Abstract
The histone-like protein H-NS is a major component of the bacterial nucleoid and plays a crucial role in global gene regulation of enteric bacteria. It is known that the expression of a variety of genes is repressed by H-NS, and mutations in hns result in various phenotypes, but the role of H-NS in the drug resistance of Escherichia coli has not been known. Here we present data showing that H-NS contributes to multidrug resistance by regulating the expression of multidrug exporter genes. Deletion of the hns gene from the ΔacrAB mutant increased levels of resistance against antibiotics, antiseptics, dyes, and detergents. Decreased accumulation of ethidium bromide and rhodamine 6G in the hns mutant compared to that in the parental strain was observed, suggesting the increased expression of some drug exporter(s) in this mutant. The increased drug resistance and decreased drug accumulation caused by the hns deletion were completely suppressed by deletion of the multifunctional outer membrane channel gene tolC. At least eight drug exporter systems require TolC for their functions. Among these, increased expression of acrEF, mdtEF, and emrKY was observed in the Δhns strain by quantitative real-time reverse transcription-PCR analysis. The Δhns-mediated multidrug resistance pattern is quite similar to that caused by overproduction of the AcrEF exporter. Deletion of the acrEF gene greatly suppressed the level of Δhns-mediated multidrug resistance. However, this strain still retained resistance to some compounds. The remainder of the multidrug resistance pattern was similar to that conferred by overproduction of the MdtEF exporter. Double deletion of the mdtEF and acrEF genes completely suppressed Δhns-mediated multidrug resistance, indicating that Δhns-mediated multidrug resistance is due to derepression of the acrEF and mdtEF drug exporter genes.
The emergence of bacterial multidrug resistance has become an increasing problem in the treatment of infectious diseases. Multidrug resistance often results from the overexpression of multidrug efflux transporters. Recent genome sequence analysis has revealed that bacteria have many intrinsic putative and proven drug transporter genes. We previously cloned all of the gene clusters encoding putative and known drug transporters of Escherichia coli and revealed that 20 genes actually encode the transporters of some drugs and/or toxic compounds (30). Since the substrate spectra of these multidrug transporters partially overlap, we are intrigued by the question of why bacteria, with their economically organized genomes, harbor such large sets of multidrug efflux genes. The key to understanding how bacteria utilize these multiple transporters lies in analysis of the regulation of transporter expression. In the present study, we analyzed the relationship between the regulation of drug transporters and the E. coli nucleoid-associated protein H-NS (histone-like nucleoid structuring protein).
H-NS, one of the most abundant proteins in the E. coli nucleoid, is widely distributed within gram-negative bacteria (4). H-NS was initially described as a transcription factor (10) and was later shown to play roles in the structure and function of chromosomal DNA (2, 40). H-NS is involved in the condensation of the bacterial chromosome and regulates the expression of many genes (∼5% of the open reading frames of the E. coli genome). Most of these genes are related to bacterial adaptation to environmental conditions and/or virulence (9). H-NS modulates transcription through the formation of large nucleoprotein structures (6, 13, 39). Mutations in hns result in various phenotypes, because H-NS is involved in the regulation of a variety of genes. However, the role of H-NS in the drug resistance of E. coli is unknown. In this paper, we report that H-NS controls the multidrug resistance of E. coli by regulating the expression of drug exporter genes.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this work were E. coli K-12 derivatives (Table 1). They were grown at 37°C in Luria-Bertani (LB) broth (34). Cells were rapidly collected for total RNA extraction when the cultures reached an optical density at 600 nm of 0.6.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| DH5α | recA endA1 hsdR17 supE4 gyrA96 relA1 Δ(lacZYA-argF)U169 (φ80 lacZΔM15) | 34 |
| W3104 | Wild type | 41 |
| W3104ΔacrAB | Derivative of W3104 that lacks acrAB | 15 |
| W3104ΔacrABΔemrKY | Derivative of W3104ΔacrAB that lacks emrKY | This study |
