Abstract
Copper plays a fundamental role in the biochemistry of all aerobic organisms. The delivery of this metal to specific intracellular targets is mediated by metallochaperones. To elucidate the role of the metallochaperone Atox1, we analyzed mice with a disruption of the Atox1 locus. Atox1−/− mice failed to thrive immediately after birth, with 45% of pups dying before weaning. Surviving animals exhibited growth failure, skin laxity, hypopigmentation, and seizures because of perinatal copper deficiency. Maternal Atox1 deficiency markedly increased the severity of Atox1−/− phenotype, resulting in increased perinatal mortality as well as severe growth retardation and congenital malformations among surviving Atox1−/− progeny. Furthermore, Atox1-deficient cells accumulated high levels of intracellular copper, and metabolic studies indicated that this defect was because of impaired cellular copper efflux. Taken together, these data reveal a direct role for Atox1 in trafficking of intracellular copper to the secretory pathway of mammalian cells and demonstrate that this metallochaperone plays a critical role in perinatal copper homeostasis.
Copper is a transition element that plays an essential role in the metabolic pathways required for cellular respiration, iron homeostasis, pigment formation, neurotransmitter production, peptide biogenesis, connective tissue biosynthesis, and antioxidant defense (1). Recognition of the inherited disorders of copper transport, Menkes and Wilson disease, dramatically underscored both the essential need for copper and the toxicity of this metal (2). Characterization of the molecular genetic basis of these disorders has provided the opportunity to elucidate the molecular mechanisms of cellular copper metabolism, including the recent identification in Saccharomyces cerevisiae of a family of cytoplasmic proteins termed metallochaperones, proposed to play a role in intracellular copper trafficking (3, 4).
Genetic and biochemical studies in yeast suggest that metallochaperones function to protect copper from intracellular scavenging while facilitating the appropriate partnerships with specific cupro-protein targets (5). The yeast metallochaperone Atx1 plays a role in copper delivery to the secretory pathway in this organism via interaction with the copper transport ATPase Ccc2 (6–8). Structural and functional analysis of the mammalian Atx1 homologue, ATOX1 (or HAH1), indicates that this protein interacts with the Menkes and Wilson copper-transporting ATPases located in the trans-Golgi network of cells (9, 10). Analysis of Wilson disease-associated mutations in the amino terminus of the Wilson ATPase suggests a critical role for ATOX1–ATPase interaction in hepatocyte copper homeostasis (10). Nevertheless, despite these experiments in yeast and in mammalian cell lines, the physiologic role of ATOX1 in mammals remains to be determined. This current study was undertaken to elucidate the function of ATOX1 in mammalian cells and to examine its role in cellular copper homeostasis by using a genetic mouse model.
Materials and Methods
Generation of Atox1−/− Null Mice.
R1 embryonic stem cells were cultured and electroporated, and neomycin-resistant clones assayed positive for β-galactosidase activity were aggregated with morula-stage embryos to generate chimeric mice as described (11). The genomic structure of mouse Atox1 has been reported (12), and Southern blot and 5′-rapid amplification of cDNA ends analysis of genomic DNA from these embryonic stem cells confirmed the presence of the β-geo gene trap at the Atox1 locus. Chimeric mice were mated to CD-1 animals to obtain germ-line transmission of the disrupted Atox1 allele. Mice heterozygous for the trapped allele were backcrossed six times (N6) to C57BL/6 mice and intercrossed seven times (F7). All subsequent analyses were performed with N6F7 generation mice. All mice were housed at the Washington University School of Medicine Vivarium under a 12-h light/12-h dark cycle. Food and water were provided ad libitum, and all care was given in compliance with National Institutes of Health guidelines on the use of laboratory and experimental animals.
Genotyping and Analysis of Mice.
