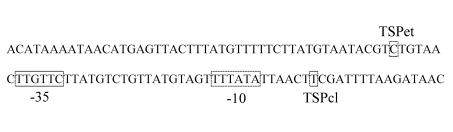Abstract
The regulatory systems controlling expression of the ctxAB genes encoding cholera toxin (CT) in the classical and El Tor biotypes of pathogenic Vibrio cholerae have been characterized and found to be almost identical. Notwithstanding this, special in vitro conditions, called AKI conditions, are required for El Tor bacteria to produce CT. The AKI conditions involve biphasic cultures. In phase 1 the organism is grown in a still tube for 4 h. In phase 2 the medium is poured into a flask to continue growth with shaking. Virtually no expression of CT occurs if this protocol is not followed. Here we demonstrated that CT expression takes place in single-phase still cultures if the volume-to-surface-area ratio is decreased, both under air and under an inert atmosphere. The expression of key genes involved in the regulation of CT production was analyzed, and we found that the expression pattern closely resembles the in vivo expression pattern.
Strains of Vibrio cholerae El Tor and classical biotypes are causative agents of cholera, and both biotypes produce the virulence factor cholera toxin (CT), which is a powerful enterotoxin considered the main toxin responsible for the profuse loss of fluid that characterizes the disease. Although the El Tor and classical biotypes are very similar, they have distinguishing properties that include disparate needs for efficient in vitro production of CT. The classical strains are relatively permissive, and they produce CT under different conditions, but induction of CT synthesis in the El Tor strains is more demanding.
In order to produce CT from the El Tor biotype, both for characterization of the toxin and for eventual use of the toxin in immunization, ways to induce the in vitro expression of the El Tor CT were investigated. The so-called AKI conditions were discovered through trial-and-error experimentation (10). The AKI conditions involve biphasic cultures; vibrios are first grown in a tube for 4 h, and then the culture is poured into a flask whose capacity is approximately 10-fold greater than that of the tube to continue growth with shaking. Why these conditions are required to induce production of CT and how they influence the complex regulatory cascade involved in CT expression are unknown. The aim of the present study was to contribute to the elucidation of these matters.
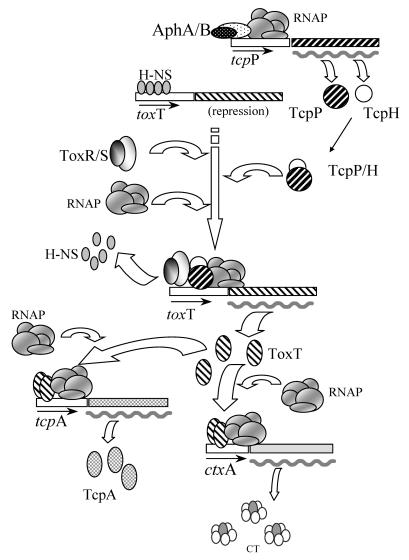
The ctxAB genes code for the CT protein, which is made up of the CTA and CTB subunits. The regulation of ctxAB in V. cholerae is elaborate, and it involves a multistep cascade. In response to the external environment, diverse regulators can influence expression of ctxAB (17). In a working model, in which the regulators at the core of the cascade are included, the control of ctxAB is thought to occur as follows (Fig. 1). The AphA and AphB proteins activate transcription of the tcpPH operon (12) that codes for the TcpP and TcpH proteins (Fig. 1). The TcpP-TcpH couple interacts with the ToxR protein (positive regulator), which acts in conjunction with the ToxS protein (ToxR-ToxS) (Fig. 1). The TcpP-TcpH-ToxR-ToxS complex then promotes transcription of toxT (3, 6-8) (Fig. 1), and this leads to production of the ToxT protein (Fig. 1). The ToxT protein directly activates transcription of the ctxAB operon (2), and this results in the synthesis of CT (Fig. 1). At the same time that ToxT activates expression of ctxAB, it also activates expression of the toxin-coregulated pilus (TCP) operon (25); this operon codes for another virulence factor, designated the TCP, which is a surface structure involved in colonization of the intestine (30). The first gene downstream from the ToxT-activated promoter is tcpA, and this gene codes for the TcpA protein (Fig. 1). The toxT gene lies downstream from tcpA and within the TCP operon itself (not shown in Fig. 1). Thus, upon induction of the TCP operon by ToxT, a transcript is produced that includes the toxT mRNA, and from this transcript additional synthesis of ToxT can occur (1, 32). In this study we monitored expression of toxT from the nearby TcpP-TcpH-ToxR-ToxS-induced promoter and not from the distant ToxT-activated tcpA promoter.
