Abstract
The genetic organization of the idn genes that encode the pathway for l-idonate catabolism was characterized. The monocistronic idnK gene is transcribed divergently from the idnDOTR genes, which were shown to form an operon. The 215-bp regulatory region between the idnK and idnD genes contains promoters in opposite orientation with transcription start sites that mapped to positions −26 and −29 with respect to the start codons. The regulatory region also contains a single putative IdnR/GntR binding site centered between the two promoters, a CRP binding site upstream of idnD, and an UP element upstream of idnK. The genes of the l-idonate pathway were shown to be under catabolite repression control. Analysis of idnD- and idnK-lacZ fusions in a nonpolar idnD mutant that is unable to interconvert l-idonate and 5-ketogluconate indicated that either compound could induce the pathway. The l-idonate pathway was first characterized as a subsidiary pathway for d-gluconate catabolism (GntII), which is induced by d-gluconate in a GntI (primary gluconate system) mutant. Here we showed that the idnK and idnD operons are induced by d-gluconate in a GntI system mutant, presumably by endogenous formation of 5-ketogluconate from d-gluconate. Thus, the regulation of the GntII system is appropriate for this pathway, which is primarily involved in l-idonate catabolism; the GntII system can be induced by d-gluconate under conditions that block the GntI system.
For three decades, there was thought to be two systems for d-gluconate catabolism, GntI and GntII (1). The GntI system consists of gntT, gntU, and gntK, which encode high- and low-affinity d-gluconate transporters and a thermoresistant gluconate kinase, respectively (19-21, 29). GntR negatively controls the GntI genes, as well as edd and eda of the Entner-Doudoroff pathway. The GntII system is composed of a thermosensitive gluconate kinase and a gluconate transporter, which provide for subsidiary catabolism of gluconate in GntI mutants (3, 16). Recently, we discovered that the GntII system is, in fact, a pathway for catabolism of l-idonate, which proceeds via a d-gluconate intermediate (3). The discovery of this novel pathway solved the longstanding question of why there are two pathways for gluconate; GntI is primarily involved in gluconate catabolism, and GntII is responsible for idonate catabolism.
The catabolic sequence for l-idonate is as follows: l-idonate is transported by the l-idonate transporter, IdnT; l-idonate is oxidized to 5-keto-gluconate (5KG) by l-idonate 5-dehydrogenase, IdnD; 5KG is reduced to d-gluconate by 5-keto-d-gluconate 5-reductase, IdnO; and d-gluconate is phosphorylated by a thermosensitive gluconate kinase, IdnK, to make 6-phosphogluconate (6PG), which is further catabolized via the Entner-Doudoroff pathway. Thus, IdnD and IdnO allow for the redox-coupled interconversion of l-idonate to d-gluconate via 5KG. The l-idonate catabolic pathway overlaps d-gluconate catabolism through the common intermediates d-gluconate and 6PG.
While the biochemistry of the l-idonate pathway is firmly established, the organization and regulation of the corresponding genes have not been characterized. Sequence annotation indicates that idnK is monocistronic and is divergently transcribed from a putative operon consisting of idnD, idnO, and idnT along with idnR, which encodes a repressor of the GalR-LacI family. In this report, we confirm the operon arrangement, transcription start sites, and regulation of the l-idonate genes. The results indicate that both l-idonate and 5KG act as inducers of the idonate pathway. Furthermore, the subsidiary role of the GntII system for gluconate catabolism was investigated in a GntI system mutant and shown to result from induction of the idnD operon and idnK by gluconate, presumably caused by accumulation of an endogenous inducer (e.g., 5KG). Lastly, functional genomic analyses with DNA arrays and two-dimensional (2-D) protein gels were used to characterize the global gene expression—and hence the physiology—of cells grown with l-idonate as the sole carbon source.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Escherichia coli strains used in this study are listed in Table 1. E. coli W1485 was the wild-type strain. All mutant and chromosomal lacZ fusion strains were derived from E. coli W1485 (2). E. coli DH5α and XL1-Blue were used for propagation of plasmids. Strains were grown at 37°C in Luria broth (LB) (13) with or without added carbohydrate (0.4%), in morpholinepropanesulfonic acid (MOPS) minimal medium with added carbohydrate (0.2%) (17), or in MOPS complete medium with added carbohydrate (0.2%) (32). MOPS complete medium contains amino acid, vitamin, purine, and pyrimidine supplements. When appropriate, ampicillin (100 μg/ml) and kanamycin (25 μg/ml) were included in the growth medium. All cultures (50-ml volume) were grown in 250-ml Erlenmeyer flasks and aerated by gyratory shaking at 300 rpm. Cell growth was monitored spectrophotometrically at 600 nm with a DU 530 Life Science UV/Vis spectrophotometer (Beckman Coulter, Inc., Fullerton, Calif.). Cultures in the early and late logarithmic phases of growth were harvested at optical densities of 0.3 and 0.7, respectively. Phenotypes of E. coli strains were determined on MacConkey indicator medium (14), tryptone-yeast extract agar (6), or LB plates (13).
TABLE 1.
E. coli strains used in this study
| Strain | Relevant genotype, phenotype, or characteristics | Reference or source |
|---|---|---|
| W1485 | K-12 wild type (λ−rph-1) | CGSCa |
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 Δ(araBAD)AH33 Δ(rhaBAD)LD78 | 6 |
| BW25141 | lacIqrrnBT14 ΔlacZWJ16hsdR514 Δ(araBAD)AH33 Δ(rhaBAD)LD78galU95 endABT333 uidA [(ΔmluI)::pir+recA1] | 6 |
| CB130 | λ−rph-1 Δ(lacI′b-PlacZ)c PidnidnK-lacZd; Kmr | This study |
| CB131 | λ−rph-1 Δ(lacI′-PlacZ)c PidnidnD-lacZd; Kmr | This study |
| CB360 | λ−rph-1 idnD::kan | This study |
| CB361 | λ−rph-1 ΔidnD | This study |
| CB361Z | λ−rph-1 ΔidnD Δ(lacI′-PlacZ)cidnD-lacZd | This study |
| CB366 | λ−rph-1 ΔidnR | This study |
| CB370 | λ−rph-1 Δcrp | This study |
| CB371 | λ−rph-1 ΔgntR | This study |
| DH5α | supE44 ΔlacU169(lacZΔM15Δ)hsdR17 endA1 gyrA96 thi-1 relA1 | BRLe |
| MD5 | λ−rph-1 idnK::kan | Fernando Valle |
| MD5E | λ−rph-1 idnK::kan gntK::cat | Fernando Valle |
| NP202 | λ−rph-1 Δ(gntRKU) | This study |
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
CGSC, E. coli Genetic Stock Collection.
Truncated gene (′).
Δ(lacI′-PlacZ) removes the 3′ terminus of lacI, the entire intergenic regulatory region upstream of lacZ, and the 5′-terminal region of lacZ.
The lacZ coding region is fused in frame to the 5′ region of the indicated idnK or idnD gene, forming a translation fusion. (see Materials and Methods).
BRL, Bethesda Research Laboratories.
Plasmid construction, DNA modification, and transformation.
Standard methods were used for DNA restrictions, ligations, and transformations and other DNA manipulations (23). PCR amplification was performed with Platinum high-fidelity Taq DNA polymerase (Invitrogen Life Technologies, Carlsbad, Calif.). The plasmids used in this study are listed in Table 2. Primers specific to this study are listed as supplementary material found on our website (http://www.ou.edu/microarray). Gene-specific deletions were carried out with E. coli W1485 by the method reported by Datsenko and Wanner (6). PCR-generated products were purified with a QIAquick PCR Purification Kit (Qiagen Inc., Valencia, Calif.). Electroporation was performed on a Gene Pulser II with 0.2-cm-gap cuvettes (Bio-Rad Laboratories, Hercules, Calif.). Colony PCR was accomplished by scraping cells from agar plates, thoroughly washing the cells five times with water, and amplifying PCR products by using sequence-specific primers and HotStarTaq DNA polymerase (Qiagen Inc., Valencia, Calif.).