Southern blot of wild-type (wt) and mutant genomic DNA digested with multiple restriction enzymes and analyzed with a 5′ Atox1 probe confirmed that the β-geo insertion is at the Atox1 locus. Southern blot with a neomycin DNA probe revealed a single insertion of the β-geo cassette at the Atox1 locus. PCR was performed by two-allele, three-primer analysis by using genomic DNA template prepared from tail clips or embryonic tissues. PCR of the wt allele with primers (available on request) resulted in a 700-bp product, whereas PCR of the mutated allele resulted in a 1,300-bp fragment. Initial PCR results were verified by Southern analysis of genomic DNA from the same mice. RNA blot analyses were performed from total RNA isolated from E12.5 embryonic fibroblasts and hybridized with Atox1 or 18S DNA probe using Ultrahyb (Ambion, Austin, TX). Immunoblot analyses were performed by using ATOX1 antisera and chemiluminescent techniques (12). No detectable Atox1 protein was observed in multiple tissues obtained from surviving Atox1−/− animals. Growth was monitored by weighing animals starting from P0 to P30 every 24 h. Hypothermia was evaluated by the ability of mice to maintain body temperature at 4°C within a 3-h period by using a rectal digital thermometer. Milk spots were determined by examining the stomach contents of dissected P1 pups. For blood values, P1 pups were decapitated, and blood drawn into heparinized capillary tubes was used immediately to obtain hematocrits, sodium, and glucose values.
Radiolabeled Copper Studies.
Placental copper transport studies were initiated in E14.5 timed pregnant dams by i.v. tail injection of 1 mCi of 64Cu that was preequilibrated with 50 μg of copper (chloride salt) in 100 μl of saline (13, 14). Twenty-four hours later, the individual placenta and embryo were dissected from the maternal tissues and rinsed three times with PBS before quantifying the amount of tissue 64Cu incorporation in a Packard Gamma Counter. Each data point was calculated as the ratio of the amount of 64Cu (cpm) retained within that tissue to the total amount of 64Cu (cpm) injected into the mother. For cell culture experiments, mouse embryonic fibroblasts (MEFs) from E12.5 embryos were isolated and cultured in DMEM with 10% FCS as described (15). After the first passage, the MEFs were genotyped by PCR analysis. Mouse fibroblast cell lines (802–5 and 802–1) were cultured as described (16). For copper retention studies, 2 × 105 cells were allowed to adhere onto 35-mm Petri dishes before incubation for 68 h with 8 × 106 cpm of 64Cu in culture medium. The cells were then washed three times with ice-cold PBS, lysed with PBS containing 1% SDS, and analyzed for 64Cu retention by using the gamma counter. For copper uptake and efflux experiments, 8 × 106 cpm of 64Cu was premixed with 200 nM copper (chloride salt) and 400 nM L-histidine (Sigma) for 1 h at 37°C in serum-free OPTIMEM (Life Technologies, Grand Island, NY). Copper uptake was initiated by incubating MEFs with 200 nM 64Cu per 4 × 104 cells at 37°C, and at different time points the uptake was terminated by rapid aspiration of 64Cu media and incubation in an ice-water bath. For efflux studies, cells were pulsed with 200 nM 64Cu for 1 h as described above, followed by three quick rinses with prewarmed PBS to remove any residual copper, and then incubated with prewarmed OPTIMEM alone for several chase periods. Cells were harvested at different time intervals and processed for quantification as described for copper retention.
Copper and Enzyme Activity Measurements.
Copper concentrations and enzymatic activities were measured from wt and Atox1−/− P2 organs obtained from 5 litters (38 pups) of Atox1+/− matings. Total tissue copper content was measured by acid digestion of tissue and atomic absorption spectrophotometry (17). Cytochrome c oxidase activity was measured spectrophotometrically on whole homogenates by monitoring loss of ferrocytochrome c (17). Tyrosinase activity was measured in whole-skin homogenates as an in-gel dopa oxidase assay as described (18). Succinate dehydrogenase activity was measured by the reduction of 2,6-dichlorophenol indophenol at 600 nm as described (19). Limited tissue prohibited direct assay of lysyl oxidase or dopamine monooxygenase activities.
Results
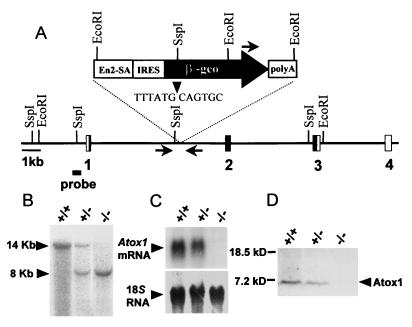
To determine the physiologic role of ATOX1, we characterized a mouse Atox1 locus that was disrupted by gene-trap insertion of a β-galactosidase-neomycin marker (β-geo) between the first and second exons of one allele of Atox1 in embryonic stem cells (Fig. 1A) (12, 20). Germ-line chimeras derived from morula aggregation with the mutated cells were used to obtain heterozygous Atox1+/− mice that were bred to homozygosity and ascertained by Southern blot analysis of mouse genomic DNA by using a 5′ Atox1-specific probe (Fig. 1B) and a neomycin DNA probe (data not shown). Analysis of mouse fibroblasts from E12.5 embryos indicated that the β-geo insertion resulted in an Atox1 null allele, as no Atox1 mRNA nor protein was detected in Atox1−/− homozygous animals based on RNA blots and immunoblots, respectively (Fig. 1 C and D).
