Abstract
An enzymatic assay was developed to measure tetrahydromethanopterin (H4MPT) levels in wild-type and mutant cells of Methylobacterium extorquens AM1. H4MPT was detectable in wild-type cells but not in strains with a mutation of either the orf4 or the dmrA gene, suggesting a role for these two genes in H4MPT biosynthesis. The protein encoded by orf4 catalyzed the reaction of ribofuranosylaminobenzene 5′-phosphate synthase, the first committed step of H4MPT biosynthesis. These results provide the first biochemical evidence for H4MPT biosynthesis genes in bacteria.
Methylobacterium extorquens AM1 is a facultative methylotrophic bacterium capable of growth on succinate and one-carbon (C1) compounds. Growth on C1 compounds requires several clusters of genes found on the chromosomal DNA (5, 6), and a number of these genes code for enzymes which have archaeal homologs that depend on tetrahydromethanopterin (H4MPT) or structurally related coenzymes (6, 7, 24, 25). Previously, these coenzymes had been found only in methanogenic or hyperthermophilic sulfur-dependent archaea (9, 19, 22, 29, 32).
M. extorquens cells contain a form of H4MPT called dephospho-H4MPT (7). Although it has been assumed that this bacterium produces dephospho-H4MPT biosynthetic enzymes, these proteins have not yet been identified, and their evolutionary relationship to archaeal enzymes is unknown. In archaea, the genes encoding only 4 of the 18 putative H4MPT biosynthesis enzymes have been identified (14, 15, 28, 33, 34). One of these enzymes, ribofuranosylaminobenzene 5′-phosphate (RFAP) synthase, catalyzes the first committed step of H4MPT biosynthesis (26, 28). In M. extorquens, a gene encoding an RFAP synthase homolog (orf4, also called mptG) has been found clustered among several genes encoding H4MPT-dependent enzymes (6, 7). The orf4 gene product is 29% identical to RFAP synthase from Archaeoglobus fulgidus (28). The protein encoded by a second putative H4MPT biosynthesis gene (dmrA) shows homology to bacterial dihydrofolate reductases and has been proposed by Marx et al. (21) to encode dihydromethanopterin reductase, which would catalyze the final step of H4MPT biosynthesis. The dmrA mutant cannot grow on C1 compounds and exhibits a methanol- and formaldehyde-sensitive phenotype characteristic of mutants deficient in H4MPT-dependent metabolism.
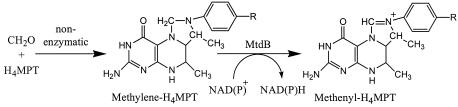
To test the hypotheses that orf4 and dmrA encode H4MPT biosynthesis enzymes, we have developed an enzymatic assay to measure H4MPT levels in M. extorquens mutants. The assay is based on the NAD+-reducing activity of methylene-H4MPT dehydrogenase B (MtdB) (16) (Fig. 1). Here, we provide the initial biochemical evidence for two H4MPT biosynthetic genes in M. extorquens and demonstrate that the protein encoded by orf4 has RFAP synthase activity.
FIG. 1.
Reaction of MtdB. The R group represents the side chain of H4MPT, which consists of ribitol, ribofuranosyl phosphate, and hydroxglutaryl groups. H4SPT from Methanosarcina thermophila contains an additional glutamate residue, while dephospho-H4MPT from M. extorquens lacks the phosphate and hydroxyglutaryl groups of H4MPT. Formaldehyde addition can occur nonenzymatically; however, in cells of M. extorquens, the reaction is catalyzed enzymatically by the formaldehyde-activating enzyme (31).
Methods.
Methanosarcina thermophila cells were grown anaerobically on acetate as previously described (28). M. extorquens AM1 wild-type and mutant strains were generously provided by the laboratory of Mary Lidstrom. It has previously been shown that the orf4, dmrA, and fae mutants are unable to grow on methanol and that complementation of each mutant with the corresponding plasmid-borne gene restores the wild-type phenotype, indicating that the mutant phenotype is not due to a polar effect (7, 21, 31). Wild-type M. extorquens cells were grown at 30°C on modified minimal medium at pH 7.0 with 20 mM succinate or 0.5% (vol/vol) methanol as previously described (1) except that the concentration of CaCl2 · 2H2O was 2.5 mg per liter. M. extorquens AM1 is naturally resistant to rifamycin, which was routinely added to wild-type and mutant cultures at 50 μg per ml to prevent contamination by other microorganisms. Cultures of the orf4, dmrA, and fae mutants were grown on succinate, rifamycin, and kanamycin (50 μg per ml). When the cultures reached an optical density at 600 nm (OD600) of 0.6, either 10 ml of 1 M succinate (pH 7.0) or 5 ml of 100% methanol was added. At an OD600 between 0.8 and 1.0, the cells were harvested by centrifugation and washed with 50 mM TES [tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] (pH 7.0; Fisher Scientific, Suwanee, Ga.), 10 mM MgCl2, and either 10 mM succinate (for cells grown on succinate) or 1% methanol. Cells were stored in liquid N2.
