Abstract
The molecular hallmarks of adult T cell leukemia (ATL) comprise outstanding deregulations of signaling pathways that control the cell cycle, resistance to apoptosis, and proliferation of leukemic cells, all of which have been identified by early excellent studies. Nevertheless, we are now confronted the therapeutic difficulties of ATL that is a most aggressive T cell leukemia/lymphoma. Using next-generation strategies, emerging molecular characteristics such as specific surface markers and an additional catalog of signals affecting the fate of leukemic cells have been added to the molecular hallmarks that constitute an organizing principle for rationalizing the complexities of ATL. Although human T cell leukemia virus type 1 is undoubtedly involved in ATL leukemogenesis, most leukemic cells do not express the viral protein Tax. Instead, cellular gene expression changes dominate homeostasis disorders of infected cells and characteristics of ATL. In this review, we summarize the state of the art of ATL molecular pathology, which supports the biological properties of leukemic cells. In addition, we discuss the recent discovery of two molecular hallmarks of potential generality; an abnormal microRNA pattern and epigenetic reprogramming, which strongly involve the imbalance of the molecular network of lymphocytes. Global analyses of ATL have revealed the functional impact of crosstalk between multifunctional pathways. Clinical and biological studies on signaling inhibitory agents have also revealed novel oncogenic drivers that can be targeted in future. ATL cells, by deregulation of such pathways and their interconnections, may become masters of their own destinies. Recognizing and understanding of the widespread molecular applicability of these concepts will increasingly affect the development of novel strategies for treating ATL.
Keywords: HTLV-1, ATL, genome, epigenetics, miRNA, signal transduction
Introduction: Current Status of Adult T Cell Leukemia Research
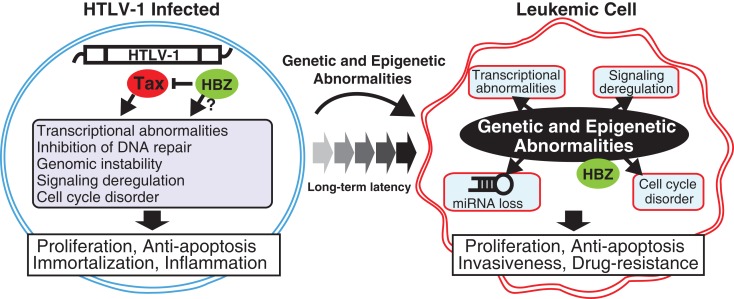
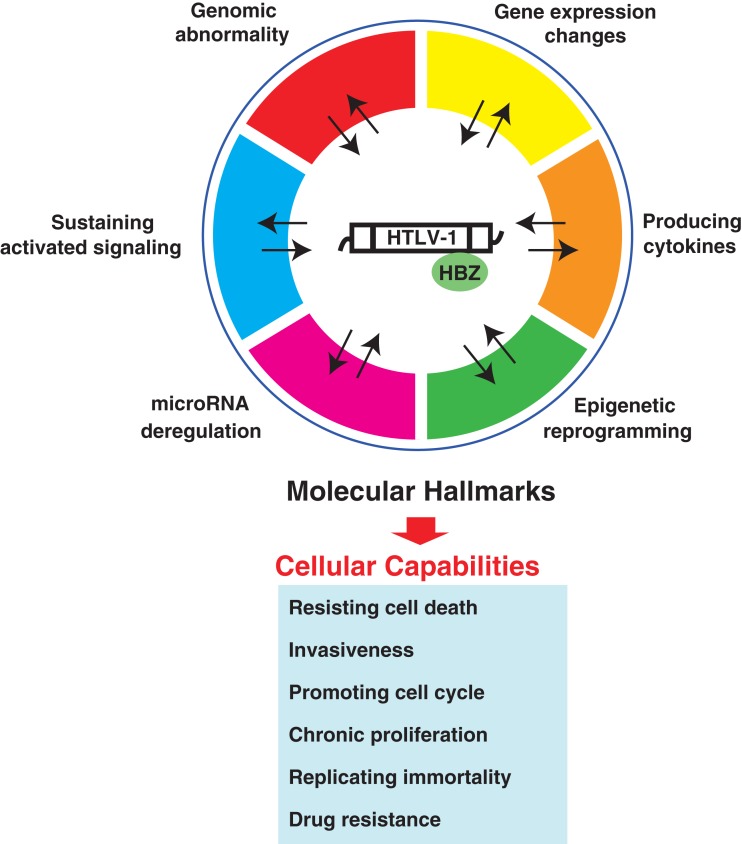
Adult T cell leukemia (ATL), which is derived from human T cell leukemia virus type 1 (HTLV-1)-infected CD4+ T cells, is an aggressive T cell leukemia/lymphoma with the worst poor prognosis (Yamaguchi and Watanabe, 2002). Endemic expansion of HTLV-1 infection and related ATL onset have been observed in Japan (Iwanaga et al., 2010). The diverse clinical features of ATL have led to its sub-classification into acute, lymphoma, chronic, and smoldering subtypes. ATL has a poor prognosis with a mean survival time of 13 months and is refractory to currently available combination chemotherapy (Yamada et al., 2001). It is therefore essential to develop a novel treatment strategy, in particular, a molecular targeting therapy. ATL is incurable because we do not have complete understanding of its molecular basis, leading to the lack of molecular targeting. Although HTLV-1 is an apparent causative agent of ATL, several studies have demonstrated that viral gene expression is rare, except for the expression of the HTLV-1 antisense gene product HBZ (Gaudray et al., 2002). Mounting evidence has shown that ATL does not contain the somatic mutant genes that can explain its aggressiveness. However, it is evident that ATL cells possess multiple deregulations of genome and gene regulation, namely the molecular hallmarks of ATL, which should be targeted (Figure 1). We believe that normal cells acquire a succession of molecular hallmarks as they progressively evolve into a neoplastic state and that the multistep process of human pathogenesis can be rationalized by the need of incipient cancer cells for acquiring traits that enable them to become leukemogenic and ultimately malignant. In this review, we first summarize the essence of each molecular hallmarks of ATL cells. Basic molecular analyses and next-generation global analyses of ATL samples have revealed the molecular traits of ATL. We address new developments that broaden the scope of the conceptualization and describe recent advances of science to acquisition of the molecular mechanistic underpinnings. Finally, we discuss the possibility and future direction of treating ATL.
Figure 1.
Transition from HTLV-1-infected cell to transformed leukemic cell. In early and latent clinical phases, the HTLV-1 Tax and HBZ mainly act as driving forces for the molecular hallmarks of infected cells. After a long-term latency, leukemic cells have acquired genetic and epigenetic abnormalities, which lead to deregulations of gene expression pattern, cell cycling, signaling activation, and miRNA expression. These molecular changes consequently induce cellular hallmark capabilities of ATL such as chronic proliferation, apoptotic resistance, multiple organ invasion, and drug resistance.
Molecular Hallmarks Controlled by Tax and HBZ
Since 1985, numerous excellent studies have indentified signaling abnormalities in HTLV-1-associated cells, mainly induced by HTLV-1 Tax. In general, T cell disorders include several deregulations of cellular processes that regulates the cell cycle, cell proliferation, and cell survival. Tax has been shown to disrupt all these cellular processes. The classical oncogenic function of Tax was first demonstrated in a study of cell cycle regulation. Tax was found to inhibit the cyclin-dependent kinase (CDK) inhibitor (CKI) CDKN2A (p16INK4A) by physical interaction (Suzuki et al., 1996). Several subsequent studies also revealed the mitogenic activity of Tax exerted through the stimulation of G1 to S phase transition (Akagi et al., 1996; Neuveut et al., 1998; Schmitt et al., 1998; Suzuki et al., 1999; Iwanaga et al., 2001; Haller et al., 2002; Liang et al., 2002). Tax also affects a cohort of cell cycle-related proteins, including CDKs, CDKN1A, CDKN1B, and CDKN2A, by regulating their expression or by physical interaction. Tax also participates in genetic damage (Jeang et al., 1990; Ressler et al., 1997; Kao and Marriott, 1999). Because of particular functions of Tax that can interact with many host factors (Interactome; Boxus et al., 2008; Simonis et al., 2012), Tax can activate several signaling pathways and lead to abnormal gene expression (a Tax-dependent molecular hallmark) and overproduction of several cytokines. Especially, IL-2 and its receptor are important for T cell activation. IL-2 signals through its receptor are primarily delivered by two molecular families, the Janus tyrosine kinases (JAKs) and signal transducers and activators of transcription (STATs), whose activation leads to lymphocyte proliferation. Tax can also activate the NF-κB and NFAT pathways responsible for the predominant expression of IL-2 and the IL-2 receptor (Ballard et al., 1988; Ruben et al., 1988; Hoyos et al., 1989; McGuire et al., 1993; Good et al., 1996). These findings implicate the IL-2–IL-2 receptor autocrine loop in ATL; however, several studies have shown that alterations of the loop alone are not sufficient to ensure the maintenance and proliferation of ATL cells because most Tax- or HTLV-1-immortalized T cells still require exogenous IL-2 and do not express detectable amounts of either IL-2 mRNA or protein (Akagi and Shimotohno, 1993; Chung et al., 2003; Chen et al., 2010). Similarly, IL-15 and its receptor and IL-13 and its receptor have been associated with Tax-expressing cells because similar to IL-2, they activate the STAT pathways (Azimi et al., 1999; Mariner et al., 2001; Chung et al., 2003; Wäldele et al., 2004). IL-6 is also transduced by Tax through the NF-κB pathway (Villiger et al., 1991; Muraoka et al., 1993). Because IL-6 mainly participates in inflammatory signaling, HTLV-1 infection can induce cytokine-dependent inflammation, which is frequently observed in ATL as well as HTLV-1-associated myelopathy/Tropical spastic paraparesis (HAM/TSP; Oh et al., 2011). OX40, a member of the TNF-receptor superfamily, is specifically expressed in HTLV-1-infected cells, whose expression is induced by the Tax-NF-κB pathway. Tax may play a role in leukemic cell infiltration in addition to cell adhesion in vivo (Imura et al., 1997). Tax affects not only the abovementioned signaling pathways but also the TGF-β pathway (Kim et al., 1990; Höllsberg et al., 1994; Arnulf et al., 2002; Lee et al., 2002). It has been recently shown that TGF-β signaling is activated by HBZ by binding with Smad 2/3 (Zhao et al., 2011). TP53 is the master regulator of the cell cycle that guards against DNA damage by inducing the transcription of several genes. Tax can inhibit TP53 functioning in multiple ways (Grassmann et al., 2005).
Strong NF-κB activation is the outstanding hallmark provided by Tax. NF-κB represents a family of inducible transcription factors that regulate diverse biological processes, including the growth and survival of both T cells and non-lymphoid cells. Transcriptional activation of genes such as several cytokines and apoptosis-resistance factors plays an important role in immunity. Tax acts as an intracellular stimulator of IKK by physical interaction, leading to persistent activation of NF-κB-mediated transcription. The Tax/IKK complex formation relies on the physical interaction between Tax and the IKK regulatory subunit IKKγ. The Tax/IKKγ interaction is required for recruiting Tax to the IKK catalytic subunits and for Tax-mediated IKK activation (Sun and Yamaoka, 2005). Recent studies have identified cellular proteins that are important for Tax-mediated NF-κB activation, such as NRP/Optineurin and TAX1BP1 (Journo et al., 2009; Shembade et al., 2011), and the ubiquitin-specific peptidase USP20 (Yasunaga et al., 2011). Subcellular localization of Tax also predominantly controls Tax-mediated NF-κB activation (Fryrear et al., 2009). Given that NF-κB governs the expression of a large array of cellular genes that control various cellular functions, the phenotypes of HTLV-1-infected cells are dominated by Tax-mediated abnormal activation.
Tax also activates several signaling pathways through key transcriptional factors such as CREB, SRF, and AP-1. It does not directly bind to promoter or enhancer DNA, however, disruption of these pathways causes serious gene expression disorders (Grassmann et al., 2005).
It should be also noted that HTLV-1 antisense product HBZ seems to be involved in leukemogenesis; its expression is sustained in leukemic cells. In vitro and in vivo studies have demonstrated that the growth-promoting activity of HBZ RNA may play an important role in oncogenesis by HTLV-I (Satou et al., 2006). Furthermore, transgenic expression of HBZ in CD4+ T cells induces T cell lymphomas and systemic inflammation in mice. HBZ directly induces Foxp3 gene transcription, and the increased CD4+Foxp3+ Treg cells in HBZ transgenic mice are functionally impaired, suggesting that the expression of HBZ in CD4+ T cells may be a key mechanism of HTLV-1-induced neoplastic and inflammatory diseases (Satou et al., 2011).
Taking together with these mounting evidences, Tax and HBZ undoubtedly contribute to leukemogenesis in HTLV-1-infected T cells. However, as a low rate of incidence, clinical observation implies that HTLV-1 itself does not have a strong capacity of leukemogenesis in contrast with other animal leukemia viruses.
Chromosomal Changes and Gene Alterations in ATL
Tax is not expressed in most ATL cases because HTLV-1 provirus is substantially silenced by proviral defect and/or epigenetic mechanism (Tamiya et al., 1996; Koiwa et al., 2002; Taniguchi et al., 2005). However, leukemic cells possess very similar traits to Tax-expressing cells (Figure 1). The paradoxical truth, i.e., memory of Tax, still remains to be elucidated. Investigation of established ATL cell lines and primary ATL samples has led to the identification of the molecular hallmarks of leukemic cells, which may partially explain their malignant characteristics.
