Abstract
The syndrome of chest pain, abnormal stress test, and nonflow limiting coronary artery disease (CAD) is common and is attributed to coronary microvascular disease (µVD). It is associated with increased hospital admissions and health care costs. But its impact on long-term survival is not known. Of the 9941 consecutive patients who had an exercise stress test for evaluation of chest pain between May 1991 and July 2007, 935 had both a positive stress test and a coronary angiogram within 1 year of their stress test forming the study cohort. Significant angiographic CAD defined as ≥70% stenosis of an epicardial coronary artery or ≥50% stenosis of the left main coronary artery was present in 324 patients. Rest (n = 611) were considered to have coronary µVD. Compared with patients with significant epicardial CAD, patients with coronary µVD were younger (63 ± 11 vs. 65 ± 10 years, p = 0.002), and had lower left ventricular wall thickness (p < 0.02), systolic blood pressure (BP; p = 0.002), pulse pressure (0.0008), systolic BP with exercise (p = 0.0001), and pulse pressure with exercise (p < 0.0001). Those with coronary µVD had a better survival compared with those with significant epicardial CAD, but worse than that expected for age- and gender-matched population (p < 0.0001). Coronary µVD as a cause of chest pain and positive stress test is common. All-cause mortality in patients with coronary µVD is worse than in an age- and gender-matched population control, but better than those with significant epicardial CAD.
Keywords: chest pain, coronary artery disease, microvascular disease, survival, prognosis
The triad of chest pain, evidence of ischemia, and nonobstructive epicardial coronary arteries is common and is attributed to coronary microvascular dysfunction (µVD).1,2,3,4,5 It results in increased hospital admissions, repeated cardiac testing, and increased health care costs. Smaller studies (n = 157 to 163) have suggested an increased risk of adverse short-term outcomes and significant decrease in quality of life and high health care costs.6,7,8,9,10,11,12,13,14 However, its impact on survival is not known. We evaluated survival of this group of patients in comparison to age- and gender-matched population and those with significant epicardial coronary artery disease (CAD).
Patients and Methods
Study Population
This is a retrospective cohort study performed at a large university medical center. The study was approved by our institutional review board which waived the need for informed consent. Our exercise stress test database was queried for patients receiving a stress test for chest pain. This yielded 9941 consecutive patients from May 1991 to July 2007. Of these, 1534 patients had a positive stress test defined as ≥1 mm stress induced ST depression in the absence of baseline ST-T changes. Of these patients with a positive stress test, coronary angiogram was performed on 935 patients within 1 year of the stress test forming the study cohort. Administrative clinical, echocardiographic, and angiographic data were compiled.
Exercise Stress Tests
All patients had exercise treadmill stress testing with either Bruce or Ramp protocol, directly supervised by a cardiologist. Heart rate and blood pressures at rest and stress were recorded. The electrocardiograms were analyzed and entered into the database at the time of the examination. Symptoms during the stress test were recorded and stress level in terms of metabolic equivalents (METs) reached from standard reference tables depending on the stress protocol used.
Coronary Angiograms
These were performed for clinical indications as judged by the treating physicians and interpreted by performing invasive cardiologist visually. The data were entered into a database at the time of study. Patients were considered to have significant epicardial CAD if the coronary angiogram demonstrated ≥70% diameter stenosis of a major epicardial coronary artery or ≥50% left main stenosis. Those with positive stress tests but nonobstructive CAS were considered to have µVD. Based on these definitions, 611 patients had coronary µVD and 324 had significant epicardial CAD.
Echocardiograms
In total, 800 patients had a standard two-dimensional echocardiographic examination with interpretation by a level 3-trained echocardiographer. Of these 800 patients, 505 were diagnosed with µVD and 295 with significant CAD. The left ventricular (LV) ejection fraction was assessed visually and entered into a database at the time of the examination. Anatomic and Doppler measurements were performed according to American Society of Echocardiography recommendations.15
Mortality Data
The primary end point of this study was all-cause mortality. Mortality data were collected from the Social Security Death Index.
Statistical Analysis
Data were imported into StatView 5.01 (SAS Institute Inc., Cary, NC) for analysis. Kaplan-Meier survival curves were computed for patients with coronary µVD and patients with epicardial CAD using log rank test. These were compared with age- and gender-matched U.S. population controls obtained from U.S. census data. Characteristics of patients with microvascular versus epicardial disease were compared using unpaired t test, or chi-squared test as appropriate. Cox regression analysis was used for multivariate survival analysis. A p value of ≤0.05 was considered statistically significant.