| W3104ΔacrABΔmdtEF | Derivative of W3104ΔacrAB that lacks mdtEF | This study |
| W3104ΔacrABΔacrEF | Derivative of W3104ΔacrAB that lacks acrEF | This study |
| W3104ΔacrABΔacrEFΔemrKY | Derivative of W3104ΔacrAB that lacks emrKY | This study |
| W3104ΔacrABΔacrEFΔmdtEF | Derivative of W3104ΔacrABΔacrEF that lacks mdtEF | This study |
| W3104ΔacrABΔacrEFΔmdtEFΔemrKY | Derivative of W3104ΔacrABΔacrEFΔmdtEF that lacks emrKY | This study |
| W3104ΔacrABΔtolC | Derivative of W3104ΔacrAB that lacks tolC | This study |
| W3104Δhns | Derivative of W3104 that lacks hns | This study |
| W3104ΔacrABΔhns | Derivative of W3104ΔacrAB that lacks hns | This study |
| W3104ΔacrABΔhnsΔtolC | Derivative of W3104ΔacrABΔhns that lacks tolC | This study |
| W3104ΔacrABΔhnsΔemrKY | Derivative of W3104ΔacrABΔhns that lacks emrKY | This study |
| W3104ΔacrABΔhnsΔmdtEF | Derivative of W3104ΔacrABΔhns that lacks mdtEF | This study |
| W3104ΔacrABΔhnsΔacrEF | Derivative of W3104ΔacrABΔhns that lacks acrEF | This study |
| W3104ΔacrABΔhnsΔacrEFΔemrKY | Derivative of W3104ΔacrABΔhnsΔacrEF that lacks emrKY | This study |
| W3104ΔacrABΔhnsΔacrEFΔmdtEF | Derivative of W3104ΔacrABΔhnsΔacrEF that lacks mdtEF | This study |
| W3104ΔacrABΔhnsΔacrEFΔmdtEFΔemrKY | Derivative of W3104ΔacrABΔhnsΔacrEFΔmdtEF that lacks emrKY | This study |
| W3104ΔacrABΔhnsΔevgAS | Derivative of W3104ΔacrABΔhns that lacks evgAS | This study |
| W3104ΔacrABΔhnsΔacrEFΔevgAS | Derivative of W3104ΔacrABΔhnsΔacrEF that lacks evgAS | This study |
| W3104ΔacrABΔydeO | Derivative of W3104ΔacrAB that lacks ydeO | This study |
| W3104ΔacrABΔhnsΔydeO | Derivative of W3104ΔacrABΔhns that lacks ydeO | This study |
RNA extraction.
Total RNA from bacterial cultures was isolated by using an RNeasy Protect bacterial minikit and RNase-free DNase (both from Qiagen) in accordance with the manufacturer's instructions. The absence of genomic DNA in DNase-treated RNA samples was confirmed by inspecting nondenaturing agarose electrophoresis gels and also by performing PCR with primers known to target the genomic DNA. RNA concentrations were determined spectrophotometrically (35).
Determination of specific transcript levels by quantitative real-time reverse transcription-PCR (qRT-PCR).
Bulk cDNA samples were synthesized from total RNA derived from E. coli cells by using TaqMan reverse transcription reagents (Perkin-Elmer [PE] Applied Biosystems) and random hexamers as primers. Specific primer pairs were designed with ABI PRISM Primer Express software (PE Applied Biosystems). rrsA of the 16S rRNA gene was chosen as the normalizing gene. Real-time PCR was performed with each specific primer pair by using SYBR Green PCR Master Mix (PE Applied Biosystems). Reactions were performed with an ABI PRISM 7000 sequence detection system (PE Applied Biosystems); during the reactions, the fluorescence signal due to SYBR Green intercalation was monitored to quantify the double-stranded DNA product formed in each PCR cycle.
Susceptibility testing.
The antibacterial activities of the agents were determined on L agar (1% tryptone, 0.5% yeast extract, and 0.5% NaCl) plates containing various compounds (oxacillin, erythromycin, novobiocin, doxorubicin, acriflavine, crystal violet, ethidium bromide, methylene blue, rhodamine 6G, tetraphenylphosphonium bromide, benzalkonium chloride, sodium dodecyl sulfate, and sodium deoxycholate) at various concentrations, as indicated. Agar plates were made by the twofold agar dilution technique recommended by the Japan Society of Chemotherapy (11, 12). Organisms were tested at a final inoculum size of 105 CFU/spot, with the use of a multipoint inoculator (Sakuma Seisakusyo, Tokyo, Japan), and were incubated at 37°C for 18 h in air. MICs of drugs and toxic compounds were determined as the concentrations that severely inhibited bacterial cell growth.
Construction of in-frame deletion mutants.
To construct gene deletion mutants from E. coli W3104 cells (41), precise in-frame deletions were generated by crossover PCR. Four sets of oligonucleotide primers (designations ending in -No, -Ni, -Ci, and -Co [Table 2]) were used for each gene. The fragment containing the deletion was then cloned into the BamHI site of the pKO3 vector (18), a gene replacement vector that contains a temperature-sensitive origin of replication and markers for positive and negative selection for chromosome integration and excision. The deletion was introduced into the chromosome by use of the pKO3 gene replacement protocol, as described previously (18).
TABLE 2.
