Abstract
Background
The prevalence of tobacco use, both cigarette smoking and smokeless, including iqmik (homemade smokeless tobacco prepared with dried tobacco leaves mixed with alkaline ash), and of tobacco-related cancer is high in Alaskan Native people (AN). To investigate possible mechanisms of increased cancer risk we studied levels of nicotine and tobacco-specific nitrosamines (TSNA) in tobacco products and biomarkers of tobacco toxicant exposure in Southwestern AN people.
Methods
Participants included 163 cigarette smokers, 76 commercial smokeless tobacco, 20 iqmik, 31 dual cigarette smokers and smokeless tobacco, and 110 nontobacco users. Tobacco use history, samples of tobacco products used, and blood and urine samples were collected.
Results
Nicotine concentrations were highest in cigarette tobacco and TSNAs highest in commercial smokeless tobacco products. The AN participants smoked on average 7.8 cigarettes per day. Nicotine exposure, assessed by several biomarker measures, was highest in iqmik users, and similar in smokeless tobacco and cigarette smokers. TSNA exposure was highest in smokeless tobacco users, and polycyclic aromatic hydrocarbon exposure was highest in cigarette smokers.
Conclusions
Despite smoking fewer cigarettes per day, AN cigarette smokers had similar daily intake of nicotine compared to the general U.S. population. Nicotine exposure was greatest from iqmik, likely related to its high pH due to preparation with ash, suggesting high addiction potential compared to other smokeless tobacco products. TSNA exposure was much higher with smokeless tobacco than other product use, possibly contributing to the high rates of oral cancer.
Impact
Our data contribute to an understanding of the high addiction risk of iqmik use and of the cancer-causing potential of various forms of tobacco use among AN people.
Introduction
The prevalence of tobacco use among Alaskan Native (AN) people is much higher than that of Alaskan non-Native people and the U.S. general population. More than 40% of AN people smoke cigarettes and 11% use smokeless tobacco, including both commercial smokeless tobacco and iqmik (1). Iqmik is a homemade preparation of tobacco leaves or commercial twist of leaf chewing tobacco combined with ash from a fungus (punk ash) or ash from willow bushes or drift wood (2). The tobacco leaves and ash are mixed and either prechewed in the mouth (premasticated) or placed in a bowl with water and stirred with a knife (nonpremasticated). After mixing, iqmik is stored in a can for future use. While AN smokers consume fewer cigarettes per day than the U.S. population of smokers (3, 4), the incidence of tobacco-related cancers, including lung cancer, oral, gastric, and esophageal cancer are higher among AN people (5). To better understand possible mechanisms of higher cancer rates, we studied the levels of nicotine and carcinogenic tobacco-specific nitrosamines (TSNA) in the cigarettes and smokeless tobacco products used by AN people and measured biomarkers of tobacco toxicant exposure among AN tobacco users.
Methods
Participants
The details of the study procedures and smoking behaviors of the participants are described in another article (6). The participants were 400 residents of the Bristol Bay region of southwest Alaska. Participants had to be an AN person, 18 years of age or older, not pregnant, not taking medications that would interfere with nicotine metabolism, and not using marijuana in the last 7 days (to avoid the potential confounding effects of marijuana use as a source of exposure to combustion products). Participants were required to not have used other illicit drugs in the last 30 days, not to be enrolled in a tobacco cessation program or to be taking nicotine medications, and not to have consumed alcohol on the day of enrollment. Tobacco users must have used tobacco in the 24 hours before enrollment. For comparison to tobacco users, we also included nontobacco users who reported not using any tobacco for the past 12 months. AN ethnicity was defined by self-report, with information collected on the ethnic group of the grandparents. Three hundred and eighty-five of the participants had 3 or 4 grandparents who were AN people; the remaining 15 had 2 grandparents who were AN. Eighty-nine percent of the subjects were Yupik, 5% Aleut, 3% Athabascan, 1% Inupiaq, and 2% others.
Procedures
Participants who volunteered for the study signed a consent form and responded to a detailed questionnaire on health status, history of tobacco use, as well as a dietary history and history of exposure to secondhand smoke (6). Samples of cigarettes and smokeless tobacco products, including iqmik, were requested from participants for chemical analysis of nicotine and TSNAs. Blood was collected from participants for measurement for plasma nicotine, cotinine and trans-3′ hydroxycotinine (3HC). A urine sample was collected for measurement of nicotine and 5 of its metabolites, and urinary biomarkers of carcinogen exposure: total 4-(methylnitrsoamino)-4-(3-pyridyl)-1-butanol (NNAL), a major metabolite of the TSNA and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK); total N′-nitrosonornicotine (NNN), another carcinogenic TSNA; and polycyclic aromatic hydrocarbons (PAH) metabolites (7). The study was approved by the ethics committee and full board at the Bristol Bay Area Health Corporation and the Institutional Review Boards of the Alaska Area Institutional Review Board and the University of California San Francisco (San Francisco, CA).