TABLE 2.
Plasmids used in this study
| Plasmid name | Description | Reference or source |
|---|---|---|
| pBluescriptIISK+ | Cloning vector; Apr | Stratagene |
| pSP72 | Cloning vector; Apr | Promega |
| pRS551 | Transcription fusion vector containing the MCS, EcoRI-SmaI-BamHI-lacZYA′a | 27 |
| pRS552 | Translation fusion vector containing the MCS, EcoRI-SmaI-BamHI-lacZYA′ | 27 |
| pRS577 | Translation fusion vector containing the MCS, BamHI-SmaI-EcoRI-lacZYA′ | 27 |
| pNP202 | Replaced a 1.3-kb StuI-BglII fragment on pTC221 with the Kanr gene cassette | This study |
| pCB92 | pBSKs+ containing 300-bp idnK′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB100 | pBSKs+ containing 300-bp idnD′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB200 | pBSKs+ containing 300-bp idnO′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB620 | pBSKs+ containing 300-bp idnT′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB700 | pBSKs+ containing 300-bp yigR′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB900 | pBSKs+ containing 300-bp idnR′ PstI-KpnI DNA fragment generated by PCR | This study |
| pCB108 | pSP72 containing 542-bp lacI′ PstI DNA fragment generated by PCR | This study |
| pCB551 | pCB108 containing 2,223-bp neo rrnB T14lacZ′ DNA fragment from pRS551 | This study |
| pCB552 | pCB108 containing 2,223-bp neo rrnB T14lacZ′ DNA fragment from pRS552 | This study |
| pCB577 | pCB108 containing 2,223-bp neo rrnB T14lacZ′ DNA fragment from pRS577 | This study |
| pCB120 | pCB552 containing EcoRI-BamHI fragment of 274-bp idn regulatory region | This study |
| pCB121 | pCB577 containing BamHI-EcoRI fragment of 274-bp idn regulatory region | This study |
| pCB220 | pCB551 containing EcoRI-BamHI fragment of 274-bp idn regulatory region | This study |
Truncated gene (′).
Construction of idnK-lacZ and idnD-lacZ gene fusions.
Single-copy idnK-lacZ and idnD-lacZ gene fusions were constructed by a recombinase-assisted lacZ fusion system developed for this work by combining the lacZ fusion system described by Simons et al. (27) and the system for allele replacement described by Wanner et al. (32). The fusion vectors used for recombinase-assisted lacZ fusion included pCB551, pCB552, and pCB577 (Table 2). These plasmids contain the ori and bla genes from pSP72, 542 nucleotides of the 3′ end of the E. coli W1485 lacI gene cloned from pCB108, and functional elements common to pRS551, pRS552, and pRS577 , including a selectable kanamycin resistance gene; four tandem copies of the T1 terminator from the E. coli rrnB operon; the unique multiple cloning site (MCS) containing BamHI, SmaI, and EcoRI; and the 5′ region of the lacZ gene. A DNA fragment containing the untranslated region between the idnK and idnD genes and a terminal BamHI or EcoRI site located upstream of the idnK and idnD start codons, respectively, was amplified by PCR. This DNA fragment was cloned into the BamHI-EcoRI sites of the protein fusion vector pCB552 and the transcription fusion vector pCB551, creating idnK-lacZ fusion plasmids pCB120 and pCB220, respectively. The same fragment was cloned in the opposite orientation into the protein fusion vector pCB577 at the EcoRI-BamHI site, generating an idnD-lacZ fusion (pCB121).
Integration of the lacZ fusions into the chromosome of E. coli W1485 was achieved by allelic replacement by homologous recombination of the fusion construct into the lacI-lacZ region of the genome. Linear DNA fragments for allelic replacement were amplified by PCR with pCB120 and pCB121 as the templates. This method eliminated native lacZ regulation and generated a lacZ fusion in one step. Bacterial colonies with the desired phenotype on tryptone-yeast extract agar-kanamycin plates were transferred to MacConkey plates, and the cells were screened for the lactose-negative phenotype. PCR was used to verify correct allelic replacement of the native lacZ regulatory region with the lacZ fusion; all of the lacZ fusion constructions were confirmed by DNA sequence analysis (25).
β-Galactosidase measurements.
β-Galactosidase activity was measured with the yeast β-galactosidase assay kit from Pierce Biotechnology, Inc. (Rockford, Ill.). Cell cultures were grown in triplicate, and each culture was assayed in triplicate. A 70-μl aliquot was taken from each culture, mixed with an equal volume of β-galactosidase assay solution, placed in individual wells of a 96-well assay plate (Falcon Software, Inc., Wellesley, Mass.), and then held at 4°C until the assay was performed. The β-galactosidase assay solution was a 1:1 mixture of Y-PER (yeast protein extraction reagent) and 2× β-galactosidase assay buffer. Before initiation of the assay, spectrophotometric measurements at 590 nm were made with a PowerWave X 96-well Microplate Spectrophotometer (Bio-Tek Instruments, Inc., Winooski, Vt.) to determine relative cell densities. The 96-well plate was incubated in the plate reader at 37°C. Measurements were made spectrophotometrically at 420 nm every 4 min for 1 h, and the data were analyzed with the KC4 kinetics software package (Bio-Tek Instruments, Inc.). β-Galactosidase activity was calculated when the reaction was linear and expressed in Miller units (15). The values reported for each sample are the means ± the standard deviations for nine independent measurements.
RNA isolation.
Total RNA for Northern blot assays and primer extension analysis was isolated by the hot-phenol method as described previously (21). Total RNA for gene expression profiling and reverse transcriptase PCR (RT-PCR) was isolated by pipetting an equal volume of an actively growing cell culture into ice-cold RNAlater (Ambion, Inc., Austin, Tex.). The RNA was then purified and treated with DNase with RNeasy mini kits and RNase-free DNase kits (Qiagen Inc.). RNA concentrations were determined by spectrophotometric measurements at 260 nm. RNA was stored in ethanol at −80°C.
Primer extension analysis.
Oligonucleotides complementary to the mRNA sequences upstream of the idnK and idnD start codons were end labeled by using T4 polynucleotide kinase (Invitrogen Life Technologies) and [γ-32P]ATP (>5,000 Ci mmol−1) as previously described (23). Each 5′-end-labeled primer (0.5 pmol [∼1.5 × 106 cpm]) was annealed to 30 μg of total RNA in a 10-μl reaction mixture by heating to 94°C for 2 min, followed by slow cooling to 42°C. The primers were then extended at 42°C for 5 h by using Moloney murine leukemia virus RT (Ambion, Inc.). The reaction was stopped by addition of 10 μl of sequence loading buffer. The reaction mixtures were boiled for 3 min, and 4-μl aliquots were run on 6% polyacrylamide gels with size reference ladders generated by dideoxy sequencing of pNP204 with the same primers used for primer extension.
Northern blot analysis.