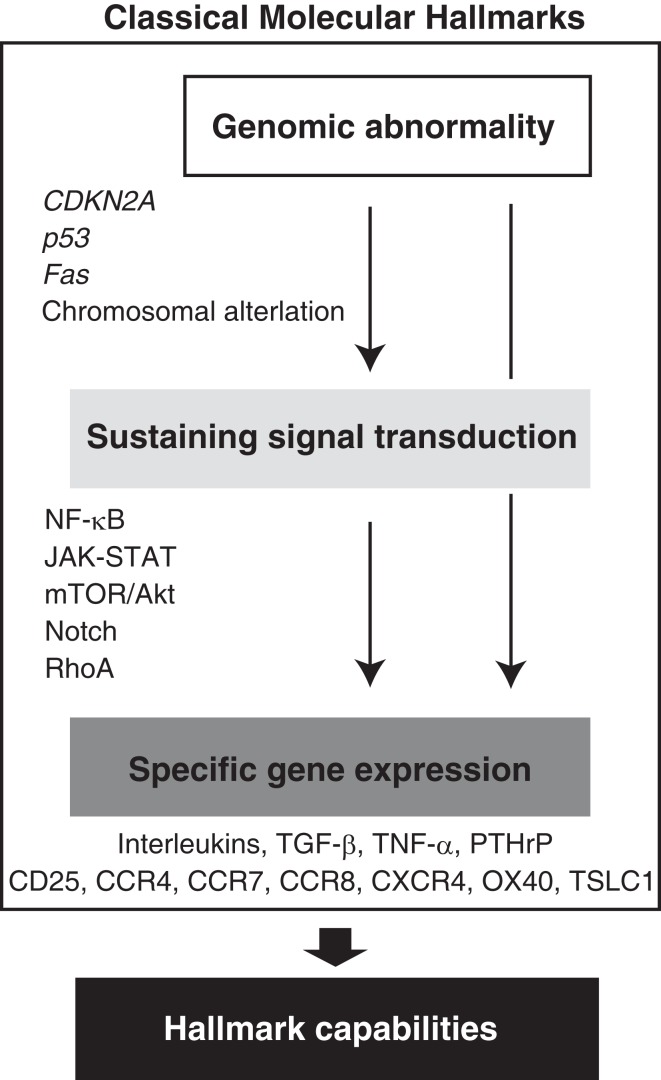
From 1980s, chromosomal analyses of clinical cases were reported. Therefore, we know that ATL is characterized by various abnormal chromosomes. Kamada et al. (1992) reported that 96% of ATL cases had an abnormal chromosome pattern, suggesting that a genomic catastrophe underlies the clinical and molecular characteristics of ATL, which is consistent with all other cancers. In 2000s, global analysis has been available and whole genomic analysis could be challenged. Comparative genomic hybridization (CGH) revealed the genomic distinctions between the clinical subtypes; patients with aggressive ATL (acute and lymphoma) have a higher number of chromosomal imbalances, losses, and gains than those with indolent ATL (chronic and smoldering). Thus, genomic abnormalities are prognostic factors (Tsukasaki et al., 2001a). High-resolution analyses based on microarrays unveiled the genomic properties of ATL (Oshiro et al., 2006) and shed light on the genomic characteristics that affect the gene expression pattern (Figure 2).
Figure 2.
Acquired molecular hallmarks of ATL. From the beginning of ATL discovery, indicated molecular hallmarks have been suggested. Hierarchical regulation of the gene expression has been expected. Abnormal expressions of these cytokines and receptors as well as various proteins that act as anti-apoptotic factors or proliferation agents are responsible for the malignant phenotypes as hallmark capabilities.
In cancer cells, genetic mutations and deletions are generally observed in genes important for cell cycle regulation, cell survival, and cell proliferation. In ATL, the accumulation of genetic deletions in the gene encoding the cell cycle regulator CDKN2A have been reported (Hatta et al., 1995; Uchida et al., 1996). Southern blotting analyses of samples from large cohort studies (114 patients) also demonstrated that the CDKN2 family (p15 and p16) is lost in more frequently observed in the acute type (33.8%) than in the chronic type of ATL (5.4%; Yamada et al., 1997). Patients with these genetic deletions showed reduced survival compared with those without deletions. CDKN2A transcription is also regulated in an epigenetic manner. Abnormal DNA methylation has been during ATL progression (Nosaka et al., 2000).
Alterations of other cell cycle-regulating genes are unusual. Mutations of CDKN1B (p27Kip1) is rare in non-Hodgkin’s lymphoma and ATL (Morosetti et al., 1995). Mutations of Rb gene have also been observed in a few ATL patients (Hatta et al., 1997). Extensive analyses have revealed that 26–40% of ATL cases have a p53 gene mutation, which is much less than that observed in other cancers (Nagai et al., 1991; Cesarman et al., 1992; Sakashita et al., 1992; Nishimura et al., 1995). Gene expression analysis of ATL samples showed that CDKN1A (p21Cip1) is commonly down regulated in ATL (Watanabe et al., 2010). In contrast, Tax activates p21Cip1 expression in HTLV-1-infected cells, which may be an important mechanism for stopping the host at the G1/S boundary and repairing damaged DNA before entering the S phase entry (Cereseto et al., 1996; de La Fuente et al., 2000). Although p21Cip1 transcription may be activated by the Tax–CREB pathway in infected cells, it seems to be epigenetically silenced in several cancers, including ATL. Thus, these gene alterations and related expression patterns are important molecular hallmarks of ATL as well as other cancers. Of note, no ATL cell has been shown to have both mutations, suggesting that either p16 or p53 mutation may be sufficient to promote the more aggressive phenotype of leukemic cells (Tawara et al., 2006). In addition, as many ATL patients carry a wild-type p53, TP53 activation by antagonists such as Nutlin-3a may be a promising strategy for ATL therapy (Hasegawa et al., 2009). The ability to resist apoptotic cell death is conferred by Fas gene mutation (Tamiya et al., 1998; Maeda et al., 1999). Indeed, Fas-negative ATL cells are resistant to adriamycin-induced apoptosis in vitro, which is consistent with the finding that ATL in this case is resistant to chemotherapy.
Single nucleotide polymorphism (SNP) is another possible cause for developing ATL. TNF-α polymorphism may be associated with increased susceptibility to development of ATL in HTLV-1 carriers (Tsukasaki et al., 2001b). An immunological study also suggested that the HLA haplotype is related to ATL progression (Yashiki et al., 2001). Currently, whole SNP can be detected by next-generation sequencing and other array-based techniques. Global understanding of SNPs in ATL patients may provide us with the genetic basis of the familial clustering of ATL.
By utilizing global survey techniques for analyzing the gene copy number, several research groups have detected specific gene copy number patterns in ATL. CGH methods first demonstrated gains at 14q32 and 2p16-22 in ATL cell lines (Ariyama et al., 1999). Fluorescence in situ hybridization (FISH) analysis with several yeast artificial chromosome (YAC), BAC, and PAC clones also mapped the breakpoints in ATL cell lines with 6q aberrations (Tagawa et al., 2002). Clinical subtype-specific genomic alterations in aggressive ATL were also identified by array-based CGH (Oshiro et al., 2006). The lymphoma type of ATL had significantly more frequent gains at 1q, 2p, 4q, 7p, and 7q and losses at 10p, 13q, 16q, and 18p, whereas the acute type showed a gain of 3/3p. CARMA1 is also a possible target gene for 7p22 amplification in the lymphoma type but not in the acute type of ATL. In contrast, BCL11B is expressed in the acute type of ATL, regardless of 14q32 gain/amplification; however, there is no or low expression of the gene in the lymphoma type of ATL. Taken together, these findings suggest that the acute and lymphoma types of ATL are genomically distinct. The physiological molecular hallmarks of ATL have been identified by integrating the gene copy number and gene expression analyses findings, and these are described below.
Global Gene Expression Analyses of ATL
Genetic alterations and other modulations enhance specific gene expression signatures. Classically, based on a comparison between normal T cells and ATL cell lines as well as primary leukemic cells, ATL has been shown to have a distinct gene expression pattern that may reflect its clinical pathogenesis. In addition, global expression analyses, mainly based on microarray, have identified emerging molecular hallmarks that can possibly be targeted in ATL therapies.
First, Harhaj et al. (1999) performed gene array analysis of HTLV-1-immortalized cells and reported up- and downregulated genes. Ruckes et al. (2001) performed suppressive subtractive hybridization that enabled the isolation of novel sequences derived from unknown genes. They identified a number of genes linked to Tax transformation and ATL leukemogenesis. Systematic comparison of gene expression between cultured cells from patients with acute ATL and that of stimulated peripheral T lymphocytes revealed 346 cDNA clones, which included the genes encoding IL-2 receptor α chain and p21Cip1. They also included a dual-specific protein phosphatase (PAC1), an interferon-inducible factor (ISG15), a basic helix–loop–helix transcription factor (DEC-1), and the secreted anti-apoptotic chemokine I-309. Pise-Masison et al. (2002) also reported the results of analyses performed using an Affymetrix Hu6800 GeneChip. They identified 763 genes with differentially regulated expression in HTLV-1 cell lines.
Tsukasaki et al. (2004) directly analyzed the gene expression patterns of fresh ATL samples (three acute types and one chronic type) using high-density oligonucleotide DNA arrays. A total of 203 genes that included ribosomal proteins, proteasome subunits, translation factors, immunophilins, heat shock proteins, and genes important for DNA replication were found to be upregulated in ATL cases, and 91 genes were downregulated. Sasaki et al. (2005) also determined the expression profiles of more than 12,000 genes in eight cases of the acute type of ATL using microarray analysis, and they found that 192 genes were upregulated more than twofold compared with healthy CD4+ and CD4+/CD45RO+ T cells. In particular, the expression of tumor suppressor in lung cancer 1 (TSLC1), caveolin 1, and prostaglandin D2 synthase was increased by more than 30-fold. Interestingly, comparison of gene expression between established cell lines with or without HTLV-1 infection led to the identification of the specific HTLV-1-related cell surface marker CD70, which is also expressed in freshly isolated leukemic cells (Baba et al., 2008).
Integrated analyses of the genome and its expression revealed more detailed data for the acquisition of molecular and physiological information. Choi et al. (2007) isolated CD4+ T cells from ATL patients and profiled their gene expression using DNA microarrays containing >44,000 probe sets. Changes in the chromosomal copy number were examined for 24 cell specimens using microarrays harboring approximately 50,000 probe sets. Coordinated analysis revealed a novel molecular target, the HGF–MET pathway. Ligation of MET by increased plasma HGF levels may confer a growth advantage on ATL cells through the phosphatidylinositol 3-kinase (PI3K) and Ras pathways.
We performed further global integrated analyses of samples from ATL patients using mRNA expression array (n = 52), microRNA (miRNA) microarray (n = 40), and DNA copy number analysis using SNP-based array (n = 168; Yamagishi et al., 2012). Each analysis revealed many novel molecular characteristics of ATL (data available in the Gene Expression Omnibus database). Analysis of this massive dataset derived from primary ATL samples led to the conclusion that genetic and epigenetic imbalances are involved in ATL leukemogenesis through miRNA and the signaling pathways responsible for leukemic cell survival, proliferation, and invasiveness. Taken together, these findings clearly indicate that disrupted gene expression is a molecular hallmark of ATL. Diverse abnormalities have been found in each of these comprehensive studies; however, several gene alterations and other critical events have been commonly implicated as determinants of the gene expression pattern. Abnormal expression of different cytokines, their receptors, and various proteins that act as anti-apoptotic factors or proliferation agents is responsible for malignant phenotypes as hallmark capabilities (Figure 2). From the spreading intelligence, we have to acquire the genuine molecular targets harboring in the bare bones of ATL.
Signaling Networks in ATL: Main Driving Forces
The cellular complexity of signaling networks confers robustness to specific gene expression and biological functions. This is supported by the fact that the inhibition of a signaling pathway can strictly suppress abnormal gene expression. The functional characteristics of ATL cells, such as chronic proliferation, abnormal survival, and penetrating multiple organs, is supported by aberrant gene expression, as described above.
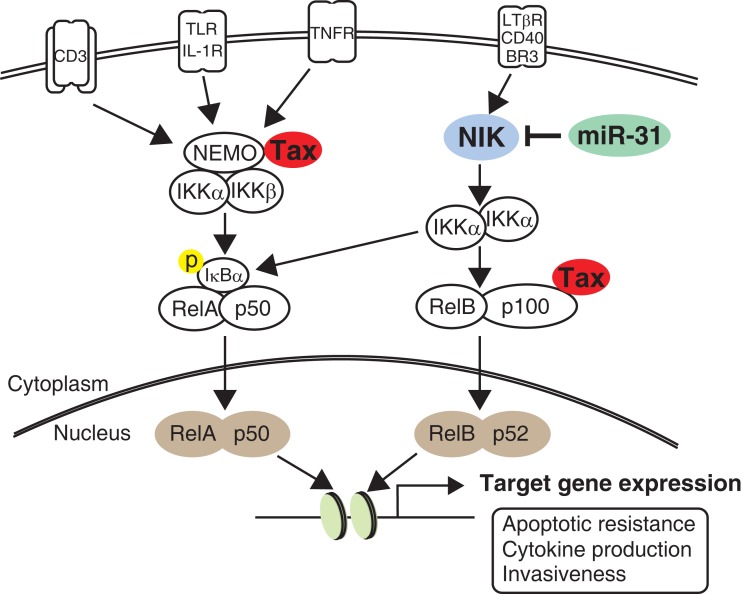
Activation of NF-κB signaling is the most outstanding hallmark of both HTLV-1-infected cells and leukemic cells. Abnormal activation and deficient negative control of the NF-κB pathway strongly contributes to prolonged survival, proliferation, and invasiveness, all of which are observed in ATL cells. Ruben et al. (1988) found that Tax can activate IL-2 receptor gene expression in a NF-κB-dependent manner. Following this, several studies clearly demonstrated that Tax can activate NF-κB activity through a multistep (Sun and Yamaoka, 2005). Strong and persistent activation of NF-κB signaling was then demonstrated in leukemic cell lines that did not express Tax (Mori et al., 1999). In transformed ATL cells, both the canonical and non-canonical NF-κB pathways are persistently activated because NF-κB inducing kinase (NIK) is aberrantly expressed in ATL (Saitoh et al., 2008). NIK plays a central role in non-canonical NF-κB signaling by IKKα phosphorylation (Thu and Richmond, 2010). Very recently, we found a novel linkage between NF-κB activation and miRNA deregulation. Comprehensive gene expression analysis and in vitro experiments revealed that miRNA-31 (miR-31) can regulate NIK expression through the 3′ untranslated region (UTR). In ATL cells, miR-31 expression is genetically and epigenetically silenced, which in turn induces constitutive NF-κB activation through NIK expression (Figure 3; Yamagishi et al., 2012). Because current evidence clearly indicates that miR-31 dominates NF-κB activity in T cells, manipulating cellular miR-31 levels may be a novel molecular approach to reduce NF-κB activity and induce apoptosis.
