170-180. [Google Scholar]
- 4.Angadi, C. V. 1978. In vivo production of slime toxin in bacterial blight infected rice plants. Phytopathol. Z. 92:193-201. [Google Scholar]
- 5.Astua-Monge, G., G. V. Minsavage, R. E. Stall, M. J. Davis, U. Bonas, and J. B. Jones. 2000. Resistance of tomato and pepper to T3 strains of Xanthomonas campestris pv. vesicatoria is specified by a plant-inducible avirulence gene. Mol. Plant-Microbe Interact. 13:911-921. [DOI] [PubMed] [Google Scholar]
- 6.Bonas, U. 1994. hrp genes of phytopathogenic bacteria. Curr. Top. Microbiol. Immunol. 192:79-98. [DOI] [PubMed] [Google Scholar]
- 7.Bonas, U., R. Schulete, S. Fenselau, G. V. Minsavage, B. J. Staskawicz, and R. E. Stall. 1991. Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol. Plant-Microbe Interact. 4:81-88. [Google Scholar]
- 8.Büttner, D., D. Nennstiel, B. Klüsener, and U. Bonas. 2002. Functional analysis of HrpF, a putative type III translocon protein from Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 184:2389-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva, A. C. R., J. A. Ferro, F. C. Reinach, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. Van Sluys, N. F. Almeida, L. M. C. Alves, A. M. Do Amaral, M. C. Bertolini, L. E. A. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Ciapina, R. M. B. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, A. J. S. Ferreira, R. C. C. Ferreira, M. I. T. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. M. Lemos, M. V. F. Lemos, E. C. Locali, M. A. Machado, A. M. B. N. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. M. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. M. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. D. Sena, C. Silva, R. F. de Souza, L. A. F. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. D. Tezza, M. Trindade dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-463. [DOI] [PubMed] [Google Scholar]
- 10.Dow, J. M., D. E. Miligan, L. Jaison, C. E. Barber, and M. J. Daniels. 1987. A gene cluster in Xanthomonas campestris required for pathogenicity controls the excretion of polygalacturonate lyase and other enzymes. Physiol. Mol. Plant Pathol. 31:261-271. [Google Scholar]
- 11.Ezuka, A., and O. Horino. 1974. Classification of rice varieties and Xanthomonas oryzae strains on the basis of their differential interactions. Bull. Tokai Kinki Natl. Agric. Exp. Stn. 27:1-19. [Google Scholar]
- 12.Fenselau, S., and U. Bonas. 1995. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion system. Mol. Plant-Microbe Interact. 8:845-854. [DOI] [PubMed] [Google Scholar]
- 13.Furutani, A., S. Tsuge, T. Oku, K. Tsuno, Y. Inoue, H. Ochiai, H. Kaku, and Y. Kubo. 2003. Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae. J. Gen. Plant Pathol. 69:271-278.
- 14.He, S. Y., M. Lindeberg, A. K. Chatterjee, and A. Collmer. 1991. Cloned Erwinia chrysanthemi out genes enable Escherichia coli to selectively secrete a diverse family of heterologous proteins to its milieu. Proc. Natl. Acad. Sci. USA 88:1079-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, N.-T., M.-N. Hung, S.-J. Chiou, F. Tang, D.-C. Chiang, H.-Y. Huang, and C.-Y. Wu. 1992. Cloning and characterization of a gene required for the secretion of extracellular enzymes across the outer membrane by Xanthomonas campestris pv. campestris. J. Bacteriol. 174:2679-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huguet, E., and U. Bonas. 1997. hrpF of Xanthomonas campestris pv. vesicatoria encodes an 87-kDa protein with homology to NolX of Rhizobium fredii. Mol. Plant-Microbe Interact. 10:488-498. [DOI] [PubMed] [Google Scholar]
- 17.Innes, R. W., M. A. Hirose, and P. L. Kuempel. 1988. Induction of nitrogen-fixing nodules on clover requires only 32 kilobase pairs of DNA from the Rhizobium trifolii symbiosis plasmid. J. Bacteriol. 170:3793-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keen, N. T., C. Boyd, and B. Henrissat. 1996. Cloning and characterization of a xylanase gene from corn strains of Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 9:651-657. [DOI] [PubMed] [Google Scholar]
- 19.Kim, J.-G., B. K. Park, C.-H. Yoo, E. Jeon, J. Oh, and I. Hwang. 2003. Characterization of the Xanthomonas axonopodis pv. glycines Hrp pathogenicity island. J. Bacteriol. 185:3155-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 21.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins Struct. Funct. Genet. 11:95-110. [DOI] [PubMed] [Google Scholar]