FIG. 1.
Schematic representation of the genetic regulatory cascade controlling CT expression. From top to bottom the diagram shows activation of tcpP by AphAB, basal repression of toxT by H-NS, activation of toxT by TcpP-TcpH and ToxR-ToxS with displacement of H-NS, activation of tcpA and ctxA by ToxT, and synthesis of TcpA and CT. Different patterns indicate the various regulators. RNAP, RNA polymerase. Small arrows beneath boxes indicate promoters, as well as the direction of transcription. A wavy thick line indicates mRNA and thus indicates transcriptional activation. Additional potential basal repression of tcpA and ctxA by H-NS (24, 33) is not shown.
The molecular bases of the differential expression of CT by El Tor and classical strains have essentially been detemined. First, it was demonstrated that there is biotype-specific control over expression of toxT (4). Then it was shown that the functionalities of the classical and El Tor TcpP and TcpH were not substantially different and thus that regulatory differences between the two biotypes were not due to the activity of these proteins but to their levels of expression (22, 23). Subsequently, it was shown that differential expression of tcpP and tcpH in the two biotypes was due to an affinity of the AphB activator that was lower for the El Tor tcpP promoter than for the classical tcpP promoter (13, 14). This difference in affinity was apparently the result of a single-nucleotide mutation (13, 14). Notwithstanding this mutation, the affinity of AphB for the El Tor tcpP promoter is thought to be sufficient for activation of tcpP, but only under permissive (i.e., AKI) conditions. Under nonpermissive conditions the mutation is critical and the affinity of AphB for the tcpP promoter is not great enough for induction. Without production of TcpP the regulatory cascade is not activated, and no expression of CT occurs.
When one examines how the AKI conditions might work, one natural assumption regarding the shaking phase is that it could function by increasing oxygen availability. In preliminary experiments (unpublished data) we explored this and other possibilities. When we replaced air with nitrogen or helium in the shaking culture phase, we found that CT synthesis was not reduced as expected if oxygen or even another component of air was acting as an inducer. We then investigated whether mechanical motion without aeration induced expression of CT, but expression did not occur. Subsequently, we explored whether an increase in the surface area of the exposed culture stimulated production of CT and observed stimulation under these conditions.
In this paper we describe experiments which demonstrated that, in the El Tor vibrios, CT synthesis is induced in single-phase still cultures if the surface area of the exposed culture is increased. These results partially explain why CT is produced under AKI conditions. To determine the effect of these new conditions on the CT genetic regulatory cascade, we examined the transcription of tcpP, toxT, ctxA, and tcpA at 1-h time intervals by primer extension. The patterns of expression of these genes were found to closely resemble those observed under in vivo conditions (18, 19).
MATERIALS AND METHODS
Strain and cultures.
V. cholerae El Tor strain E7946 was used in this study. This strain was maintained at −70°C in Luria-Bertani medium containing 20% glycerol. For cultures the strain was plated onto Luria-Bertani agar directly from the frozen glycerol stock. After overnight growth at 37°C under an air atmosphere, a suspension of bacteria in saline solution (0.9% NaCl), adjusted to an optical density at 600 nm (OD600) of 1.0, was produced. Two microliters of this suspension per 10 ml of medium was used to start each liquid culture, which was incubated at 37°C. All bacterial suspensions were fresh and used within 10 min after preparation. The medium used for all cultures was AKI (1.5% Bacto-Peptone [Difco], 0.4% yeast extract, 0.5% NaCl) without sodium bicarbonate (11, 20).
Growth under different atmospheres.