Oligonucleotides used for construction and verification of deletion strains
| Oligonucleotide | Oligonucleotide sequence (5′ to 3′) |
|---|---|
| acrA-No | CGCGGATCCATTCGCATTTGTGGAATATAATCTCCATCA |
| acrA-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATATGTAAACCTCGAGTGTCCG |
| acrB-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTGATACAACGTGTAATCACTAAGGCC |
| acrB-Co | CGCGGATCCATGGAAAAAACTTACTGACCTGGAC |
| emrK-No | CGCGGATCCTGGATACCGTTAACTCCGGGG |
| emrK-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCACTATTATCTCTCATTTCTCATAGAT |
| emrY-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTGATGATAAAAGGAGGGGGTTATAGCG |
| emrY-Co | CGCGGATCCTGTTGGCGGTGGTCCTGGTGG |
| mdtE-No | CGCGGATCCCAGTTCAAAATTATGCAACTGATTCTG |
| mdtE-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATTTTAGTCCCTGAAAATTCTTGAG |
| mdtF-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTAACGTGTAAATGAGAGTAAGGTTGA |
| mdtF-Co | CGCGGATCCCGTCAAATTCCTCTGCATACTATTGC |
| acrE-No | CGCGGATCCCGTCGTCTTGCTTACGCCAT |
| acrE-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATTACTATTCCTCAAAAAACCAAAAG |
| acrF-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTAAATCAGAAACATAAAGGCGCTTTCG |
| acrF-Co | CGCGGATCCCGTCGTCTTGCTTACGCCAT |
| tolC-No | CGCGGATCCTCATCCCGGCAACCATCTC |
| tolC-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATTCCTTGTGGTGAAGCAGTAT |
| tolC-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTGATGACGACGACGGGG |
| tolC-Co | CGCGGATCCGCTGGATTGCTGGGCC |
| hns-No | CGCGGATCCCTCCCTTACGAAGCCTTGCATAATCC |
| hns-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATTGTAGTAATCTCAAACTTATAT |
| hns-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTAATCTTTTGTAGATTGCACTTGC |
| hns-Co | CGCGGATCCGTTGAATTAGCGCCGGGTGAAAGCGTAC |
| evgA-No | CGCGGATCCGAAAACGCAATAAATAAAACTACCGCC |
| evgA-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATAGATTATTCCCTTTGCAATGA |
| evgS-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTAAATAGCGGCTCCCACAATGTTC |
| evgS-Co | CGCGGATCCCATGGCACCTTTTGATGTTTTCAATACT |
| ydeO-No | CGCGGATCCTATTCCGGTTGAATTAGAACC |
| ydeO-Ni | CACGCAATAACCTTCACACTCCAAATTTATAACCATTTTATCTCCTTAAAACAATAAAGT |
| ydeO-Ci | GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTGATTATTTGCTAACGAGTAGTCAACC |
| ydeO-Co | CGCGGATCCGTCGGCGTAAAGTCTGAGAAA |
Observation of drug accumulation in E. coli cells.
E. coli cells were spotted onto L-agar plates containing a low concentration of ethidium bromide (1 μg/ml) or rhodamine 6G (0.5 μg/ml) at a final inoculum size of 105 CFU/spot, by use of a multipoint inoculator (Sakuma Seisakusyo), and were incubated at 37°C for 18 h in air. Drug accumulation in E. coli cells was observed as the fluorescence of ethidium bromide or rhodamine 6G in cells under UV light, by use of an Electronic U.V. Transilluminator FAS-II (TOYOBO, Osaka, Japan).
RESULTS
Deletion of the hns gene increases resistance to multiple antibiotics and toxic compounds.
Deletion of hns did not change the drug susceptibility of wild-type E. coli W3104 (41) (Table 3), because the intrinsic multidrug efflux transporter AcrAB masks the effect of hns deletion (Table 3). In addition, qRT-PCR analysis did not detect any changes in the expression levels of acrA and acrB in the hns deletion strain relative to the wild-type strain (data not shown). We therefore used a host strain lacking the acrAB gene (W3104ΔacrAB) (15). AcrAB is constitutively expressed in E. coli and is largely responsible for the intrinsic resistance of E. coli to dyes, detergents, and most lipophillic antibiotics (38). E. coli W3104ΔacrAB showed hypersensitivity to these compounds (Table 3). Deletion of hns increased the drug resistance of the acrAB deletion strain to multiple structurally unrelated compounds such as antibiotics, antiseptics, dyes, and detergents, as shown in Table 3.
TABLE 3.