Figure 1.
Generation of the Atox1 null allele. (A) The genomic locus of the mouse Atox1 gene disrupted by the promoterless β-galactosidase-neomycin (β-geo) cassette. The relevant restriction sites, location of the β-geo insertion (arrowhead) between exons 1 and 2, the 5′ probe for Southern analysis (horizontal bar), and location of sequences used as primer sets for genotyping (horizontal arrows) are indicated (En2-SA, engrailed 2 splice acceptor site; IRES, internal ribosome entry site). (B) Southern blot analysis of genomic DNA digested with EcoRI from Atox1+/+, Atox1+/−, and Atox1−/− embryos and probed with the 5′ probe. (C) RNA blot analysis of 10 μg of total RNA isolated from E12.5 embryonic fibroblasts and probed with the Atox1 or 18S (Ambion) DNA probe. (D) Immunoblot analysis of Atox1 protein in E12.5 embryonic fibroblasts. One-hundred-microgram cell lysates were separated by 10–20% Tricine SDS/PAGE gel and probed with ATOX1 antisera followed by chemiluminescent detection.
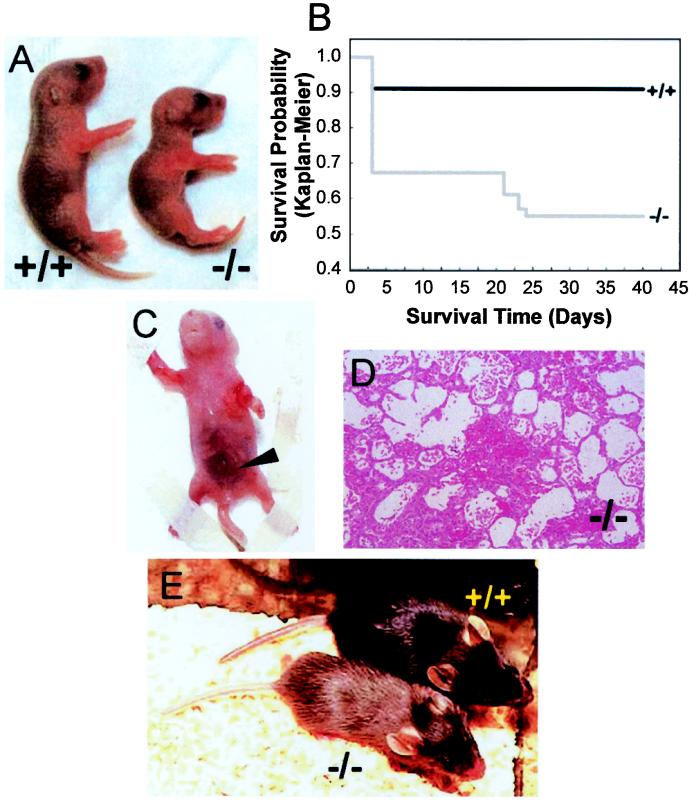
Analysis of staged offspring from Atox1+/− matings showed the expected Mendelian ratio until birth, but revealed a growth retardation phenotype in Atox1−/− neonatal mice that was apparent in most pups by postnatal day 3 (P3) (Fig. 2A). Organ weight to body length comparison of mutant and wt littermates indicated that this growth impairment was symmetrical (data not shown). Despite normal sucking behavior, the presence of milk spots and normal red cell hemoglobin (hematocrit; +/+ 37.83 ± 2.75%, +/− 40.6 ± 3.84, −/− 40.13 ± 2.1%), serum glucose (+/+ 60 ± 4.58 mg/dl, +/− 47.27 ± 17.84 mg/dl, −/− 34.5 ± 7.55 mg/dl), and electrolyte values (sodium; +/+ 143 ± 1.73 mmol/liter, +/− 141.53 ± 4.85 mmol/liter, −/− 140.25 ± 0.96 mmol/liter), in comparison with wt littermates, P3 Atox1−/− pups displayed decreased activity and considerable mortality (Fig. 2B). Despite the trend toward lower serum glucose values in Atox1−/− pups, these values were within the normal range observed in newborn mice. Atox1−/− pups displayed skin laxity, and some animals exhibited peripartum hemorrhaging, which contributed to the overall early mortality (Fig. 2 C and D). Genotyping of a total of 166 progeny from Atox1+/− intercrosses identified 49 Atox1−/− pups, of which 33 survived beyond P3. Before weaning, an additional six animals died from prolonged seizure activity, giving an overall perinatal mortality of 45%. Unlike the Atox1−/− mice, the growth, mortality, and gross phenotype of Atox1+/− mice were found to be indistinguishable from their wt littermates (data not shown). Beyond weaning, surviving Atox1−/− mice remained growth-retarded, hypothermic, and hypopigmented (Fig. 2E), suggesting long-term effects of perinatal Atox1 deficiency.