Nitrogen, helium, and air atmospheres were used for cultures. In all cases the cultures were grown in vials or Erlenmeyer flasks fitted with gas-tight rubber caps (catalog no. DX3043C A [Daigger & Co. Inc., Linconshire, Ill.] or catalog no. Z10145-1 [Aldrich Chemical Co., St. Louis, Mo.]). The air initially present in vials and flasks was replaced by another atmosphere by repetitive displacement. This was done by passing two needles through the cap, one for filling and the other for emptying. Filling was done by using a small latex balloon that contained the gas being assayed (50 to 200 ml), which was connected via a syringe to the filling needle. After repetitive displacement the filling needle and balloon were left in situ. The low positive pressure exerted by the balloon ensured that there was constant replenishment of gas and guarded against the potential entry of external air in the event of minor leaks. The purity of the nitrogen and helium used (Praxair Technology, Inc., Danbury, Conn.) was 99.999%. The initial tests to determine the effects of the variable volumes on CT expression were performed in vials. For these tests 15-ml cultures were first grown under the typical AKI conditions. Screw-cap vials (19 by 70 mm) were filled to the top with medium and then inoculated. Following inoculation the vials were incubated under still conditions for 4 h. After this, 200-, 400-, 600-, 800-, or 1,600-μl aliquots of the growing cultures were transferred to 7.5-ml vials (15 by 60 mm). The atmosphere in the vials was then quickly replaced (with air or nitrogen), and the vials were incubated under still conditions for 3 h. In the experiments in which there was a constant exposed surface there was no pregrowth under AKI conditions, and cultures were started directly in 125-ml Erlenmeyer flasks (single-phase cultures). For these single-phase cultures a constant volume (3 ml) of preinoculated medium was used. After the preinoculated medium was placed in the flasks, the atmosphere was replaced with air, nitrogen, or helium. Once the atmosphere was replaced, the flasks were incubated under still conditions for 3, 4, 5, 6, or 7 h.
To obtain an improved appreciation of the culture conditions, we estimated surface areas, depths, and volume-to-surface ratios. For the 7.5-ml vials the calculated exposed surface area was 1.54 cm2. In these vials the 1.6-ml cultures were approximately 1 cm deep, while the 0.2-ml cultures were approximately 1.25 mm deep. The estimated volume-to-surface-area ratios for the 1.6-ml cultures were approximately 1 ml/cm2, while the estimated volume-to-surface-area ratios for the 0.2-ml cultures were 0.13 ml/cm2. In the 125-ml flasks the calculated exposed culture surface area was 26 cm2, the depth of the cultures (3 ml) was 1.2 mm, and the volume-to-surface-area ratio was 0.12 ml/cm2.
RNA isolation, primer extension analysis, and DNA sequencing.
Total RNA was isolated with the TRIZOL reagent from GIBCO-BRL (Bethesda, Md.). The concentrations of RNA were adjusted to the same value by using the OD260 and were verified by visual estimation of the intensity of the 16S and 23S RNA bands in ethidium bromide-stained UV-illuminated agarose gels. In general, duplicate cultures were prepared for each sample time, and the bacterial cell pellets from these duplicate cultures were pooled before isolation of RNA. However, for the shorter times (3 and 4 h) pooling of pellets from up to five replicate cultures was necessary to obtain sufficient amounts of RNA. To ensure that conditions were as similar as possible for replicate cultures, the preparation of inocula, the inoculation time, the incubation period, and the point at which cultures were harvested were all done with prearranged time delays. In addition, the OD600 of each replicate culture was verified to ensure that the values were within the expected range.
Primer extension reactions were performed by using the C. therm. polymerase for reverse transcription (Roche Diagnostics, Indianapolis, Ind.) according to the manufacturer's instructions. The following primers were used: tcpP oligo (GCA TAA TAG ACT TGA TTA GTG CAT TC), toxT oligo (5′ CCA ATC ATT GCG TTC TAC TCT GAA G 3′), tcpA oligo (5′ CGA GTA ATG TCA TAC CCT CTT GAC 3′), and ctxA oligo (5′ CTG CCC GAT ATA ACT TAT CAT CAT TTG C 3′).
For DNA sequencing reactions a ThermoSequenase kit (Amersham Life Sciences Corp., Cleveland, Ohio) was used with [γ-32P]ATP (Amersham International) as the substrate for end labeling.
Enzyme-linked immunosorbent assay.
The GM1 enzyme-linked immunosorbent assay (28) was used for semiquantitative determination of CT in culture supernatants; whole CT (Calbiochem, Inc., Darmstadt, Germany) at a concentration of 1 μg/ml was used as a standard. Monoclonal antibody LT39 directed towards the B subunit of CT (29), kindly provided by A.-M. Svennerholm (Göteborg University, Göteborg, Sweden), was used for detection.