Total cellular RNA (5 μg) was denatured by incubation for 10 min at 68°C in formaldehyde-MOPS gel loading buffer (Ambion, Inc.) and electrophoresed through a 1.5% agarose gel containing formaldehyde and MOPS buffer. RNA was transferred to Nytran SuPerCharged superior nylon transfer membranes (Schleicher & Schuell, Inc., Keene, N.H.) by using a rapid downward transfer system. Antisense RNA probes were generated by reverse transcription from plasmids pCB92, pCB100, pCB200, pCB620, pCB700, and pCB900, containing the truncated genes idnK′, idnD′, idnO′, idnT′, yjgR′, and idnR′, respectively. These plasmids were constructed by cloning PCR products generated with nested gene-specific primers into pBluescript II SK+ (Table 2). All plasmids were linearized at the 3′ end of the truncated gene at the BamHI site, and a 32P-labeled RNA probe was synthesized by transcription with T7 RNA polymerase (Cloned; Ambion, Inc.) in the presence of [α-32P]UTP (23). Probe hybridization to the membrane-bound RNA and stripping from the membranes were done as described previously (29). Hybridized membranes were visualized by exposure to X-ray film or phosphorimaging screens, which were scanned with a STORM 820 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
RT-PCR.
RT-PCR products were prepared by using the SuperScript One-Step RT-PCR system with Platinum Taq DNA polymerase (Invitrogen Life Technologies) as instructed by the manufacturer. Total RNA was isolated at an optical density of 0.7 from E. coli W1485 grown in MOPS complete medium containing 0.2% l-idonic acid. The primers were checked for performance in PCRs by using E. coli W1485 genomic DNA as the template. RNA samples were tested for contaminating genomic DNA by using each RNA sample as a template for PCR; RNA samples contaminated with DNA were not used. The RT-PCR products were separated by electrophoresis through 1% agarose gels stained with ethidium bromide and documented with an Epi Chemi II Darkroom (UVP, Inc., Upland, Calif.).
Transcriptome profiling and treatment of data.
The methods used to handle whole-genome E. coli arrays and data analysis are described in detail on our website (http://www.ou.edu/microarray) and by Conway et al. (5). The C-terminal primer set (Sigma-GenoSys, The Woodlands, Tex.) was used to transcribe radioactively labeled cDNA (first-strand synthesis) with [α-32P]dCTP and SuperScript II RNase H− RT (Invitrogen Life Technologies) from samples of total cellular RNA. Duplicate Panorama E. coli Gene Array membranes (Sigma-GenoSys) from consecutive printings were used. Hybridization and stripping of membranes were done as described previously (28). Phosphorimages of hybridized membranes were analyzed with ArrayVision (Imaging Research Inc., St. Catharines, Ontario, Canada) to obtain raw spot intensity data. The raw data were normalized by expressing individual spot intensities as a fraction of the sum of all gene-specific spot intensities in each image, and the data were analyzed as previously described by using semiautomated Microsoft Visual Basic programs in Microsoft Excel (5).
2-D polyacrylamide gel electrophoresis (PAGE).
Cells were harvested by centrifugation and washed twice in a 10 mM MgCl-50 mM HEPES solution at pH 6.5 and then transferred to a lysis buffer that contained 9 M urea, 40 mM Tris-HCl, 4% 3-[(cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), and 1% dithiothreitol (DTT). After sonication on ice for 5 × 1 min with 30-s cooling intervals, cell debris was removed by centrifugation at 3,000 × g for 10 min at 4°C. The protein concentration of the supernatant was determined by the Bradford assay (4).
A 200-μg sample of cell extract was loaded onto 7-cm immobilized pH gradient strips that had a nonlinear pH range of 3 to 10 (Amersham Biosciences, Uppsala, Sweden). A rehydration solution that contained 8 M urea, 2% CHAPS, 1% DTT, and 0.5% immobilized pH gradient buffer (Amersham Biosciences) was added to the extract to a final volume of 120 μl. Rehydration was carried out for 10 h at 20°C as described by Sanchez et al. (24). Isoelectric focusing (IEF) was carried out with an Ettan IPGphor IEF unit (Amersham Biosciences) for 1 h at 100 V, 30 min at 500 V, 30 min at 1 kV, 1 h at 3 kV, 1 h at 5 kV, and 2 h at 8 kV. The temperature was held at 20°C throughout IEF. After IEF, the strips were incubated in a 50 mM Tris-HCl solution (pH 8.8) that contained 6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, and 1% DTT for 30 min. The strips were then placed on top of 15% PAGE gels containing 2% sodium dodecyl sulfate and attached with a 0.5% agarose solution that contained a trace amount of bromophenol blue (American Bioanalytical, Natick, Mass.). Electrophoresis was then carried out with a Mini-PROTEAN II system (Bio-Rad Laboratories) at 20 mA for ∼4 h, until the bromophenol blue front reached the bottom of the gel, and the gel was then stained with Coomassie brilliant blue.
In situ digestion, nano-electrospray MS-MS/MS, and data analysis.
The stained gels were compared visually, and differentially expressed spots of interest were excised and prepared by trypsin digestion in accordance with the protocol of Devreese et al. (7). Nano-electrospray mass spectrometry (MS) and tandem mass spectrophotometry (MS/MS) were carried out on a Q-Tof mass spectrometer (Micromass, Manchester, United Kingdom) under conditions similar to these described by Devreese et al. (7). In situ digests were washed with C18 ZipTip pipette tips (Millipore Corp., Bedford, Mass.). Extracts thus prepared were loaded into a coated fused-silica capillary tip (New Objective, Inc., Woburn, Mass.) and then placed into the nanospray source on the mass spectrometer. The capillary tube voltage was held at 0.9 kV, and spraying was initiated with a flow of N2 (∼3 lb/in2) at the back of the capillary tubing. Spectra were taken in the 100-to-2,000 mass range with 2-s scans, and data were collected for 2 min. Several of the most prominent doubly and triply charged molecular ions were manually identified and selected for collision-induced dissociation fragmentation with Ar as the collision gas, with the collision energy adjusted between 22 to 33 eV, depending on the optimum for fragmentation of the peptide.
The MS/MS spectra were interpreted with MassLynx 4.0 software as described by the manufacturer (Micromass). The MaxEnt3 tool was used to convert multiply charged fragment ions to singly charged species, and the PepSeq tool was used to determine the amino acid sequence after finding the fragment ion series. Sequences were matched to an E. coli protein database with version 3.4 of the FASTA search program (18).
Chemicals and enzymes.
Restriction enzymes and DNA-modifying enzymes were purchased from Invitrogen Life Technologies, Qiagen Inc., and Promega Corp. (Madison, Wis.). The T7 Sequenase version 2.0 kit and radioactive [α-32P]UTP and [γ-32P]ATP were purchased from Amersham Biosciences, Inc. (Piscataway, N.J.). Biochemicals were purchased from Sigma-Aldrich Corp. (St. Louis, Mo.). Panorama E. coli gene arrays were obtained from Sigma-GenoSys. Sodium l-idonate was received as a generous gift from Alisha S. Jarnagin (Genencor International, Inc., Palo Alto, Calif.).
RESULTS
Annotation of the idn promoter region.
Examination of the 215-bp sequence between the idnD and idnK genes revealed two putative −10 and −35 RNA polymerase binding sites on opposite strands. Both genes contain conserved Shine-Dalgarno sequences located 4 and 7 bp upstream from the IdnD and IdnO translation start sites, respectively (26). In addition, a single putative cyclic AMP (cAMP) receptor protein (CRP) binding site (ATTTGTGA-TGAAGA-TCACGTCA) was identified upstream of the idnD gene. A putative IdnR operator site (ATGTTA-CGCA-TAACGT) with homology to the GntR consensus binding sequence (ATGTTA-[N4]-TAACAT) (21) is centered between the two promoters, −78.5 and −83.5, with respect to the idnK and idnD transcription start sites, respectively, suggesting that this site may function as a regulatory element for both promoters. The position of the putative IdnR binding site is interesting because this location is atypical of negative control, despite the fact that IdnR and GntR belong to the GalR-LacI family of negative regulators (29). Slightly upstream of the idnK gene (−38 to −59) is a putative A-T-rich UP element sequence (8) that could be involved in stabilization of RNA polymerase-promoter interactions.