Results
Patient Characteristics
The baseline features are summarized in Table 1. For the entire cohort, the age was 63 ± 11 years, 70% were men. When compared with patients with epicardial CAD, patients with coronary µVD were younger (63 ± 11 vs. 65 ± 10 years, p = 0.002). They also had lower LV free wall thickness (10.7 ± 1.9 vs. 11.1 ± 2.9 mm, p = 0.003), systolic blood pressure (BP; p = 0.002), pulse pressure (0.0008), systolic BP with stress (p = 0.0001), and pulse pressure with stress (p < 0.0001). Both groups were similar in terms of gender, LV ejection fraction and MET levels achieved with exercise.
Table 1. Patient Characteristics.
| Variables | All Patients | Microvascular (n = 611) | Macrovascular (n = 324) | p Value |
|---|---|---|---|---|
| Age in years | 63 ± 11 | 63 ± 11 | 65 ± 10 | 0.002 |
| Men | 70% | 69% | 70% | 0.61 |
| Ejection fraction (%) | 58 ± 14 | 57 ± 154 | 59 ± 13 | 0.06 |
| LV end-diastolic diameter (mm) | 49 ± 7 | 49 ± 7 | 49 ± 7 | 0.69 |
| LV end-systolic diameter (mm) | 33 ± 8 | 33 ± 8 | 33 ± 8 | 0.87 |
| Ventricular septum (mm) | 11.8 + 2 | 11.6 + 2.5 | 12.0 + 2 | 0.02 |
| LV posterior wall (cm) | 10.8 + 1.6 | 10.7 + 1.9 | 11.1 + 2.9 | 0.003 |
| Pulmonary artery systolic pressures (mm Hg) | 33 ± 9 | 33 ± 9 | 34 ± 9 | 0.63 |
| Baseline heart rate (bpm) | 73 ± 13 | 73 ± 13 | 74 ± 12 | 0.21 |
| Baseline systolic blood pressure (mm Hg) | 131 ± 19 | 129 ± 18 | 133 ± 19 | 0.002 |
| Baseline diastolic blood pressure (mm Hg) | 79 ± 10 | 79 ± 10 | 79 ± 13 | 0.96 |
| Baseline pulse pressure (mm Hg) | 52 ± 18 | 51 ± 17 | 55 ± 18 | 0.0008 |
| Stress heart rate (bpm) | 132 ± 24 | 131 ± 24 | 133 ± 23 | 0.31 |
| Stress systolic blood pressure (mm Hg) | 172 ± 27 | 170 ± 25 | 176 ± 28 | 0.0001 |
| Stress diastolic blood pressure (mm Hg) | 79 ± 11 | 79 ± 12 | 78 ± 10 | 0.31 |
| Delta systolic blood pressure (mm Hg) | 41 ± 21 | 40 ± 20 | 42 ± 23 | 0.21 |
| Stress pulse pressure (mm Hg) | 93 ± 25 | 91 ± 24 | 98 ± 25 | <0.0001 |
| Chest pain on stress | 38% | 42% | 34% | 0.009 |
| MET level achieved | 8.4 ± 3.4 | 8.3 ± 3.4 | 8.3 ± 3 | 0.98 |
| Follow-up duration (years) | 7.4 ± 4.4 | 7.9 ± 4.3 | 6.9 ± 4.3 | 0.002 |
| Number of deaths (%) | 210 (20%) | 117 (19%) | 80 (25%) | 0.05 |
LV, left ventricular; bpm, beats per minute; MET, metabolic equivalent.
Survival as a Function of Significant CAD
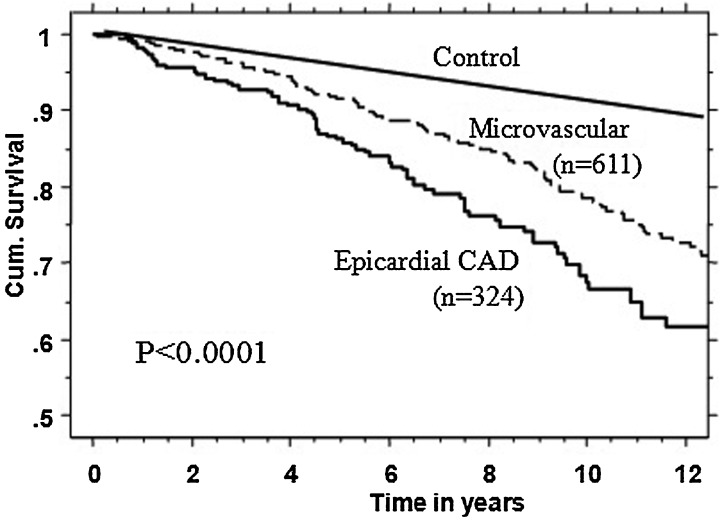
Using Kaplan-Meier analysis, as shown in Fig. 1, the survival in those with coronary µVD was significantly better compared with those with significant CAD, but worse compared with age- and gender-matched U.S. population (p < 0.0001).