RESULTS AND DISCUSSION
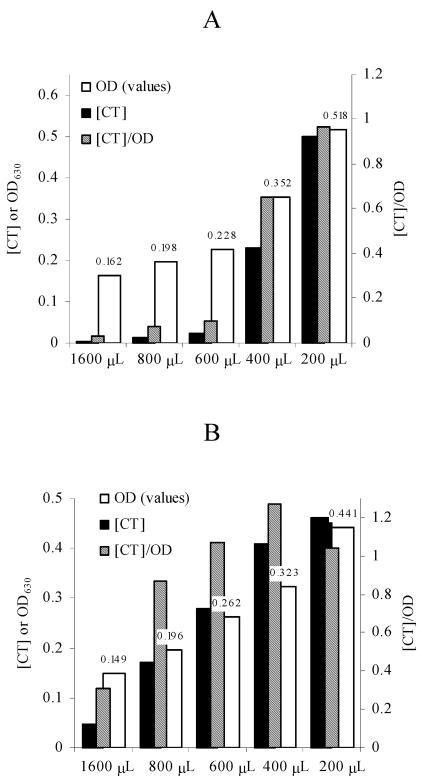
In preliminary experiments (unpublished data) aimed at discriminating between the various potential factors that could induce expression of CT in AKI cultures, we discovered that the exposed surfaces of cultures were a key stimulatory factor under AKI conditions. Following up on these results, we found that in still cultures and when the size of the container was constant, the production of CT increased as the volume of the culture was reduced under air (Fig. 2A), nitrogen (Fig. 2B), and helium (data not shown) atmospheres. Expression under anaerobic atmospheres indicates that the shaking phase of AKI cultures induces production primarily because it involves an increase in the relative culture surface area and not because of increased aeration.
FIG. 2.
Decreasing the culture volume in vials that are the same size induces expression of CT. (A) Expression under air; (B) expression under nitrogen. In both panels volume is plotted on the x axis, and both the CT concentration (in micrograms per milliliter) and OD630 are plotted on the left y axis. On the right y axis the [CT]/optical density (OD) ratio is plotted. The values above the open bars correspond to the OD630s of cultures. The OD630s were obtained with a microtiter plate reader (200 μl/well).
How these conditions induce expression of CT is not clear, but it is possible that vibrios are somehow able to sense a relative increase in the gas-liquid interphase. It could be that the increase in the exposed culture surface area simply promotes the loss of some volatile metabolite that inhibits induction of CT production. Devising practical ways to determine if vibrios can respond to gas-liquid (or analogous) interphases and analyses to detect and characterize volatile metabolites are experiments that remain to be done.
Progressively reducing the culture volume in vials that were a constant size had a dual effect because as the levels of CT increased, the bacterial densities of cultures also increased (Fig. 2). To estimate the contribution of the progressively larger numbers of bacteria to the concentrations of CT, [CT]/optical density ratios were calculated. The results (Fig. 2) demonstrated that the [CT]/optical density ratios increased continually and therefore that there was induction of CT production in response to the reduction in culture volume.
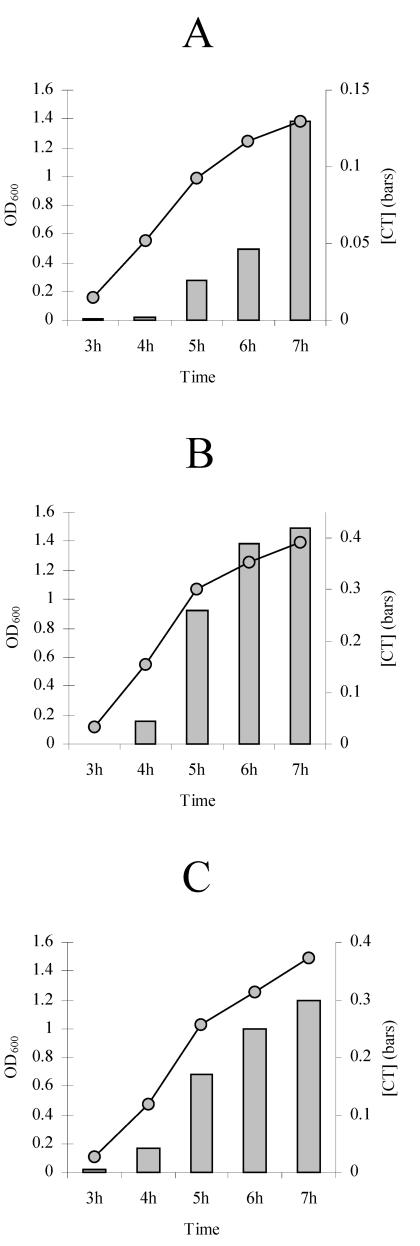
Based on the results described above, a constant volume of 3 ml in a 125-ml flask was used for subsequent experiments. These proportions were approximately equivalent to those of the 200-μl cultures in the 7.5-ml vials. Single-phase still cultures were then incubated under air, nitrogen, and helium atmospheres, and CT expression was monitored at 1-h intervals. Under the three atmospheres increases in CT occurred in a time-related or growth-associated manner (Fig. 3), just as they did under other culture conditions. The expression of CT in these time course experiments may also be attributed to induction because as the growth curve progressed, the [CT]/OD600 ratios also increased (data not shown).