Analytical chemistry
Tobacco samples were analyzed in the Tobacco Smoke Analysis laboratory at the Centers for Disease Control and Prevention (CDC). Concentrations of nicotine and TSNAs were measured in each tobacco product, in triplicate if a sufficient sample was available. The target analytes included nicotine, N′-nitrosoanabasine (NAB), N′-nitrosoanatabine (NAT), NNN, NNK, and NNAL. A measured amount of the finely ground tobacco product was spiked with a solution containing isotopically labeled TSNAs and nicotine as internal standards, and then solvent was extracted with 20 mmol/L aqueous ammonium acetate solution. The extracts were filtered and diluted 10-fold for high-performance liquid chromatography/tandem mass spectrometric (HPLC/MS-MS) analysis for TSNA analysis and further diluted 1,000-fold for nicotine analysis. The HPLC/MS-MS analytical run time for each sample was 8 minutes at a flow rate of 1 mL/min using a Waters Xterra C18 MS column (50 mm × 4.6 mm i.d. × 5 um) at 60°C. Ionization of all analytes was achieved by using electrospray ionization in positive ion mode. Mass spectral data on precursor and product ions were collected in multiple reaction monitoring (MRM) mode on an Applied Biosystems API 4000 mass spectrometer. All chromatographic data were processed with Analyst 1.4 software from Applied Biosystems.
Plasma cotinine and 3HC, and the urine total NNAL and PAH metabolites were measured by the Clinical Pharmacology Laboratory at San Francisco General Hospital by published liquid chromatography/tandem mass spectrometric (LC/MS-MS) assays (8–10). Urine nicotine and 5 nicotine metabolites (nicotine glucuronide, cotinine, cotinine glucuronide, 3HC, and 3HC glucuronide) were measured by the Tobacco Exposure Biomarkers Laboratory at CDC with an LC/MS-MS method that has been previously described (11). Urine total NNN was measured at the University of Minnesota (Minneapolis, MN) by a published liquid chromatography/electrospray ionization/tandem mass spectrometric assay (12).
Data analysis
Summary statistics were computed for the nicotine and nitrosamine content of the various types of tobacco products and included the mean and 95% confidence interval (CI). Comparisons were carried out across products using the Kruskal–Wallis test. The Wilcoxon rank sum test compared the products pairwise using a P value of less than 0.01 for significance to control for multiple comparisons.
Exposure of AN people to nicotine was examined using 3 biomarkers; plasma cotinine, the sum of plasma cotinine plus 3HC, and the molar sum of nicotine and 5 metabolites in urine, normalized for creatinine. Cotinine is the proximate metabolite of nicotine. Plasma cotinine is widely used as a marker for nicotine exposure in populations; however the relationship between plasma cotinine and nicotine intake is influenced by individual differences in the rate of metabolism of nicotine to cotinine and the rate of cotinine clearance (13). The sum of 3HC plus cotinine appears to be superior to cotinine alone as a biomarker of nicotine exposure based on a recent empirical study (14). The best biomarker of nicotine exposure is the sum of nicotine and its metabolites in urine, the molar sum of which is termed urine nicotine equivalents (14).
Biomarker levels were computed as arithmetic means (nicotine and metabolites) or geometric means (NNAL, NNN, and PAH metabolities) with 95% CIs. Values were compared between groups using nonparametric tests (Wilcoxon rank sum test and the Kruskal–Wallis test). The statistical analysis first compared nonusers to each of the 4 tobacco user groups, and then the 4 tobacco user groups were compared with one another. For both sets of analyses a lower P value cutoff of P < 0.01 was used to account for multiple pairwise comparisons. Spearman correlations among biomarkers within tobacco use groups were also determined.
Results
Chemical constituents of tobacco products
Constituents of tobacco products were measured in 26 cigarettes, 22 commercial spit tobacco samples, and 23 iqmik samples. Table 1 presents nicotine and nitrosamine content of tobacco in the various types of products. The nicotine concentration was highest in cigarette tobacco. Concentrations of NNK, NNN, and other TSNAs in commercial smokeless tobacco products were substantially higher than in cigarettes. Levels of NNK and other TSNAs in iqmik were considerably lower than that of cigarettes or commercial smokeless tobacco.
Table 1.