For high-level expression of MtdB, the mtdB gene (16) was amplified by the PCR (27) for cloning into the NdeI and BamHI sites of pET28b (Novagen, Inc., Madison, Wis.). This vector introduces an N-terminal six-histidine (His6) tag. The template was plasmid pALS8 (7), and the primers were 5′-GGACGTCCATATGGCCCGCTCGATCCTGCACA and 5′-GAAGGATCCTCATCCGGCGATCTCGAC. After amplification with Pfu polymerase (Stratagene, La Jolla, Calif.), the PCR product was purified with a PCR purification kit (QIAGEN, Valencia, Calif.), cut with NdeI and BamHI (New England Biolabs, Beverly, Mass.), and ligated (T4 DNA ligase; New England Biolabs) into pET28b cut with the same enzymes. The DNA was used to transform electrocompetent Escherichia coli DH1. The sequence of the insert was verified by dideoxy sequencing (27), and the plasmid was transformed into E. coli BL21(DE3):RIL cells (Stratagene). The expression cell line was called SW11.
For overproduction of His6-MtdB, SW11 cells were grown in Luria-Bertani medium with kanamycin (50 μg per ml) at 37°C. When cells reached an OD600 of 0.8, expression was induced with isopropylthiogalactoside (IPTG; Inalco Pharmaceuticals, San Luis Obispo, Calif.) at 1 mM. Cells were harvested after 3 h, washed with 50 mM MOPS (morpholinepropanesulfonic acid) (pH 7.0) and 10 mM MgCl2, suspended in the same buffer (2 ml of buffer per g of cells), disrupted by French pressure cell lysis at 20,000 lb/in2, and centrifuged at 27,000 × g for 60 min. The supernatant (cell extract) was stored in 400-μl portions at −80°C. Because H4MPT is oxygen sensitive, His6-MtdB was partially purified in an anaerobic chamber by using Ni-nitriloacetic acid (NTA) spin columns (QIAGEN). The protein was eluted with 250 mM imidazole (pH 8.0) according to the manufacturer's instructions.
For determination of H4MPT concentrations, M. extorquens cells (10 to 16 g) were thawed in an anaerobic chamber (Coy Products, Inc., Grass Lake, Mich.) containing 2% H2 and 98% N2. Breakage buffer (50 mM TES [pH 7.0], 10 mM MgCl2, 20 mM 2-mercaptoethanol) with DNase I (Sigma Chemical Co., St. Louis, Mo.) was added at a ratio of 1 ml of buffer per g of cells. Cells were disrupted anaerobically by two passages through a French pressure cell and centrifuged for 2 h at 27,000 × g (4°C). The supernatant was filtered through a 0.45-μm-pore-size filter (Millipore, Bedford, Mass.). Proteins were removed by using a Centricon-3 filtration device (Millipore) in the absence of O2. The filtrate (filtered cell extract) was stored anaerobically in a glass vial covered with foil to protect H4MPT from light inactivation.
H4MPT was partially purified from filtered M. extorquens cell extracts by using ion-exchange and hydrophobic-interaction chromatography in an anaerobic chamber (10). To filtered cell extract (12 to 16 ml), an equal volume of buffer A (50 mM MOPS [pH 6.8], 1% [vol/vol] 2-mercaptoethanol) was added. The mixture was loaded onto a 1-ml column of DEAE-Sephadex A25-125 (Sigma). Although H4MPT did not bind to the column, some contaminants bound to the column and were removed. H4MPT was concentrated on a 0.5-ml Serdolit Pad I column (Serva, Heidelberg, Germany) equilibrated with buffer B (1.4% [vol/vol] formic acid [pH 3], 10 mM 2-mercaptoethanol.) The column was washed with 2 ml of buffer B, followed by a methanol gradient of 1 ml each of 15, 25, and 50% (vol/vol) in buffer B. The pH of each fraction was adjusted to 7. Formaldehyde (2 μl of a 37% [vol/vol] solution) was added to 800 μl of the fractions, and the mixtures were incubated at room temperature for 10 min. After the solutions were transferred to a 3-ml glass cuvette, 1.1 ml of assay buffer (120 mM KH2PO4 [pH 6.8], 3 mM formaldehyde) and 20 μl of Ni-NTA-purified His6-MtdB were added. The absorbance at 340 nm (A340) was monitored for 25 s, and the reaction was initiated with 100 μl of 2 mM NAD+. The amount of NADH produced was estimated by using an extinction coefficient at 340 nm of 6.22 per mM NADH per cm (8).