Transcription start sites for the idn genes.
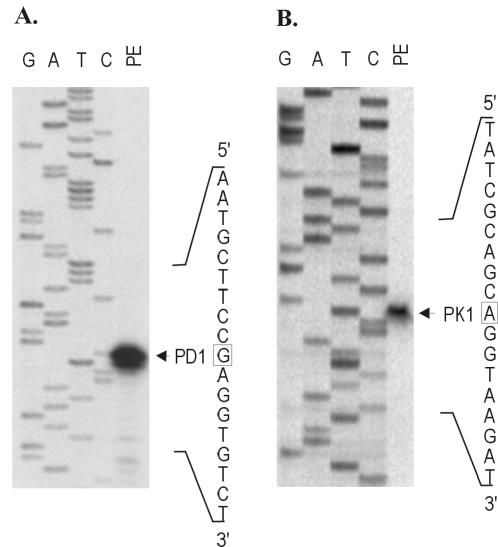
Primer extension analysis was used to map the transcription start sites for idnK and idnD with RNA extracted from cells grown in the presence of l-idonate (Fig. 1). Growth on 5KG resulted in the same transcription start sites (data not shown). The idnD transcript start site (PD1) was located 29 bp upstream of the idnD start codon (Fig. 1A), and the idnK transcript start site (PK1) was located 26 bp upstream of the idnK start codon (Fig. 1B). These transcription start sites are consistent with the locations of the putative idnD and idnK promoter sequence elements.
FIG. 1.
Primer extension of the transcription start sites for idnD and idnK. (A) Extension of the idnD transcript (PD1). (B) Extension of the idnK transcript (PK1). Lanes: PE, primer extension products; G, A, T, and C, corresponding sequence ladders.
Organization of idn transcription.
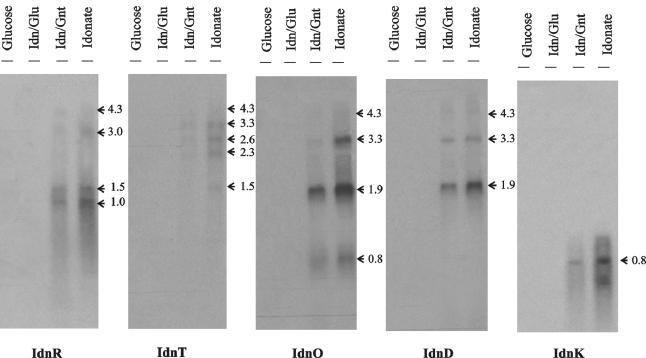
The organization of the idn genes suggested that idnD, -O, -T, and -R might be transcribed as a polycistronic message. The idnK transcript is monocistronic, as indicated by a 0.8-kb band of the expected length (Fig. 2). Northern blot analysis also suggested that idnD, -O, -T, and -R are cotranscribed (Fig. 2). Transcripts that hybridized with the idnD and idnO probes were observed at 1.9, 3.3, and 4.3 kb, although the latter transcript hybridized with very low intensity. An individual transcript for idnD was not observed, but there was an idnO-specific transcript of 0.8 kb. The most abundant transcript for idnD and idnO was 1.9 kb. The idnT probe hybridized to a 3.3-kb transcript, suggesting cotranscription with idnD and idnO. In addition, Northern hybridization revealed a 1.5-kb idnT transcript of sufficient length to encode idnT alone. The 4.3-kb transcript that hybridized to all four idnD, idnO, idnT, and idnR probes is consistent with the predicted transcript length of the idnDOTR operon. However, this transcript was apparently unstable and only a very faint band was observed. Overall, the results of Northern blot analysis supported the hypothesis that idnD, idnO, idnT, and idnR are cotranscribed and that the primary transcript is processed to form several gene-specific transcripts, which are more stable than the primary idnDOTR message.
FIG. 2.
Northern blot analysis of the idnK, idnD, idnO, idnT, and IdnR transcripts in E. coli W1485. Total RNA was isolated from late-log-phase cultures grown on MOPS minimal medium containing the carbohydrate listed above each lane. A total of 5 μg of RNA was loaded per lane. Estimated transcript sizes (in kilobases) are shown to the right of each blot and were determined from an RNA Millennium Marker (Ambion, Inc.) run with each independent RNA gel (data not shown). Hybridizations were carried out with 300-nucleotide probes specific for the gene encoding the protein indicated under each blot.
Computer analysis of predicted mRNA secondary structures in the idn regulatory region suggested the presence of stem-loop terminator-like structures at the 3′ ends of idnO, idnT, and idnK, but not idnR (data not shown). The strong intensity of the putative 1.9-kb idnD-idnO transcript in Northern blot assays implies that the predicted stem-loop structure at the end of idnO functions as a terminator. It is also likely that the stem-loop structures positioned after idnT and idnK function as transcription terminators, since transcripts ending after the predicted coding region of both genes were resolved in Northern blot assays.
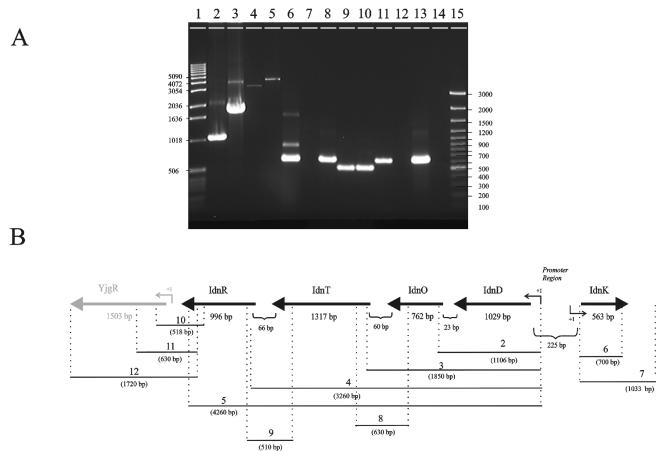
RT-PCR with RNA obtained from cells grown on l-idonate confirmed cotranscription of the idnDOTR operon (lanes 2 to 5, 8 and 9, Fig. 3). The monocistronic idnK transcript observed by Northern blot analysis was also confirmed by RT-PCR (lanes 6 and 7, Fig. 3). RT-PCR indicated that transcription did not terminate immediately downstream of idnR, but rather extended at least 500 bp into the yjgR gene (lanes 10 and 11, Fig. 3). However, this transcript did not appear to extend beyond the carboxy terminus of the yjgR structural gene, as downstream primers failed to yield a product (lane 12, Fig. 3). Further, Northern hybridization with a probe specific for yjgR revealed a 1.5-kb transcript that was not induced by l-idonate or d-gluconate (data not shown). A yjgR knockout grew well on l-idonate, confirming that YjgR is not required for l-idonate catabolism (data not shown).
FIG. 3.
RT analysis of the idnDOTR and idnK transcripts. (A) A 1.5% agarose gel showing the RT-PCR products with template RNA isolated from cells grown on MOPS compete medium containing 0.2% l-idonate. Lanes 2 to 12 correspond to regions 2 to 12 in the schematic representation (B). The RT-PCR products shown in lanes 2 to 12 were generated with primer pairs that flanked the corresponding regions depicted in the schematic. The length of each predicted RT-PCR product is shown in the schematic (in base pairs). Lanes: 1, 1-kb DNA ladder; 15, 100-bp DNA ladder; 13, control PCR product obtained from E. coli W1485 genomic DNA with a primer set that generated a 620-bp DNA fragment; 14, control PCR product obtained from total RNA with the same primer set as in lane 13. The values on the left and right are sizes in base pairs.