Figure 2.
Atox1 deficiency results in growth failure and mortality. (A) Images of Atox1+/+ (Left) and Atox1−/− pups (Right) depicting growth differences at P3. (B) Kaplan–Meier plot of survival in progeny from Atox1+/− ♂ × Atox1+/− ♀ mating (n = 166). Note initial drop by P3, followed by another decrease because of seizures around weaning age. Abdominal hemorrhage (arrowhead) (C) and micrograph (100×) depicting pulmonary alveolar hemorrhage (D) observed in some Atox1−/− newborn pups. (E) Twenty-three-day-old weanling Atox1+/+ (Right) and Atox1−/− (Left) mice. Note size difference and the hypopigmentation of tail and coat color.
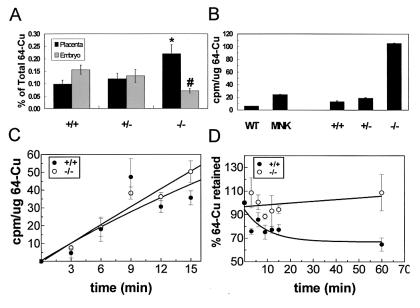
The phenotypes observed in Atox1−/− animals were in part similar to phenotypes observed in copper-deprived animals, indicating that perinatal copper homeostasis may be perturbed in the absence of Atox1. To examine copper homeostasis in Atox1−/− mice, 64Cu was administered intravenously to pregnant females from Atox1+/− intercrosses; 24 h later, the amount of 64Cu distributed in individual placentas and their corresponding embryos was determined. Significantly less copper was transported into Atox1−/− embryos as compared with Atox1+/+ and Atox1+/− embryos. Most of the copper in the Atox1−/− fetuses remained in the placenta (Fig. 3A). Transplacental nutrient uptake from the maternal circulation is mediated by syncytiotrophoblasts and capillary endothelium cells, which are embryonic in origin and line the maternal blood vessels within the placenta. Because the aberration in placental copper transport corresponds specifically to Atox1−/− genotype, the data suggest that Atox1-deficient cells are unable to maintain normal copper status from maternally derived nutrients. To test this hypothesis, MEFs isolated from E12.5 embryos were incubated with 64Cu containing media, and the total amount of copper remaining within the cells was determined after 68 h. Atox1−/− MEFs retained significantly greater amounts of copper compared with cells derived from Atox1+/+ or Atox1+/− littermates. A similar phenotype of copper retention was observed when these studies were repeated with fibroblasts that were deficient in the Menkes ATPase (Fig. 3B) (21). The difference in the amount of copper accumulated by the Menkes and Atox1−/− fibroblasts may be because of genetic strain differences or may reflect the fact that the Menkes (Mobr-J) defect results in a partial loss-of-function of this protein. The increase in copper accumulation within Atox1−/− cells was not because of differences in copper uptake, as the initial rate for copper uptake was identical in wt and Atox1−/− MEFs (Fig. 3C). To further characterize the copper-retaining phenotype of Atox1−/− cells, MEFs were pulse labeled with 64Cu for 1 h, and cells were analyzed at specific chase periods for 64Cu retained. Atox1-deficient MEFs revealed a severe impairment in copper efflux compared with wt cells. Thus, Atox1−/− cells accumulate copper because of defective copper efflux from the cell (Fig. 3D).
Figure 3.