Nicotine and nitrosamine content of tobacco products
| Biomarker mean | Cigarette (N = 24) | Commercial smokeless tobacco (N = 22) | Iqmik nonpremasticated (N = 19) | Iqmik premasticated (N = 4) | Pd |
|---|---|---|---|---|---|
| Nicotine, mg/g | 18.68c (17.80–19.56) | 12.54b (11.79–13.30) | 12.98b (11.71–14.26) | 12.61 (4.48–20.75) | <0.001 |
| NAB, ng/g | 79.7b (69.6–89.9) | 181.2a (139.8–222.5) | 62.5b (48.0–77.0) | 104.0e (0–257.8) | <0.001 |
| NAT, ng/g | 1421.6b (1,206.2–1,637.0) | 2,453.5a (1,616.6–3,290.5) | 938.3c (733.5–1,143.2) | 1,580.8e (0–4,025.9) | <0.001 |
| NNK, ng/g | 536.9b (467.7–606.2) | 829.0a (679.6–978.4) | 81.8c (56.5–107.0) | 565.3e (0–2,108.3) | <0.001 |
| NNN, ng/g | 2,028.1b (1707.1–2,349.1) | 2,874.3a (2,207.6–3,541.0) | 846.8c (628.7–1,065.0) | 1,065.8e (0–2,466.4) | <0.001 |
| NNAL, ng/g | 52.4b (45.5–59.3) | 166.5a (113.3–219.6) | 24.7c (10.9–38.4) | 59.9e (0–155.9) | <0.001 |
NOTE: Values in parenthesis indicate 95% CI.
Values with the same letter are not significantly different; different letters indicate significant differences compared with other products, P < 0.01; comparisons only among commercial cigarettes, commercial ST, and Iqmik nonmasticated.
The P value was derived from the nonparametric Kruskal–Wallis test. “Iqmik premasticated” was excluded from the comparisons between groups due to a very small sample size.
The lower limit of the CI was truncated at zero.
Subjects and tobacco use behavior
The participants included 163 smokers (71 men and 92 women; average age, 36.0 years; SD, 14.4), 76 smokeless tobacco users, (35 men and 41 women; average age, 39.2 years; SD, 12.8), 20 iqmik users (6 men and 14 women; average age, 42.9 years; SD, 17.0), 31 dual cigarette smokers and smokeless tobacco users (16 men and 15 women; average age, 28.8 years; SD, 10.4), and 110 nontobacco users (52 men and 58 women; average age, 38.9; SD, 14.7). Three self-reported nontobacco users were excluded from the analysis because their plasma cotinine concentrations were above 14 ng/mL, a level indicating active tobacco use (15). Additional demographic data on subjects are reported elsewhere (6).
On average the smokers smoked 7.8 cigarettes per day, whereas dual users smoked 5.7 cigarettes per day (Table 2). The Fagerstrom test of nicotine dependence score averaged 2.6, SD 2.1; 1.9, SD 1.9 for smokers and dual users, respectively (16). The smokeless tobacco users used on average 1.6 tins per week; iqmik users used 1.2 tins per week; and dual users used on average either 1.3 tins of smokeless tobacco or 0.5 tins of iqmik per week. The Severson smokeless tobacco dependence score averaged 4.0, SD 3.3; 5.5, SD 3.3; and 3.0, SD 4.1 for smokeless, iqmik, and dual users, respectively (17). Nonsmokers reported that 97.3% had a smoking ban in their homes and 96.4% had no secondhand smoke exposure at home or in steam baths.
Table 2.
Tobacco use and exposure biomarker levels
| Cigarette smoke (N = 163) | Spit tobacco (N = 76) | Iqmik (N = 20) | Dual use (N = 31) | Nonuser (N = 107) | |
|---|---|---|---|---|---|
| Tobacco use | |||||
| Cigarettes/d | 7.8 (5.21) | — | — | 5.7 (3.1) | — |
| Tins/wk | — | 1.6 (1.5) | 1.2 (1.3) | 1.3 (1.0)e 0.5 (0.6)f |
|
| Plasma | |||||
| Cotinine, ng/mL | 170.6c (154.5–186.6) | 221.0b (190.7–251.2) | 343.2a (251.6–434.8) | 187.1b,c (141.1–233.1) | 0.3d (0.1–0.5) |