To prepare samples containing tetrahydrosarcinapterin (H4SPT) from Methanosarcina thermophila TM1, cells (5 g) were sealed in a stoppered serum vial and purged with H2 gas for 5 min. H2 treatment was required for the enzymatic reduction of the oxidized forms of sarcinapterin to H4SPT. Anaerobic acetate buffer (10 ml of 30 mM sodium acetate [pH 4.0], 200 mM 2-mercaptoethanol) was added, and the cells were autoclaved for 15 min. The autoclaved cell extract was centrifuged anaerobically at 13,000 × g for 20 min to remove precipitated proteins. The supernatant containing H4SPT was stored in anaerobic vials at −80°C. For the measurement of H4SPT, the assay mixture contained 1.8 ml of assay buffer (120 mM KH2PO4 [pH 6.8], 3 mM formaldehyde), 20 μl of Ni-NTA-purified His6-MtdB, and 100 μl of heat-treated cell extract. The reaction was initiated with 100 μl of 2 mM NAD+.
PCR was used to amplify the orf4 gene from plasmid pALS8 (7). The primers (5′-GATCCATATGAGACCGTGGCCCGAGGTCCCG and 5′-CATGGGATCCCTAAACTTCCGCAACCGAG; Genosys) introduced a 5′ NdeI site and a 3′ BamHI site for cloning into pET15b (Novagen), which provides an N-terminal His6 tag. The plasmid (pCL1) was transformed into chemically competent DH1 cells, and the sequence of the insert was verified. The plasmid was transformed into BL21(DE3) cells (Novagen) containing the pG-Tf2 plasmid for expression of a chaperone to assist in protein folding (HSP Research Institute, Hayashibara Biochemical Laboratories, Inc., Okayama, Japan) (23). Expression of the His6-orf4 gene was induced as previously described for the RFAP synthase gene from Methanothermobacter thermautotrophicus (2) except that ampicillin (125 μg per ml) was used instead of kanamycin.
RFAP synthase activity was measured as previously described (28) except that the reaction mixtures were incubated for 16 h at 30°C in 50 mM TES (pH 7.0). Protein concentrations were measured by using the Bradford assay (Bio-Rad) (3) with bovine serum albumin as the standard. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue R-250 (Bio-Rad) (12). Phosphoribosylpyrophosphate (PRPP) was obtained from Sigma. All other chemicals were obtained from Fisher Scientific.
Development of an enzymatic assay to measure H4MPT.
To facilitate the discovery of H4MPT biosynthetic genes, an enzymatic assay was developed to enable the rapid screening of mutants deficient in H4MPT production. In this assay, formaldehyde is added to protein-free cell extracts to chemically convert H4MPT to methylene-H4MPT (Fig. 1). The oxidation of methylene-H4MPT is coupled to the reduction of NAD+ via MtdB from M. extorquens, producing an increase in A340. MtdB is highly specific for H4MPT and does not react with tetrahydrofolate (16). Thus, the enzyme can be used to distinguish between H4MPT and tetrahydrofolate in bacterial cells. The production of a histidine-tagged version of the enzyme (His6-MtdB) allowed for the rapid purification of large quantities of the enzyme by nickel affinity chromatography.