FIG. 3.
Time course of expression of CT with a large exposed culture surface when different atmospheres were used. (A) Expression under air; (B) expression under nitrogen; (C) expression under helium. A constant volume of 3 ml in a 125-ml flask was used in all cases. Individual cultures were used for each of the sample times. The incubation time is plotted on the x axis. The concentration of CT (in micrograms per milliliter) is plotted on the right y axis. The OD600 of the culture is plotted on the left y axis.
The use of single-phase still cultures to induce expression of CT may represent a step forward for gene expression studies of V. cholerae El Tor because this system is simpler than the biphasic AKI system. Moreover, as discussed below, the gene expression patterns suggest that this type of culture may have more potential to mimic the in vivo situation.
Under our culture conditions expression under a helium or nitrogen atmosphere was higher than expression under an air atmosphere. To profit from this effect and at the same time avoid potential contributions of the used atmosphere, we used the inert gas helium for subsequent analyses. We also reasoned that an atmosphere lacking oxygen would be more similar to the microaerophilic intestinal milieu and therefore that the data obtained could eventually be more useful for comparisons with in vivo results.
To determine how the classical strains responded to these culture conditions, the O395 strain was grown under the optimal stimulating conditions and under a helium atmosphere. The time course CT expression pattern for the O395 strain was practically the same as that for the El Tor E7946 strain, although the CT concentrations were approximately threefold higher (data not shown). These results suggest that there was an equivalent response of the genetic regulatory cascade. Therefore, our culture system may provide the opportunity to compare side by side and under exactly the same growth conditions the gene expression patterns of the classical and El Tor strains.
To survey how the regulatory molecular cascade controlling CT expression in El Tor strain E7946 was affected by the new conditions, we analyzed the patterns of expression of tcpP, tcpA, toxT, and ctxA by the primer extension method. In these experiments the transcriptional start point (TSP) was also determined. The TSP for the tcpP gene in El Tor strains has been mapped, and our data coincided with the previously reported site (23). Our TSPs for tcpA and ctxA also coincided with the previously proposed El Tor TSPs (21, 25, 31). However, the TSP for the toxT gene from El Tor strains has not been reported, and our TSP did not coincide with a previously proposed TSP for the classical O395 strain (7); our TSP was located 42 bases upstream (Fig. 4). This was not an additional TSP because close inspection of the region where the classical TSP was expected revealed no detectable bands at any of the sample times (data not shown). The DNA sequence upstream from our TSP was found to be identical to the classical sequence. Therefore, the TSP identified does not appear to be the result of a new promoter generated by mutation, at least not in the near vicinity. We are currently experimentally reviewing the TSPs for classical and El Tor strains under various in vitro growth conditions.
FIG. 4.
TSPs for classical and El Tor toxT genes. Two TSPs are shown: the TSP mapped in this work for El Tor V. cholerae strain E7946 (TSPet) and the previously reported (7) TSP for classical V. cholerae strain O395 (TSPcl). The TSPs for strains E7946 and O395 are enclosed in boxes, as are the proposed −10 and −35 transcriptional signals for the strain O395 TSP (7).