Susceptibilities of E. coli strains to toxic compounds
| Strain | MIC (μg/ml)a of:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OXA | EM | NOV | DXR | ACR | CV | EBR | MB | R6G | TPP | BENZ | SDS | DOC | |
| W3104 | >256 | 128 | >512 | >128 | 256 | 128 | 512 | >512 | 256 | 512 | 64 | >512 | >40,000 |
| W3104Δhns | >256 | 64 | >512 | >128 | 256 | 128 | 512 | >512 | 256 | 512 | 64 | >512 | >40,000 |
| W3104ΔacrAB | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔemrKY | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔmdtEF | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔacrEF | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔacrEFΔemrKY | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔacrEFΔmdtEF | 1 | 4 | 32 | 4 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔacrEFΔmdtEFΔemrKY | 1 | 4 | 32 | 4 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔtolC | 0.5 | 2 | 2 | 2 | 8 | 1 | 8 | 8 | 4 | 8 | 4 | 16 | 156 |
| W3104ΔacrABΔhns | 64 | 32 | 128 | >128 | 64 | 128 | 512 | 512 | 256 | 256 | 32 | >512 | >40,000 |
| W3104ΔacrABΔhnsΔtolC | 0.5 | 2 | 2 | 2 | 8 | 1 | 8 | 8 | 4 | 8 | 4 | 32 | 156 |
| W3104ΔacrABΔhnsΔemrKY | 64 | 32 | 128 | >128 | 64 | 128 | 512 | 512 | 256 | 256 | 32 | >512 | >40,000 |
| W3104ΔacrABΔhnsΔmdtEF | 64 | 16 | 128 | >128 | 64 | 64 | 512 | 512 | 256 | 256 | 16 | >512 | >40,000 |
| W3104ΔacrABΔhnsΔacrEF | 4 | 16 | 32 | >128 | 32 | 8 | 64 | 32 | 128 | 8 | 8 | 128 | 10,000 |
| W3104ΔacrABΔhnsΔacrEFΔemrKY | 4 | 16 | 32 | >128 | 32 | 8 | 64 | 32 | 128 | 8 | 8 | 128 | 10,000 |
| W3104ΔacrABΔhnsΔacrEFΔmdtEF | 1 | 2 | 16 | 2 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 2,500 |
| W3104ΔacrABΔhnsΔacrEFΔmdtEFΔemrKY | 1 | 2 | 16 | 2 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 2,500 |
| W3104ΔacrABΔhnsΔevgAS | 32 | 32 | 128 | >128 | 64 | 128 | 512 | 512 | 256 | 256 | 32 | >512 | >40,000 |
| W3104ΔacrABΔhnsΔacrEFΔevgAS | 2 | 16 | 32 | >128 | 32 | 8 | 64 | 16 | 128 | 8 | 8 | 128 | 10,000 |
| W3104ΔacrABΔydeO | 1 | 4 | 32 | 8 | 16 | 1 | 16 | 8 | 4 | 8 | 4 | 64 | 5,000 |
| W3104ΔacrABΔhnsΔydeO | 64 | 32 | 128 | >128 | 64 | 128 | 512 | 512 | 256 | 256 | 32 | >512 | >40,000 |
MIC determinations were repeated at least three times. Boldfaced values differ from the MIC of W3104ΔacrAB by a factor of more than 4. Abbreviations: OXA, oxacillin; EM, erythromycin; NOV, novobiocin; DXR, doxorubicin; ACR, acriflavine; CV, crystal violet; EBR, ethidium bromide; MB, methylene blue; R6G, rhodamine 6G; TPP, tetraphenylphosphonium bromide; BENZ, benzalkonium chloride; SDS, sodium dodecyl sulfate; DOC, sodium deoxycholate.
Effect of deletion of hns on drug accumulation in E. coli cells.
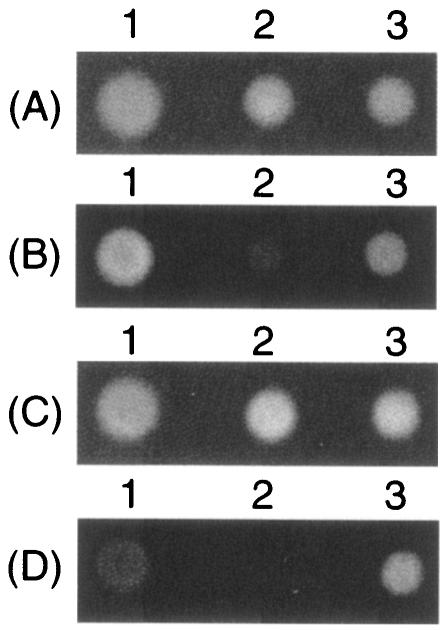
One of the major mechanisms of bacterial multidrug resistance is active drug efflux. Therefore, we investigated drug efflux in the hns-deficient mutant. E. coli W3104ΔacrAB and W3104ΔacrABΔhns cells were spotted onto agar plates containing 1 μg of ethidium bromide/ml or 0.5 μg of rhodamine 6G/ml, and the plates were then incubated at 37°C for 18 h. Since the concentrations of the drugs were eightfold lower than their MICs for W3104ΔacrAB, these compounds did not inhibit cell growth (Fig. 1A and C). Accumulation of these drugs in E. coli cells was observed from the fluorescence of ethidium bromide (Fig. 1B) and rhodamine 6G (Fig. 1D) under UV light. As shown in Fig. 1B and D, hns deletion resulted in a drastic decrease in fluorescence, clearly indicating the active efflux of these drugs from Δhns cells.