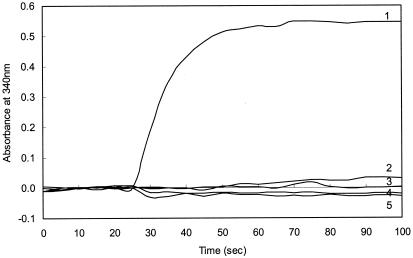
Because M. extorquens cells contain low concentrations of H4MPT relative to those of methanogens (7, 13), the assay conditions were first optimized by using extracts of the methanogen Methanosarcina thermophila. This organism produces H4SPT, an H4MPT analog (20). When H2-reduced Methanosarcina thermophila extracts were heated to remove proteins and combined with formaldehyde, NAD+, and His6-MtdB, an increase in A340, corresponding to the production of NADH, was observed (Fig. 2, line 1). No increase in A340 was observed if any of the reaction components (formaldehyde, heated methanogen cell extract, His6-MtdB, and NAD+) were omitted (Fig. 2, lines 2 to 5). These results demonstrate that methylene-H4SPT is a substrate for His6-MtdB and that His6-MtdB can be used to detect H4MPT analogs in cell extracts.
FIG. 2.
Detection of H4MPT in methanogen cell extracts by the MtdB assay. The complete reaction mixture (line 1) contained 120 mM KH2PO4 (pH 6.8), 3 mM formaldehyde, 20 μl of Ni-NTA-purified His6-MtdB (15 μg of protein), and 100 μl of Methanosarcina thermophila heat-treated cell extract. After a stable baseline was established at 340 nm for 25 s, the reaction was initiated with 100 μl of 2 mM NAD+. Control assays contained all of the components except His6-MtdB (line 2), NAD+ (line 3), heated cell extract (line 4), or formaldehyde (line 5).
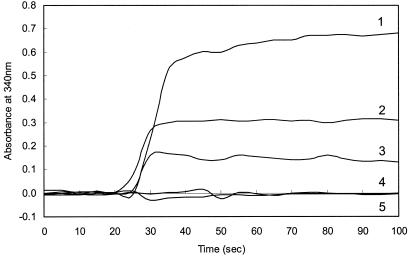
The MtdB assay was then used to measure H4MPT levels in wild-type M. extorquens extracts. Initial attempts to measure H4MPT levels in M. extorquens extracts were unsuccessful due to the high background A340. To decrease the absorbance due to contaminating molecules, H4MPT was partially purified by DEAE-Sephadex and hydrophobic-interaction chromatography. By this procedure, H4MPT was detected at a concentration of 44 μM in wild-type M. extorquens cells grown on methanol (Fig. 3, line 1). When cells were grown on succinate, the H4MPT concentration was about half the level found in methanol-grown cells (Fig. 3, line 2). This result was expected based on the report that H4MPT-dependent cyclohydrolase activity in M. extorquens is lower during growth on succinate than during growth on methanol (30). This finding may indicate that the H4MPT-dependent pathway is inducible during growth on methanol.
FIG. 3.
Detection of dephospho-H4MPT in extracts of M. extorquens AM1. Cell extracts were prepared and concentrated as described in the text. The assay components were the same as those described in the legend to Fig. 2. Cell extracts were from wild-type AM1 grown on 0.5% methanol (line 1), wild-type AM-1 grown on 20 mM succinate (line 2), fae mutant cells grown on succinate (line 3), orf4 mutant cells grown on succinate (line 4), or dmrA mutant cells grown on succinate (line 5).
Evidence for the role of two genes in bacterial H4MPT biosynthesis.
The orf4 and dmrA genes of M. extorquens have previously been proposed to encode bacterial H4MPT biosynthetic enzymes (21, 28). To test these hypotheses, the enzymatic assay was used to determine whether the orf4 and dmrA deletion mutants grown on succinate were capable of producing H4MPT. When the orf4 mutant was tested by using the His6-MtdB assay, no increase in A340 was detected (Fig. 3, line 4), indicating the absence of H4MPT in orf4 mutant extracts. Similarly, no H4MPT was detected in extracts of the dmrA mutant (Fig. 3, line 5). This result is consistent with roles for orf4 and dmrA as H4MPT biosynthetic genes.
As an additional control, we measured the level of H4MPT in a mutant for a gene that is not involved in H4MPT biosynthesis. The fae gene codes for the formaldehyde-activating enzyme (31), which catalyzes the reaction between formaldehyde and H4MPT to produce methylene-H4MPT. This enzyme is not required for H4MPT biosynthesis. As predicted, H4MPT was detected in extracts of the fae mutant (Fig. 3, line 3) at about two-thirds the level found in wild-type cells grown on succinate. We suspect that this difference may be due to the inefficiency of the fae mutant in converting formaldehyde and H4MPT to methylene-H4MPT, the substrate for His6-MtdB. In support of this hypothesis, we found that the complete nonenzymatic conversion of formaldehyde and H4MPT to methylene-H4MPT required 10 min in wild-type cell extracts but 2 h in fae mutant extracts, suggesting that a smaller proportion of the H4MPT in fae mutants was originally present as methylene-H4MPT.
