Abstract
Purpose
We examined the success of early endoscopic realignment of pelvic fracture associated urethral injury after blunt pelvic trauma.
Materials and Methods
A retrospective review was performed of patients with pelvic fracture associated urethral injury who underwent early endoscopic realignment using a retrograde or retrograde/antegrade approach from 2004 to 2010 at a Level 1 trauma center. Followup consisted of uroflowmetry, post-void residual and cystoscopic evaluation. Failure of early endoscopic realignment was defined as patients requiring urethral dilation, direct vision internal urethrotomy, posterior urethroplasty or self-catheterization after initial urethral catheter removal.
Results
A total of 19 consecutive patients (mean age 38 years) with blunt pelvic fracture associated urethral injury underwent early endoscopic realignment. Twelve cases of complete urethral disruption, 4 of incomplete disruption and 3 of indeterminate status were noted. Mean time to realignment was 2 days and mean duration of urethral catheterization after realignment was 53 days. One patient was lost to followup after early endoscopic realignment. Using an intent to treat analysis early endoscopic realignment failed in 15 of 19 patients (78.9%). Mean time to early endoscopic realignment failure after catheter removal was 79 days. The cases of early endoscopic realignment failure were managed with posterior urethroplasty (8), direct vision internal urethrotomy (3) and direct vision internal urethrotomy followed by posterior urethroplasty (3). Mean followup for the 4 patients considered to have undergone successful early endoscopic realignment was 2.1 years.
Conclusions
Early endoscopic realignment after blunt pelvic fracture associated urethral injury results in high rates of symptomatic urethral stricture requiring further operative treatment. Close followup after initial catheter removal is warranted, as the mean time to failure after early endoscopic realignment was 79 days in our cohort.
Keywords: urethra; wounds, nonpenetrating; urethral stricture; fractures, bone; pelvis
Pelvic fracture associated urethral injury is an uncommon yet debilitating sequela of blunt pelvic trauma. The published rate of posterior urethral injury associated with pelvic fracture varies from 5% to 25% in small series.1–3 However, a recent review of the National Trauma Data Bank reported a lower incidence of 1.54%.4 The initial management of these devastating injuries involves EER or placement of a suprapubic cystostomy tube followed by delayed urethroplasty. The cited advantages of EER include an earlier return to voiding, the possibility of no future operative interventions, and if a urethral stricture develops EER may better align the distracted urethral segments during formal urethroplasty.5,6
The reported success of EER is variable, with rates of clinically significant stricture formation ranging from 14% in a single institution series to 53% in a large multicenter review. Our primary aim was to analyze the success of EER after blunt PFAUI in a subset of consecutive patients who were treated from initial injury to potential urethral reconstruction at our Level 1 trauma hospital. A secondary aim was to assess incontinence and erectile dysfunction during followup clinic appointments.
METHODS
A retrospective review was performed of consecutive patients with blunt PFAUI who underwent EER from January 2004 through July 2010 at Harborview Medical Center, a Level 1 trauma center serving the Pacific Northwest. No patients undergoing EER were excluded from analysis. An intent to treat analysis was used for patients who did not return for followup after EER. Patients with clinical suspicion of PFAUI after initial blunt pelvic injury underwent a retrograde urethrogram and/or flexible cystoscopy to confirm the diagnosis. EER was performed once patients were clinically stable. Delay to EER was commonly the result of clinical instability at presentation to the emergency department or an unstable pelvis that required stabilization before EER could safely proceed. For those patients who required EER delay, bladder drainage was achieved with a SPT as a temporizing measure. EER was performed in the emergency department using a flexible cystoscope or in the operating room with fluoroscopic guidance, with or without the use of a second flexible cysto-scope through a suprapubic tract.7,8 The SPT was removed after successful EER. The operative records were reviewed to determine the duration of the EER procedure. All patients were maintained on antibiotics from the time of presentation until the completion of EER.
Urethral catheterization was maintained for a minimum of 3 weeks for urethral lacerations and 6 weeks for complete disruption. Catheters were left longer if necessary as part of the polytrauma recovery. A pericatheter retrograde urethrogram or voiding cystourethrogram was performed at urethral catheter removal. Contrast was injected around the urethral catheter using a 5 or 8Fr feeding tube to obtain the pericatheter study. Patients were closely followed with uroflowmetry and post-void residual during the first month after initial urethral catheter removal. Additional followup included uroflowmetry, post-void residual and/or cystoscopic evaluation at 3, 12 and 24 months.
Endoscopic realignment was deemed successful if no further procedures, including self-catheterization, were necessary and no stricture recurrence was noted at followup cystoscopy. For patients in whom EER failed, DVIU was considered if the stricture was less than 1 to 2 cm and not completely obliterative, and otherwise posterior urethroplasty was performed in lieu of initial DVIU. Cut to the light DVIU was not performed. Stricture length was determined by intraoperative surgeon assessment. Increased operative time and the need for additional maneuvers such as inferior pubectomy, corporal splitting or crural rerouting were considered markers of increased difficulty during posterior urethroplasty. Postoperative erectile dysfunction and incontinence were assessed through the subjective evaluation of patients on followup clinic visits.