Atox1 is essential for normal copper homeostasis. (A) Placental copper transport was determined in E14.5 pregnant females from Atox1+/− ♂ × Atox1+/− ♀ mating. Fifty micrograms of 64Cu was injected intravenously into pregnant dams, and 24 h later the placenta and embryo were dissected from maternal tissue and 64Cu incorporation quantified in a gamma counter. Data represent the mean ± SEM from four separate litters and at least 10 animals of each genotype. Each data point is calculated as the amount of 64Cu retained by the tissue relative to the total amount of 64Cu injected into the mother (*, P < 0.05 among the +/+ and −/− placenta and +/+ and −/− embryos; #, P < 0.01 between −/− placenta and −/− embryos; P values were calculated by using a one-way ANOVA with Student–Newman–Keuls multiple comparison test). (B) 64Cu retention was determined in cultured fibroblasts derived from Atox1 mouse embryos (E12.4 +/+, +/−, and −/−) or mouse cell lines (wt, 802–5; MNK, 802–1). Cells (2 × 105) were incubated with 8 × 106 cpm of 64Cu. After 68 h, the cells were washed, lysed, and analyzed for 64Cu retained by using a gamma counter. Each data point represents the mean ± SEM from three separate experiments performed in triplicate and expressed as cpm/μg of total cell protein. Copper uptake (C) and copper efflux (D) were performed by incubating MEFs with 200 nM 64Cu per 4 × 104 cells. For efflux, cells were pulsed with 64Cu for 1 h followed by several chase periods with unsupplemented culture media. Cells were harvested at time intervals indicated and processed as described above.
A reduction in placental copper transport should result in newborn mice with decreased copper content in biosynthetically active tissues such as the neonatal liver and brain. Consistent with this concept, P2 Atox1−/− pups had 50% less copper in the liver (mean ± SD: +/+, 20.2 ± 4.12 μg/g; −/−, 10.1 ± 1.71 μg/g, P < 0.01) and in the brain (+/+, 1.37 ± 0.35 μg/g; −/−, 0.685 ± 0.174 μg/g, P < 0.01). Furthermore, the activity of essential cupro-enzymes including brain cytochrome oxidase (COX; +/+, 0.42 ± 0.02 unit/mg; −/−, 0.267 ± 0.03 unit/mg, P < 0.01) and tyrosinase (skin tissue from −/− pups showed ≈25% of tyrosinase activity compared with +/+ littermates) was significantly decreased in P2 Atox1−/− mice. The diminution in brain COX activity was not because of differences in mitochondrial number or integrity, as the activity of the inner mitochondrial membrane protein, succinate dehydrogenase, was normal (+/+, 0.013 ± 0.002 unit/mg, −/−, 0.014 ± 0.003 unit/mg).
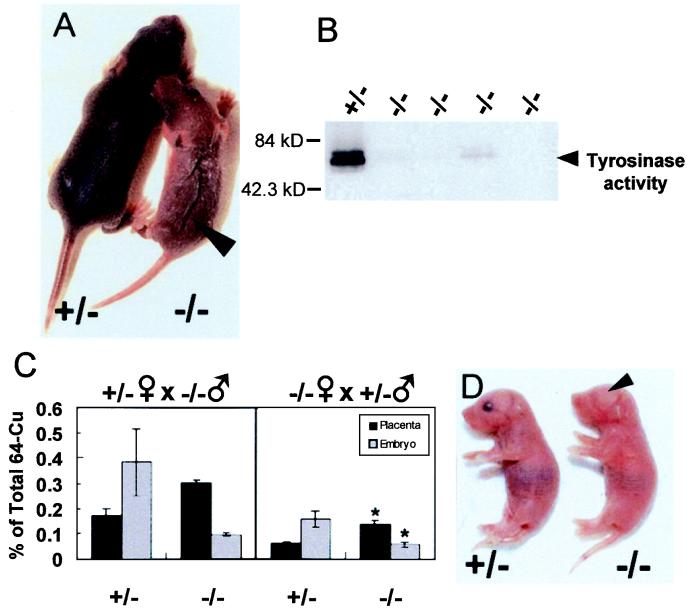
As placental transfer of nutrients is often dependent on both maternal and fetal components, progeny from Atox1+/− ♂ × Atox1−/− ♀ and Atox1−/− ♂ × Atox1+/− ♀ intercrosses were examined. Atox1−/− mice derived from Atox1−/− mothers exhibited a striking phenotype of growth retardation, skin laxity, and hypopigmentation as compared with its Atox1+/− littermates (Fig. 4A). The phenotype in these Atox1−/− mice was more severe than that observed previously in Atox1−/− mice derived from heterozygote mothers, as evidenced by a near complete lack of tyrosinase enzyme activity in skin tissue (Fig. 4B), despite the presence of normal amounts of tyrosinase protein in those samples (data not shown). Atox1−/− pups showed increased mortality within the first day of life. Six Atox1−/− pups (46%) died within 24 h after birth from Atox1+/− ♂ × Atox1−/− ♀ mating (three litters; 13 −/−, 12 +/−), whereas