Figure 3.
NF-κB signaling pathway in ATL. Compared to normal T cell, HTLV-1-infected cell shows intense activity of NF-κB pathway through the Tax interactome. In contrast, leukemic cell sustains strong NF-κB activity without Tax by acquisition of miR-31-dependent NIK expression. Activation of the non-canonical pathway ultimately joins canonical pathway, resulting in transactivation of numerous target genes important for cell survival, cytokine production, and invasiveness.
Numerous efforts have demonstrated that NF-κB inhibitory agents may be suitable for treating ATL. Inhibitors such as bortezomib (Satou et al., 2004), Bay 11-7082 (Mori et al., 2002), DHMEQ (Watanabe et al., 2005), ACHP (Sanda et al., 2006), and IMD0354 (Uota et al., 2012) have the ability to kill leukemic cells. Fludarabine, a purine analog that has significant activity in B cell malignancies, also inhibits the NF-κB activity in ATL (Nishioka et al., 2007). Tax degradation by arsenic trioxide and IFN-α treatments is also useful for inhibiting NF-κB in HTLV-1-infected cells (El-Sabban et al., 2000). In the near future, gene manipulation for example by siRNA targeting NIK may be a promising strategy for inhibiting NF-κB activity with strict specificity (Yamagishi et al., 2012). Because NF-κB prevention showed good results in a xenograft model of cell lines with or without Tax (Dewan et al., 2003; Watanabe et al., 2005; Ohsugi et al., 2007a), molecular targeting therapy based on the NF-κB pathway is a promising new ATL treatment. Of note, NF-κB inhibition by DHMEQ can also remove virus-carrying cells from carrier peripheral blood mononuclear cell (PBMC) samples (Watanabe et al., 2005).
Why is NF-κB important for ATL cell survival? One of the target genes is BCL-XL, which is expressed when NF-κB is stimulated. In HTLV-1-infected cell lines, BCL-XL is expressed through the Tax-NF-κB pathway. Interestingly, fresh ATL samples show BCL-XL overexpression (Nicot et al., 2000). Given that BCL-XL is a principal anti-apoptotic protein as a BCL-2 family member, persistent BCL-XL expression is one of the molecular capability of apoptotic resistance, which may contribute to clinical chemoresistance. Indeed, inhibition of the NF-κB pathway by NIK depletion leads to impaired BCL-XL levels and apoptotic cell death in ATL (Yamagishi et al., 2012). In addition, a recent study has implicated that both BFL1 (BCL2A1) and BCL-XL are responsible for ATL cell survival. BFL1 expression is regulated by Tax and HBZ through the NF-κB and AP-1 pathways, respectively (Macaire et al., 2012).
The JAK–STAT pathway, which is universal and essential to cytokine receptor signaling, is also one of the best understood signal transduction cascades in several cell types, including ATL cells. The JAK–STAT pathway mediates signaling by cytokines, which control cell survival, proliferation, and differentiation. Constitutive JAK activation leads to persistent activation of STAT transcription factors, and several cancers exhibit constitutive STAT activation in the absence of JAK- or STAT-activating mutations. JAK–STAT activation in HTLV-1-infected cells was first reported in 1995 (Migone et al., 1995). Takemoto et al. (1997) demonstrated that STAT-3 and STAT5 are activated in freshly isolated ATL cells. However, functional redundancy was also reported. Blockade of the JAK3–STAT5 pathway by AG490 failed to inhibit the proliferation of HTLV-1-transformed T cell lines, despite activation of the pathway (Kirken et al., 2000). Recently, a JAK2–STAT5 inhibitor, AZ960, was shown to have an anti-proliferative effect on HTLV-1-infected and ATL cell lines (Yang et al., 2010). However, which gene is transactivated by STAT remains unknown. The biological relevance of the JAK–STAT pathway in ATL remains to be elucidated.
Emerging Signaling Pathways and Inhibitory Agents
In addition to the abovementioned pathways, several signaling pathways, including the AP-1, NFAT, and CREB pathways, may be involved in the functional characteristics of ATL (Hall and Fujii, 2005). These are activated by Tax. Leukemic cells may have certain activities in each of these pathways.
The RhoA family also participates in Tax-mediated immortalization. A recent study employing a proteomic approach to identify Tax-binding proteins in a HTLV-1-infected T cell line identified direct interactions between Tax and several small GTPases, including RhoA, Rac1, and Cdc42, all of which are involved in a wide variety of cellular processes, including cytoskeleton organization and transcriptional activation (Wu et al., 2004). Recently, our global gene expression analysis as well as miRNA study strongly suggested that RhoA is overexpressed in ATL cells. A decreased miR-31 level may contribute to RhoA expression and migration activity (Yamagishi et al., 2012). The gene expression signature determined from patient samples could identify the additional signaling pathways involved in leukemogenesis (authors’ unpublished data).
Recently, several studies have demonstrated that ATL shows emerging signaling activation, which is well established as oncogenic signaling in other types of cancer. Typically, the Notch pathway, which is the signaling pathway implicated in T cell acute lymphoblastic leukemia (T-ALL), is also activated in ATL (Pancewicz et al., 2010). More than 30% of ATL cases show activating mutations in NOTCH1, leading to reduced CDC4/Fbw7-mediated degradation and stabilization of the intracellular cleaved form of Notch1 (ICN1). Inhibition of Notch signaling by a γ-secretase inhibitor reduced tumor cell proliferation and tumor formation in ATL-engrafted mice. It is therefore suggested that activated Notch may be important for ATL pathogenesis.
TGF-β signaling is also reportedly important. Increased TGF-β production was observed in freshly isolated ATL cells, and this was at least partially mediated by AP-1 (Niitsu et al., 1988; Kim et al., 1990). Nevertheless TGF-β could inhibit the growth of normal T lymphocytes, HTLV-1-infected cells were resistant to this inhibition (Höllsberg et al., 1994). In ATL cells, transcription factor 8 (TCF8) is frequently disrupted by several mechanisms, primarily by epigenetic dysregulation. TCF8 mutant mice frequently develop invasive CD4+ T cell lymphomas in vivo. Downregulation of TCF8 expression in ATL cells in vitro is associated with resistance to TGF-β1, suggesting that escape from TGF-β1-mediated growth inhibition is important in the pathogenesis of ATL (Hidaka et al., 2008). The same research group also reported that ZEB1 downregulation and Smad7 overexpression contribute to resistance to TGF-β1-mediated growth suppression in ATL (Nakahata et al., 2010). ZEB1 plays a critical role in regulating the epithelial–mesenchymal transition (EMT) in several solid cancers. The general function of ZEB1 in lymphocytes, particularly in association with leukemogenesis and TGF-β signaling, is a very intriguing issue.
If a chemical drug that can inhibit cellular signaling can induce apoptosis specifically, it is probable that leukemic cell survival is supported by the targeted signaling. Recent studies of inhibitors have indicated that mTOR signaling may be involved in ATL. mTOR is a serine/threonine-specific protein kinase that is located downstream of the PI3K/Akt pathway. Deregulation of mTOR signaling is implicated in a range of cancers due to its roles in cell survival and proliferation, protein synthesis and breakdown, membrane trafficking, and protein kinase C signaling (Zoncu et al., 2011). Ikezoe et al. (2007) used several inhibitors to show that the PI3K/Akt/mTOR pathway is activated in ATL cell lines as well as fresh ATL cells. In addition, a resent study reported that the mTOR complex 1 (mTORC1) inhibitor everolimus has a dramatic inhibitory effect on the growth of HTLV-1-positive and HTLV-1-negative malignant T cells, whereas normal resting or activated T cells are resistant to it (Darwiche et al., 2011). Furthermore, in addition to HTLV-1-infected cells in which Tax activates the Akt pathway (Liu et al., 2001), transformed leukemic cells in which Tax is not expressed may have some Akt activity. The most important point is how these pathways are constitutively activated in ATL. The functional importance of this pathway and its therapeutic potential will be addressed in the near future.
In the context of inhibitory agents, a higher response rate following azidothymidine/interferon α (AZT/IFNα) treatment of ATL patients has been reported in several human trials (Gill et al., 1995; Bazarbachi et al., 2010). AZT treatment of ATL patients inhibits telomerase activity and results in progressive telomere shortening and increased p14ARF expression. A functional relationship between p53 and sensitivity to AZT has also been suggested (Datta et al., 2006). Ritonavir, developed as a protease against HIV-1, also has an anti-ATL effect. Ritonavir decreases NF-κB activity linked to the inhibition of IκBα phosphorylation and induces the apoptosis of ATL cells. In addition, it very efficiently prevents tumor growth and leukemic infiltration in various organs of NOG mice when administered at the same dose as that used in the treatment of patients with AIDS (Dewan et al., 2006). Together, several signaling networks are deregulated in leukemic cells, and these are a specific molecular feature of ATL (Figure 2). At present, signal interception is the most effective strategy to treat ATL. At the same time, the occurrence of incidental side effects should be carefully considered because these signaling pathways are essential for normal cell function and survival.
Cytokines Production and ATL
CD4+ T cells play a central role in the immune response by controlling cells such as B cells, dendritic cells, and cytotoxic cells and their responses through various cytokines. Deregulation of the associated signaling pathways leads to abnormal gene expressions, including that of several cytokines. ATL has been implicated in the production of various cytokines, including IL-1 (Wano et al., 1987), TGF-β (Niitsu et al., 1988), TNF-α, IFN-γ, GM-CSF (Yamada et al., 1996), and PTHrP (Watanabe et al., 1990). Especially, an elevated serum C-terminal PTHrP level is a characteristic marker of the HTLV-1 carrier status, and the determination of this level in ATL patients could be useful for assessing the prognosis (Yamaguchi et al., 1994). SCID mice model of ATL also showed clearly elevated serum levels of calcium and C-terminal PTHrP, resulting in the development of hypercalcemia (Takaori-Kondo et al., 1998). The high frequency of hypercalcemia is one of the notable clinical characteristics of ATL, in particular, the aggressive types of ATL (Kiyokawa et al., 1987). Besides PTHrP, which plays an important role in bone resorption by stimulating osteoclasts, abnormal expression of the RANK ligand (RANKL) has also been demonstrated in ATL with hypercalcemia (Nosaka et al., 2002). Recent studies have revealed that a central region of HTLV-1 gp46 acts as an antagonist for osteoprotegerin and leads to hypercalcemia (Sagara et al., 2007, 2009).
SCID mice engrafted with cells from Tax-transgenic mice that develop lymphoma produced TNF-α, PDGF-BB, sICAM-1, and sVCAM-1 as factors that may contribute to high levels of organ infiltration (Watters et al., 2010). PDGF, in particular, the BB isoform, is a well-known potent osteotropic factor that stimulates the osteoclasts and osteoblasts functions (Yi et al., 2002). High IL-2 production was not observed in previous ATL studies or in our microarray data (Yamagishi et al., 2012). Nevertheless, IL-2 is an HTLV-1-induced cytokine associated with the NF-κB pathway (Hoyos et al., 1989). In contrast, receptor subsets for IL-2 (IL2Rs) are generally overexpressed in ATL cells.
Interestingly, smoldering/chronic ATL PBMCs spontaneously proliferate ex vivo in an IL-12-, IL-9-, and IL-15-dependent manner, whereas acute type ATL PBMCs do not proliferate or proliferate independent of cytokines. Furthermore, purified leukemic cells from indolent ATL cases produce IL-2/IL-9 and the downstream JAK–STAT pathway is activated. Thus, autocrine/paracrine cytokine stimulation of leukemic cell proliferation may occur in patients with smoldering/chronic ATL (Chen et al., 2010).
Cell Surface Markers and Their Functions
IL-2 receptor α (CD25) was the first marker of ATL and HTLV-1-infected cells. CD25 expression is dependent on NF-κB activity (Ruben et al., 1988). Global gene expression analysis has also validated the high CD25 mRNA level in ATL patient samples (Yamagishi et al., 2012).
Chemokines and their receptors mainly function in the migration and tissue localization of lymphocytes. The expression of the following ATL-specific chemokine receptors has been identified: CCR4 (Yoshie et al., 2002), CCR7 (Hasegawa et al., 2000), CCR8 (Ruckes et al., 2001), and CXCR4 (Twizere et al., 2007). In addition, other cell surface proteins such as OX40 (Imura et al., 1997) and TSLC1 (Sasaki et al., 2005) are highly expressed in ATL cells and are thus the molecular hallmarks of ATL; they may also participate in leukemogenesis. For example, TSLC1, a well-known tumor suppressor in various carcinomas, is overexpressed in ATL. The cytoplasmic domain of TSLC1 directly interacts with the PDZ domain of TIAM1 and induces the formation of lamellipodia through Rac activation in HTLV-1-transformed and ATL cell lines. TIAM1 may integrate signals from TSLC1 to regulate the actin cytoskeleton through Rac activation (Masuda et al., 2010).
Some ligands are also expressed by ATL cells; therefore, autocrine/paracrine stimulation is implicated. Tax develops a strategy based on the activation of the SDF-1α/CXCR4 axis in infected cells (Twizere et al., 2007). In Tax-transgenic mice and their transplantation model, AMD3100, a CXCR4 antagonist, inhibits the infiltration of lymphomatous cells into tissues in vivo, indicating the involvement of the SDF-1α/CXCR4 interaction in leukemic cell migration (Kawaguchi et al., 2009). CCR4 expression is clinically considerable. The defucosylated anti-CCR4 monoclonal antibody KW-0761 induces CCR4-specific antibody-dependent cellular cytotoxicity (ADCC) against CCR4-positive ATL cells. In view of its molecular functions, CCR4 expression may also account for the frequent infiltration of ATL cells into the skin and lymph nodes (Yoshie et al., 2002). Specific surface markers are therefore worthy of attention to identify concentrations of leukemic cells as well as minor infected cells in asymptomatic carriers. A recent study reported the development of a new method for concentrating leukemic cells by multi-color flow cytometry. The majority of leukemic cells are included in the CD4+, CD3-dim, and CD7-low subpopulations (Tian et al., 2011). Consequently, characteristic expression of cytokines and their receptors is clearly required for leukemic cell behavior, which in turn may be used as landmarks and/or therapeutic motifs.