FIG. 1.
Effects of deletion of hns and tolC on drug accumulation in E. coli cells. Strains W3104ΔacrAB (lanes 1), W3104ΔacrABΔhns (lanes 2), and W3104ΔacrABΔhnsΔtolC (lanes 3) were spotted onto L-agar plates containing 1 μg of ethidium bromide/ml (A and B) or 0.5 μg of rhodamine 6G/ml (C and D). After incubation at 37°C for 18 h, E. coli colonies were observed under visible white light (A and C) and UV light (B and D).
Effect of tolC deletion on the effect of hns deletion.
The results described above indicate that the expression of a multidrug exporter(s) may be increased by hns deletion. In a previous study, we revealed that at least 20 intrinsic drug efflux transporters are encoded in the E. coli chromosome (30). Among these, RND (resistance nodulation cell division)-family transporters play major roles in both intrinsic and elevated resistance of gram-negative bacteria to a wide range of noxious compounds (22, 26, 27). RND transporters need two other proteins for their function: a membrane fusion protein (MFP) and an outer membrane channel. In E. coli, all of the five RND drug exporter systems (AcrAB, AcrD, AcrEF, MdtEF, and MdtABC) require the common outer membrane channel TolC for their functions (5, 7, 8, 25, 29-31). (YhiUV has been renamed MdtEF according to the systematic nomenclature available at the EcoGene website [33; http://bmb.med.miami.edu/ecogene/ecoweb/].) In addition, two major facilitator superfamily drug transporter systems (EmrAB and EmrKY) and one ABC drug transporter system (MacAB) also need TolC for their functions (15, 16, 19, 31).
In order to determine whether or not hns deletion-mediated multidrug resistance is due to the TolC-dependent drug exporter(s), we investigated the effect of tolC deletion on the drug resistance of the Δhns strain. Deletion of tolC from strain W3104ΔacrAB increased the susceptibilities of cells to some compounds, particularly novobiocin, sodium dodecyl sulfate, and sodium deoxycholate. This increase is probably due to prevention of the leaking of compounds through TolC or inactivation of some TolC-dependent drug exporter(s). tolC deletion completely inhibited hns deletion-mediated multidrug resistance. tolC deletion from W3104ΔacrABΔhns increased susceptibilities to all the compounds listed in Table 3. tolC deletion restored the accumulation of ethidium bromide and rhodamine 6G in the Δhns strain (Fig. 1B and D, lanes 3). These results indicated that hns deletion-mediated multidrug resistance is due to increased expression of a TolC-dependent drug exporter(s) caused by hns deletion.
Determination of the amounts of TolC-dependent drug exporter transcripts by qRT-PCR.
In order to determine which drug exporters' expression is increased by hns deletion, we investigated hns deletion-dependent changes in the amounts of mRNAs of drug exporter genes by qRT-PCR. Total RNAs from exponential-phase cultures of W3104ΔacrAB and W3104ΔacrABΔhns were isolated, and cDNA samples were then synthesized by using TaqMan reverse transcription reagents (PE Applied Biosystems) and random hexamers as primers. Then real-time PCR of the cDNAs was performed with each specific primer pair by using SYBR Green PCR Master Mix (PE Applied Biosystems). The expression levels of TolC-dependent drug exporter genes (except for AcrAB), typical TolC-independent drug exporter genes (mdfA, emrE, and mdtK [ydhE has been renamed mdtK according to the systematic nomenclature available at the EcoGene website]), and the tolC gene in W3104ΔacrABΔhns were compared with those in W3104ΔacrAB. The results are shown in Table 4. The expression levels of three exporter genes (acrE, mdtE, and emrK) were significantly increased (more than fourfold in comparison with basal levels) by hns deletion: 4.1-, 12-, and 6.7-fold increases were observed for acrE, mdtE, and emrK, respectively. Deletion of hns did not increase the expression levels of other drug exporter genes or of the tolC gene (Table 4).
TABLE 4.
Fold induction of specific transcripts attributed to hns deletion as determined by qRT-PCR
| Gene | Fold change |
|---|---|
| TolC-dependent transporter genes | |
| acrD | 1.0 |
| acrE | 4.1 |
| mdtE | 12.0 |
| mdtA | 0.5 |
| emrK | 6.7 |
| macA | 2.3 |
| emrA | 0.5 |
| TolC-independent transporter genes | |
| mdfA | 0.7 |
| emrE | 1.1 |
| mdtK | 1.5 |
| Outer membrane channel gene (tolC) | 0.9 |
Effects of deletion of drug exporter genes on hns deletion-mediated multidrug resistance.