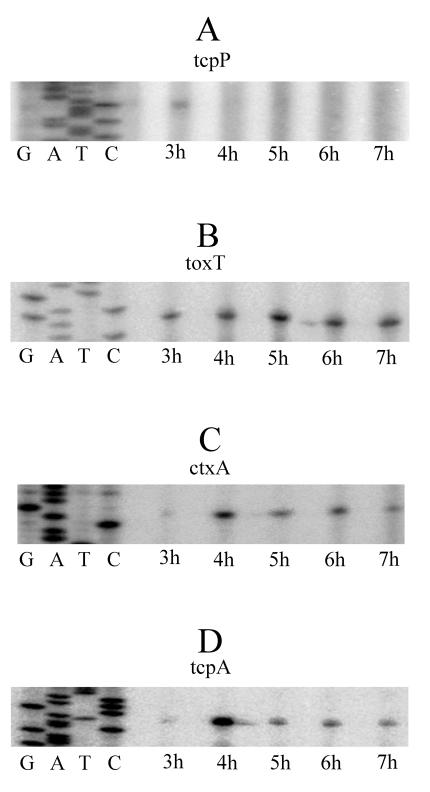
In agreement with the observed induction of CT (Fig. 2 and 3) and as proposed by the regulatory cascade model (Fig. 1), we detected expression of tcpP under our conditions. Transcription of tcpP took place early in the culture and at h 3 (Fig. 5A), but the expression was ephemeral, so that at subsequent times the tcpP message became undetectable (Fig. 5A). Repeat experiments with alternate primers and/or overexposure of film to reveal potential weak signals (data not shown) suggested that this result was not due to technical reasons but to true suppression of tcpP expression. Induction of tcpP may have started almost exactly at 3 h because sampling at 2.5 h did not result in a detectable signal. Therefore, tcpP transcription appeared to take place in the form of a single pulse that lasted about 1 h.
FIG. 5.
Temporal expression patterns of tcpP, toxT, ctxA, and tcpA, as determined by primer extension. Cultures were grown as described in the legend to Fig. 3 by using a helium atmosphere. Expression of genes was determined hourly. (A) Expression pattern of tcpP; (B) expression pattern of toxT; (C) expression pattern of ctxA; (D) expression pattern of tcpA. In each case a DNA sequence ladder (GATC) was included to identify the putative transcriptional start site.
The expression of toxT is positively affected by TcpP (Fig. 1), and therefore, it was important to determine how the ephemeral expression of tcpP affected toxT transcription. In addition, the pattern of expression of toxT was of interest because we have previously found that under AKI conditions toxT is expressed in a transient manner and only during an approximately 2-h time window (20). In the present experiments toxT was not expressed transiently but was expressed continuously and from the earliest time sampled (Fig. 5B). This pattern of expression was also found under in vivo conditions; under these conditions toxT transcription was estimated to start at h 3 and to be continuous after this (19). In brief, unlike expression of toxT under the AKI conditions, expression of toxT under our conditions was continuous instead of transient, and the expression pattern closely resembled the in vivo expression pattern.
The continuous expression of toxT, despite the ephemeral transcription of tcpP, is an intriguing observation given that activation of toxT is thought to depend on the TcpP protein. To explain continuous expression of toxT in the apparent absence of TcpP, direct induction of toxT by ToxR could be postulated. However, this possibility seems improbable because ToxR appears to act by providing the promoter recognition function for a productive interaction of TcpP with the RNA polymerase rather than by directly inducing toxT (15, 16). One alternative explanation is that the regulatory cascade is primed by the transient presence of TcpP and that an additional regulator(s) is responsible for the continuous expression of toxT. It is also possible that after priming by TcpP the H-NS protein, which normally represses toxT (24) (Fig. 1), no longer binds to the promoter region and that this causes constitutive transcription of toxT. Whatever the conditions responsible for continuous expression of toxT, the presence of ToxR seems to be essential because ToxR-negative mutants are highly defective for toxT expression (3). The hypothesis that ToxR is crucial for the expression of toxT is also supported by the in vivo observation that a double ΔtcpPH ΔtoxR mutant was incapable of inducing tcpA (18), most likely because in the absence of ToxR no ToxT was produced. A theoretical scenario in which the constant presence of TcpP (unlike ToxR) is not strictly necessary for induction of toxT is consistent with the observation that a ΔtcpPH mutant was able to induce (in vivo) both ctxA and tcpA (18). This scenario presupposes the presence of ToxT in the cell and hence provides evidence of TcpPH-independent induction of the toxT gene, most likely mediated by ToxR. It seems possible that in the absence of continuous synthesis of TcpP, constant expression of toxT can occur.