Transcription regulation of the idn genes.
The enzymes of the l-idonate pathway are induced by l-idonate (3). To confirm that the idn transcripts are similarly induced, we measured carbon source-dependent transcription of the l-idonate pathway genes (Fig. 2). Northern blot hybridization analysis indicated strong induction of idn transcripts in the presence of l-idonate and no induction with d-glucose. This result suggests that l-idonate functions to induce the idn genes.
To determine if 5KG also acts as an inducer, we tested induction of idn transcription in a strain containing a nonpolar idnD mutation that blocks the interconversion of l-idonate and 5KG without affecting expression of the other idn genes. An idnD-lacZ fusion in the idnD nonpolar mutant strain (CB361Z) was induced by 5KG and l-idonate, suggesting that both sugars can induce the l-idonate pathway (Table 3). This result was confirmed by Northern analysis, which showed that transcription of idnO was induced by growth on either 5KG or l-idonate in CB361Z (data not shown).
TABLE 3.
Expression of idnD-lacZ in the idnD nonpolar mutant and the wild-type background
| Strain | Relevant genotype | β-Galactosidase activity (Miller unitsa) with indicated carbon sourceb
|
||||
|---|---|---|---|---|---|---|
| None | Idonate | 5-Ketogluconate | Gluconate | Glucose | ||
| CB130 | Wild type | 76 ± 3.5 | 930 ± 27 | 580 ± 33 | 260 ± 40 | 40 ± 9.7 |
| CB361Z | ΔidnD | 39 ± 1.2 | 1,477 ± 105 | 530 ± 20 | 255 ± 38 | 20 ± 7.7 |
Reported values are means and standard deviations of three independent experiments performed in triplicate.
Cells were grown in LB medium containing the carbon sources indicated at 0.2%.
Transcriptional regulation of the idn regulon was further analyzed with lacZ gene fusions. Because the idnD and idnK genes are divergently transcribed from the same 215-bp region of DNA, gene fusions were constructed with the same promoter-containing fragment cloned in opposite orientations—one in the direction of idnK transcription and the other in the direction of idnD transcription. These fusions were integrated into the genome as single copies, because multicopy fusions expressed from plasmids did not appropriately reflect regulation. The idnD- and idnK-lacZ fusions were remarkably similar in expression, suggesting that regulation of the two promoters is coordinated (Table 4). Both fusions were induced by l-idonate and 5KG and slightly induced by d-gluconate, whereas d-glycerol, d-glucose, and succinate did not cause induction. Sugars related to the l-idonate pathway in other eubacteria (30), 2-ketogluconate, 2,5-diketogluconate, iduronate, and 2-ketogulonate, did not cause induction of the idn genes in E. coli W1485.
TABLE 4.
β-Galactosidase activity of CB130 (idnK-lacZ) and CB131 (idnD-lacZ) grown in MOPS complete medium
| Carbon sourceb | β-Galactosidase activity (Miller unitsa) with strain:
|
|
|---|---|---|
| CB130 | CB131 | |
| Idonate + cAMPc | 1,667 ± 169 | 1,816 ± 146 |
| Idonate | 750 ± 28 | 714 ± 33 |
| Idonate + gluconate | 441 ± 40 | 418 ± 33 |
| Gluconate | 279 ± 20 | 342 ± 19 |
| Gluconate + cAMPc | 405 ± 31 | 383 ± 43 |
| 5-Ketogluconate | 499 ± 62 | 495 ± 57 |
| 2,5-Diketogluconate | 157 ± 51 | 119 ± 22 |
| 2-Ketogluconate | 169 ± 25 | 108 ± 25 |
| Iduronate | 140 ± 10 | 155 ± 16 |
| 2-Ketogulonate | 25 ± 13 | 45 ± 11 |
| Glycerol | 104 ± 3.6 | 115 ± 18 |
| Glucose | 123 ± 8.2 | 116 ± 25 |
| Succinate | 155 ± 18 | 123 ± 34 |
Reported values are means and standard deviations of three independent experiments performed in triplicate.
Cells were grown in MOPS complete medium containing the carbon sources indicated at 0.2%.
Exogenous cAMP added to growth medium at 4mM.
Catabolite repression of the idn genes was observed in cells growing on a combination of l-idonate and d-gluconate or l-idonate and d-glucose; greater repression was observed with the addition of d-glucose (Fig. 2 and Table 4). Moreover, addition of cAMP (4 mM) to cells harboring the lacZ reporter fusions caused a 2.5-fold increase in reporter activity when the cells were grown on l-idonate, and a similar response was also observed for cells grown on d-gluconate (Table 4). A crp mutant strain (CB370) was unable to grow on MOPS minimal medium containing l-idonate (Table 5). As reported previously, the crp mutant demonstrated very poor growth on d-gluconate (19). Taken together, these results indicate that the idn promoters are subject to cAMP-CRP-dependent catabolite repression.
TABLE 5.
Specific growth rates of mutant strains on MOPS minimal medium
| Strain | Genotype | Specific growth rate (h−1)a
|
|||
|---|---|---|---|---|---|
| Glu | Gnt | 5KG | Idn | ||
| W1485 | Wild type | 1.3 | 1.4 | <0.1 | 1.0 |
| CB370 | Δcrp | 1.3 | <0.1 | NGb | NG |
| CB366 | ΔidnR | 1.3 | 1.3 | NG | NG |
| CB371 | ΔgntR | 1.4 | 1.3 | NG | 1.1 |
| MD5 | idnK::kan | 1.3 | 1.2 | NG | NG |
| MDE5 | idnK::kan gntK::cat | 1.4 | NG | NG | NG |
| NP202 | ΔgntRKU | 1.4 | 1.0c | NG | 0.9 |
Growth rate experiments were performed in triplicate, and all standard deviations were less than ± 0.1. Abbreviations: d-glucose, Glu; d-gluconate, Gnt; 5-ketogluconate, 5KG; l-idonate, Idn.
NG, no growth.
Growth after >24-h lag.
Growth physiology of GntI and GntII system mutants.
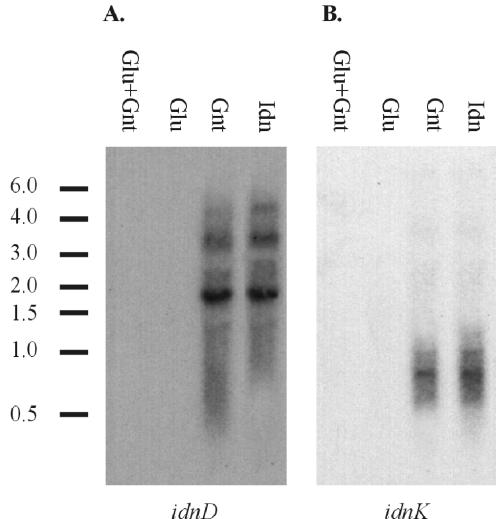
To understand the role of the GntI and GntII systems in growth on sugar acids, we used mutational analysis to evaluate growth on MOPS minimal medium supplemented with either d-glucose, d-gluconate, 5-ketogluconate, or l-idonate (Table 5). The wild-type E. coli strain, W1485, grew well on all of the carbon sources used except 5KG, which has been described previously (3). The idnR mutant (CB366) was unable to grow on l-idonate. By comparison, the gntR mutant (CB371) was unaffected for growth on l-idonate. Failure of the idnK mutant (MD5) to grow on MOPS minimal medium containing l-idonate is consistent with its role in phosphorylation of d-gluconate, an intermediate of the l-idonate pathway. The idnK gntK double mutant (MDE5) failed to grow on d-gluconate, as well as l-idonate. Interestingly, a gntRKU deletion mutant (NP202) can grow on d-gluconate after a lag phase of 24 h. Northern blot analysis of the gntRKU mutant, NP202, revealed that the idnD and idnK transcripts were fully induced when cells were grown on d-gluconate (Fig. 4). In the wild-type strain, induction of the idn genes by d-gluconate is minimal compared to that by l-idonate (Table 4). This result suggests that growth of the gntK mutant on d-gluconate causes endogenous accumulation of the inducer of idnK and idnD.