New Paradigm from miRNA
According to the summary of previous ATL studies described above, we can fight ATL to a certain extent. However, we cannot cure ATL because of relapse with multidrug resistance, immunodeficiency, and strong invasiveness. In addition to the previously proposed molecular hallmarks (Figure 2), we urgently need a conceptual advance that can promote understanding of the source of disrupted gene expression. Indeed, in the course of our remarkable progress in researching ATL and other malignancies, new observations have helped in clarifying and modifying the original formulations of the hallmark capabilities.
One of the most significant recent advances in biomedical research has been the discovery of the 22-nt-long class of non-coding RNA designated miRNA that posttranscriptionally regulates gene expression by binding to the target mRNAs. miRNA is expressed by all metazoans and plants, as well as by several DNA viruses; it regulates cellular processes such as development, differentiation, growth, homeostasis, stress responses, apoptosis, and immune activation (Esquela-Kerscher and Slack, 2006). In ATL filed, some studies have been reported, and several miRNA aberrations have been identified in HTLV-1-infected cells and ATL samples.
Pichler et al. (2008) first identified abnormal miRNA expression in HTLV-1-infected cells. They explored the interconnections between HTLV-1 and cellular miRNAs by using several HTLV-1-transformed cell lines. miR-21, miR-24, miR-146a, and miR-155 were found to be upregulated and miR-223 was found to be deregulated in HTLV-1-infected cells. In particular, miR-146a expression was directly stimulated by Tax through the NF-κB pathway. In silico analysis predicts that many candidate genes may be deregulated by miRNA changes (Pichler et al., 2008).
Yeung et al. (2008) performed miRNA microarray analysis of 327 well-characterized human miRNAs in 7 HTLV-1-related cell lines and four acute ATL patient samples. They found that miR-18a, miR-93, and miR-130b were overexpressed in ATL samples. Of note, these miRNAs were also upregulated by PHA-mediated T cell activation. Tumor protein p53 inducible nuclear protein 1 (TP53INP1) is a gene targeted by one of miR-93 and miR-130b, and reduced TP53INP1 expression mediated by miRNA upregulation contributes to cell proliferation and survival (Yeung et al., 2008).
Bellon et al. (2009) also reported the result of miRNA array analysis of 7 ATL samples and normal PBMC and CD4+ T cells and revealed that miR-150, miR-155, miR-223, miR-142-3p, and miR-142-5p are upregulated, whereas miR-181a, miR-132, miR-125a, and miR-146b are downregulated in ATL. They discussed that miRNAs involved in normal hematopoiesis and immune responses are profoundly deregulated in ATL tumor cells ex vivo (Bellon et al., 2009).
Each of these studies has identified interesting miRNAs that are deregulated in ATL-related cells; however, no identical miRNA patterns have been observed. The amount of cellular miRNAs may be susceptible to various environmental conditions such as transcriptional activity, maturation processing, and epigenetic regulation. The end results appear to be affected by the methodology employed and the conditions and types of samples used. Very recently, we established global gene expression analyses of a large cohort ATL study that included analyses of mRNA expressions, miRNA levels, and genomic copy number (Yamagishi et al., 2012). A strict threshold (p < 1 × 10−5) and two-dimensional hierarchical clustering analysis revealed 61 miRNAs with significantly altered expression levels in ATL cells (n = 40) compared with control CD4+ T cells (n = 22). It is most important that primary ATL samples show global miRNA downregulation, similar to observations in other cancer researches (Lu et al., 2005; Gaur et al., 2007). Fifty-nine of the 61 miRNA (96.7%) showed decreased expression in ATL. The amount of cellular miRNA may be susceptible to various environments such as transcriptional activity, maturation processing, and also epigenetic regulation. Among them, miR-31 is the most profoundly repressed miRNA in all ATL individuals (fold change, 0.00403). It is a known tumor suppressor that may also be associated with metastatic breast cancer (Valastyan et al., 2009). Other downregulated miRNAs found in ATL patients may also be involved in the hallmark capabilities of ATL, since they are uniformly decreased in tested ATL samples and each miRNA may regulate a large number of genes.
Several predictions and experimental approaches have defined a novel miR-31 target gene, MAP3K14 (also called NIK), which is a persistent NF-κB activator in various malignancies, including B cell lymphoma (Pham et al., 2011), multiple myeloma (Annunziata et al., 2007), breast cancer (Yamamoto et al., 2010), pancreatic cancer (Nishina et al., 2009), and ATL (Saitoh et al., 2008). Interestingly, all these malignancies have low miR-31 levels. Manipulation of the miR-31 level clearly indicated that the miR-31 level was negatively correlated with cellular NF-κB activity. Importantly, enforced miR-31 expression in B cells attenuated both BAFF and CD40L-mediated NIK accumulation and subsequent canonical and non-canonical NF-κB signaling. As discussed above, NF-κB activity dominates the regulation of apoptosis and subsequent cell survival. Induced miR-31 expression or NIK knockdown reduces apoptotic resistant proteins such as BCL-XL and XIAP, resulting in strong apoptosis in ATL cell lines as well as in primary leukemic cells from ATL patients (Yamagishi et al., 2012). Several lines of evidence definitively support two notions: (1) miR-31 acts as a tumor suppressor in T cells and (2) NIK-regulated NF-κB is of pivotal importance to cancer cell survival (Uribesalgo et al., 2012).
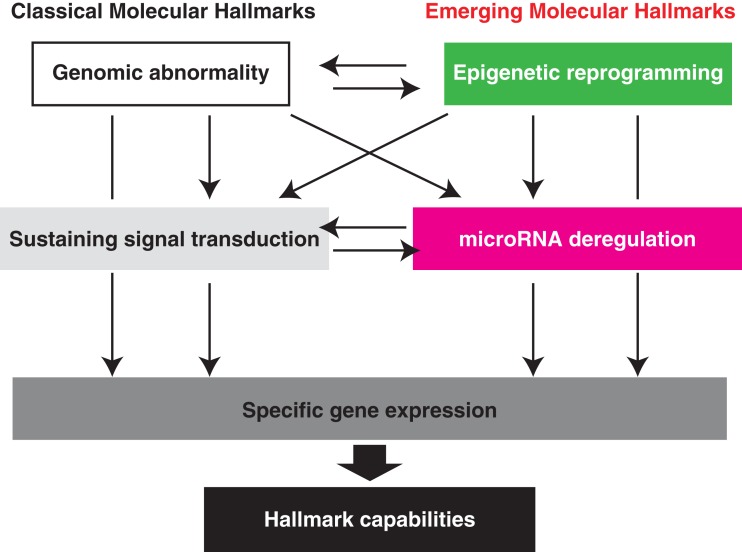
The fact that deregulated miRNA expression predominates NF-κB activity is a conceptual advance. Regulation of global miRNA downregulation and each regulatory network may shed light on our understanding of the next-generation molecular hallmarks of ATL and of molecules suitable for therapeutic targeting (Figure 4). Since a single miRNA can regulate the expression of multiple genes, pleiotropic miRNA may have potential as molecular therapy. Profound miR-31 loss is a characteristic of ATL; however, decreased miR-31 expression seems to be commonly observed in various malignancies. The regulatory mechanism of miR-31 had not been identified until our discovery. In general, down modulation of gene expression is coordinated by some contents of transcriptional factors and an epigenetic regulatory mechanism.
Figure 4.
Emerging molecular hallmarks, microRNA deregulation and epigenetic reprogramming. An increasing body of research suggests that two additional molecular hallmarks are involved in the pathogenesis of ATL. In addition to the genomic abnormality, epigenetic imbalance widely governs the downstream molecular capabilities. Deregulation of the cellular miRNA levels directly influence hundreds of genes expression. Importantly, cross talking among each category can attain more complex gene regulatory network that is indispensable for exercise of various functions at appropriate timing.
Epigenetic Deregulation Observed in ATL
Technological advances in genomics and epigenomics have supplied new methods to distinguish one cell type from another. The epigenetic code consists of the combined on–off states of hundreds of genes, which coordinately dictate cellular identity and function. Increasing attention is being paid to global regulatory factors and molecular mechanisms by which control gene transcription. This genome programming operates fundamentally through DNA methylation, histone chemical modification, and protein complex binding in these environments.
Cancer-associated epigenetic reprogramming has been suggested because DNA methylation is a transcriptional regulator. In ATL, no attempt has been made to determine global epigenetic statements that can explain deregulated gene expression. Analysis of epigenetic factors such as DNA methylation and related gene silencing has been reported, particularly in some tumor suppressor genes such as the CDK inhibitor family.
The CpG island of CDKN2A gene is more frequently methylated in fresh tumor cells isolated from patients with acute ATL (47%) or lymphoma type ATL (73%) than in fresh tumor cells isolated from patients with chronic (17%) and smoldering (17%) ATL, which are relatively less malignant (Nosaka et al., 2000). No CDKN2A gene is methylated in asymptomatic carriers or uninfected individuals. A possible inverse correlation between CDKN2A mRNA expression and gene methylation status is suggested. Methylation-specific polymerase chain reaction (MSP) also suggested the presence of an additional DNA methylation in CDKN2B gene (20%; Hofmann et al., 2001). In addition to the cell cycle regulators, multifunctional factors involved in cell proliferation, differentiation, and apoptosis, e.g., bone morphogenetic protein (BMP) is deregulated by aberrant DNA methylation in malignant lymphomas (Daibata et al., 2007) and also ATL (Taniguchi et al., 2008). Above all, the BMP6 promoter is hypermethylated in ATL: acute (96%), lymphoma (94%), chronic (44%), and smoldering (20%). BMP6 promoter methylation seems to be a common epigenetic event at later stages of ATL. The adenomatous polyposis coli (APC) gene is also a tumor suppressor, and its mRNA level is at least partially regulated by DNA methylation (Tsuchiya et al., 2000). In ATL, 48% of primary samples have methylated promoter DNA in the APC region (Yang et al., 2005).
The methylated CpG island amplification/representational difference analysis method revealed 53 aberrantly hypermethylated DNA sequences in ATL (Yasunaga et al., 2004). Among them, kruppel-like factor 4 (KLF4) and early growth response 3 (EGR3) were found to be responsible for apoptotic resistance in ATL cell lines, implicating that DNA methylation is involved in leukemogenesis. Abnormal DNA demethylation may also be involved. MEL1S, an alternatively spliced form of MEL1, is frequently expressed in ATL cells because of DNA hypomethylation at an alternative transcriptional start site. Aberrant MEL1S expression is associated with dysregulation of TGF-β-mediated signaling (Yoshida et al., 2004). Thus, altered DNA methylation pattern, including DNA demethylation, is one of the molecular hallmarks of ATL linking leukemogenesis to gene transcription control.
Histone modifications such as histone acetylation and specific methylations confer dynamic exchanges of transcription. Although a global survey of histone modification in ATL (such as by ChIP-on-chip analysis) has not been reported, experimental evidence with epigenetic drugs strongly suggests that epigenetic reprogramming is the background of the molecular hallmarks of ATL. Histone deacetylase (HDAC) inhibitors effectively inhibit the proliferation of several cancers (Spiegel et al., 2012) as well as that of HTLV-1-infected cell lines and primary ATL samples (Nishioka et al., 2008). Analysis of signaling cascades suggested that HDAC inhibition can block nuclear translocation of NF-κB components. Paradoxically, another study implicated that the HDAC inhibitors can actively modulate the NF-κB pathway through RelA acetylation (Chiechio et al., 2009). Anyway, abnormal histone deacetylation may be involved in cell survival and cell cycle regulation in ATL cells.
Histone acetylation and DNA methylation actually cooperate in regulating a cohort of genes during multiple processes of leukemogenesis. For example, thioredoxin-binding protein-2 (TBP-2) expression is lost during the transformation step in HTLV-1-infected T cells (Nishinaka et al., 2004). TBP-2 seems to play a crucial role in the growth regulation of T cells. Sequential treatment with a DNA methylation inhibitor, 5-Aza-dC, and an HDAC inhibitor can restore the TBP-2 expression, suggesting that loss of TBP-2 expression is caused by both DNA methylation and histone deacetylation in transformed infected cell lines (Ahsan et al., 2006).
Besides acetylation, the N-terminus of histone proteins contains several residues that can be methylated. Integrated histone modification consequently decides the degrees of chromatin condensation and subsequent transcriptional sensitivity. Trimethylation of the histone H3 Lys27 (H3K27me3) mark plays a central role in the repression of transcription, mainly in the euchromatin region. The Polycomb family is a master regulator of the H3K27me3 level by inducing and maintaining the histone mark. Progress over the past decade has defined two main protein complexes: Polycomb repressive complex 1 (PRC1) and PRC2, with fundamental roles in Polycomb-mediated gene silencing (Schuettengruber et al., 2007). PRC2 methylates the histone histone 3 lysine 27 (H3K27). PRC1 is commonly viewed as an important, direct executor of silencing at target genes. Although H3K27 methylation is a key chromatin mark, there is ongoing debate about its molecular consequences. In the context of cancer research, deregulation by the Polycomb family confers a specific gene expression pattern responsible for chronic proliferation, survival, peculiar development, and cancer-associated stemness in various cancer types, including ATL (Sparmann and van Lohuizen, 2006).
The involvement of the Polycomb family in ATL was first revealed by global gene expression analysis. Significantly higher levels of enhancer of zeste homolog 2 (EZH2) as well as RING1 and YY1 binding protein (RYBP) transcripts with enhanced H3K27me3 levels were found in ATL cells compared with normal CD4+ T cells (Sasaki et al., 2011). EZH2 serves as the catalytic subunit in the PRC2 and mediates gene silencing by catalyzing the trimethylation of H3K27 at the promoters of target genes. EZH2 is highly expressed in many cancer types, including breast and prostate cancer and lymphomas, and it is often correlated with advanced stages of tumor progression and a poor prognosis. Importantly, EZH2 inhibition by 3-deazaneplanocin A and the HDAC inhibitor panobinostat showed a synergistic effect in killing the ATL cell lines. Because the Polycomb family generally contributes to silencing of tumor suppressor genes, e.g., the CDKN2 family, the genes silenced in ATL should be addressed to elucidate the functional significance of the Polycomb family in the leukemogenic process.