| 3HC, ng/mL | 75.5b (65.4–85.5) | 95.1b (76.6–113.5) | 207.1a (120.0–294.3) | 76.1b (54.5–97.7) | 0.1c (0.05–0.2) |
| Cotinine + 3HC, nmol/mL | 1.36c (1.23–1.49) | 1.75b (1.50–1.99) | 3.03a (2.16–3.89) | 1.46b,c (1.10–1.81) | 0.002d (0.001–0.003) |
| Urine | |||||
| Nicotine equivalents, pmol/mg creatinine | 61.4b (54.8–68.1) | 72.8b (59.6–86.0) | 116.8a (96.3–137.2) | 55.2b (41.4–69.0) | 0.1c (0–0.001) |
| NNAL, pmol/mg creatinine | 0.89c,d (0.76–1.04) | 4.14a (3.40–5.04) | 0.59c (0.40–0.86) | 1.08d (0.67–1.74) | 0.01b (0.01–0.02) |
| NNNg, pmol/mg creatinine | 0.00059a (0.00048–0.000073) | 0.00070a (0.00053–0.00092) | 0.00074a (0.00028–0.00196) | 0.00114a (0.00071–0.00183) | 0.00008b (0.00004–0.00016) |
| 2-Napthol, pmol/mg creatinine | 103.2a (89.4–119.2) | 45.2b,c (36.6–55.9) | 29.5b (23.1–37.7) | 63.1c (48.0–82.8) | 47.3b,c (37.6–59.6) |
| Sum of hydroxyfluorenes, pmol/mg creatinine | 13.2a (11.6–15.1) | 6.1b (5–7.4) | 5.8b (4.7–7.2) | 7.8b (4.9–12.4) | 6.2b (5.1–7.6) |
| Sum of hydroxyphenanthrenes, pmol/mg creatinine | 2.8a (2.4–3.2) | 2.4a (1.8–3.2) | 1.9a (1.4–2.6) | 2.2a (1.3–3.6) | 2.3a (1.9–2.9) |
| 1-Hydroxypyrene, pmol/g creatinine | 1.25a (1.08–1.44) | 1.05a,b (0.75–1.46) | 1.05a,b (0.65–1.71) | 0.89a,b (0.69–1.14) | 0.83b (0.64–1.08) |
NOTE: Tobacco use parameters shown as mean (SD); plasma cotinine, 3HC, (cotinine + 3HC), and urine nicotine equivalents shown as arithmetic mean (95% CI); urine NNAL, NNN, and PAH metabolites shown as geometric mean (95% CI). Values in parenthesis indicate 95% CI.
Values with the same letter are not significantly different; different letters indicates significant differences between groups, P < 0.01.
Smokeless.
Iqmik
N: cigarette smokers, 140; spit tobacco, 72; iqmik, 20; dual use, 28; nonusers, 23.
Exposure to nicotine, NNAL, NNN, and PAH metabolites
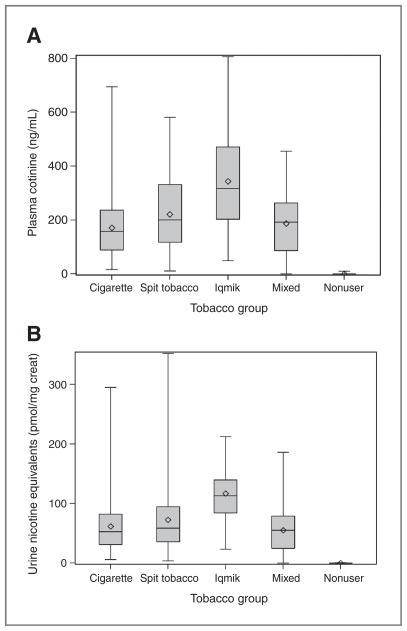
Nicotine exposure data in our subjects are provided in Table 2 and Fig. 1. Nicotine intake was estimated using 3 different measures: plasma cotinine, the molar sum of plasma cotinine and 3HC, and urine nicotine equivalents. All nicotine intake measures were twice as high in iqmik users than in cigarette smokers. Nicotine intake was also greater on average in commercial spit tobacco users than in smokers and greater in iqmik users than in spit tobacco users. The average plasma cotinine concentration in tobacco nonusers was 0.3 ng/mL.
Figure 1.
Box and whisker plots of biomarkers of nicotine exposure in different tobacco use groups. A, plasma cotinine. B, urine nicotine equivalents. The solid line inside the box is the median, the diamond is the mean, the top and bottom of the box represent the 25% to 75% interquartile range, and the vertical line indicates minimum and maximum values.
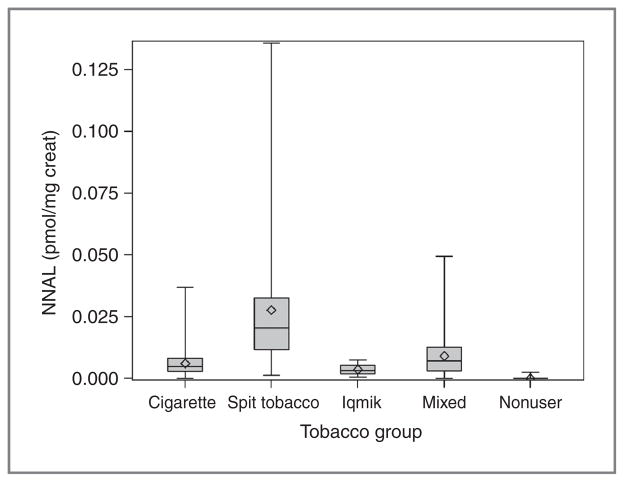
Concentrations of total NNAL and total NNN in urine are shown in Table 2 and Fig. 2. The highest levels of NNAL were seen in commercial smokeless tobacco users followed by dual users and then cigarette smokers. NNAL levels in iqmik users were on average much lower than levels in commercial smokeless tobacco users but not significantly different from those in cigarette smokers. Urine NNN levels were not significantly different comparing the various tobacco user groups, but were significantly lower in nonusers.