two Atox1−/− pups (20%) died within that time period from Atox1−/− ♂ × Atox1+/− ♀ mating (three litters; 10 −/−, 10 +/−). These findings suggest an influence of maternal Atox1 deficiency on perinatal copper metabolism, which, although not sufficient to result in overt signs or symptoms in Atox1+/− pups (Fig. 4A), significantly exacerbated the copper deficiency in Atox1−/− pups. The concept of maternal Atox1 influence was supported by placental copper transport studies. As noted previously in Fig. 3A, the difference in 64Cu distribution between the placenta and embryo was found to be dependent on the embryonic genotype (Fig. 4C). However, now an overall reduction of 60% in copper availability for placental transport was observed in Atox1−/− fetuses derived from Atox1−/− mothers compared with Atox1−/− fetuses derived from Atox1+/− mothers (Fig. 4C). Furthermore, some Atox1−/− pups born to Atox1−/− mothers demonstrated severe congenital eye defects (Fig. 4D). Microphthalmia was observed in six Atox1−/− pups obtained from Atox1+/− ♂ × Atox1−/− ♀ mating (11 litters, 68 pups), of which only one mouse survived past P3. This phenotype was not due to the mouse genetic background because Atox1−/− progeny from the same number of litters from Atox1−/− ♂ × Atox1+/− ♀ and Atox1+/− ♂ × Atox1+/− ♀ mating did not show microphthalmia. In addition, occasional Atox1−/− mice born to Atox1−/− mothers also demonstrated central nervous system malformations and other developmental abnormalities not observed in control littermates.
Figure 4.
Atox1−/− progeny exhibit a maternal effect. (A) Images of Atox1+/− (Left) and Atox1−/− (Right) P8 pups from Atox1+/− ♂ × Atox1−/− ♀ mating. Note skin laxity on the dorsal surface (arrowhead), hypopigmentation, and growth retardation (body weight: +/− = 3.31 g, −/− = 1.74 g). (B) Tyrosinase activity was determined in 100 μg of skin tissue extract separated on a 4–20% SDS/PAGE gel and assayed for dopa oxidase activity. Each lane represents a P8 skin sample from Atox1+/− ♂ × Atox1−/− ♀ progeny. (C) Placental copper transport was determined in Atox1+/− and Atox1−/− pregnant females mated to the indicated males. Data represent the mean ± SEM from three separate litters and at least four animals of each genotype (*, P < 0.01 for −/− placentas and −/− embryos derived from Atox1+/− versus Atox1−/− mothers). (D) Image of Atox1−/− P2 pup showing microphthalmia (Left) and Atox1+/− littermate (Right) from Atox1+/− ♂ × Atox1−/− ♀ mating.
Discussion
The data in this study demonstrate a critical role for the metallochaperone ATOX1 in mammalian copper homeostasis. The striking phenotypes observed in Atox1−/− mice are in part similar to those observed in copper-deprived animals where diminished activity of the cupro-enzymes lysyl oxidase, dopamine monooxygenase, and tyrosinase results in impaired connective tissue integrity, temperature regulation, and pigment formation (1, 2). The copper-retaining phenotype of Atox1-deficient MEFs reveals the cellular mechanism underlying the impaired placental copper transfer to Atox1−/− embryos, where copper retention within the placenta, due to defective cellular copper efflux, results in decreased copper availability for the fetal circulation. These data, taken together with the demonstrated interaction between ATOX1 and the copper-transporting ATPases (9, 10), definitively reveal a role for ATOX1 in the pathway of copper trafficking from the cytoplasm to the secretory pathway in mammalian cells.