We recently identified a notable gene silenced by Polycomb. A human gene that encodes miR-31, hsa-miR-31, is located at 9p21.3, which is adjacent to clusters of the CDKN2 and IFNA families. In addition to the genetic loss (12.5% of ATL cases), transcription of the miR-31 precursor is completely lost in ATL cells. Computational predictions and experimental evidence clearly demonstrated that an assembly of YY1 binding motifs upstream of the miR-31 region is responsible for the occupancy of the Polycomb family at the target region, which leads to H3K27me3-dependent transcriptional repression. Overexpression of EZH2 and suppressor of zeste 12 (SUZ12) homolog, components of PRC2, in ATL cells can induce and maintain the epigenetic silencing of miR-31. Of note, given that miR-31 is a master regulator of the ATL-specific gene expression pattern described above, Polycomb-mediated loss can influence gene expression downstream of miR-31 (Figure 4). Indeed, the amount of EZH2 and SUZ12 directly strengthens cellular miR-31 depletion, which in turn activates the NF-κB pathway through NIK induction and confers anti-apoptotic features to T cell (Yamagishi et al., 2012). It is noteworthy that the molecular and biological interconnections between Polycomb–miR-31–NF-κB are conserved in breast cancer cells and B lymphocytes. By organizing the new principle, various cell types may realize the more complex gene regulatory network required for maintenance and execution of cellular functions. Imbalance of this network probably switches the cell fate from one to another.
The origin of epigenetic reprogramming observed in ATL cells remains elusive. In addition to self-dysfunction of the epigenetic machinery, a possible mechanism is viral hijacking; HTLV-1 Tax can physically associate with the key histone modifiers HDAC1 (Ego et al., 2002), SUV39H1 (Kamoi et al., 2006), and SMYD3 (Yamamoto et al., 2011). However, at present, the possible influence of the Tax-epigenetic association on gene regulation is unknown. Governing the epigenetic system by Tax may disrupt gene expression, leading to chronic proliferation and abnormal survival of HTLV-1-infected cells. In the context of viral gene regulation, epigenetic changes, mainly DNA methylation, in the HTLV-1 provirus may facilitate ATL cell evasion of the host immune system by suppressing viral gene transcription (Koiwa et al., 2002; Taniguchi et al., 2005). Recent studies using in vivo models strongly suggested that Tax and also other viral proteins are directly linked to leukemogenesis, despite viral gene expression being rare in circulating leukemic cells in patients (Hasegawa et al., 2006; Ohsugi et al., 2007b; Banerjee et al., 2010; Satou et al., 2011). Furthermore, not only histone methylation but also other histone modifications such as phosphorylation and ubiquitination are intriguing for understanding the molecular and physiological hallmarks of ATL.
Therapeutic Targeting of ATL
To establish more effective molecular-targeted therapies for ATL, we need to understand the exact molecular underpinnings of ATL. In addition to classical molecular characteristics, the emerging hallmarks of miRNA deregulation and epigenetic reprogramming broaden the scope of conceptualization of the responsible molecular mechanism (Figure 4). As highlighted in this review, ATL possesses six molecular hallmarks: genomic abnormality, specific changes in gene expression, sustaining activated signaling, producing cytokines, miRNA deregulation, and epigenetic reprogramming. These molecular hallmarks confer robustness to leukemic cell hallmark capabilities: resisting cell death, promoting cell cycle, invasiveness, chronic proliferation, replicating immortality, and drug resistance (Figure 5). Consideration of hallmark principles should aid in developing future therapeutics. Several studies with inhibitory agents have clearly indicated that blockade of signaling drivers appears to be both practical and feasible for inducing leukemic cell apoptosis. However, common clinical traits of ATL include relapse and drug resistance. Importantly, each of the core hallmark capabilities is regulated by a partially redundant signaling pathways. Consequently, a targeted therapeutic agent that inhibits only one key pathway in ATL may not completely shut off another hallmark capability, allowing some ATL cells to survive with residual function until they or their progeny eventually adapt to the selective pressure imposed by the therapy. In this case, given that the number of parallel signaling pathways supporting a given hallmark is limited, it may become possible to target all of these supporting pathways therapeutically, thereby preventing the development of adaptive resistance. However, it is possible that this could involve critical side effects. Alternatively, most upstream elements that can act pleiotropically in leukemic cells, e.g., miRNA and epigenetics, may be heralded as one of the fruits of remarkable progress into understanding the ATL mechanism. Moreover, selective co-targeting of multiple core and emerging molecular hallmarks in mechanism-guided combinations therapies will result in more effective and durable therapies for aggressive ATL.
Figure 5.
Conceptual illustration of the molecular hallmarks of ATL. This illustration encompasses the six molecular hallmarks of ATL. These organized principles provide characteristics of ATL itself. Because they may be directly associated with the clinical traits of ATL, targeting the one outstanding hallmark or co-targeting of multiple molecular hallmarks in mechanism-guided combinations will result in more effective and durable therapies for aggressive ATL.
Conclusion and Future Direction
We have explored our present understanding of the molecular aspects of ATL to refine and extend the six specific traits, the molecular hallmarks of ATL, which have provided a useful conceptual framework for understanding the complex biology of ATL (Figure 5).
Other areas are currently in rapid flux. In recent years, the biological importance of several elaborate ATL models, including the Tax-transgenic model (Hasegawa et al., 2006), HBZ transgenic model (Satou et al., 2011), and HTLV-1-infected humanized SCID mice (Banerjee et al., 2010), has been proposed. ATL-initiating stem cell theory has also been developed (Yamazaki et al., 2009; El Hajj et al., 2010). Similar to other lymphomas and solid cancers, leukemic cells in tissues may be encompassed by a tumor microenvironment that contributes to leukemogenesis. The organized principles of the molecular basis of ATL may be helpful in the coming decade of ATL study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work is supported by JSPS KAKENHI Grant Number 24790436 (Makoto Yamagishi), 23390250 (Toshiki Watanabe), NEXT KAKENHI Grant Number 221S0001 (Toshiki Watanabe), and Grants-in-Aid from the Ministry of Health, Labour and Welfare H24-G-004 (Makoto Yamagishi and Toshiki Watanabe).
References
- Ahsan M. K., Masutani H., Yamaguchi Y., Kim Y. C., Nosaka K., Matsuoka M., Nishinaka Y., Maeda M., Yodoi J. (2006). Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene 25, 2181–2191 10.1038/sj.onc.1209256 [DOI] [PubMed] [Google Scholar]
- Akagi T., Ono H., Shimotohno K. (1996). Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 12, 1645–1652 [PubMed] [Google Scholar]
- Akagi T., Shimotohno K. (1993). Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata C. M., Davis R. E., Demchenko Y., Bellamy W., Gabrea A., Zhan F., Lenz G., Hanamura I., Wright G., Xiao W., Dave S., Hurt E. M., Tan B., Zhao H., Stephens O., Santra M., Williams D. R., Dang L., Barlogie B., Shaughnessy J. D., Kuehl W. M., Staudt L. M. (2007). Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 10.1016/j.ccr.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyama Y., Mori T., Shinomiya T., Sakabe T., Fukuda Y., Kanamaru A., Yamada Y., Isobe M., Seto M., Nakamura Y., Inazawa J. (1999). Chromosomal imbalances in adult T-cell leukemia revealed by comparative genomic hybridization: gains at 14q32 and 2p16-22 in cell lines. J. Hum. Genet. 44, 357–363 10.1007/s100380050178 [DOI] [PubMed] [Google Scholar]
- Arnulf B., Villemain A., Nicot C., Mordelet E., Charneau P., Kersual J., Zermati Y., Mauviel A., Bazarbachi A., Hermine O. (2002). Human T-cell lymphotropic virus oncoprotein Tax represses TGF-beta 1 signaling in human T cells via c-Jun activation: a potential mechanism of HTLV-I leukemogenesis. Blood 100, 4129–4138 10.1182/blood-2001-12-0372 [DOI] [PubMed] [Google Scholar]
- Azimi N., Jacobson S., Leist T., Waldmann T. A. (1999). Involvement of IL-15 in the pathogenesis of human T lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis: implications for therapy with a monoclonal antibody directed to the IL-2/15R beta receptor. J. Immunol. 163, 4064–4072 [PubMed] [Google Scholar]
- Baba M., Okamoto M., Hamasaki T., Horai S., Wang X., Ito Y., Suda Y., Arima N. (2008). Highly enhanced expression of CD70 on human T-lymphotropic virus type 1-carrying T-cell lines and adult T-cell leukemia cells. J. Virol. 82, 3843–3852 10.1128/JVI.02013-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. (1988). HTLV-I Tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science 241, 1652–1655 10.1126/science.2843985 [DOI] [PubMed] [Google Scholar]
- Banerjee P., Tripp A., Lairmore M. D., Crawford L., Sieburg M., Ramos J. C., Harrington W., Jr., Beilke M. A., Feuer G. (2010). Adult T-cell leukemia/lymphoma development in HTLV-1-infected humanized SCID mice. Blood 115, 2640–2648 10.1182/blood-2009-10-246959 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bazarbachi A., Plumelle Y., Carlos Ramos J., Tortevoye P., Otrock Z., Taylor G., Gessain A., Harrington W., Panelatti G., Hermine O. (2010). Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J. Clin. Oncol. 28, 4177–4183 10.1200/JCO.2010.32.1760 [DOI] [PubMed] [Google Scholar]
- Bellon M., Lepelletier Y., Hermine O., Nicot C. (2009). Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 113, 4914–4917 10.1182/blood-2008-11-189845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxus M., Twizere J. C., Legros S., Dewulf J. F., Kettmann R., Willems L. (2008). The HTLV-1 Tax interactome. Retrovirology 5, 76. 10.1186/1742-4690-5-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereseto A., Diella F., Mulloy J. C., Cara A., Michieli P., Grassmann R., Franchini G., Klotman M. E. (1996). p53 Functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood 88, 1551–1560 [PubMed] [Google Scholar]
- Cesarman E., Chadburn A., Inghirami G., Gaidano G., Knowles D. M. (1992). Structural and functional analysis of oncogenes and tumor suppressor genes in adult T-cell leukemia/lymphoma shows frequent p53 mutations. Blood 80, 3205–3216 [PubMed] [Google Scholar]
- Chen J., Petrus M., Bryant B. R., Nguyen V. P., Goldman C. K., Bamford R., Morris J. C., Janik J. E., Waldmann T. A. (2010) , Autocrine/paracrine cytokine stimulation of leukemic cell proliferation in smoldering and chronic adult T-cell leukemia. Blood 116, 5948–5956 10.1182/blood-2009-12-259614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiechio S., Zammataro M., Morales M. E., Busceti C. L., Drago F., Gereau R. W., IV, Copani A., Nicoletti F. (2009). Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol. Pharmacol. 75, 1014–1020 10.1124/mol.108.054346 [DOI] [PubMed] [Google Scholar]
- Choi Y. L., Tsukasaki K., O’Neill M. C., Yamada Y., Onimaru Y., Matsumoto K., Ohashi J., Yamashita Y., Tsutsumi S., Kaneda R., Takada S., Aburatani H., Kamihira S., Nakamura T., Tomonaga M., Mano H. (2007). A genomic analysis of adult T-cell leukemia. Oncogene 26, 1245–1255 10.1038/sj.onc.1209957 [DOI] [PubMed] [Google Scholar]
- Chung H. K., Young H. A., Goon P. K., Heidecker G., Princler G. L., Shimozato O., Taylor G. P., Bangham C. R., Derse D. (2003). Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 102, 4130–4136 10.1182/blood-2003-04-1043 [DOI] [PubMed] [Google Scholar]
- Daibata M., Nemoto Y., Bandobashi K., Kotani N., Kuroda M., Tsuchiya M., Okuda H., Takakuwa T., Imai S., Shuin T., Taguchi H. (2007). Promoter hypermethylation of the bone morphogenetic protein-6 gene in malignant lymphoma. Clin. Cancer Res. 13, 3528–3535 10.1158/1078-0432.CCR-06-2766 [DOI] [PubMed] [Google Scholar]
- Darwiche N., Sinjab A., Abou-Lteif G., Chedid M. B., Hermine O., Dbaibo G., Bazarbachi A. (2011). Inhibition of mammalian target of rapamycin signaling by everolimus induces senescence in adult T-cell leukemia/lymphoma and apoptosis in peripheral T-cell lymphomas. Int. J. Cancer 129, 993–1004 10.1002/ijc.25716 [DOI] [PubMed] [Google Scholar]