Figure 2.
Box and whisker plot of urine total NNAL in different tobacco use groups.
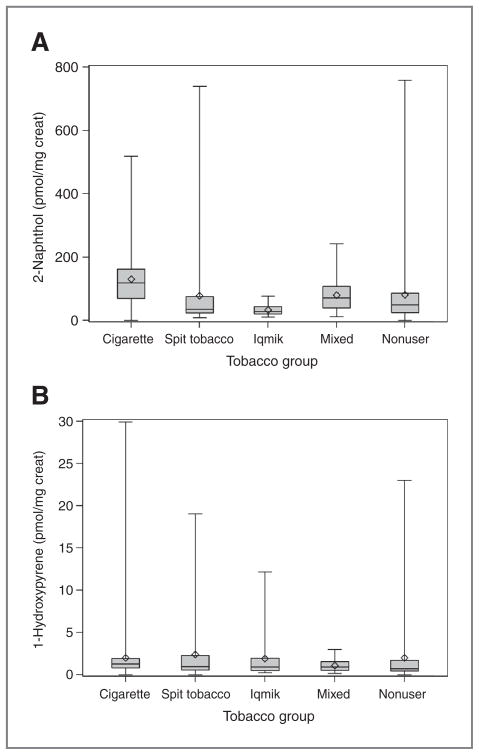
Urine PAH metabolite concentrations are shown in Table 2. Levels for 2 metabolites of PAHs, 2-naphthol and 1-hydroxypyrene, are shown in Fig. 3. Urine 2-napthol and fluorene metabolite concentrations were highest on average in cigarette smokers. Average urine hydroxyphenanthrenes and 1-hydroxpyrene levels were similar across all groups, except levels of 1-hydroxypyrene in nonusers were significantly lower than cigarette smokers.
Figure 3.
Box and whisker plots of urine PAH metabolites in different tobacco use groups. 2-Naphthol (A) and 1-hydroxypyrene (B).
Correlations among biomarkers and tobacco consumption measures
Among cigarette smokers there were significant correlations between urine nicotine equivalents and plasma cotinine (r = 0.73), urine NNAL (r = 0.73), urine NNN (r = 0.58), urine 2-napthol (r = 0.70), urine 1-hydroxypyrene (r = 0.53), and cigarettes per day (r = 0.49), with all P < 0.0001. Among commercial smokeless tobacco users there were significant correlations between urine nicotine equivalents and plasma cotinine (r = 0.72), urine NNAL (r = 0.87) and urine NNN (r = 0.66), all P < 0.001; urine 2-napthol (r = 0.33, P < 0.01) and tins/wk (r = 0.26, P < 0.05). There was no significant correlation with 1-hydroxypyrene. For iqmik users there was a significant correlation between urine nicotine equivalents and plasma cotinine (r =0.78, P <0.001) and urine NNN (r =0.48, P <0.05); but not with urine NNAL or PAH metabolites or with tins/wk. Urine NNAL and urine NNN were significantly correlated in smokers (r = 0.59) and spit tobacco users (r = 0.67), both P < 0.0001.
Discussion
Our study provides several novel findings. To the best of our knowledge, these are the first data on biomarkers of tobacco exposure in a regional population of AN people. Furthermore, we provide novel data on tobacco toxicant exposure in users of the regional smokeless tobacco product iqmik, as well as dual cigarette and smokeless tobacco users.
Our measurements of nicotine and TSNAs in the tobacco products confirm prior research that nicotine concentrations are higher in general in cigarette tobacco than in smokeless tobacco (18). Of note is that nicotine concentrations in iqmik and commercial smokeless tobacco were similar. However, because of the addition of ash that is quite alkaline, the pH is much higher in iqmik (average, 10.9) than in commercial smokeless tobacco (5.4–8.6; refs. 2, 19). As reported previously, the levels of NNK and other TSNAs are higher in commercial smokeless tobacco than in cigarette tobacco (18). In contrast to commercial smokeless tobacco, TSNA levels are much lower in iqmik. Presumably, this is because iqmik is made from dried tobacco leaves that are fire cured and not fermented, resulting in less generation of NNK from nicotine. Our observation agrees with data in the IARC monograph where chewing tobacco (leaf or twist used for iqmik) has substantially lower NNN and NNK levels than the commercial moist snuff varieties sold in the United States (18). The lower levels of NNK in iqmik might also be due to decomposition under the alkaline conditions (pH 10.9), via base-induced condensations involving the relatively acidic methylene protons adjacent to the carbonyl group of NNK.