RFAP synthase activity of the orf4 gene product.
To provide biochemical evidence that the orf4 gene codes for RFAP synthase, we measured the RFAP synthase activity of M. extorquens wild-type and orf4 mutant cells. However, because of the low activity of the enzyme in M. extorquens cell extracts, it was necessary to incubate the assay solutions for an extended time period (16 h) to obtain reliable results. Extracts of wild-type M. extorquens cells contained a low level of RFAP synthase activity (0.49 nmol of RFAP produced in 16 h with 4 mg of protein) (Table 1). This value is about 100 times lower than the specific activity of RFAP synthase in methanogen cells (28). RFAP synthase activity was not observed when the substrate PRPP was omitted from the assay. Furthermore, the RFAP synthase activity of M. extorquens cells was inhibited by a known RFAP synthase inhibitor, p-methylaminobenzoic acid, under conditions that inhibit RFAP synthase from methanogens (26). In contrast, no RFAP synthase activity was detectable in extracts of the orf4 mutant (Table 1).
TABLE 1.
RFAP synthase activity of M. extorquens AM1 strains and E. coli BL21(DE3) producing His6-Orf4
| Cell extract | Amt of protein used in assay (mg) | Amt of RFAP produced (nmol)a |
|---|---|---|
| M. extorquens wild type | ||
| + PABAb (6.4 mM), + PRPP (8.8 mM) | 4 | 0.49 ± 0.17 |
| + PABA (6.4 mM), without PRPP | 4 | ND |
| + PABA (85 μM), + PRPP (8.8 mM) | 6 | 0.38 ± 0.06 |
| + PABA (85 μM), + PRPP (8.8 mM), + p-methylaminobenzoic acid (5 mM) | 6 | ND |
| M. extorquens orf4 mutant | 6 | ND |
| E. coli BL21(DE3) producing His6-Orf4 | 1.5 | 4.3 ± 1.7 |
Average ± standard error for three readings. Cell extracts (140 to 180 μl) (1.5 to 6 mg of protein) were incubated for 16 h at 30°C. The product (RFAP) was converted to the pink azo-dye derivative, and RFAP synthase activity was measured as described in the text. Samples with activity showed a pink color, while samples with no detectable activity were clear. The spectrophotometric detection limit for the assay was 0.3 nmol of RFAP. ND, none detected.
PABA, p-aminobenzoic acid.
Attempts to purify RFAP synthase from M. extorquens cells were unsuccessful because of enzyme instability. Therefore, the orf4 gene was cloned into the pET15b vector for expression in E. coli. Initial attempts to express orf4 at 37°C with or without a His6 tag resulted in large amounts of insoluble protein. Both the soluble and the insoluble fractions from the cells lacked RFAP synthase activity (data not shown). A similar difficulty was previously encountered in expressing RFAP synthase from Methanothermobacter thermautotrophicus (2). This problem was overcome by coexpressing the RFAP synthase gene with a plasmid-encoded chaperone at 20°C. Under these same conditions, a small proportion of the His6-Orf4 protein was produced as soluble RFAP synthase. Over a period of 16 h, cell extract (1.5 mg of protein) produced 4.3 nmol of RFAP (Table 1). The His6-Orf4 protein was partially purified (23-fold) by nickel affinity chromatography; however, this procedure did not result in pure protein because of the low level of enzyme produced in the soluble form. RFAP synthase activity was undetectable in extracts of cells containing the pET15b vector without orf4. Taken together, these results demonstrate that M. extorquens cells contain RFAP synthase activity and that orf4 functions in H4MPT biosynthesis as a bacterial RFAP synthase gene.
Discussion.