- Datta A., Bellon M., Sinha-Datta U., Bazarbachi A., Lepelletier Y., Canioni D., Waldmann T. A., Hermine O., Nicot C. (2006). Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood 108, 1021–1029 10.1182/blood-2006-01-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Fuente C., Santiago F., Chong S. Y., Deng L., Mayhood T., Fu P., Stein D., Denny T., Coffman F., Azimi N., Mahieux R., Kashanchi F. (2000). Overexpression of p21(waf1) in human T-cell lymphotropic virus type 1-infected cells and its association with cyclin A/cdk2. J. Virol. 74, 7270–7283 10.1128/JVI.74.16.7270-7283.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M. Z., Terashima K., Taruishi M., Hasegawa H., Ito M., Tanaka Y., Mori N., Sata T., Koyanagi Y., Maeda M., Kubuki Y., Okayama A., Fujii M., Yamamoto N. (2003). Rapid tumor formation of human T-cell leukemia virus type 1-infected cell lines in novel NOD-SCID/gammac(null) mice: suppression by an inhibitor against NF-kappaB. J. Virol. 77, 5286–5294 10.1128/JVI.77.9.5286-5294.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M. Z., Uchihara J. N., Terashima K., Honda M., Sata T., Ito M., Fujii N., Uozumi K., Tsukasaki K., Tomonaga M., Kubuki Y., Okayama A., Toi M., Mori N., Yamamoto N. (2006). Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood 107, 716–724 10.1182/blood-2005-02-0735 [DOI] [PubMed] [Google Scholar]
- Ego T., Ariumi Y., Shimotohno K. (2002). The interaction of HTLV-1 Tax with HDAC1 negatively regulates the viral gene expression. Oncogene 21, 7241–7246 10.1038/sj.onc.1205701 [DOI] [PubMed] [Google Scholar]
- El Hajj H., El-Sabban M., Hasegawa H., Zaatari G., Ablain J., Saab S. T., Janin A., Mahfouz R., Nasr R., Kfoury Y., Nicot C., Hermine O., Hall W., de Thé H., Bazarbachi A. (2010). Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 207, 2785–2792 10.1084/jem.20101095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sabban M. E., Nasr R., Dbaibo G., Hermine O., Abboushi N., Quignon F., Ameisen J. C., Bex F., de Thé H., Bazarbachi A. (2000). Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood 96, 2849–2855 [PubMed] [Google Scholar]
- Esquela-Kerscher A., Slack F. J. (2006). Oncomirs – microRNAs with a role in cancer. Nat. Rev. Cancer 6, 259–269 10.1038/nrc1840 [DOI] [PubMed] [Google Scholar]
- Fryrear K. A., Durkin S. S., Gupta S. K., Tiedebohl J. B., Semmes O. J. (2009). Dimerization and a novel Tax speckled structure localization signal are required for Tax nuclear localization. J. Virol. 83, 5339–5352 10.1128/JVI.00232-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudray G., Gachon F., Basbous J., Biard-Piechaczyk M., Devaux C., Mesnard J. M. (2002). The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 76, 12813–12822 10.1128/JVI.76.24.12813-12822.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A., Jewell D. A., Liang Y., Ridzon D., Moore J. H., Chen C., Ambros V. R., Israel M. A. (2007). Characterization of microRNA expression levels and their biological correlates in human cancer cell lines. Cancer Res. 67, 2456–2468 10.1158/0008-5472.CAN-06-2698 [DOI] [PubMed] [Google Scholar]
- Gill P. S., Harrington W., Jr., Kaplan M. H., Ribeiro R. C., Bennett J. M., Liebman H. A., Bernstein-Singer M., Espina B. M., Cabral L., Allen S., Kornblau S., Pike M. C., Levine A. M. (1995). Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N. Engl. J. Med. 332, 1744–1748 10.1056/NEJM199506293322603 [DOI] [PubMed] [Google Scholar]
- Good L., Maggirwar S. B., Sun S. C. (1996). Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 15, 3744–3750 [PMC free article] [PubMed] [Google Scholar]
- Grassmann R., Aboud M., Jeang K. T. (2005). Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 24, 5976–5985 10.1038/sj.onc.1208978 [DOI] [PubMed] [Google Scholar]
- Hall W. W., Fujii M. (2005). Deregulation of cell-signaling pathways in HTLV-1 infection. Oncogene 24, 5965–5975 10.1038/sj.onc.1208975 [DOI] [PubMed] [Google Scholar]
- Haller K., Wu Y., Derow E., Schmitt I., Jeang K. T., Grassmann R. (2002). Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22, 3327–3338 10.1128/MCB.22.10.3327-3338.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj E. W., Good L., Xiao G., Sun S. C. (1999). Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene 18, 1341–1349 10.1038/sj.onc.1202405 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Nomura T., Kohno M., Tateishi N., Suzuki Y., Maeda N., Fujisawa R., Yoshie O., Fujita S. (2000). Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood 95, 30–38 [PubMed] [Google Scholar]
- Hasegawa H., Sawa H., Lewis M. J., Orba Y., Sheehy N., Yamamoto Y., Ichinohe T., Tsunetsugu-Yokota Y., Katano H., Takahashi H., Matsuda J., Sata T., Kurata T., Nagashima K., Hall W. W. (2006). Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat. Med. 12, 466–472 10.1038/nm1389 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Yamada Y., Iha H., Tsukasaki K., Nagai K., Atogami S., Sugahara K., Tsuruda K., Ishizaki A., Kamihira S. (2009). Activation of p53 by Nutlin-3a, an antagonist of MDM2, induces apoptosis and cellular senescence in adult T-cell leukemia cells. Leukemia 23, 2090–2101 10.1038/leu.2009.171 [DOI] [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Miller C. W., Yamada Y., Tomonaga M., Koeffler H. P. (1995). Homozygous deletions of the p15 (MTS2) and p16 (CDKN2/MTS1) genes in adult T-cell leukemia. Blood 85, 2699–2704 [PubMed] [Google Scholar]
- Hatta Y., Yamada Y., Tomonaga M., Koeffler H. P. (1997). Extensive analysis of the retinoblastoma gene in adult T cell leukemia/lymphoma (ATL). Leukemia 11, 984–989 10.1038/sj.leu.2400686 [DOI] [PubMed] [Google Scholar]
- Hidaka T., Nakahata S., Hatakeyama K., Hamasaki M., Yamashita K., Kohno T., Arai Y., Taki T., Nishida K., Okayama A., Asada Y., Yamaguchi R., Tsubouchi H., Yokota J., Taniwaki M., Higashi Y., Morishita K. (2008). Down-regulation of TCF8 is involved in the leukemogenesis of adult T-cell leukemia/lymphoma. Blood 112, 383–393 10.1182/blood-2008-01-131185 [DOI] [PubMed] [Google Scholar]
- Hofmann W. K., Tsukasaki K., Takeuchi N., Takeuchi S., Koeffler H. P. (2001). Methylation analysis of cell cycle control genes in adult T-cell leukemia/lymphoma. Leuk. Lymphoma 42, 1107–1109 10.3109/10428190109097731 [DOI] [PubMed] [Google Scholar]
- Höllsberg P., Ausubel L. J., Hafler D. A. (1994). Human T cell lymphotropic virus type I-induced T cell activation. Resistance to TGF-beta 1-induced suppression. J. Immunol. 153, 566–573 [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. (1989). Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science 244, 457–460 10.1126/science.2497518 [DOI] [PubMed] [Google Scholar]
- Ikezoe T., Nishioka C., Bandobashi K., Yang Y., Kuwayama Y., Adachi Y., Takeuchi T., Koeffler H. P., Taguchi H. (2007). Longitudinal inhibition of PI3K/Akt/mTOR signaling by LY294002 and rapamycin induces growth arrest of adult T-cell leukemia cells. Leuk. Res. 31, 673–682 10.1016/j.leukres.2006.08.001 [DOI] [PubMed] [Google Scholar]
- Imura A., Hori T., Imada K., Kawamata S., Tanaka Y., Imamura S., Uchiyama T. (1997). OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood 89, 2951–2958 [PubMed] [Google Scholar]
- Iwanaga M., Watanabe T., Utsunomiya A., Okayama A., Uchimaru K., Koh K. R., Ogata M., Kikuchi H., Sagara Y., Uozumi K., Mochizuki M., Tsukasaki K., Saburi Y., Yamamura M., Tanaka J., Moriuchi Y., Hino S., Kamihira S., Yamaguchi K., Joint Study on Predisposing Factors of ATL Development Investigators (2010). Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood 116, 1211–1219 10.1182/blood-2009-12-257410 [DOI] [PubMed] [Google Scholar]
- Iwanaga R., Ohtani K., Hayashi T., Nakamura M. (2001). Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20, 2055–2067 10.1038/sj.onc.1204304 [DOI] [PubMed] [Google Scholar]
- Jeang K. T., Widen S. G., Semmes O. J. T., Wilson S. H. (1990). HTLV-1 trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247, 1082–1084 10.1126/science.2309119 [DOI] [PubMed] [Google Scholar]
- Journo C., Filipe J., About F., Chevalier S. A., Afonso P. V., Brady J. N., Flynn D., Tangy F., Israël A., Vidalain P. O., Mahieux R., Weil R. (2009). NRP/optineurin cooperates with TAX1BP1 to potentiate the activation of NF-kappaB by human T-lymphotropic virus type 1 tax protein. PLoS Pathog. 5, e1000521. 10.1371/journal.ppat.1000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada N., Sakurai M., Miyamoto K., Sanada I., Sadamori N., Fukuhara S., Abe S., Shiraishi Y., Abe T., Kaneko Y. (1992). Chromosome abnormalities in adult T-cell leukemia/lymphoma: a karyotype review committee report. Cancer Res. 52, 1481–1493 [PubMed] [Google Scholar]
- Kamoi K., Yamamoto K., Misawa A., Miyake A., Ishida T., Tanaka Y., Mochizuki M., Watanabe T. (2006). SUV39H1 interacts with HTLV-1 Tax and abrogates Tax transactivation of HTLV-1 LTR. Retrovirology 3, 5. 10.1186/1742-4690-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Marriott S. J. (1999). Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 73, 4299–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A., Orba Y., Kimura T., Iha H., Ogata M., Tsuji T., Ainai A., Sata T., Okamoto T., Hall W. W., Sawa H., Hasegawa H. (2009). Inhibition of the SDF-1alpha-CXCR4 axis by the CXCR4 antagonist AMD3100 suppresses the migration of cultured cells from ATL patients and murine lymphoblastoid cells from HTLV-I Tax transgenic mice. Blood 114, 2961–2968 10.1182/blood-2008-11-189308 [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kehrl J. H., Burton J., Tendler C. L., Jeang K. T., Danielpour D., Thevenin C., Kim K. Y., Sporn M. B., Roberts A. B. (1990). Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J. Exp. Med. 172, 121–129 10.1084/jem.172.1.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirken R. A., Erwin R. A., Wang L., Wang Y., Rui H., Farrar W. L. (2000). Functional uncoupling of the Janus kinase 3-Stat5 pathway in malignant growth of human T cell leukemia virus type 1-transformed human T cells. J. Immunol. 165, 5097–5104 [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Yamaguchi K., Takeya M., Takahashi K., Watanabe T., Matsumoto T., Lee S. Y., Takatsuki K. (1987). Hypercalcemia and osteoclast proliferation in adult T-cell leukemia. Cancer 59, 1187–1191 [DOI] [PubMed] [Google Scholar]
- Koiwa T., Hamano-Usami A., Ishida T., Okayama A., Yamaguchi K., Kamihira S., Watanabe T. (2002). 5’-Long terminal repeat-selective CpG methylation of latent human T-cell leukemia virus type 1 provirus in vitro and in vivo. J. Virol. 76, 9389–9397 10.1128/JVI.76.18.9389-9397.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. K., Kim B. C., Brady J. N., Jeang K. T., Kim S. J. (2002). Human T-cell lymphotropic virus type 1 tax inhibits transforming growth factor-beta signaling by blocking the association of Smad proteins with Smad-binding element. J. Biol. Chem. 277, 33766–33775 10.1074/jbc.M202078200 [DOI] [PubMed] [Google Scholar]
- Liang M. H., Geisbert T., Yao Y., Hinrichs S. H., Giam C. Z. (2002). Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J. Virol. 76, 4022–4033 10.1128/JVI.76.23.12312-12319.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang Y., Yamakuchi M., Masuda S., Tokioka T., Yamaoka S., Maruyama I., Kitajima I. (2001). Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene 20, 2514–2526 10.1038/sj.onc.1204771 [DOI] [PubMed] [Google Scholar]
- Lu J., Getz G., Miska E. A., Alvarez-Saavedra E., Lamb J., Peck D., Sweet-Cordero A., Ebert B. L., Mak R. H., Ferrando A. A., Downing J. R., Jacks T., Horvitz H. R., Golub T. R. (2005). MicroRNA expression profiles classify human cancers. Nature 435, 834–838 10.1038/nature03702 [DOI] [PubMed] [Google Scholar]
- Macaire H., Riquet A., Moncollin V., Biémont-Trescol M. C., Duc Dodon M., Hermine O., Debaud A. L., Mahieux R., Mesnard J. M., Pierre M., Gazzolo L., Bonnefoy N., Valentin H. (2012). Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J. Biol. Chem. 287, 21357–21370 10.1074/jbc.M112.340992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Yamada Y., Moriuchi R., Sugahara K., Tsuruda K., Joh T., Atogami S., Tsukasaki K., Tomonaga M., Kamihira S. (1999). Fas gene mutation in the progression of adult T cell leukemia. J. Exp. Med. 189, 1063–1071 10.1084/jem.189.7.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner J. M., Lantz V., Waldmann T. A., Azimi N. (2001). Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. J. Immunol. 166, 2602–2609 [DOI] [PubMed] [Google Scholar]
- Masuda M., Maruyama T., Ohta T., Ito A., Hayashi T., Tsukasaki K., Kamihira S., Yamaoka S., Hoshino H., Yoshida T., Watanabe T., Stanbridge E. J., Murakami Y. (2010). CADM1 interacts with Tiam1 and promotes invasive phenotype of human T-cell leukemia virus type I-transformed cells and adult T-cell leukemia cells. J. Biol. Chem. 285, 15511–15522 10.1074/jbc.M109.059790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire K. L., Curtiss V. E., Larson E. L., Haseltine W. A. (1993). Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J. Virol. 67, 1590–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migone T. S., Lin J. X., Cereseto A., Mulloy J. C., O’Shea J. J., Franchini G., Leonard W. J. (1995). Constitutively activated JAK-STAT pathway in T cells transformed with HTLV-I. Science 269, 79–81 10.1126/science.7604283 [DOI] [PubMed] [Google Scholar]