The regulator ToxT directly activates the ctxAB operon (Fig. 1). To monitor this effect, we examined transcription of ctxA. Expression of ctxA was found to start at h 4 and to be essentially constant after this, although the level of expression at the starting point was higher (Fig. 5C). Under AKI conditions expression of ctxA was similar except that transcription started 1 h later and when the cultures were in the shaking phase (20). When the pattern obtained under in vivo conditions was compared with our ctxA expression pattern, the patterns coincided totally in terms of timing because in the mouse model expression of ctxA also occurred at h 4 of growth (18).
Expression of tcpA was found to occur synchronously with expression of ctxA and with a basically identical pattern, including higher expression at the initial time of induction (Fig. 5D). Very similar expression patterns for ctxA and tcpA would be in full agreement with the proposal that ToxT directly activates both genes (Fig. 1). As for ctxA, there was a 1-h disparity in the time of expression of tcpA when the data were compared with the data obtained under AKI conditions. When the data were compared with the data obtained under in vivo conditions, the expression pattern of tcpA also coincided; however, the similarities could be more limited in this case because in vivo transcription of tcpA was bimodal, with a weak expression peak at h 1 and a much stronger peak at h 4 (18). The latter peak exactly coincided with our tcpA induction time, but we could not test for expression of this gene at 1 h due to the low bacterial densities at this point.
When we compared the expression patterns of ctxA, tcpA, and toxT under our conditions, transcription of ctxA and tcpA occurred about 1 h later than transcription of toxT (Fig. 5). Analogous delays have been observed under AKI conditions (20) and in the in vivo model (18, 19). Our culture procedure is new; therefore, the persistent occurrence of a delay suggests that this could be an obligatory step common to the three systems. Perhaps in every case and soon after synthesis, ToxT is mainly quiescent, and activation of the protein is required for induction of its target genes. This hypothesis could be supported by the observation that the activity of ToxT can be modulated by environmental signals (27).
We believe that our culture system could help identify the natural external stimuli that induce (or suppress) expression of CT, as well as other virulence factors, in V. cholerae. This may be true because except for the potential absence of the early (1-h) peak of expression of tcpA, the El Tor in vivo temporal expression patterns and our expression patterns basically coincided perfectly. It is therefore possible that our system furnishes signals that, at least to some extent, successfully mimic the in vivo situation. This may have been partly due to the fact that we used anaerobic atmospheres, which presumably are conditions that are similar to in vivo conditions. Although air allowed expression of CT, we noticed that the toxin levels were lower, and this suggests that the conditions were unfavorable. A positive effect of low-oxygen conditions would agree with the nature of AKI conditions because cultures are grown first in tubes, and this creates a microaerophilic environment, especially after dissolved oxygen is depleted by bacterial growth. These similarities could explain why both systems successfully induce CT production.
Obviously, until fully perfected, in vitro systems will necessarily lack stimuli present in the in vivo models. However, to obtain partial yet potentially highly relevant responses, our method and variations of this method could offer alternatives for studying gene expression in V. cholerae. In this respect, it may be important that shallow standing cultures rather than shaking cultures lead to differential expression of at least 45 proteins in Mycobacterium bovis (5). For V. cholerae gene expression studies and to better imitate the bacterial surroundings during infection, our in vitro conditions could be combined with in vivo stimuli, such as intestinal fluid (26).
Acknowledgments
J.S. acknowledges financial support provided by SILANES, S.A. de C.V., México. G.S.-C., T.B., and J.S. acknowledge membership in SNI, CONACyT, México. T.B. gratefully acknowledges financial support provided by CONACyT México (research grant 34236-E).
We gratefully acknowledge Leticia López Escobar and Diana Diaz de Anda for skillful and dedicated technical assistance.