We provide several novel observations related to nicotine exposure of AN smokers and tobacco users. First, the average plasma cotinine level of 170 ng/mL in AN smokers is only slightly lower than the 200 ng/mL average for U.S. smokers in a representative population sample (20). However, the average number of cigarettes consumed by U.S. smokers overall is about 15 cigarettes per day, whereas the average in our AN population was 7.8 cigarettes per day. Thus the AN smokers who participated were on average taking in much more nicotine (and presumably other tobacco smoke toxicants) per cigarette than the average U.S. smoker. Similar observations have been made comparing other groups of light smokers (20–22). Nicotine intake from commercial smokeless tobacco was on average similar to that of cigarette smokers, which has been observed in other U.S. and European populations (23, 24).
Nicotine intake from iqmik was strikingly higher than that from commercial smokeless tobacco. Because the nicotine content of iqmik, the number of dips used per day and duration of use was similar to commercial smokeless tobacco products, the greater systemic dose is most likely due to greater oropharyngeal absorption of nicotine from the iqmik product related to the addition of alkaline ash. Nicotine is a weak base, and in an alkaline environment more nicotine is in the free or unionized state than in the ionized state. Alkaline iqmik (pH 10.9) results in 99.9% unionized nicotine, which is expected to be absorbed more rapidly through the buccal mucosa than in nicotine from commercial smokeless tobacco which has a pH of 5.4 to 8.6 (2, 19). Anecdotal reports provided by our participants indicated that iqmik frequently results in very intense symptoms of nicotine effects and toxicity. Because the dose of nicotine and the rapidity of absorption of nicotine are thought to influence the risk of addiction, one would predict that iqmik would be more highly addictive than commercial smokeless tobacco products. Not only is it likely that iqmik use would be harder to quit, but iqmik addiction may also serve as a gateway to cigarette addiction (25). Our findings are consistent with those of Hurt and colleagues, who found that among pregnant women plasma nicotine and cotinine levels were higher in iqmik users than in users of other forms of tobacco (26).
Exposure to NNK, as indicated by urine levels of NNAL, was higher in smokeless tobacco users than in smokers, which has been reported by other researchers in different regions of the United States (23). The difference reflects the higher level of NNK in commercial smokeless tobacco than in cigarette tobacco, presumably related to the smokeless tobacco curing process. NNK exposure in iqmik users was much lower than in commercial smokeless tobacco, consistent with lower levels in the product. This is most likely because iqmik is made with leaf or twist chewing tobacco with relatively low moisture content, unlike popular snuff products that contain much higher water content and are often fermented which may increase the nitrosation reactions leading to higher TSNA content. We did not observe significant differences in urinary NNN among tobacco user groups, which may be due, at least in part, to the contribution of endogenous formation of NNN to total NNN (12).
NNK and NNAL are potent pulmonary carcinogens (7). Smokeless tobacco use is not associated with increase of lung cancer, but is associated with increased risk of pancreatic cancer and for some products an increased risk of oral and esophageal cancer (27). Presumably these cancers are caused at least in part by exposure to TSNAs. Dual cigarette smokers and smokeless tobacco users had, on average, higher exposure to NNK than cigarette smokers alone, which suggests that a combination of smoking and smokeless tobacco use might be more harmful than smoking alone. On the basis of the higher TSNA levels in smokeless tobacco than in iqmik users, one might predict a higher cancer risk from the former. However the possible contribution of components of ash to cancer risk from iqmik also needs to be considered. Thus, it would be premature to conclude that iqmik poses a lower cancer risk than commercial smokeless tobacco.
PAH analysis confirmed prior research that cigarette smokers are exposed on average to higher levels of some PAHs than nonsmokers (9). This is particularly true of the more volatile PAHs such a naphthalene and fluorene, which are most specific to cigarette smoking (9). Differences in urine 1-hydroxypyrene excretion between smokers and nonsmokers were much less prominent, consistent with other environmental sources of exposure to pyrene. Although smokeless tobacco made with fire cured tobacco also contains PAHs (28), we did not observe significant differences in PAH biomarker levels comparing users of smokeless tobacco, iqmik users, or nonusers.
Analysis of within-participant correlations between self-reported product use and biomarkers of exposure revealed moderately strong correlations with number of cigarettes smoked per day and weak correlations with tins per week for commercial smokeless tobacco users, but no significant correlations between amount of iqmik use and exposures. The latter may be due to variability in the composition of the homemade product, including variability in pH. Among smokers there were strong correlations between nicotine intake and exposure to NNK and PAHs, as expected from previous studies (29). For commercial smokeless tobacco users there was a strong correlation between nicotine intake and NNK exposures, but weaker correlations with PAH exposure. This observation is consistent with the fact that nicotine is a precursor of TSNAs, but that PAH levels in smokeless tobacco are very dependent on the nature of the curing process and the length and condition of storage of the product. Among iqmik users there were no significant correlations between nicotine intake and carcinogen exposure, most likely related to wide variability in product constituents, including pH.