M. extorquens contains several clusters of genes required for C1 metabolism, including genes that encode homologs of archaeal H4MPT-dependent and methanofuran-dependent enzymes (6, 7). The functions of many of the C1 metabolism genes are unknown, but some have been proposed to play roles in H4MPT and methanofuran biosynthesis (6). In this work, the production of a His6-tagged form of MtdB enabled us to develop an enzymatic assay to measure H4MPT levels in cell extracts and assign H4MPT biosynthetic functions to two of the uncharacterized C1 gene products. The orf4 mutant lacked RFAP synthase activity, while the recombinant His6-Orf4 protein catalyzed the RFAP synthase reaction (Table 1). This is the first biochemical evidence for an RFAP synthase gene outside the archaea. The proposed role of dmrA as a dihydromethanopterin reductase (21) is supported by the inability of the dmrA mutant to produce H4MPT (Fig. 3) and by additional evidence obtained in our laboratory that the DmrA protein catalyzes the NAD(P)H-dependent reduction of H2MPT to H4MPT (M. A. Caccamo, C. S. Malone, and M. E. Rasche, Abstr. 103rd Gen. Meet. Am. Soc. Microbiol., abstr. K-065, 2003). The His6-MtdB assay described here will be used to identify additional genes of the H4MPT biosynthesis pathway in methylotrophic bacteria.
The distribution of H4MPT-dependent pathways among bacteria and archaea is becoming clearer in light of the many prokaryotic genomes being sequenced. H4MPT-dependent enzymes have been found in autotrophic Xanthobacter strains, in methanotrophs, and in methylotrophic bacteria that use the serine pathway or the ribulose monophosphate (RuMP) pathway to assimilate formaldehyde (30). Genome sequencing indicates that the aerobic hyperthermophilic archaeon Aeropyrum pernix and other diverse microorganisms contain RFAP synthase homologs (4, 11, 17, 18, 28). These organisms may contain previously unidentified forms of H4MPT. At least six derivatives of H4MPT have been characterized by structural analyses (7, 19, 20, 32), and the MtdB enzyme used in this work reacts with at least three of these analogs (H4MPT from Methanothermobacter marburgensis [16], H4SPT from Methanosarcina thermophila [Fig. 2], and dephospho-H4MPT from M. extorquens [Fig. 3]). Thus, the enzymatic assay for H4MPT may offer a convenient method for detecting previously uncharacterized forms of H4MPT as well as for identifying the remaining H4MPT biosynthetic genes of bacteria and archaea.
Acknowledgments
We are grateful to Mary Lidstrom, Ludmila Chistoserdova, and Christopher Marx for their generosity in sharing the plasmid pALS8 and the M. extorquens wild-type, orf4, dmrA, and fae mutant strains. We thank Jack Shelton for sequencing the mtdB and orf4 genes and Vicki Kopf and Chi Bissett for their research contributions.
This work was supported by National Science Foundation grant numbers MCB-9876212 and MCB-9815924 and the Florida Agricultural Experiment Station.
Footnotes
Florida Agricultural Experiment Station journal series no. R-09891.
REFERENCES
- 1.Attwood, M. M., and W. Harder. 1972. A rapid and specific enrichment procedure for Hyphomicrobium spp. Antonie Leeuwenhoek 38:369-377. [DOI] [PubMed] [Google Scholar]
- 2.Bechard, M. E., S. Chhatwal, R. E. Garcia, and M. E. Rasche. 2003. Application of a colorimetric assay to identify putative ribofuranosylaminobenzene 5′-phosphate synthase genes expressed with activity in Escherichia coli. Biol. Proced. Online 5:69-77. [Online.] http://www.biologicalprocedures.com/bpo/general/home.htm. [DOI] [PMC free article] [PubMed]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Bult, C. J., O. White, G. J. Olsen, L. Zhou, R. D. Fleischmann, G. G. Sutton, J. A. Blake, L. M. FitzGerald, R. A. Clayton, J. D. Gocayne, A. R. Kerlavage, B. A. Dougherty, J. F. Tomb, M. D. Adams, C. I. Reich, R. Overbeek, E. F. Kirkness, K. G. Weinstock, J. M. Merrick, A. Glodek, J. L. Scott, N. S. Geoghagen, and J. C. Venter. 1996. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273:1058-1073. [DOI] [PubMed] [Google Scholar]
- 5.Chistoserdova, L. 1996. Metabolism of formaldehyde in M. extorquens AM1, p. 16-24. In M. E. Lidstrom and F. R. Tabita (ed.), Microbial growth on C1 compounds. Kluwer, Dordrecht, The Netherlands.