FIG. 4.
Northern blot analysis of a GntI system mutant. idnD (A) and idnK (B) transcription is shown. Total RNA was isolated from late-log-phase cultures of E. coli NP202 (W1485 ΔgntRKU) grown on LB medium containing the carbohydrate listed above each lane. An aliquot of 5 μg of RNA was loaded per lane, and the bars and corresponding values to the left of each blot show the locations and sizes (in kilobases) of RNA standards.
Functional genomic analysis of cells grown on idonate.
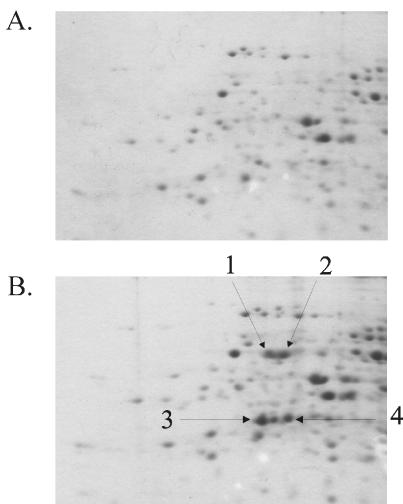
Very little is known about the physiology of cells growing on rarely studied sugar acids, such as l-idonate. Therefore, we used whole-genome DNA arrays to identify genes induced by growth on MOPS complete medium containing l-idonate and d-glucose. These data sets are available on the Internet (http://www.ou.edu/microarray). The five idn genes were among the most strongly induced genes in cells grown on l-idonate compared to d-glucose, including idnD and idnO, which topped the list (Table 6). The expression profile of the idn genes in cells grown on l-idonate was qualitatively similar to the relative induction observed in Northern blot assays (compare Fig. 2 and Table 6). When cells were grown on l-idonate, the percentage of total transcripts in the cells was highest for idnD, followed by idnO, idnT, idnR, and idnK (Table 6). Of all of the transcripts in E. coli cells grown on l-idonate, the idnD and idnO transcripts were the 36th and 58th most highly expressed, respectively (data not shown). These levels are typical of highly expressed genes in fast-growing bacteria (12). To confirm that changes in the transcript levels of idnD and idnO directly correlated with the changes in the protein levels, proteins found to be specifically induced by growth on l-idonate were cut out of 2-D gels (Fig. 5), digested with trypsin, and identified by MS/MS. Four spots thus analyzed were identified as being IdnD and IdnO. Two modified forms of each protein were present on the gels.
TABLE 6.
The fifty most highly induced E. coli genes in cells grown on l-idonate
| Gene | Gene product | Intensitya | Ratiob | P valuec |
|---|---|---|---|---|
| idnD | l-Idonate dehydrogenase | 0.254 | 1.6 | 1.4 × 10−6 |
| idnO | 5-Ketogluconate:gluconate oxidoreductase | 0.187 | 1.3 | 1.9 × 10−7 |
| b2790 | ORFd hypothetical protein | 0.172 | 0.97 | 2.4 × 10−4 |
| yheH | Putative export protein for general secretion pathway | 0.041 | 0.93 | 9.1 × 10−4 |
| idnK | Gluconate kinase | 0.039 | 0.83 | 2.1 × 10−3 |
| yfeK | ORF, hypothetical protein | 0.055 | 0.81 | 4.8 × 10−4 |
| yhfP | ORF, hypothetical protein | 0.022 | 0.79 | 5.9 × 10−4 |
| yicM | Putative transport protein | 0.104 | 0.78 | 8.9 × 10−4 |
| yhdT | ORF, hypothetical protein | 0.035 | 0.78 | 5.0 × 10−5 |
| narW | Cryptic nitrate reductase 2, delta subunit | 0.019 | 0.73 | 5.4 × 10−4 |
| flhA | Flagellar biosynthesis; possible export function | 0.108 | 0.72 | 2.9 × 10−3 |
| ymfL | ORF, hypothetical protein | 0.021 | 0.72 | 1.6 × 10−4 |
| rimJ | Acetylation of N-terminal alanine protein S5 | 0.124 | 0.69 | 2.6 × 10−8 |
| ymfO | ORF, hypothetical protein | 0.050 | 0.68 | 2.0 × 10−3 |
| b2459 | ORF, hypothetical protein | 0.108 | 0.66 | 6.2 × 10−4 |
| yggC | Putative kinase | 0.031 | 0.60 | 3.8 × 10−4 |
| narJ | Nitrate reductase 1, delta subunit, assembly function | 0.068 | 0.60 | 8.9 × 10−5 |
| narU | Nitrite extrusion protein 2 | 0.014 | 0.59 | 9.7 × 10−4 |
| yjiW | ORF, hypothetical protein | 0.017 | 0.58 | 5.0 × 10−4 |
| yiaC | ORF, hypothetical protein | 0.041 | 0.58 | 1.1 × 10−4 |
| thiM | Hydoxyethylthiazole kinase | 0.089 | 0.57 | 2.0 × 10−3 |
| b1600 | Possible chaperone | 0.017 | 0.56 | 5.9 × 10−4 |
| ycal | ORF, hypothetical protein | 0.032 | 0.56 | 7.3 × 10−4 |
| b1152 | ORF, hypothetical protein | 0.015 | 0.55 | 2.3 × 10−3 |
| hyaF | Nickel incorporation into hydrogenase 1 proteins | 0.045 | 0.54 | 2.5 × 10−5 |
| b2460 | ORF, hypothetical protein | 0.061 | 0.54 | 2.5 × 10−3 |
| ymfA | ORF, hypothetical protein | 0.020 | 0.53 | 4.7 × 10−4 |
| nrfE | Formate-dependent nitrite reductase, assembly function | 0.381 | 0.53 | 2.3 × 10−3 |
| yibK | ORF, hypothetical protein | 0.027 | 0.53 | 2.8 × 10−3 |
| aceE | Pyruvate dehydrogenase (decarboxylase component) | 0.046 | 0.52 | 5.8 × 10−4 |
| evgS | Putative sensor for regulator EvgA | 0.205 | 0.51 | 7.4 × 10−7 |
| ycjV | Putative ATP binding component of a transport system | 0.088 | 0.51 | 6.9 × 10−5 |
| queA | Synthesis of queuine in tRNA | 0.080 | 0.51 | 2.7 × 10−3 |
| idnR | l-Idonate operon regulator | 0.045 | 0.51 | 1.2 × 10−3 |
| idnT | l-Idonate transporter | 0.064 | 0.51 | 6.0 × 10−5 |
| yafV | Putative EC 3.5 amidase-type enzyme | 0.023 | 0.51 | 1.7 × 10−3 |
| yi21-2 | IS2 hypothetical protein | 0.057 | 0.50 | 1.3 × 10−3 |
| cynT | Carbonic anhydrase | 0.034 | 0.50 | 2.2 × 10−5 |
| yhgG | ORF, hypothetical protein | 0.019 | 0.50 | 1.5 × 10−4 |
| b1565 | ORF, hypothetical protein | 0.011 | 0.50 | 6.6 × 10−10 |
| yi21-6 | IS2 hypothetical protein | 0.046 | 0.49 | 1.3 × 10−3 |
| yihN | Putative resistance protein (transport) | 0.020 | 0.49 | 1.8 × 10−4 |
| b2931 | Putative oxidoreductase | 0.097 | 0.48 | 2.7 × 10−3 |
| ychM | ORF, hypothetical protein | 0.160 | 0.48 | 3.2 × 10−5 |
| b1141 | ORF, hypothetical protein | 0.080 | 0.48 | 2.3 × 10−3 |
| trpD | Anthranilate synthase component II | 0.177 | 0.48 | 1.2 × 10−5 |
| yrdB | ORF, hypothetical protein | 0.013 | 0.47 | 1.2 × 10−3 |
| yhfY | ORF, hypothetical protein | 0.019 | 0.47 | 4.0 × 10−5 |
| entA | 2,3-dihydroxybenzoate dehydrogenase, enterochelin | 0.017 | 0.47 | 2.4 × 10−4 |
| yjgH | ORF, hypothetical protein | 0.085 | 0.46 | 1.4 × 10−4 |
Intensity normalized to transcript levels in cells grown on l-idonate and expressed as a percentage of the sum of the transcript levels of all of the genes in the arrays (5).