Figure 1.
Kaplan-Meier survival curves in patients with significant symptomatic coronary microvascular disease compared with age- and gender-matched U.S. population (control) and those with significant epicardial coronary artery disease (CAD).
Other Predictors of Survival and Multivariate Survival Model
Table 2 summarizes both the univariate and multivariate predictors of survival using Cox regression analysis in all the 935 patients with positive exercise stress tests. All the variables from Table 1 were used in the analysis and significant ones are listed. The significant univariate predictors of reduced survival included age (p < 0.0001), presence of significant CAD (p = 0.005), lower LV ejection fraction (p = 0.007), higher LV end-systolic dimension (p = 0.0001), greater ventricular wall thickness (p = 0.0008), higher pulmonary artery systolic pressure (p < 0.0001), higher resting blood pressure (p = 0.002), higher resting pulse pressure (p < 0.0001), lower METs achieved (p < 0.0001), lower stress heart rate (p < 0.0001), lower delta systolic blood pressure with exercise (p = 0.0001), and lower delta heart rate with exercise (p < 0.0001). All these were entered into a multivariate model which showed age (p < 0.0001), significant CAD (p = 0.001), METs achieved (p = 0.02), and stress heart rate (p = 0.002) to be independent predictors of survival. Gender was not predictive of survival.
Table 2. Predictors of Survival in Patients with Positive Stress Tests.
| Variables | Univariate p Value | Univariate Hazard Ratio with 95% Confidence Interval | Multivariate p Value | Multivariate Hazard Ratio with 95% Confidence Interval |
|---|---|---|---|---|
| Age (years) | <0.0001 | 1.09 (1.07–1.10) | <0.0001 | 1.08 (1.05–1.11) |
| Significant CAD | 0.005 | 1.51 (1.15–2) | 0.001 | 1.96 (1.30–2.80) |
| LV ejection fraction (%) | 0.007 | 0.987 (0.978–0.996) | – | – |
| LV end-systolic diameter (cm) | 0.0001 | 1.37 (1.17–1.61) | – | – |
| Ventricular septal thickness (cm) | 0.0008 | 2.0 (1.3–3.1) | – | – |
| PA systolic pressures (mm Hg) | <0.0001 | 1.05 (1.04–1.07) | – | – |
| Resting systolic blood pressures (mm Hg) | 0.002 | 1.011 (1.004–1.019) | – | – |
| Resting pulse pressure (mm Hg | <0.0001 | 1.02 (1.01–1.03) | – | – |
| Stress heart rate (bpm) | <0.0001 | 0.98 (0.97–0.99) | 0.002 | 0.98 (0.97-0.99) |
| MET level reached | <0.0001 | 0.87 (0.83–0.91) | 0.02 | 0.91 (0.83–0.99) |
| Delta heart rate (bpm) | <0.0001 | 0.98 (0.97–0.99) | – | – |
| Delta systolic blood pressures (mm Hg) | 0.0001 | 0.987 (0.981-0.994) | – | – |
CAD, coronary artery disease; LV, left ventricular; PA, pulmonary artery; bpm, beats per minute; MET, metabolic equivalent.
Predictors of Survival in Patients with Coronary µVD
As in the entire cohort, age (p < 0.0001), presence of any CAD (p = 0.004), higher resting pulse pressure (p = 0.003), and lower exercise tolerance as reflected by METs achieved (p = 0.0005) were associated with lower survival. Gender, presence or absence of chest pain on stress test, and LV ejection fraction were not predictive of survival.
Discussion
Our study confirms that coronary µVD, defined as chest pain, a positive stress test, and nonobstructive CAD, is associated with higher all-cause mortality than age- and gender-matched controls, though lower than those with significant epicardial CAD causing chest pain and an abnormal stress test. Our series is larger than other reported studies, with longer follow-up, additional echocardiographic data, and hard mortality data.