- 22.Noda, T., Z. Sato, H. Kobayashi, S. Iwasaki, and S. Okuda. 1989. Isolation and structural elucidation of phytotoxic substances produced by Xanthomonas campestris pv. oryzae (Ishiyama) Dye. Ann. Phytopathol. Soc. Jpn. 46:663-666. [Google Scholar]
- 23.Oku, T., A. M. Alvarez, and C. I. Kado. 1995. Conservation of the hypersensitivity-pathogenicity regulatory gene hrpX of Xanthomonas campestris and X. oryzae. DNA Sequence 5:245-249. [DOI] [PubMed] [Google Scholar]
- 24.Rahme, L. G., M. N. Mindrinos, and N. J. Panopoulos. 1991. Genetic and transcriptional organization of the hrp cluster of Pseudomonas syringae pv. phaseolicola. J. Bacteriol. 173:575-586. (Erratum, 174:3840.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., F. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 27.Suvendra, K. R., R. Rajeshwari, and R. V. Sonti. 2000. Mutants of Xanthomonas oryzae pv. oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol. Plant-Microbe Interact. 13:394-401. [DOI] [PubMed] [Google Scholar]
- 28.Swings, J., M. Van den Mooter, L. Vauterin, B. Hoste, M. Gillis, T. W. Mew, and K. Kersters. 1990. Reclassification of causal agents of bacterial blight (Xanthomonas campestris pv. oryzae) and bacterial leaf streak (Xanthomonas campestris pv. oryzicola) of rice as pathovars of Xanthomonas oryzae (ex Ishiyama 1922) sp. nov., nom. rev. Int. J. Syst. Bacteriol. 40:309-311. [Google Scholar]
- 29.Tsuchiya, K., T. W. Mew, and S. Wakimoto. 1982. Bacteriological and pathological characteristics of wild types and induced mutants of Xanthomonas campestris pv. oryzae. Phytopathology 72:43-46. [Google Scholar]
- 30.Tsuge, S., A. Furutani, R. Fukunaka, Y. Kubo, and O. Horino. 2001. Growth complementation of hrpXo mutants of Xanthomonas oryzae pv. oryzae by virulent strains in rice cultivars resistant and susceptible to the parental strain. J. Gen. Plant Pathol. 67:51-57. [Google Scholar]
- 31.Tsuge, S., A. Furutani, R. Fukunaka, T. Oku, K. Tsuno, H. Ochiai, Y. Inoue, H. Kaku, and Y. Kubo. 2002. Expression of Xanthomonas oryzae pv. oryzae hrp genes in a novel synthetic medium, XOM2. J. Gen. Plant Pathol. 68:363-371. [Google Scholar]
- 32.Vidaver, A. K. 1967. Synthetic and complex media for the rapid detection of phytopathogenic pseudomonds: effect of the carbon source. Appl. Microbiol. 15:1523-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vieira, J., and J. Messing. 1987. Production of single-stranded plasmid DNA. Methods Enzymol. 153:3-11. [DOI] [PubMed] [Google Scholar]
- 34.Watabe, M., M. Yamaguchi, I. Furusawa, and O. Horino. 1993. Virulence, and bacterial multiplication and movement in rice leaves of Xanthomonas campestris pv. oryzae mutants impaired in productivity of extracellular polysaccharide. Ann. Phytopathol. Soc. Jpn. 59:544-550. [Google Scholar]
- 35.Wei, Z. M., R. J. Laby, C. H. Zumoff, D. W. Bauer, Y. He, A. Collmer, and S. V. Beer. 1992. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257:85-88. [DOI] [PubMed] [Google Scholar]
- 36.Wengelnik, K., and U. Bonas. 1996. HrpXv, an AraC-type regulator, activates expression of five out of six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J. Bacteriol. 178:3462-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wengelnik, K., G. Van den Ackerveken, and U. Bonas. 1996. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant-Microbe Interact. 9:704-712. [DOI] [PubMed] [Google Scholar]
- 38.Willis, D. K., J. J. Rich, and E. M. Hrabak. 1991. hrp genes of phytopathogenic bacteria. Mol. Plant-Microbe. Interact. 4:132-138. [Google Scholar]
- 39.Xiao, Y., Y. Lu, S. Heu, and S. W. Hutcheson. 1992. Organization and environmental regulation of the Pseudomonas syringae pv. syringae 61 hrp cluster. J. Bacteriol. 174:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu, G. W., and C. F. Gonzalez. 1989. Evaluation of TN4431-induced protease mutants of Xanthomonas campestris pv. oryzae for growth in plants and pathogenicity. Phytopathology 79:1210-1215. [Google Scholar]
- 41.Zhu, W., M. M. Magbanua, and F. F. White. 2000. Identification of two novel hrp-associated genes in the hrp gene cluster of Xanthomonas oryzae pv. oryzae. J. Bacteriol. 182:1844-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]