- Mori N., Fujii M., Ikeda S., Yamada Y., Tomonaga M., Ballard D. W., Yamamoto N. (1999). Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood 93, 2360–2368 [PubMed] [Google Scholar]
- Mori N., Yamada Y., Ikeda S., Yamasaki Y., Tsukasaki K., Tanaka Y., Tomonaga M., Yamamoto N., Fujii M. (2002). Bay 11-7082 inhibits transcription factor NF-kappaB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100, 1828–1834 10.1182/blood-2002-01-0151 [DOI] [PubMed] [Google Scholar]
- Morosetti R., Kawamata N., Gombart A. F., Miller C. W., Hatta Y., Hirama T., Said J. W., Tomonaga M., Koeffler H. P. (1995). Alterations of the p27KIP1 gene in non-Hodgkin’s lymphomas and adult T-cell leukemia/lymphoma. Blood 86, 1924–1930 [PubMed] [Google Scholar]
- Muraoka O., Kaisho T., Tanabe M., Hirano T. (1993). Transcriptional activation of the interleukin-6 gene by HTLV-1 p40tax through an NF-kappa B-like binding site. Immunol. Lett. 37, 159–165 10.1016/0165-2478(93)90026-X [DOI] [PubMed] [Google Scholar]
- Nagai H., Kinoshita T., Imamura J., Murakami Y., Hayashi K., Mukai K., Ikeda S., Tobinai K., Saito H., Shimoyama M., Shimotohno K. (1991). Genetic alteration of p53 in some patients with adult T-cell leukemia. Jpn. J. Cancer Res. 82, 1421–1427 10.1111/j.1349-7006.1991.tb01815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata S., Yamazaki S., Nakauchi H., Morishita K. (2010). Downregulation of ZEB1 and overexpression of Smad7 contribute to resistance to TGF-beta1-mediated growth suppression in adult T-cell leukemia/lymphoma. Oncogene 29, 4157–4169 10.1038/onc.2010.172 [DOI] [PubMed] [Google Scholar]
- Neuveut C., Low K. G., Maldarelli F., Schmitt I., Majone F., Grassmann R., Jeang K. T. (1998). Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18, 3620–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot C., Mahieux R., Takemoto S., Franchini G. (2000). Bcl-X(L) is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood 96, 275–281 [PubMed] [Google Scholar]
- Niitsu Y., Urushizaki Y., Koshida Y., Terui K., Mahara K., Kohgo Y., Urushizaki I. (1988). Expression of TGF-beta gene in adult T cell leukemia. Blood 71, 263–266 [PubMed] [Google Scholar]
- Nishimura S., Asou N., Suzushima H., Okubo T., Fujimoto T., Osato M., Yamasaki H., Lisha L., Takatsuki K. (1995). p53 Gene mutation and loss of heterozygosity are associated with increased risk of disease progression in adult T cell leukemia. Leukemia 9, 598–604 [PubMed] [Google Scholar]
- Nishina T., Yamaguchi N., Gohda J., Semba K., Inoue J. (2009). NIK is involved in constitutive activation of the alternative NF-kappaB pathway and proliferation of pancreatic cancer cells. Biochem. Biophys. Res. Commun. 388, 96–101 10.1016/j.bbrc.2009.07.125 [DOI] [PubMed] [Google Scholar]
- Nishinaka Y., Nishiyama A., Masutani H., Oka S., Ahsan K. M., Nakayama Y., Ishii Y., Nakamura H., Maeda M., Yodoi J. (2004). Loss of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 in human T-cell leukemia virus type I-dependent T-cell transformation: implications for adult T-cell leukemia leukemogenesis. Cancer Res. 64, 1287–1292 10.1158/0008-5472.CAN-03-0908 [DOI] [PubMed] [Google Scholar]
- Nishioka C., Ikezoe T., Yang J., Koeffler H. P., Taguchi H. (2007). Fludarabine induces apoptosis of human T-cell leukemia virus type 1-infected T cells via inhibition of the nuclear factor-kappaB signal pathway. Leukemia 21, 1044–1049 10.1038/sj.leu.2404647 [DOI] [PubMed] [Google Scholar]
- Nishioka C., Ikezoe T., Yang J., Komatsu N., Bandobashi K., Taniguchi A., Kuwayama Y., Togitani K., Koeffler H. P., Taguchi H. (2008). Histone deacetylase inhibitors induce growth arrest and apoptosis of HTLV-1-infected T-cells via blockade of signaling by nuclear factor kappaB. Leuk. Res. 32, 287–296 10.1016/j.leukres.2007.05.026 [DOI] [PubMed] [Google Scholar]
- Nosaka K., Maeda M., Tamiya S., Sakai T., Mitsuya H., Matsuoka M. (2000). Increasing methylation of the CDKN2A gene is associated with the progression of adult T-cell leukemia. Cancer Res. 60, 1043–1048 [PubMed] [Google Scholar]
- Nosaka K., Miyamoto T., Sakai T., Mitsuya H., Suda T., Matsuoka M. (2002). Mechanism of hypercalcemia in adult T-cell leukemia: overexpression of receptor activator of nuclear factor kappaB ligand on adult T-cell leukemia cells. Blood 99, 634–640 10.1182/blood.V99.2.634 [DOI] [PubMed] [Google Scholar]
- Oh U., McCormick M. J., Datta D., Turner R. V., Bobb K., Monie D. D., Sliskovic D. R., Tanaka Y., Zhang J., Meshulam J., Jacobson S. (2011). Inhibition of immune activation by a novel nuclear factor-kappa B inhibitor in HTLV-I-associated neurologic disease. Blood 117, 3363–3369 10.1182/blood-2010-10-306571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsugi T., Kumasaka T., Okada S., Ishida T., Yamaguchi K., Horie R., Watanabe T., Umezawa K. (2007a). Dehydroxymethylepoxyquinomicin (DHMEQ) therapy reduces tumor formation in mice inoculated with tax-deficient adult T-cell leukemia-derived cell lines. Cancer Lett. 257, 206–215 10.1016/j.canlet.2007.07.017 [DOI] [PubMed] [Google Scholar]
- Ohsugi T., Kumasaka T., Okada S., Urano T. (2007b). The Tax protein of HTLV-1 promotes oncogenesis in not only immature T cells but also mature T cells. Nat. Med. 13, 527–528 10.1038/nm0507-527 [DOI] [PubMed] [Google Scholar]
- Oshiro A., Tagawa H., Ohshima K., Karube K., Uike N., Tashiro Y., Utsunomiya A., Masuda M., Takasu N., Nakamura S., Morishita Y., Seto M. (2006). Identification of subtype-specific genomic alterations in aggressive adult T-cell leukemia/lymphoma. Blood 107, 4500–4507 10.1182/blood-2005-09-3801 [DOI] [PubMed] [Google Scholar]
- Pancewicz J., Taylor J. M., Datta A., Baydoun H. H., Waldmann T. A., Hermine O., Nicot C. (2010). Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia. Proc. Natl. Acad. Sci. U.S.A. 107, 16619–16624 10.1073/pnas.1010722107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham L. V., Fu L., Tamayo A. T., Bueso-Ramos C., Drakos E., Vega F., Medeiros L. J., Ford R. J. (2011). Constitutive BR3 receptor signaling in diffuse, large B-cell lymphomas stabilizes nuclear factor-κB-inducing kinase while activating both canonical and alternative nuclear factor-κB pathways. Blood 117, 200–210 10.1182/blood-2010-06-290437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler K., Schneider G., Grassmann R. (2008). MicroRNA miR-146a and further oncogenesis-related cellular microRNAs are dysregulated in HTLV-1-transformed T lymphocytes. Retrovirology 5, 100. 10.1186/1742-4690-5-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pise-Masison C. A., Radonovich M., Mahieux R., Chatterjee P., Whiteford C., Duvall J., Guillerm C., Gessain A., Brady J. N. (2002). Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 62, 3562–3571 [PubMed] [Google Scholar]
- Ressler S., Morris G. F., Marriott S. J. (1997). Human T-cell leukemia virus type 1 Tax transactivates the human proliferating cell nuclear antigen promoter. J. Virol. 71, 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. (1988). Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science 241, 89–92 10.1126/science.2838905 [DOI] [PubMed] [Google Scholar]
- Ruckes T., Saul D., Van Snick J., Hermine O., Grassmann R. (2001). Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98, 1150–1159 10.1182/blood.V98.4.1150 [DOI] [PubMed] [Google Scholar]
- Sagara Y., Inoue Y., Ohshima K., Kojima E., Utsunomiya A., Tsujimura M., Shiraki H., Kashiwagi S. (2007). Antibody to the central region of human T-lymphotropic virus type 1 gp46 is associated with the progression of adult T-cell leukemia. Cancer Sci. 98, 240–245 10.1111/j.1349-7006.2007.00467.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara Y., Inoue Y., Sagara Y., Kashiwagi S. (2009). Involvement of molecular mimicry between human T-cell leukemia virus type 1 gp46 and osteoprotegerin in induction of hypercalcemia. Cancer Sci. 100, 490–496 10.1111/j.1349-7006.2008.01061.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y., Yamamoto N., Dewan M. Z., Sugimoto H., Martinez Bruyn V. J., Iwasaki Y., Matsubara K., Qi X., Saitoh T., Imoto I., Inazawa J., Utsunomiya A., Watanabe T., Masuda T., Yamamoto N., Yamaoka S. (2008). Overexpressed NF-kappaB-inducing kinase contributes to the tumorigenesis of adult T-cell leukemia and Hodgkin Reed-Sternberg cells. Blood 111, 5118–5129 10.1182/blood-2007-09-110635 [DOI] [PubMed] [Google Scholar]
- Sakashita A., Hattori T., Miller C. W., Suzushima H., Asou N., Takatsuki K., Koeffler H. P. (1992). Mutations of the p53 gene in adult T-cell leukemia. Blood 79, 477–480 [PubMed] [Google Scholar]
- Sanda T., Asamitsu K., Ogura H., Iida S., Utsunomiya A., Ueda R., Okamoto T. (2006). Induction of cell death in adult T-cell leukemia cells by a novel IkappaB kinase inhibitor. Leukemia 20, 590–598 10.1038/sj.leu.2404129 [DOI] [PubMed] [Google Scholar]
- Sasaki D., Imaizumi Y., Hasegawa H., Osaka A., Tsukasaki K., Choi Y. L., Mano H., Marquez V. E., Hayashi T., Yanagihara K., Moriwaki Y., Miyazaki Y., Kamihira S., Yamada Y. (2011). Overexpression of enhancer of zeste homolog 2 with trimethylation of lysine 27 on histone H3 in adult T-cell leukemia/lymphoma as a target for epigenetic therapy. Haematologica 96, 712–719 10.3324/haematol.2010.028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Nishikata I., Shiraga T., Akamatsu E., Fukami T., Hidaka T., Kubuki Y., Okayama A., Hamada K., Okabe H., Murakami Y., Tsubouchi H., Morishita K. (2005). Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood 105, 1204–1213 10.1182/blood-2004-03-1222 [DOI] [PubMed] [Google Scholar]
- Satou Y., Nosaka K., Koya Y., Yasunaga J. I., Toyokuni S., Matsuoka M. (2004). Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia 18, 1357–1363 10.1038/sj.leu.2403400 [DOI] [PubMed] [Google Scholar]
- Satou Y., Yasunaga J., Yoshida M., Matsuoka M. (2006). HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc. Natl. Acad. Sci. U.S.A. 103, 720–705 10.1073/pnas.0507631103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satou Y., Yasunaga J., Zhao T., Yoshida M., Miyazato P., Takai K., Shimizu K., Ohshima K., Green P. L., Ohkura N., Yamaguchi T., Ono M., Sakaguchi S., Matsuoka M. (2011). HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. 7, e1001274. 10.1371/journal.ppat.1001274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt I., Rosin O., Rohwer P., Gossen M., Grassmann R. (1998). Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72, 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B., Chourrout D., Vervoort M., Leblanc B., Cavalli G. (2007). Genome regulation by polycomb and trithorax proteins. Cell 128, 735–745 10.1016/j.cell.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Shembade N., Pujari R., Harhaj N. S., Abbott D. W., Harhaj E. W. (2011). The kinase IKKα inhibits activation of the transcription factor NF-κB by phosphorylating the regulatory molecule TAX1BP1. Nat. Immunol. 12, 834–843 10.1038/ni.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonis N., Rual J. F., Lemmens I., Boxus M., Hirozane-Kishikawa T., Gatot J. S., Dricot A., Hao T., Vertommen D., Legros S., Daakour S., Klitgord N., Martin M., Willaert J. F., Dequiedt F., Navratil V., Cusick M. E., Burny A., Van Lint C., Hill D. E., Tavernier J., Kettmann R., Vidal M., Twizere J. C. (2012). Host-pathogen interactome mapping for HTLV-1 and -2 retroviruses. Retrovirology 9, 26. 10.1186/1742-4690-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparmann A., van Lohuizen M. (2006). Polycomb silencers control cell fate, development and cancer. Nat. Rev. Cancer 6, 846–856 10.1038/nrc1991 [DOI] [PubMed] [Google Scholar]
- Spiegel S., Milstien S., Grant S. (2012). Endogenous modulators and pharmacological inhibitors of histone deacetylases in cancer therapy. Oncogene 31, 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Yamaoka S. (2005). Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene 24, 5952–5964 10.1038/sj.onc.1208391 [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kitao S., Matsushime H., Yoshida M. (1996). Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity towards CDK4. EMBO J. 15, 1607–1614 [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Narita T., Uchida-Toita M., Yoshida M. (1999). Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 259, 384–391 10.1006/viro.1999.9760 [DOI] [PubMed] [Google Scholar]
- Tagawa H., Miura I., Suzuki R., Suzuki H., Hosokawa Y., Seto M. (2002). Molecular cytogenetic analysis of the breakpoint region at 6q21-22 in T-cell lymphoma/leukemia cell lines. Genes Chromosomes Cancer 34, 175–185 10.1002/gcc.10057 [DOI] [PubMed] [Google Scholar]
- Takaori-Kondo A., Imada K., Yamamoto I., Kunitomi A., Numata Y., Sawada H., Uchiyama T. (1998). Parathyroid hormone-related protein-induced hypercalcemia in SCID mice engrafted with adult T-cell leukemia cells. Blood 91, 4747–4751 [PubMed] [Google Scholar]