In order to determine whether or not hns deletion-mediated multidrug resistance is due to increased expression of the acrEF, mdtEF, and/or emrKY drug exporter genes, we investigated the effects of these gene deletions on drug resistance levels of W3104ΔacrAB and W3104ΔacrABΔhns (Table 3). When the acrEF, mdtEF, and emrKY genes were deleted one by one or simultaneously from W3104ΔacrAB, resistance levels did not change, suggesting that these genes are not expressed under normal conditions. Single deletion of emrKY or mdtEF did not change the increased multidrug resistance of W3104ΔacrABΔhns. On the other hand, deletion of acrEF from W3104ΔacrABΔhns drastically decreased the levels of hns deletion-mediated multidrug resistance, except for resistance to erythromycin, doxorubicin, and rhodamine 6G, indicating that hns deletion-mediated drug resistance is mainly due to AcrEF. However, this strain still retained some resistance to several compounds. That is, strain W3104ΔacrABΔhnsΔacrEF showed decreased but significant resistance to oxacillin, erythromycin, doxorubicin, crystal violet, ethidium bromide, methyl viologen, and rhodamine 6G. The remaining drug resistance pattern was similar to that conferred by overproduction of MdtEF (YhiUV) (30). Double deletion of acrEF-mdtEF from W3104ΔacrABΔhns completely prevented hns deletion-mediated multidrug resistance, clearly indicating that hns deletion-mediated multidrug resistance is due to increased expression of these two drug exporter genes. The reason why the single deletion of mdtEF from W3104ΔacrABΔhns did not change hns deletion-mediated resistance levels may be that increased expression of AcrEF masks the effect of mdtEF deletion. Deletion of emrKY from W3104ΔacrABΔhnsΔacrEF and W3104ΔacrABΔhnsΔacrEFΔmdtEF did not affect the drug susceptibilities of these strains.
Effects of hns deletion on the expression levels of other genes located near emrKY, mdtEF, and acrEF.
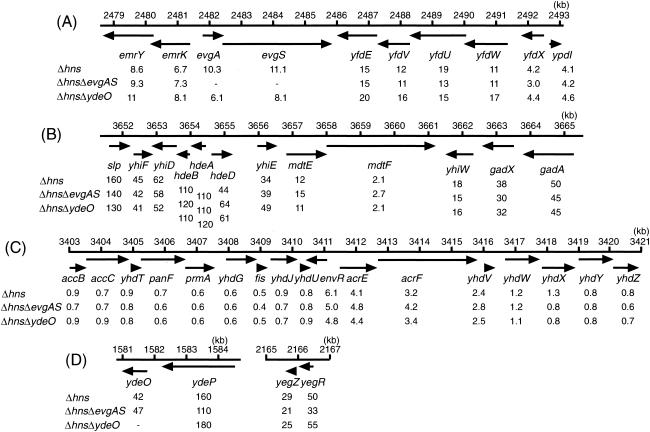
We investigated the effects of hns deletion on the expression levels of genes located near emrK, mdtE, and acrE by qRT-PCR analysis. The results are shown in Fig. 2. hns deletion increased the expression of genes near emrK (Fig. 2A). Expression of emrY, emrK, evgA, evgS, yfdE, yfdV, yfdU, yfdW, yfdX, and ypdI increased by factors of 8.6, 6.7, 10, 11, 15, 12, 19, 11, 4.2, and 4.2, respectively. hns deletion also increased the expression of genes near mdtE (Fig. 2B). Expression of slp, yhiF, yhiD, hdeB, hdeA, hdeD, yhiE, mdtF, yhiW, gadX, and gadA increased by factors of 160, 45, 62, 110, 110, 44, 34, 2.1, 18, 38, and 50, respectively. The effects of hns deletion on the expression of genes around acrE were lower than those on the expression of genes around emrK and mdtE (Fig. 2C). Deletion of hns increased the expression of one gene upstream of acrE (envR) and two downstream genes (acrF and yhdV) by factors of 6.1, 3.2, and 2.4, respectively. It is thought that envR is a repressor of the acrEF operon (3, 14, 30). However, although the expression level of envR was increased by hns deletion, the expression level of acrEF was also increased. This result indicates that the Δhns effect overcomes the inhibitory effect of EnvR.
FIG. 2.
Effects of deletion of hns, evgAS, and ydeO on the expression levels of genes near emrKY, mdtEF, and acrEF. (A) Gene clusters around emrKY; (B) gene clusters around mdtEF; (C) gene clusters around acrEF; (D) genes regulated by the EvgA response regulator. Arrows indicate the direction of transcription. Total RNAs from exponential-phase cultures of W3104ΔacrAB, W3104ΔacrABΔhns, W3104ΔacrABΔhnsΔevgAS, and W3104ΔacrABΔhnsΔydeO were isolated, and the expression level of each gene was then determined by qRT-PCR. Values below diagrams are fold changes in mRNA levels from those in W3104ΔacrAB, as determined by qRT-PCR. Minus signs indicate gene deletion. Positions on E. coli chromosomal DNA (given above the diagrams in kilobase pairs) correspond to those on the Colibri website (http://genolist.pasteur.fr/Colibri/).