Our data suggest that this syndrome is not benign. Morbidity and cost data have been available from earlier studies. Schächinger et al reported an increased risk of cardiovascular events over a median follow-up of 7.7 years in 147 patients with abnormal coronary reactivity to acetylcholine after adjusting for other comorbidities.13 Kaski et al, had no deaths in a cohort of 99 patients with coronary syndrome X, with a mean follow-up of 7 ± 4 years.10 The Women and Ischemia Syndrome Evaluation (WISE) group reported that impaired coronary vasomotor response to acetylcholine, chest pain, or abnormal myocardial energy metabolism detected with magnetic resonance spectroscopy was associated with an increased risk of coronary events, including angina, myocardial infarction, heart failure, hospital admissions, and increased health care costs.6,8,12,14 But there are no mortality data and WISE study is restricted to women.
Our study is consistent with the one by From et al.9 The authors reported poor prognosis in patients with “false-positive” stress echocardiograms defined as positive stress with nonflow limiting coronary angiograms in a large series of 1477 patients.9 The survival in those with “true” and “false” positive stress echocardiograms was similar. This suggests that some patients with “noncardiac” chest pain or “false-positive” stress echocardiograms may be experiencing µVD or true myocardial ischemia, and that the long-term prognosis is not entirely benign. Hence, both of these categories of patients need further attention in terms of risk factor modification and understanding of their biology that leads to higher mortality.
Our data and that from From et al suggest that positive stress test itself, irrespective of severity of coronary anatomy, is a strong predictor of higher mortality.9 Our data suggest in addition, based on multivariate analysis, that higher grades of coronary stenosis, age, and stress duration are important prognostic factors in addition to stress positivity. These findings are intuitive, but have practical implications in managing these patients. Significant CAD groups were predictably associated with higher age, higher systolic blood pressure, and pulse pressures at rest as well as stress. All these factors are of greater atherosclerotic burden.
Study Limitations
The major limitation of this study is its retrospective nature using administrative databases and lack of availability of reliable pharmacological data which may potentially affect outcomes. However, our study population is large with additional comprehensive echocardiographic data in addition to exercise and angiographic data.
Conclusions
Coronary µVD as a cause of chest pain and positive stress test is common. All-cause mortality in patients with coronary µVD is worse than an age- and gender-matched population, but better than those with significant epicardial CAD.
Funding Sources
None
Disclosures
None
Conflicts of Interest
None
References
- 1.Crea F, Lanza G A. Angina pectoris and normal coronary arteries: cardiac syndrome X. Heart. 2004;90(4):457–463. doi: 10.1136/hrt.2003.020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanza G A, Buffon A, Sestito A. et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51(4):466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Cannon R O III, Camici P G, Epstein S E. Pathophysiological dilemma of syndrome X. Circulation. 1992;85(3):883–892. doi: 10.1161/01.cir.85.3.883. [DOI] [PubMed] [Google Scholar]
- 4.el-Tamimi H, Davies G J, Sritara P, Hackett D, Crea F, Maseri A. Inappropriate constriction of small coronary vessels as a possible cause of a positive exercise test early after successful coronary angioplasty. Circulation. 1991;84(6):2307–2312. doi: 10.1161/01.cir.84.6.2307. [DOI] [PubMed] [Google Scholar]
- 5.Kaski J C. Pathophysiology and management of patients with chest pain and normal coronary arteriograms (cardiac syndrome X) Circulation. 2004;109(5):568–572. doi: 10.1161/01.CIR.0000116601.58103.62. [DOI] [PubMed] [Google Scholar]
- 6.von Mering G O, Arant C B, Wessel T R. et al. National Heart, Lung, and Blood Institute . Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 7.Suwaidi J A, Hamasaki S, Higano S T, Nishimura R A, Holmes D R Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 8.Pepine C J, Anderson R D, Sharaf B L. et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55(25):2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.From A M, Kane G, Bruce C, Pellikka P A, Scott C, McCully R B. Characteristics and outcomes of patients with abnormal stress echocardiograms and angiographically mild coronary artery disease (<50% stenoses) or normal coronary arteries. J Am Soc Echocardiogr. 2010;23(2):207–214. doi: 10.1016/j.echo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Kaski J C, Rosano G M, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson P A. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25(4):807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 11.Bugiardini R. Women, 'non-specific' chest pain, and normal or near-normal coronary angiograms are not synonymous with favourable outcome. Eur Heart J. 2006;27(12):1387–1389. doi: 10.1093/eurheartj/ehi758. [DOI] [PubMed] [Google Scholar]
- 12.Johnson B D, Shaw L J, Pepine C J. et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women's Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27(12):1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 13.Schächinger V, Britten M B, Zeiher A M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 14.Buchthal S D, den Hollander J A, Merz C N. et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342(12):829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 15.Lang R M, Bierig M, Devereux R B. et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]