- 6.Chistoserdova, L., S.-W. Chen, A. Lapidus, and M. E. Lidstrom. 2003. Methylotrophy in Methylobacterium extorquens AM1 from a genomic point of view. J. Bacteriol. 185:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chistoserdova, L., J. A. Vorholt, R. K. Thauer, and M. E. Lidstrom. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic archaea. Science 281:99-102. [DOI] [PubMed] [Google Scholar]
- 8.Dawson, R. M. C., D. C. Elliot, W. H. Elliot, and K. M. Jones. 1986. Data for biochemical research, 3rd ed. Clarendon Press, Oxford, England.
- 9.DiMarco, A. A., T. A. Bobik, and R. S. Wolfe. 1990. Unusual coenzymes of methanogenesis. Annu. Rev. Biochem. 59:355-394. [DOI] [PubMed] [Google Scholar]
- 10.Escalante-Semerana, J. C., J. A. Leigh, K. L. Rinehart, and R. S. Wolfe. 1984. Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme of methanogenesis. Proc. Natl. Acad. Sci. USA 81:1976-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galagan, J. E., C. Nusbaum, A. Roy, M. G. Endrizzi, P. Macdonald, W. FitzHugh, S. Calvo, R. Engels, S. Smirnov, D. Atnoor, A. Brown, N. Allen, J. Naylor, N. Stange-Thomann, K. DeArellano, R. Johnson, L. Linton, P. McEwan, K. McKernan, J. Talamas, A. Tirrell, W. Ye, A. Zimmer, R. D. Barber, I. Cann, D. E. Graham, D. A. Grahame, A. M. Guss, R. Hedderich, C. Ingram-Smith, H. C. Kuettner, J. A. Krzycki, J. A. Leigh, W. Li, J. Liu, B. Mukhopadhyay, J. N. Reeve, K. Smith, T. A. Springer, L. A. Umayam, O. White, R. H. White, E. Conway de Macario, J. G. Ferry, K. F. Jarrell, H. Jing, A. J. L. Macario, I. Paulsen, M. Pritchett, K. R. Sowers, R. V. Swanson, S. H. Zinder, E. Lander, W. W. Metcalf, and B. Birren. 2002. The genome of M. acetivorans reveals extensive metabolic and physiological diversity. Genome Res. 12:532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfin, D. E. 1990. One-dimensional gel electrophoresis. Methods Enzymol. 182:425-441. [DOI] [PubMed] [Google Scholar]
- 13.Gorris, L. G., and C. van der Drift. 1994. Cofactor contents of methanogenic bacteria reviewed. Biofactors 4:139-145. [PubMed] [Google Scholar]
- 14.Graham, D. E., H. Xu, and R. H. White. 2002. A member of a new class of GTP cyclohydrolases produces formylaminopyrimidine nucleotide monophosphates. Biochemistry 41:15074-15084. [DOI] [PubMed] [Google Scholar]
- 15.Graupner, M., H. Xu, and R. H. White. 2000. Identification of an archaeal 2-hydroxy acid dehydrogenase catalyzing reactions involved in coenzyme biosynthesis in methanoarchaea. J. Bacteriol. 182:3688-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagemeier, C. H., L. Chistoserdova, M. E. Lidstrom, R. K. Thauer, and J. A. Vorholt. 2000. Characterization of a second methylene tetrahydromethanopterin dehydrogenase from Methylobacterium extorquens AM1. FEBS Lett. 267:3762-3769. [DOI] [PubMed] [Google Scholar]
- 17.Kawarabayasi, Y., Y. Hino, H. Horikawa, S. Yamazaki, Y. Haikawa, K. Jin-no, M. Takahashi, M. Sekine, S. Baba, A. Ankai, H. Kosugi, A. Hosoyama, S. Fukui, Y. Nagai, K. Nishijima, H. Nakazawa, M. Takamiya, S. Masuda, T. Funahashi, T. Tanaka, Y. Kudoh, J. Yamazaki, N. Kushida, A. Oguchi, and H. Kikuchi. 1999. Complete genome sequence of an aerobic hyper-thermophilic crenarchaeon, Aeropyrum pernix K1. DNA Res. 6:83-101. [DOI] [PubMed] [Google Scholar]
- 18.Klenk, H. P., R. A. Clayton, J. F. Tomb, O. White, K. E. Nelson, K. A. Ketchum, R. J. Dodson, M. Gwinn, E. K. Hickey, J. D. Peterson, D. L. Richardson, A. R. Kerlavage, D. E. Graham, N. C. Kyrpides, R. D. Fleischmann, J. Quackenbush, N. H. Lee, G. G. Sutton, S. Gill, E. F. Kirkness, B. A. Dougherty, K. McKenney, M. D. Adams, B. Loftus, S. Peterson, C. I. Reich, L. K. McNeil, J. H. Badger, A. Glodek, L. Zhou, R. Overbeek, J. D. Gocayne, J. F. Weidman, L. McDonald, T. Utterback, M. D. Cotton, T. Spriggs, P. Artiach, B. P. Kaine, S. M. Sykes, P. W. Sadow, K. P. D'Andrea, C. Bowman, C. Fujii, S. A. Garland, T. M. Mason, G. J. Olsen, C. M. Fraser, H. O. Smith, C. R. Woese, and J. C. Venter. 1997. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390:364-370. [DOI] [PubMed] [Google Scholar]