Log10 ratio of normalized transcript levels on l-idonate compared to those on d-glucose.
Each P value (scientific notation) indicates the probability that the reported log10 ratio is significant.
ORF, open reading frame.
FIG. 5.
2-D PAGE of extracted proteins from cells grown in MOPS minimal medium containing 0.2% d-glucose (A) or l-idonate (B). The two modified forms of IdnD and IdnO were identified by MS/MS as described in the text and are indicated by arrows 1 and 2 and arrows 3 and 4, respectively.
The 50 genes most highly induced on l-idonate compared to d-glucose are shown in Table 6. Only 19 of these genes encode proteins with known functions, 5 of which belong to the idn operon. The remaining 31 significantly induced genes encode products with unknown functions. The induction of these genes was not confirmed by other methods used for monitoring transcription, and it is not clear that their induction is relevant to growth on l-idonate. Thus, expression profiling did not shed any additional light on the physiology of growth on l-idonate.
DISCUSSION
The organization of the genes of the l-idonate pathway, which is suggested by the arrangement of the pathway genes around a divergent regulatory region, was confirmed in these studies. Transcription start sites for the divergent promoters are positioned at −29 and −26 relative to the idnD and idnK start codons, respectively, consistent with the predicted −10 and −35 promoter elements (Fig. 1). The pathway genes are arranged in two transcription units, the idnDOTR operon, and the divergently transcribed, monocistronic idnK gene (Fig. 2 and 3). The putative regulatory elements identified within the idn regulatory region provide some interesting clues regarding the regulation of the idn genes. A putative CRP binding site is positioned at −41.5 relative to the idnD transcription start site, suggesting a CRP-dependent class II promoter (33). The UP element at −42.5 bp relative to the idnK transcription start site is in a position expected to improve transcription initiation at the idnK promoter (22). The location of the putative IdnR binding site centered between the idnD and idnK transcription start sites suggests that IdnR may coordinately regulate both promoters.
l-Idonate and 5KG both induced the l-idonate pathway, as indicated by induction of idnD and idnK reporter fusions in an idnD nonpolar mutant that cannot interconvert l-idonate and 5KG (Table 3). The induction ratios of the idnD and idnK promoters were remarkably similar, indicating that transcription from the divergent promoters is, in fact, coordinated (Table 4). This coordinated expression apparently provides a mechanism by which to balance flux through the l-idonate pathway and maintain concentrations of the pathway intermediates at levels required for induction of the pathway genes and for appropriate regulation of the closely associated GntI pathway.
The relative order of idn transcript abundance in the Northern blot and DNA array experiments (Fig. 2 and Table 6, respectively) indicates that idnD and idnO are the most highly expressed idn transcripts (in that order), followed by idnT, idnR, and idnK. Thus, their relative expression levels are correlated with their proximity to the promoters. The low level of idnR expression is consistent with the known expression level of most regulators (9). The lower level of idnT and idnK expression suggests that flux through the pathway could be limited by l-idonate transport and phosphorylation. In addition to being highly induced by growth on l-idonate, idnD and idnO were among the most highly expressed genes in the E. coli transcriptome (Table 6) and their products were among the most abundant proteins (Fig. 5).
The relative levels of gene-specific idn gene transcripts appear to be controlled by posttranscriptional processing and/or mRNA secondary structures that could act as terminators. Under inducing conditions, there was a low level of the full-length idnDOTR transcript and shorter gene-specific transcripts were observed. The relatively high abundance of 1.9-kb idnDO and 3.3-kb idnDOT transcripts suggests that the predicted mRNA stem-loop structures located at the 3′ ends of the idnO and idnT genes may function as transcriptional terminators. The alternative possibility that the gene-specific transcripts correspond to promoters within the idnDOTR operon was not tested. The 3′ end of the idnDOTR transcript does not appear to contain any secondary structure indicative of a terminator, and transcription of the operon was found to extend into the 5′ end of the downstream yjgR gene (Fig. 3). However, yjgR knockout mutants grew normally on l-idonate and yjgR was not induced in cells grown on l-idonate, indicating that YjgR is not involved in l-idonate catabolism.
Catabolite repression of the l-idonate pathway indicates that glucose and d-gluconate are preferred over l-idonate (Table 4); the slower growth rate of cells on l-idonate seems to explain this hierarchy of nutrient choice (Table 5). Hogema et al. (10) demonstrated that d-gluconate is catabolite repressing because it lowers the intracellular cAMP and CRP concentrations through a mechanism that does not involve the phosphotransferase system (PTS) EIIAGlu enzyme. This explains why the addition of cAMP did not fully relieve the repression of the idn genes caused by d-gluconate (Table 4). In the presence of catabolite-repressing sugars such as d-glucose and d-gluconate, cAMP and CRP levels are low and transcription of the idn genes is not induced. Only in the absence of catabolite-repressing sugars, when l-idonate or 5KG is present, are the idn genes fully expressed.
Failure of the idnK mutant (MDE5) to grow on l-idonate indicates that the presumed intracellular accumulation of d-gluconate formed by IdnD and IdnO did not reach levels high enough to induce the GntI system for d-gluconate catabolism, specifically gntK, the idnK paralog. This result suggests that transcription of the GntI and GntII systems is tuned to the concentrations of inducers such that the d-gluconate and l-idonate pathways are regulated appropriately (i.e., GntI is induced by gluconate and GntII is induced by l-idonate). This possibility is being explored.
The operation of GntII as a subsidiary gluconate pathway was examined in a gntRKU mutant (Fig. 4 and Table 5) that exhibits a lag before initiating growth on d-gluconate (11). It was previously suggested that the physiological reason why 5KG functions as an inducer of the l-idonate pathway could be to act as an endogenous inducer of the GntII system for subsidiary d-gluconate catabolism (31). Since cells grow poorly on 5KG, it is unlikely that 5KG is physiologically relevant as a growth substrate. Induction of the GntII system in the GntI mutant can be attributed to accumulation of d-gluconate in mutants blocked in gluconate kinase (e.g., gntK); in turn, the accumulated d-gluconate could be converted to 5KG by the basal level of IdnO, a freely reversible enzyme that converts d-gluconate to 5KG with NAD as a cofactor (3). As 5KG accumulates, it would induce the subsidiary d-gluconate kinase encoded by idnK, which can functionally substitute for GntK of the GntI pathway for d-gluconate catabolism. This same mechanism would also be expected to substitute for GntT in a gntT mutant by inducing the subsidiary d-gluconate transporter IdnT.