The seizures, hypothermia, and neurodegeneration observed in humans with Menkes disease, and in mice with mutations at the homologous mottled locus, have been attributed to regional alterations of COX activity secondary to copper deficiency in the neonatal brain (17, 22). Thus, it is reasonable to assume that the morbidity associated with Atox1-deficient mice is in part related to reduced COX activity in specific areas of the brain that are metabolically active during development. Importantly, previous observations of the phenotype of different mottled alleles (Mo) have demonstrated a correlation between the type and severity of symptoms and the amount of in utero copper transferred to the developing fetus (23). For example, dappled mice (Modp) die in utero from impaired connective tissue integrity and hemorrhage secondary to a deficiency in lysyl oxidase activity, whereas brindled mice (Mobr) have increased but variable neonatal mortality similar to that observed here in the Atox1−/− mice. The heterogeneity in the phenotype in Atox1−/− mice may potentially arise from incomplete penetrance resulting from either specific modifying genetic loci or epigenetic effects such as the amount of perinatal copper available during embryonic growth and development. The latter possibility then reveals phenotypic differences based on developmental and tissue-specific thresholds for copper requirement, a concept reinforced by data from Ctr1-deficient animals in the accompanying article (24).
The finding that Atox1−/− mice derived from Atox1−/− mothers exhibited a more severe phenotype suggests that, in part, copper deficiency in Atox1−/− mothers along with Atox1 deficiency in both maternal and fetal components within the placenta leads to a more acute deprivation of perinatal copper availability to the developing embryo, thus compounding the Atox1−/− phenotype. Accordingly, these animals showed a marked impairment in growth, higher mortality, reduced pigmentation because of little or no tyrosinase activity, and microphthalmia. Although the precise role of Atox1 in eye development is unclear, a similar phenotype of hypopigmentation and microphthalmia is observed in mice deficient in Mitf, a transcription factor required for the development and maturation of the neural crest-derived melanocytes (25). These findings then raise the possibility that copper-trafficking pathways involving Atox1 may play a role in the complex signal transduction events involved in neural crest cell development.
Our findings, together with previous biochemical studies (9, 10), are consistent with the hypothesis that ATOX1 is required for copper trafficking to the secretory pathway of mammalian cells through interaction with the Menkes and Wilson ATPase. Thus, the severe pleiotropic phenotype in Atox1 deficiency may be because of impaired in utero copper delivery to both the Menkes and Wilson proteins during development. Furthermore, Atox1 may have additional roles during cell differentiation and survival, a concept supported by recent studies implicating a direct role for Atox1 in copper-dependent antioxidant and apoptotic defense in neurons (26). The ability of excess copper to partially compensate for Atx1 deficiency in yeast (6) suggests that the phenotype observed in Atox1-deficient mice might also be affected by alterations in available copper. Preliminary studies administering i.p. copper to neonatal mice and weanling mice support this concept in that these animals demonstrate an improvement in survival and rescue of coat color. The microphthalmia and other developmental defects associated with Atox1 deficiency, and the embryonic lethality associated with loss of Ctr1 in the accompanying report (24), reiterate the essential role for copper in fetal development. Current efforts are now focused on careful delineation of Atox1-associated phenotypes during tissue morphogenesis. In this regard, these studies underscore the unique potential of genetic models to contribute new knowledge to our understanding of the complex relationship between nutrition and fetal development. The observation that maternal genotype may contribute to differences in fetal copper acquisition and fetal development highlights an important complexity in considering genetic and environmental influences on nutrient delivery to the developing fetus. As such, the approach presented here provides a useful heuristic paradigm for defining the role of specific nutrients in the pathogenesis of birth defects, an area recently highlighted by the discovery of the role of folic acid in human fetal neural tube closure (27).
Acknowledgments
We thank Dennis Thiele for sharing information before publication, Michael Welch for 64Cu, and Scott Saunders, Lou Muglia, and Dave Wilson for helpful discussions and critical review of the manuscript. This work was supported by National Institutes of Health Postdoctoral Fellowship HL07873 (to I.H.) and National Institutes of Health Research Grant DK58783 (to J.D.G.). J.D.G. is a recipient of the Burroughs Wellcome Scholar Award in Experimental Therapeutics.
Abbreviations
- COX
cytochrome oxidase
- wt
wild type
- MEFs
mouse embryonic fibroblasts
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 6543.