- Takemoto S., Mulloy J. C., Cereseto A., Migone T. S., Patel B. K., Matsuoka M., Yamaguchi K., Takatsuki K., Kamihira S., White J. D., Leonard W. J., Waldmann T., Franchini G. (1997). Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. U.S.A. 94, 13897–13902 10.1073/pnas.94.25.13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya S., Etoh K., Suzushima H., Takatsuki K., Matsuoka M. (1998). Mutation of CD95 (Fas/Apo-1) gene in adult T-cell leukemia cells. Blood 91, 3935–3942 [PubMed] [Google Scholar]
- Tamiya S., Matsuoka M., Etoh K., Watanabe T., Kamihira S., Yamaguchi K., Takatsuki K. (1996). Two types of defective human T-lymphotropic virus type I provirus in adult T-cell leukemia. Blood 88, 3065–3073 [PubMed] [Google Scholar]
- Taniguchi A., Nemoto Y., Yokoyama A., Kotani N., Imai S., Shuin T., Daibata M. (2008). Promoter methylation of the bone morphogenetic protein-6 gene in association with adult T-cell leukemia. Int. J. Cancer 123, 1824–1831 10.1002/ijc.23749 [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Nosaka K., Yasunaga J., Maeda M., Mueller N., Okayama A., Matsuoka M. (2005). Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2, 64. 10.1186/1742-4690-2-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawara M., Hogerzeil S. J., Yamada Y., Takasaki Y., Soda H., Hasegawa H., Murata K., Ikeda S., Imaizumi Y., Sugahara K., Tsuruda K., Tsukasaki K., Tomonaga M., Hirakata Y., Kamihira S. (2006). Impact of p53 aberration on the progression of adult T-cell leukemia/lymphoma. Cancer Lett. 234, 249–255 10.1016/j.canlet.2005.03.039 [DOI] [PubMed] [Google Scholar]
- Thu Y. M., Richmond A. (2010). NF-kappaB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev. 21, 213–226 10.1016/j.cytogfr.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Kobayashi S., Ohno N., Isobe M., Tsuda M., Zaike Y., Watanabe N., Tani K., Tojo A., Uchimaru K. (2011). Leukemic T cells are specifically enriched in a unique CD3(dim) CD7(low) subpopulation of CD4(+) T cells in acute-type adult T-cell leukemia. Cancer Sci. 102, 569–577 10.1111/j.1349-7006.2010.01833.x [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Tamura G., Sato K., Endoh Y., Sakata K., Jin Z., Motoyama T., Usuba O., Kimura W., Nishizuka S., Wilson K. T., James S. P., Yin J., Fleisher A. S., Zou T., Silverberg S. G., Kong D., Meltzer S. J. (2000). Distinct methylation patterns of two APC gene promoters in normal and cancerous gastric epithelia. Oncogene 19, 3642–3646 10.1038/sj.onc.1203704 [DOI] [PubMed] [Google Scholar]
- Tsukasaki K., Krebs J., Nagai K., Tomonaga M., Koeffler H. P., Bartram C. R., Jauch A. (2001a). Comparative genomic hybridization analysis in adult T-cell leukemia/lymphoma: correlation with clinical course. Blood 97, 3875–3881 10.1182/blood.V97.12.3875 [DOI] [PubMed] [Google Scholar]
- Tsukasaki K., Miller C. W., Kubota T., Takeuchi S., Fujimoto T., Ikeda S., Tomonaga M., Koeffler H. P. (2001b). Tumor necrosis factor alpha polymorphism associated with increased susceptibility to development of adult T-cell leukemia/lymphoma in human T-lymphotropic virus type 1 carriers. Cancer Res. 61, 3770–3774 [PubMed] [Google Scholar]
- Tsukasaki K., Tanosaki S., DeVos S., Hofmann W. K., Wachsman W., Gombart A. F., Krebs J., Jauch A., Bartram C. R., Nagai K., Tomonaga M., Said J. W., Koeffler H. P. (2004). Identifying progression-associated genes in adult T-cell leukemia/lymphoma by using oligonucleotide microarrays. Int. J. Cancer 109, 875–881 10.1002/ijc.20028 [DOI] [PubMed] [Google Scholar]
- Twizere J. C., Springael J. Y., Boxus M., Burny A., Dequiedt F., Dewulf J. F., Duchateau J., Portetelle D., Urbain P., Van Lint C., Green P. L., Mahieux R., Parmentier M., Willems L., Kettmann R. (2007). Human T-cell leukemia virus type-1 Tax oncoprotein regulates G-protein signaling. Blood 109, 1051–1060 10.1182/blood-2006-06-026781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Kinoshita T., Watanabe T., Nagai H., Murate T., Saito H., Hotta T. (1996). The CDKN2 gene alterations in various types of adult T-cell leukaemia. Br. J. Haematol. 94, 665–670 10.1046/j.1365-2141.1996.d01-1841.x [DOI] [PubMed] [Google Scholar]
- Uota S., Zahidunnabi Dewan M., Saitoh Y., Muto S., Itai A., Utsunomiya A., Watanabe T., Yamamoto N., Yamaoka S. (2012). An IκB kinase 2 inhibitor IMD-0354 suppresses the survival of adult T-cell leukemia cells. Cancer Sci. 103, 100–106 10.1111/j.1349-7006.2011.02110.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribesalgo I., Ballaré C., Di Croce L. (2012). Polycomb regulates NF-κB signaling in cancer through miRNA. Cancer Cell 21, 5–7 10.1016/j.ccr.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Valastyan S., Reinhardt F., Benaich N., Calogrias D., Szász A. M., Wang Z. C., Brock J. E., Richardson A. L., Weinberg R. A. (2009). A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137, 1032–1046 10.1016/j.cell.2009.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Villiger P. M., Cronin M. T., Amenomori T., Wachsman W., Lotz M. (1991). IL-6 production by human T lymphocytes. Expression in HTLV-1-infected but not in normal T cells. J. Immunol. 146, 550–559 [PubMed] [Google Scholar]
- Wäldele K., Schneider G., Ruckes T., Grassmann R. (2004). Interleukin-13 overexpression by tax transactivation: a potential autocrine stimulus in human T-cell leukemia virus-infected lymphocytes. J. Virol. 78, 6081–6090 10.1128/JVI.78.12.6081-6090.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wano Y., Hattori T., Matsuoka M., Takatsuki K., Chua A. O., Gubler U., Greene W. C. (1987). Interleukin 1 gene expression in adult T cell leukemia. J. Clin. Invest. 80, 911–916 10.1172/JCI113152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Nakahata S., Hamasaki M., Saito Y., Kawano Y., Hidaka T., Yamashita K., Umeki K., Taki T., Taniwaki M., Okayama A., Morishita K. (2010). Downregulation of CDKN1A in adult T-cell leukemia/lymphoma despite overexpression of CDKN1A in human T-lymphotropic virus 1-infected cell lines. J. Virol. 84, 6966–6977 10.1128/JVI.01616-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ohsugi T., Shoda M., Ishida T., Aizawa S., Maruyama-Nagai M., Utsunomiya A., Koga S., Yamada Y., Kamihira S., Okayama A., Kikuchi H., Uozumi K., Yamaguchi K., Higashihara M., Umezawa K., Watanabe T., Horie R. (2005). Dual targeting of transformed and untransformed HTLV-1-infected T cells by DHMEQ, a potent and selective inhibitor of NF-kappaB, as a strategy for chemoprevention and therapy of adult T-cell leukemia. Blood 106, 2462–2471 10.1182/blood-2004-09-3646 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Yamaguchi K., Takatsuki K., Osame M., Yoshida M. (1990). Constitutive expression of parathyroid hormone-related protein gene in human T cell leukemia virus type 1 (HTLV-1) carriers and adult T cell leukemia patients that can be trans-activated by HTLV-1 tax gene. J. Exp. Med. 172, 759–765 10.1084/jem.172.3.759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters K. M., Dean J., Hasegawa H., Sawa H., Hall W., Sheehy N. (2010). Cytokine and growth factor expression by HTLV-1 Lck-Tax transgenic cells in SCID mice. AIDS Res. Hum. Retroviruses 26, 593–603 10.1089/aid.2009.0212 [DOI] [PubMed] [Google Scholar]
- Wu K., Bottazzi M. E., de la Fuente C., Deng L., Gitlin S. D., Maddukuri A., Dadgar S., Li H., Vertes A., Pumfery A., Kashanchi F. (2004). Protein profile of tax-associated complexes. J. Biol. Chem. 279, 495–508 10.1074/jbc.M307049200 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Hatta Y., Murata K., Sugawara K., Ikeda S., Mine M., Maeda T., Hirakata Y., Kamihira S., Tsukasaki K., Ogawa S., Hirai H., Koeffler H. P., Tomonaga M. (1997). Deletions of p15 and/or p16 genes as a poor-prognosis factor in adult T-cell leukemia. J. Clin. Oncol. 15, 1778–1785 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Ohmoto Y., Hata T., Yamamura M., Murata K., Tsukasaki K., Kohno T., Chen Y., Kamihira S., Tomonaga M. (1996). Features of the cytokines secreted by adult T cell leukemia (ATL) cells. Leuk. Lymphoma 21, 443–447 10.3109/10428199609093442 [DOI] [PubMed] [Google Scholar]
- Yamada Y., Tomonaga M., Fukuda H., Hanada S., Utsunomiya A., Tara M., Sano M., Ikeda S., Takatsuki K., Kozuru M., Araki K., Kawano F., Niimi M., Tobinai K., Hotta T., Shimoyama M. (2001). A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br. J. Haematol. 113, 375–382 10.1046/j.1365-2141.2001.02737.x [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Nakano K., Miyake A., Yamochi T., Kagami Y., Tsutsumi A., Matsuda Y., Sato-Otsubo A., Muto S., Utsunomiya A., Yamaguchi K., Uchimaru K., Ogawa S., Watanabe T. (2012). Polycomb-mediated loss of miR-31 activates NIK-dependent NF-κB pathway in adult T cell leukemia and other cancers. Cancer Cell 21, 121–135 10.1016/j.ccr.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Kiyokawa T., Watanabe T., Ideta T., Asayama K., Mochizuki M., Blank A., Takatsuki K. (1994). Increased serum levels of C-terminal parathyroid hormone-related protein in different diseases associated with HTLV-1 infection. Leukemia 8, 1708–1711 [PubMed] [Google Scholar]
- Yamaguchi K., Watanabe T. (2002). Human T lymphotropic virus type-I and adult T-cell leukemia in Japan. Int. J. Hematol. 76, 240–245 10.1007/BF03165123 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ishida T., Nakano K., Yamagishi M., Yamochi T., Tanaka Y., Furukawa Y., Nakamura Y., Watanabe T. (2011). SMYD3 interacts with HTLV-1 Tax and regulates subcellular localization of Tax. Cancer Sci. 102, 260–266 10.1111/j.1349-7006.2010.01752.x [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Ito T., Shimizu T., Ishida T., Semba K., Watanabe S., Yamaguchi N., Inoue J. I. (2010). Epigenetic alteration of the NF-kappaB-inducing kinase (NIK) gene is involved in enhanced NIK expression in basal-like breast cancer. Cancer Sci. 101, 2391–2397 10.1111/j.1349-7006.2010.01685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki J., Mizukami T., Takizawa K., Kuramitsu M., Momose H., Masumi A., Ami Y., Hasegawa H., Hall W. W., Tsujimoto H., Hamaguchi I., Yamaguchi K. (2009). Identification of cancer stem cells in a Tax-transgenic (Tax-Tg) mouse model of adult T-cell leukemia/lymphoma. Blood 114, 2709–2720 10.1182/blood-2008-08-174425 [DOI] [PubMed] [Google Scholar]
- Yang J., Ikezoe T., Nishioka C., Furihata M., Yokoyama A. (2010). AZ960, a novel Jak2 inhibitor, induces growth arrest and apoptosis in adult T-cell leukemia cells. Mol. Cancer Ther. 9, 3386–3395 10.1158/1535-7163.MCT-09-0947 [DOI] [PubMed] [Google Scholar]
- Yang Y., Takeuchi S., Tsukasaki K., Yamada Y., Hata T., Mori N., Fukushima A., Seo H., Koeffler H. P., Taguchi H. (2005). Methylation analysis of the adenomatous polyposis coli (APC) gene in adult T-cell leukemia/lymphoma. Leuk. Res. 29, 47–51 10.1016/S0145-2126(05)80124-9 [DOI] [PubMed] [Google Scholar]
- Yashiki S., Fujiyoshi T., Arima N., Osame M., Yoshinaga M., Nagata Y., Tara M., Nomura K., Utsunomiya A., Hanada S., Tajima K., Sonoda S. (2001). HLA-A*26, HLAB* 4002, HLA-B*4006, and HLA-B*4801 alleles predispose to adult T cell leukemia: the limited recognition of HTLV type 1 tax peptide anchor motifs and epitopes to generate anti-HTLV type 1 tax CD8(+) cytotoxic T lymphocytes. AIDS Res. Hum. Retroviruses 17, 1047–1061 10.1089/088922201300343735 [DOI] [PubMed] [Google Scholar]
- Yasunaga J., Lin F. C., Lu X., Jeang K. T. (2011). Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J. Virol. 85, 6212–6219 10.1128/JVI.00079-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga J., Taniguchi Y., Nosaka K., Yoshida M., Satou Y., Sakai T., Mitsuya H., Matsuoka M. (2004). Identification of aberrantly methylated genes in association with adult T-cell leukemia. Cancer Res. 64, 6002–6009 10.1158/0008-5472.CAN-04-1422 [DOI] [PubMed] [Google Scholar]
- Yeung M. L., Yasunaga J., Bennasser Y., Dusetti N., Harris D., Ahmad N., Matsuoka M., Jeang K. T. (2008). Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res. 68, 8976–8985 10.1158/0008-5472.CAN-08-0856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B., Williams P. J., Niewolna M., Wang Y., Yoneda T. (2002). Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res. 62, 917–923 [PubMed] [Google Scholar]
- Yoshida M., Nosaka K., Yasunaga J., Nishikata I., Morishita K., Matsuoka M. (2004). Aberrant expression of the MEL1S gene identified in association with hypomethylation in adult T-cell leukemia cells. Blood 103, 2753–2760 10.1182/blood-2003-07-2482 [DOI] [PubMed] [Google Scholar]
- Yoshie O., Fujisawa R., Nakayama T., Harasawa H., Tago H., Izawa D., Hieshima K., Tatsumi Y., Matsushima K., Hasegawa H., Kanamaru A., Kamihira S., Yamada Y. (2002). Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood 99, 1505–1511 10.1182/blood.V99.5.1505 [DOI] [PubMed] [Google Scholar]
- Zhao T., Satou Y., Sugata K., Miyazato P., Green P. L., Imamura T., Matsuoka M. (2011). HTLV-1 bZIP factor enhances TGF-β signaling through p300 coactivator. Blood 118, 1865–1876 10.1182/blood-2010-12-326199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R., Efeyan A., Sabatini D. M. (2011). mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 10.1038/nrm3025 [DOI] [PMC free article] [PubMed] [Google Scholar]