We used functional genomic tools to ensure that nothing was overlooked regarding the physiology of growth on l-idonate. As predicted, the genes of the l-idonate pathway were induced by growth on idonate (Table 6). What was not anticipated was the induction of genes such as araD, narW, thiM, hyaF, and nrfE. The induction of these genes has not been confirmed by other methods used to monitor transcription, and it is not clear that their induction is relevant to growth on l-idonate. Thus, expression profiling failed to shed any additional light on the physiology of growth on l-idonate.
We investigated the translation of the idnD and idnO transcripts and determined that the protein level directly correlated with the transcript level, suggesting little, if any, translation control in expression of the idnDOTR transcript. The 2-D gel analysis revealed duplicate spots for both IdnD and IdnO, suggesting that a charged group had modified these proteins and altered their mobility in the gel (Fig. 5). The only protein-modifying enzyme that was induced by growth on l-idonate was rimJ, which encodes an N-terminal acetyltransferase that modifies ribosomal protein S5 (Table 6). It is unlikely that RimJ modifies IdnD or IdnO, since acetyl groups are generally neutral in charge. Alternatively, the negatively charged molecules l-idonate and 5KG may have remained bound to the catalytic sites of these proteins during extraction, thereby changing their overall charge.
In summary, the results presented here indicate that the idn genes are organized in two coordinately regulated operons, idnDOTR and idnK. The idn genes are specifically induced by l-idonate and 5KG and are catabolite repressed by glucose and gluconate. Whole-genome expression profiling of cells growing on l-idonate indicated that the majority of the genes induced code for proteins of unknown function and thus reveal little about the physiology of growth on l-idonate. Lastly, d-gluconate does not normally induce the idn (GntII) genes unless the GntI system is nonfunctional and does so apparently by formation of the endogenous inducer 5KG.
Acknowledgments
We thank April Anderson for critical reading of the manuscript.
Work on this project was supported by grants from the NSF (MCB-9723593) and NIH (AI48945), as well as a generous gift from Genencor International.
REFERENCES
- 1.Bachi, B., and H. L. Kornberg. 1975. Genes involved in the uptake and catabolism of gluconate by Escherichia coli. J. Gen. Microbiol. 90:321-335. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, B. J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 3.Bausch, C., N. Peekhaus, C. Utz, T. Blais, E. Murray, T. Lowary, and T. Conway. 1998. Sequence analysis of the GntII (subsidiary) system for gluconate metabolism reveals a novel pathway for l-idonic acid catabolism in Escherichia coli. J. Bacteriol. 180:3704-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Conway, T., B. Kraus, D. L. Tucker, D. J. Smalley, A. F. Dorman, and L. McKibben. 2002. DNA array analysis in a Microsoft Windows environment. BioTechniques 32:110, 112-114, 116, 118-119. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devreese, B., F. Vanrobaeys, and J. Van Beeumen. 2001. Automated nanoflow liquid chromatography/tandem mass spectrometric identification of proteins from Shewanella putrefaciens separated by two-dimensional polyacrylamide gel electrophoresis. Rapid Commun. Mass Spectrom. 15:50-56. [DOI] [PubMed] [Google Scholar]
- 8.Estrem, S. T., T. Gaal, W. Ross, and R. L. Gourse. 1998. Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. USA 95:9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottesman, S. 1984. Bacterial regulation: global regulatory networks. Annu. Rev. Genet. 18:415-441. [DOI] [PubMed] [Google Scholar]
- 10.Hogema, B. M., J. C. Arents, T. Inada, H. Aiba, K. Van Dam, and P. W. Postma. 1997. Catabolite repression by glucose 6-phosphate, gluconate, and lactose in Escherichia coli. FEMS Microbiol. 24:857-867. [DOI] [PubMed] [Google Scholar]
- 11.Istúriz, T., E. Palmero, and J. Vitelli-Flores. 1986. Mutations affecting gluconate catabolism in Escherichia coli. Genetic mapping of the locus for the thermosensitive gluconokinase. J. Gen. Microbiol. 132:3209-3212. [DOI] [PubMed] [Google Scholar]
- 12.Karlin, S., J. Mrazek, A. Campbell, and D. Kaiser. 2001. Characterizations of highly expressed genes of four fast-growing bacteria. J. Bacteriol. 183:5025-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luria, S. E., and M. Delbruck. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacConkey, A. 1905. Lactose fermenting bacteria in faces. J. Hyg. 5:333-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, J. H. 1972. Experiments in molecular genetics. Cold Springs Harbor Laboratory, Cold Springs Harbor, N.Y.
- 16.Nagel de Zwaig, R., N. Zwaig, T. Istúriz, and R. S. Sánchez. 1973. Mutations affecting gluconate metabolism in Escherichia coli. J. Bacteriol. 114:463-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peekhaus, N., and T. Conway. 1998. Positive and negative transcriptional regulation of the Escherichia coli gluconate regulon gene gntT by GntR and the cyclic AMP (cAMP)-cAMP receptor protein complex. J. Bacteriol. 180:1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peekhaus, N., S. Tong, J. Reizer, M. H. Saier, Jr., E. Murray, and T. Conway. 1997. Characterization of a novel transporter family that includes multiple Escherichia coli gluconate transporters and their homologues. FEMS Microbiol. Lett. 147:233-238. [DOI] [PubMed] [Google Scholar]
- 21.Porco, A., N. Peekhaus, C. Bausch, S. Tong, T. Isturiz, and T. Conway. 1997. Molecular genetic characterization of the Escherichia coli gntT gene of GntI, the main system for gluconate metabolism. J. Bacteriol. 179:1584-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross, W., A. Ernst, and R. L. Gourse. 2001. Fine structure of E. coli RNA polymerase-promoter interactions: alpha subunit binding to the UP element minor groove. Genes Dev. 15:491-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Sanchez, J. C., V. Rouge, M. Pisteur, F. Ravier, L. Tonella, M. Moosmayer, M. R. Wilkins, and D. F. Hochstrasser. 1997. Improved and simplified in-gel sample application using reswelling of dry immobilized pH gradients. Electrophoresis 18:324-327. [DOI] [PubMed] [Google Scholar]
- 25.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shine, J., and L. Dalgarno. 1975. Terminal-sequence analysis of bacterial ribosomal RNA. Correlation between the 3′-terminal-polypyrimidine sequence of 16-S RNA and translational specificity of the ribosome. Eur. J. Biochem. 57:221-230. [DOI] [PubMed] [Google Scholar]
- 27.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 28.Tao, H., C. Bausch, C. Richmond, F. R. Blattner, and T. Conway. 1999. Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J. Bacteriol. 181:6425-6440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong, S., A. Porco, T. Isturiz, and T. Conway. 1996. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of GntI, the main system for gluconate metabolism. J. Bacteriol. 178:3260-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Truesdell, S. J., J. C. Sims, P. A. Boerman, J. L. Seymour, and R. A. Lazarus. 1991. Pathways for metabolism of ketoaldonic acids in an Erwinia sp. J. Bacteriol. 173:6651-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsunedomi, R., H. Izu, T. Kawai, K. Matsushita, T. Ferenci, and M. Yamada. 2003. The activator of GntII genes for gluconate metabolism, GntH, exerts negative control of GntR-regulated GntI genes in Escherichia coli. J. Bacteriol. 185:1783-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanner, B. L., R. Kodaira, and F. C. Neidhardt. 1977. Physiological regulation of a decontrolled lac operon. J. Bacteriol. 130:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou, Y., T. J. Merkel, and R. H. Ebright. 1994. Characterization of the activating region of Escherichia coli catabolite gene activator protein (CAP). II. Role at class I and class II CAP-dependent promoters. J. Mol. Biol. 243:603-610. [DOI] [PubMed] [Google Scholar]