There are several potential limitations of our study. Foremost with respect to generalizability is that we studied AN people in only one region, the Bristol Bay region of southwest Alaska, who volunteered to participate. Our participants were primarily Yupik. The AN people in other areas of Alaska have different ethnic backgrounds and different cultural influences which could influence smoking behavior and exposure to tobacco toxicants. Although, the number of subjects using iqmik only was relatively small, our article provides new data on exposure to carcinogens among users of this tobacco product and indicate the potential increased addiction risk among this population. There are no other published data on human exposure to nicotine and carcinogens among iqmik users, so our data are unique.
Our findings have important implications for tobacco regulation under the auspices of the U.S. Food and Drug Administration as well globally under the Framework Convention on Tobacco Control. First, the TSNA levels of commercial smokeless tobacco are high and may be contributing to the high rate of oral cancer in AN people. TSNA levels in smokeless tobacco can be controlled and reduced to substantially lower levels than those observed in the present study (30), which could have a beneficial effect on cancer risk at the population level. Second, the very high levels of unionized (free) nicotine and the resultant high levels of nicotine absorption from iqmik is consistent with a high addiction risk. A high level of addiction is likely to increase the risk of transition to smoked products and of dual use and to impede efforts to achieve and sustain abstinence, further increasing the risk to health. Thus, the pH and levels of unionized nicotine in smokeless tobacco products need to be evaluated and potentially regulated as a way to reduce addiction and disease risk (31, 32). Finally, the use of iqmik raises challenges to the regulation of products that are prepared by the user or by small vendors. This is of particular importance because many people think that noncommercial products are less harmful than commercial products (2). Research is needed on the contribution of ash to the harmful effects of tobacco, and when regulation is not feasible, public health workers and regulators need to educate the public about the risks of additives such as ash.
Acknowledgments
The scientific team thank for the leadership and direction from the members of the Board of Directors of the Bristol Bay Area Health Corporation, the members of the Ethics Committee of that organization and the Community Advisory Board for this study, and the BBAHC Director of Community Health Services, Rose Loera and Shelley Wallace, all who contributed their time and expertise to making this study possible. The authors thank Kim Hatt, Helen Peters, and Ana Chartier who were study assistants to the project; Drs. David Ashley and Tom Bernert for advice on study design; Joni Jensen for training project personnel and data auditing; Margaret Wilson, Olivia Yturralde, Christopher Havel, James McGuffey, Liqin Zhang, and James La Valle for analytical chemistry; Faith Allen for data management; and Marc Olmsted for editorial assistance.
Grant Support
This study was supported NIDA/NCI NARCH III (Indian Health Service Grant) U26IHS30001/01 and National Institute on Drug Abuse grant DA012353.
Footnotes
Note: Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention.
Disclosure of Potential Conflicts of Interest
R.F. Tyndale has honoraria from speakers’ bureau for CPT associate editor, has ownership interest (including patents) for Nicogen research, and is the consultant/advisory board member for Novartis and McNeil. The findings and conclusions of this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. No potential conflicts of interest were disclosed by other authors.
Authors’ Contributions
Conception and design: N.L. Benowitz, C. Renner, R.F. Tyndale, C.H. Watson
Development of methodology: N.L. Benowitz, C. Renner, D.K. Hatsukami, P. Jacob III
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.):
N.L. Benowitz, C. Renner, I. Stepanov, C. Sosnoff, P. Jacob III
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): N.L. Benowitz, A. Lanier, R.F. Tyndale, D.K. Hatsukami, B.R. Lindgren, P. Jacob III
Writing, review, and/or revision of the manuscript: N.L. Benowitz, C. Renner, A. Lanier, R.F. Tyndale, D.K. Hatsukami, B.R. Lindgren, I. Stepanov, C.H. Watson, C. Sosnoff, P. Jacob III
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): N.L. Benowitz, C. Renner, D.K. Hatsukami, C.H. Watson, C. Sosnoff
Study supervision: N.L. Benowitz, C. Renner, A. Lanier, D.K. Hatsukami
References
- 1.Alaska Native Tribal Health Consortium. Alaska Native Health Status Report. Alaska: Alaska Native Epidemiology Center; 2009. [cited 2009 Mar 23]. Available from: http://www.anthc.org/chs/epicenter/upload/ANHSR.pdf. [Google Scholar]
- 2.Renner CC, Enoch C, Patten CA, Ebbert JO, Hurt RD, Moyer TP, et al. Iqmik: a form of smokeless tobacco used among Alaska natives. Am J Health Behav. 2005;29:588–94. doi: 10.5555/ajhb.2005.29.6.588. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services PHS. Tobacco use among U.S. racial/ethnic minority groups, African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, and Hispanics: a report of the Surgeon General (Executive Summary) USDHHS, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Promotion, Office of Smoking and Health; Washington, DC: Government Printing Office; 1998. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–8. [PubMed] [Google Scholar]