References
- 1.Pena M M, Lee J, Thiele D J. J Nutr. 1999;129:1251–1260. doi: 10.1093/jn/129.7.1251. [DOI] [PubMed] [Google Scholar]
- 2.Culotta V C, Gitlin J D. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. Vol. 2. New York: McGraw–Hill; 2001. pp. 3105–3126. [Google Scholar]
- 3.Valentine J, Gralla E B. Science. 1997;278:817–818. doi: 10.1126/science.278.5339.817. [DOI] [PubMed] [Google Scholar]
- 4.O'Halloran T V, Culotta V C. J Biol Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 5.Rae T D, Schmidt P J, Pufahl R A, Culotta V C, O'Halloran T V. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 6.Lin S J, Pufahl R A, Dancis A, O'Halloran T V, Culotta V C. J Biol Chem. 1997;272:9215–9220. [PubMed] [Google Scholar]
- 7.Pufahl R A, Singer C P, Peariso K L, Lin S J, Schmidt P J, Fahrni C J, Culotta V C, Penner-Hahn J E, O'Halloran T V. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig A C, Huffman D L, Hou M Y, Wernimont A K, Pufahl R A, O'Halloran T V. Struct Fold Des. 1999;7:605–617. doi: 10.1016/s0969-2126(99)80082-3. [DOI] [PubMed] [Google Scholar]
- 9.Larin D, Mekios C, Das K, Ross B, Yang A S, Gilliam T C. J Biol Chem. 1999;274:28497–28504. doi: 10.1074/jbc.274.40.28497. [DOI] [PubMed] [Google Scholar]
- 10.Hamza I, Schaefer M, Klomp L W, Gitlin J D. Proc Natl Acad Sci USA. 1999;96:13363–13368. doi: 10.1073/pnas.96.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faisst A M, Gruss P. Dev Dyn. 1998;212:293–303. doi: 10.1002/(SICI)1097-0177(199806)212:2<293::AID-AJA14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Hamza I, Klomp L W, Gaedigk R, White R A, Gitlin J D. Genomics. 2000;63:294–297. doi: 10.1006/geno.1999.6046. [DOI] [PubMed] [Google Scholar]
- 13.Gitlin D, Hughes W L, Janeway C A. Nature (London) 1960;188:150–151. doi: 10.1038/188150a0. [DOI] [PubMed] [Google Scholar]
- 14.Mann J R, Camakaris J, Danks D M. Biochem J. 1980;186:629–631. doi: 10.1042/bj1860629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spector D L, Goldman R D, Leinwand L A. Cells: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1998. [Google Scholar]
- 16.Kelly E J, Palmiter R D. Nat Genet. 1996;13:219–222. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 17.Prohaska J R. J Nutr. 1983;113:2048–2058. doi: 10.1093/jn/113.10.2048. [DOI] [PubMed] [Google Scholar]
- 18.Orlow S, Lamoreux M, Pifko-Hirst S, Zhou B. J Invest Dermatol. 1993;101:137–140. doi: 10.1111/1523-1747.ep12363621. [DOI] [PubMed] [Google Scholar]
- 19.Cooney G J, Taegtmeyer H, Newsholme E A. Biochem J. 1981;200:701–703. doi: 10.1042/bj2000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonaldo P, Chowdhury K, Stoykova A, Torres M, Gruss P. Exp Cell Res. 1998;244:125–136. doi: 10.1006/excr.1998.4208. [DOI] [PubMed] [Google Scholar]
- 21.Camakaris J, Danks D M, Ackland L, Cartwright E, Borger P, Cotton R G. Biochem Genet. 1980;18:117–131. doi: 10.1007/BF00504364. [DOI] [PubMed] [Google Scholar]
- 22.Prohaska J R. Nutrition. 2000;16:502–504. doi: 10.1016/s0899-9007(00)00308-7. [DOI] [PubMed] [Google Scholar]
- 23.Mercer J F. Am J Clin Nutr. 1998;67:1022S–1028S. doi: 10.1093/ajcn/67.5.1022S. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Prohaska J R, Thiele D J. Proc Natl Acad Sci USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goding C R. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 26.Kelner G S, Lee M, Clark M E, Maciejewski D, McGrath D, Rabizadeh S, Lyons T, Bredesen D, Jenner P, Maki R A. J Biol Chem. 2000;275:580–584. doi: 10.1074/jbc.275.1.580. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth C, Bendich A. Annu Rev Nutr. 1996;16:73–97. doi: 10.1146/annurev.nu.16.070196.000445. [DOI] [PubMed] [Google Scholar]