In a previous study, it was found that the gene clusters shown in Fig. 2A and B are positively regulated by the EvgA response regulator of the two-component signal transduction system (28). The expression level of evgA was increased by hns deletion (Fig. 2A). In order to determine whether or not the increased expression of drug exporter genes caused by hns deletion (Fig. 2A and B) is due to increased expression of the evgAS two-component system, we deleted evgAS from W3104ΔacrABΔhns. Deletion of evgAS from the hns deletion strain affected neither the increased expression levels of these genes (Fig. 2) nor the hns deletion-mediated multidrug resistance levels, even in the hns-acrEF deletion strain (Table 3).
Recently, it was reported that the gene cluster shown in Fig. 2B is positively regulated by ydeO (23) and that the level of ydeO expression is increased by hns deletion (Fig. 2D). Therefore, we investigated the effect of ydeO deletion. Deletion of ydeO affected neither the increased expression of genes shown in Fig. 2B nor hns deletion-mediated multidrug resistance. These data, together with those for evgAS deletion, clearly indicate that the hns deletion-mediated increase in the expression of drug exporter genes is independent of EvgAS- and YdeO-mediated regulation.
DISCUSSION
In this study, we found that H-NS represses the expression of some TolC-dependent multidrug exporter genes and that, as a result, deletion of the hns gene confers multidrug resistance on the acrAB-deficient strain. In addition, qRT-PCR analysis revealed that expression of genes located near the mdtEF and emrKY exporter systems was increased by hns deletion. This observation is in good agreement with the microarray data of Hommais et al. (9).
Previously, Sulavik et al. constructed E. coli strains with deletions of putative drug exporters and outer membrane channels (38). They reported that deletion of acrAB increased the drug susceptibility of E. coli cells, whereas deletion of the other drug exporter genes increased E. coli drug susceptibility slightly or not at all, indicating that most drug exporter genes are not expressed under normal conditions. Therefore, studies on the regulation of these drug exporter genes are necessary to gain further insights into the physiological roles of multidrug exporters.
We previously found that overexpression of evgA, which is a response regulator of the two-component regulatory system, conferred multidrug resistance on E. coli cells (31, 32). Later, Masuda and Church reported that overexpression of the ydeO regulatory gene also conferred multidrug resistance on E. coli (23). The hns deletion-mediated increase in expression of drug exporter genes is independent of such transcriptional regulator-mediated upregulation. hns deletion-mediated regulation is more global than two-component system-mediated regulation.
Ma et al. reported that the expression of acrAB is induced by fatty acids, sodium chloride, and ethanol (21). Lomovskaya et al. reported that the emrAB drug exporter gene is induced by salicylic acid and 2,4-dinitrophenol (20). In addition, it has been reported that the expression of mdtEF (yhiUV) is controlled by RpoS (1, 37), a conserved alternative sigma factor that is needed for E. coli to survive stresses such as heat shock (17, 24), oxidative stress (17, 24), osmotic challenge (24), and near-UV light (36). Thus, the regulation of drug exporter genes is closely related to stress responses. Hommais et al. suggested that the control of gene expression by H-NS has a strong relationship with the maintenance of intracellular homeostasis (9). In this study, we found that H-NS represses the expression of acrEF and mdtEF. Thus, it was revealed that H-NS-mediated maintenance of intracellular homeostasis has a close relationship with the expression of drug exporter genes.
Acknowledgments
We thank George M. Church for plasmid pKO3.
K. Nishino was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to K.N. and A.Y.), by a grant-in-aid from the Zoonosis Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan (to K.N.), by a grant from the COE Program in the 21st Century of the Japan Society for the Promotion of Science (to K.N.), and by a grant from the Core Research Evolutional Science and Technology (CREST) program of the Japan Science and Technology Corporation (to A.Y.).