- 19.Lin, X., and R. H. White. 1988. Structure of solfapterin (erythro-neopterin-3′-d-2-deoxy-2-aminoglucopyranoside) isolated from the thermophilic archaebacterium Sulfolobus solfataricus. J. Bacteriol. 170:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maden, B. E. H. 2000. Tetrahydrofolate and tetrahydromethanopterin compared: functionally distinct carriers in C1 metabolism. Biochem. J. 350:609-629. [PMC free article] [PubMed] [Google Scholar]
- 21.Marx, C. J., B. N. O'Brien, J. Breezee, and M. E. Lidstrom. 2003. Novel methylotrophy genes of Methylobacterium extorquens AM1 identified by using transposon mutagenesis including a putative dihydromethanopterin reductase. J. Bacteriol. 185:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möller-Zinkhan, D., G. Börner, and R. K. Thauer. 1989. Function of methanofuran, tetrahydromethanopterin, and coenzyme F420 in Archaeoglobus fulgidus. Arch. Microbiol. 152:362-368. [Google Scholar]
- 23.Nishihara, K., M. Kanemori, H. Yanagi, and T. Yura. 2000. Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl. Environ. Microbiol. 66:884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pomper, B. K., O. Saurel, A. Milon, and J. A. Vorholt. 2002. Generation of formate by the formyltransferase/hydrolase complex (Fhc) from Methylobacterium extorquens AM1. FEBS Lett. 523:133-137. [DOI] [PubMed] [Google Scholar]
- 25.Pomper, B. K., J. A. Vorholt, L. Chistoserdova, M. E. Lidstrom, and R. K. Thauer. 1999. A methenyl tetrahydromethanopterin cyclohydrolase and a methenyl tetrahydrofolate cyclohydrolase in Methylobacterium extorquens AM1. Eur. J. Biochem. 261:475-480. [DOI] [PubMed] [Google Scholar]
- 26.Rasche, M. E., and R. H. White. 1998. Mechanism for the enzymatic formation of 4-(beta-d-ribofuranosyl)aminobenzene 5′-phosphate during the biosynthesis of methanopterin. Biochemistry 37:11343-11351. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Scott, J. W., and M. E. Rasche. 2002. Purification, overproduction, and partial characterization of β-RFA-P synthase, a key enzyme in the pathway of methanopterin biosynthesis. J. Bacteriol. 184:4442-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Beelen, P., J. F. A. Labro, J. T. Keltjens, W. H. Geerts, G. D. Vogels, W. M. Laarhoven, W. Guijt, and C. A. G. Haasnoot. 1984. Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur. J. Biochem. 139:359-365. [DOI] [PubMed] [Google Scholar]
- 30.Vorholt, J. A., L. Chistoserdova, S. M. Stolyar, R. K. Thauer, and M. E. Lidstrom. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J. Bacteriol. 181:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vorholt, J. A., C. J. Marx, M. E. Lidstrom, and R. K. Thauer. 2000. Novel formaldehyde-activating enzyme in Methylobacterium extorquens AM1 required for growth on methanol. J. Bacteriol. 182:6645-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White, R. H. 1993. Structures of the modified folates in the thermophilic archaebacterium Pyrococcus furiosus. Biochemistry 32:745-753. [DOI] [PubMed] [Google Scholar]
- 33.White, R. H. 2001. Biosynthesis of the methanogenic cofactors. Vitam. Horm. 61:299-337. [DOI] [PubMed] [Google Scholar]
- 34.Xu, H., R. Aurora, G. D. Rose, and R. H. White. 1999. Identifying two ancient enzymes in Archaea using predicted secondary structure alignment. Nat. Struct. Biol. 6:750-754. [DOI] [PubMed] [Google Scholar]