REFERENCES
- 1.Brown, R. C., and R. K. Taylor. 1995. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol. Microbiol. 16:425-439. [DOI] [PubMed] [Google Scholar]
- 2.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 3.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DiRita, V. J., M. Neely, R. K. Taylor, and P. M. Bruss. 1996. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc. Natl. Acad. Sci. USA 93:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Florczyk, M. A., L. A. McCue, R. F. Stack, C. R. Hauer, and K. A. McDonough. 2001. Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins. Infect. Immun. 69:5777-5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins, D. E., and V. J. DiRita. 1994. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol. Microbiol. 14:17-29. [DOI] [PubMed] [Google Scholar]
- 8.Higgins, D. E., and V. J. DiRita. 1996. Genetic analysis of the interaction between Vibrio cholerae transcription activator ToxR and toxT promoter DNA. J. Bacteriol. 178:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulbert, R. R., and R. K. Taylor. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J. Bacteriol. 184:5533-5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwanaga, M. K., Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 11.Iwanaga, M., and T. Kuyyakanond. 1987. Large production of cholera toxin by Vibrio cholerae O1 in yeast extract peptone water. J. Clin. Microbiol. 25:2314-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacikova, G., and K. Skorupski. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J. Bacteriol. 181:4250-4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacikova, G., and K. Skorupski. 2000. Differential activation of the tcpPH promoter by AphB determines biotype specificity of virulence gene expression in Vibrio cholerae. J. Bacteriol. 182:3228-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacikova, G., and K. Skorupski. 2002. Binding site requirements of the virulence gene regulator AphB: differential affinities for the Vibrio cholerae classical and El Tor tcpPH promoters. Mol. Microbiol. 44:533-547. [DOI] [PubMed] [Google Scholar]
- 15.Krukonis, E. S., R. R. Yu, and V. J. Dirita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 16.Krukonis, E. S., and V. J. DiRita. 2003. DNA binding and ToxR responsiveness by the wing domain of TcpP, an activator of virulence gene expression in Vibrio cholerae. Mol. Cell 12:157-165. [DOI] [PubMed] [Google Scholar]
- 17.Krukonis, E. S., and V. J. DiRita. 2003. From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae. Curr. Opin. Microbiol. 6:186-190. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 19.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medrano, A. I., V. J. DiRita, G. Castillo, and J. Sanchez. 1999. Transient transcriptional activation of the Vibrio cholerae El Tor virulence regulator toxT in response to culture conditions. Infect. Immun. 67:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mekalanos, J. J., D. J. Swartz, G. D. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 22.Murley, Y. M., P. A. Carroll, K. Skorupski, R. K. Taylor, and S. B. Calderwood. 1999. Differential transcription of the tcpPH operon confers biotype-specific control of the Vibrio cholerae ToxR virulence regulon. Infect. Immun. 67:5117-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murley, Y. M., J. Behari, R. Griffin, and S. B. Calderwood. 2000. Classical and El Tor biotypes of Vibrio cholerae differ in timing of transcription of tcpPH during growth in inducing conditions. Infect. Immun. 68:3010-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogierman, M. A., E. Voss, C. Meaney, R. Faast, S. R. Attridge, and P. A. Manning. 1996. Comparison of the promoter proximal regions of the toxin-co-regulated tcp gene cluster in classical and El Tor strains of Vibrio cholerae O1. Gene 170:9-16. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez, J., G. Castillo, A. I. Medrano, A. Martinez-Palomo, and M. H. Rodriguez. 1995. In vitro growth of Vibrio cholerae in cholera stool fluid leads to differential expression of virulence factors. Arch. Med. Res. 26:S47-S53. [PubMed] [Google Scholar]
- 27.Schuhmacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svennerholm, A. M., and J. Holmgren. 1978. Identification of Escherichia coli heat labile enterotoxin by means of a ganglioside immunosorbent assay (GM1-ELISA) procedure. Curr. Microbiol. 1:19-23. [Google Scholar]
- 29.Svennerholm, A. M., M. Wikström, L. Lindholm, and J. Holmgren. 1986. Monoclonal antibodies and immunodetection methods for Vibrio cholerae and Escherichia coli, p. 77-95. In A. J. Macario et al. (ed.), Monoclonal antibodies against bacteria, vol. III. Academic Press, New York, N.Y.
- 30.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, S., S. G. Williams, and P. A. Manning. 1995. Regulation of tcp genes in classical and El Tor strains of Vibrio cholerae O1. Gene 166:43-48. [DOI] [PubMed] [Google Scholar]
- 32.Yu, R. R., and V. J. DiRita. 1999. Analysis of an autoregulatory loop controlling ToxT, cholera toxin, and toxin-coregulated pilus production in Vibrio cholerae. J. Bacteriol. 181:2584-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]