- 5.Day GE, Provost E, Lanier AP. Alaska native mortality rates and trends. Public Health Rep. 2009;124:54–64. doi: 10.1177/003335490912400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renner CC, Lanier AP, Lindgren B, Jensen J, Patten CA, Parascandola M, et al. Tobacco Use Among South Western Alaska Native People: Products, Patterns of Use and Dependence. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts137. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–22. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 8.Jacob P, III, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Sub-picogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80:8115–21. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob P, III, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–98. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 10.Jacob P, III, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–76. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernert JT, Alexander JR, Sosnoff CS, McGuffey JE. Time course of nicotine and cotinine incorporation into samples of nonsmokers’ beard hair following a single dose of nicotine polacrilex. J Anal Toxicol. 2011;35:1–7. doi: 10.1093/anatox/35.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami D, et al. Presence of the carcinogen N′-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69:8236–40. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 14.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P., III Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19:1160–6. doi: 10.1158/1055-9965.EPI-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Boyle RG, Jensen J, Hatsukami DK, Severson HH. Measuring dependence in smokeless tobacco users. Addict Behav. 1995;20:443–50. doi: 10.1016/0306-4603(95)00013-3. [DOI] [PubMed] [Google Scholar]
- 18.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2007;89:1–592. [PMC free article] [PubMed] [Google Scholar]
- 19.Richter P, Hodge K, Stanfill S, Zhang L, Watson C. Surveillance of moist snuff: total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine Tob Res. 2008;10:1645–52. doi: 10.1080/14622200802412937. [DOI] [PubMed] [Google Scholar]
- 20.O’Connor RJ, Giovino GA, Kozlowski LT, Shiffman S, Hyland A, Bernert JT, et al. Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. Am J Epidemiol. 2006;164:750–9. doi: 10.1093/aje/kwj263. [DOI] [PubMed] [Google Scholar]
- 21.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD, Richter PA, Strauss WJ, et al. Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA. 1998;280:135–9. doi: 10.1001/jama.280.2.135. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Stable EJ, Herrera B, Jacob P, III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–6. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 23.Hecht SS, Carmella SG, Murphy SE, Riley WT, Le C, Luo X, et al. Similar exposure to a tobacco-specific carcinogen in smokeless tobacco users and cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1567–72. doi: 10.1158/1055-9965.EPI-07-0227. [DOI] [PubMed] [Google Scholar]
- 24.Wennmalm A, Benthin G, Granstrom EF, Persson L, Petersson AS, Winell S. Relation between tobacco use and urinary excretion of thromboxane A2 and prostacyclin metabolites in young men. Circulation. 1991;83:1698–704. doi: 10.1161/01.cir.83.5.1698. [DOI] [PubMed] [Google Scholar]
- 25.Angstman S, Patten CA, Renner CC, Simon A, Thomas JL, Hurt RD, et al. Tobacco and other substance use among Alaska Native youth in western Alaska. Am J Health Behav. 2007;31:249–60. doi: 10.5555/ajhb.2007.31.3.249. [DOI] [PubMed] [Google Scholar]
- 26.Hurt RD, Renner CC, Patten CA, Ebbert JO, Offord KP, Schroeder DR, et al. Iqmik–a form of smokeless tobacco used by pregnant Alaska natives: nicotine exposure in their neonates. J Matern Fetal Neonatal Med. 2005;17:281–9. doi: 10.1080/14767050500123731. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Ye W, Zendehdel K, Adami J, Adami HO, Boffetta P, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet. 2007;369:2015–20. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- 28.Stepanov I, Villalta PW, Knezevich A, Jensen J, Hatsukami D, Hecht SS. Analysis of 23 polycyclic aromatic hydrocarbons in smokeless tobacco by gas chromatography-mass spectrometry. Chem Res Toxicol. 2010;23:66–73. doi: 10.1021/tx900281u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–83. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanfill SB, Connolly GN, Zhang L, Jia LT, Henningfield JE, Richter P, et al. Global surveillance of oral tobacco products: total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.037465. [DOI] [PubMed] [Google Scholar]
- 31.WHO Study Group. The scientific basis of tobacco product regulation. World Health Organ Tech Rep Ser. 2007:1–112. back cover. [PubMed] [Google Scholar]
- 32.Ashley DL, Burns D, Djordjevic M, Dybing E, Gray N, Hammond SK, et al. The scientific basis of tobacco product regulation. World Health Organ Tech Rep Ser. 2008:1–277. 1. following 277. [PubMed] [Google Scholar]