REFERENCES
- 1.Altuvia, S., D. Weinstein-Fischer, A. Zhang, L. Postow, and G. Storz. 1997. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell 90:43-53. [DOI] [PubMed] [Google Scholar]
- 2.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 3.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 4.Bloch, V., Y. Yang, E. Margeat, A. Chavanieu, M. T. Auge, B. Robert, S. Arold, S. Rimsky, and M. Kochoyan. 2003. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat. Struct. Biol. 10:212-218. [DOI] [PubMed] [Google Scholar]
- 5.Elkins, C. A., and H. Nikaido. 2002. Substrate specificity of the RND-type multidrug efflux pumps AcrB and AcrD of Escherichia coli is determined predominantly by two large periplasmic loops. J. Bacteriol. 184:6490-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconi, M., B. Colonna, G. Prosseda, G. Micheli, and C. O. Gualerzi. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates the accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17:7033-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirakawa, H., K. Nishino, T. Hirata, and A. Yamaguchi. 2003. Comprehensive studies on the drug resistance mediated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 185:1851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 40:20-36. [DOI] [PubMed] [Google Scholar]
- 10.Jacquet, M., R. Cukier-Kahn, J. Pla, and F. Gros. 1971. A thermostable protein factor acting on in vitro DNA transcription. Biochem. Biophys. Res. Commun. 45:1597-1607. [DOI] [PubMed] [Google Scholar]
- 11.Japan Society of Chemotherapy. 1981. Method for the determination of minimum inhibitory concentrations (MICs) of aerobic bacteria by the agar dilution method. Chemotherapy (Tokyo) 29:76-79. [Google Scholar]
- 12.Japan Society of Chemotherapy. 1992. Method for the determination of minimum inhibitory concentrations (MICs) by the micro-broth dilution method. Chemotherapy (Tokyo) 40:184-189. [Google Scholar]
- 13.Jordi, B. J., and C. F. Higgins. 2000. The downstream regulatory element of the proU operon of Salmonella typhimurium inhibits open complex formation by RNA polymerase at a distance. J. Biol. Chem. 275:12123-12128. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, K., N. Tsukagoshi, and R. Aono. 2001. Suppression of the hypersensitivity of the Escherichia coli acrB mutant to organic solvents by integrational activation of the acrEF operon with the IS1 or IS2 element. J. Bacteriol. 183:2646-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi, N., K. Nishino, T. Hirata, and A. Yamaguchi. 2003. Membrane topology of ABC-type macrolide antibiotic exporter MacB in Escherichia coli. FEBS Lett. 546:241-246. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, N., K. Nishino, and A. Yamaguchi. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange, R., D. Fischer, and R. Hengge-Aronis. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lomovskaya, O., K. Lewis, and A. Matin. 1995. EmrR is a negative regulator of the Escherichia coli multidrug resistance pump EmrAB. J. Bacteriol. 177:2328-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1995. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol. Microbiol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 22.Ma, D., D. N. Cook, J. E. Hearst, and H. Nikaido. 1994. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 2:489-493. [DOI] [PubMed] [Google Scholar]
- 23.Masuda, N., and G. M. Church. 2003. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 48:699-712. [DOI] [PubMed] [Google Scholar]
- 24.McCann, M. P., C. D. Fraley, and A. Matin. 1993. The putative sigma factor KatF is regulated posttranscriptionally during carbon starvation. J. Bacteriol. 175:2143-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagakubo, S., K. Nishino, T. Hirata, and A. Yamaguchi. 2002. The putative response regulator BaeR stimulates multidrug resistance of Escherichia coli via a novel multidrug exporter system, MdtABC. J. Bacteriol. 184:4161-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikaido, H. 1994. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264:382-388. [DOI] [PubMed] [Google Scholar]
- 28.Nishino, K., Y. Inazumi, and A. Yamaguchi. 2003. Global analysis of genes regulated by EvgA of the two-component regulatory system in Escherichia coli. J. Bacteriol. 185:2667-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino, K., J. Yamada, H. Hirakawa, T. Hirata, and A. Yamaguchi. 2003. Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to β-lactams. Antimicrob. Agents Chemother. 47:3030-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the yhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishino, K., and A. Yamaguchi. 2001. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 183:1455-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudd, K. E. 2000. EcoGene: a genome sequence database for Escherichia coli K-12. Nucleic Acids Res. 28:60-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 36.Sammartano, L. J., R. W. Tuveson, and R. Davenport. 1986. Control of sensitivity to inactivation by H2O2 and broad-spectrum near-UV radiation by the Escherichia coli katF locus. J. Bacteriol. 168:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schellhorn, H. E., J. P. Audia, L. I. Wei, and L. Chang. 1998. Identification of conserved, RpoS-dependent stationary-phase genes of Escherichia coli. J. Bacteriol. 180:6283-6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulavik, M. C., C. Houseweart, C. Cramer, N. Jiwani, N. Murgolo, J. Greene, B. DiDomenico, K. J. Shaw, G. H. Miller, R. Hare, and G. Shimer. 2001. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob. Agents Chemother. 45:1126-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tupper, A. E., T. A. Owen-Hughes, D. W. Ussery, D. S. Santos, D. J. Ferguson, J. M. Sidebotham, J. C. Hinton, and C. F. Higgins. 1994. The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J. 13:258-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams, R. M., and S. Rimsky. 1997. Molecular aspects of the E. coli nucleoid protein, H-NS: a central controller of gene regulatory networks. FEMS Microbiol. Lett. 156:175-185. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto, T., M. Tanaka, C. Nohara, Y. Fukunaga, and S. Yamagishi. 1981. Transposition of the oxacillin-hydrolyzing penicillinase gene. J. Bacteriol. 145:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]