RESULTS
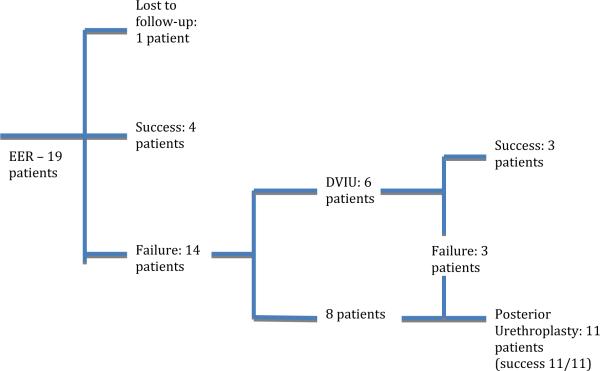
A total of 19 consecutive patients with blunt PFAUI underwent EER (see figure). Mean patient age was 38 years (median 36, range 21 to 73) with a mean followup of 40 months (range 10 to 80). The etiologies of injury were motor vehicle crash (10), crush injury (4), vehicle vs pedestrian accident (3), snow-boarding accident (1) and motorcycle crash (1). EER was performed in the operating room in 15 of 19 patients, while the reminder (4) underwent EER via retrograde urethrocystoscopy in the emergency room. At the time of EER 12 patients had complete urethral disruption, 4 had incomplete urethral disruption and in 3 the extent of urethral injury could not be determined. Mean time from injury to endoscopic realignment was 2 days (median 2, range 0 to 7). Mean duration of urethral catheterization after endoscopic realignment was 53 days (median 55, range 12 to 87). The operative reports included documentation of the time to complete EER for 13 of 19 subjects. The average procedure length for these 13 subjects was 74 minutes (median 65, range 10 to 284).
Patient management algorithm following PFAUI
One patient was lost to followup after EER. Using an intent to treat analysis we classified this case as a treatment failure and calculated followup after EER as 0. Based on this approach the overall success rate of EER was 4 of 19 (21%) with a mean followup of 3.7 years (median 3.5, range 0 to 7.1). For the patients treated successfully with EER mean followup was 2.1 years (median 2.2, range 0.86 to 3.1). The findings identified at EER in patients treated successfully were complete prostatomembranous urethral disruption (2) and partial proximal bulbar laceration (2).
The figure depicts the treatment pathway for the 15 patients in whom EER failed (78.9%). Mean time to identification of stricture formation after initial urethral catheter removal was 79 days (median 72, range 0 to 288). After initial EER failure 8 patients underwent posterior urethroplasty. Six patients were initially treated with DVIU. However, treatment failed in 3 of these patients who subsequently required posterior urethroplasty.
As shown in the figure, after EER failure 11 patients underwent posterior urethroplasty with a mean subjective stricture length of 1.8 cm (range 1 to 3). Penile revascularization before posterior urethroplasty was not required for any patient. The mean operative time for posterior urethroplasty was 225 minutes (median 220, range 131 to 307). During posterior urethroplasty additional operative maneuvers beyond urethral mobilization were required in 3 patients. The corporal bodies were split in 1 patient after complete urethral disruption and in another with a 1 cm membranous urethral tear. The third patient required inferior pubectomy and corporal splitting for successful urethroplasty. Corporal rerouting was not performed in any patient. The mean operative time for the urethroplasty procedures requiring additional urethral lengthening maneuvers was 243 minutes (median 239). One case required a combined abdominoperineal approach for repair, while all others were performed via a peri-neal approach. Posterior urethroplasty was successful in all 11 patients. Mean followup after posterior urethroplasty was 3.0 years (range 0.6 to 5.9).
Of the 4 patients treated successfully with EER alone after PFAUI none reported erectile dysfunction or SUI. Of the remaining 14 patients in whom EER failed who returned for clinical care, 4 reported erectile dysfunction and none reported subjective SUI. All 4 patients with subjective erectile dysfunction sustained a complete posterior urethral disruption. All 4 of these patients had successful treatment of erectile function with phosphodiesterase type 5 inhibitors or intracavernous injections.
Of the subjects in whom EER failed 79% had inferomedial pubic bone fractures and 50% had symphysis pubis diastasis. In contrast, 50% of subjects who underwent successful EER had inferomedial pubic bone fracture and/or symphysis pubis diastasis. A more thorough analysis of pelvic fracture patterns and EER outcomes was not performed due to low numbers.
DISCUSSION
Among the consecutive patients with blunt PFAUI treated at our Level 1 trauma center from 2004 to 2010 the success rate of EER alone was 21% (4 of 19). The severity of PFAUI did not correlate with successful EER outcomes as 2 of 12 patients with evidence of complete urethral disruption had successful EER. Subjective SUI was not reported among the 18 patients who returned after EER, while subjective erectile dysfunction was only reported in 4 of those in whom EER failed. As noted in previous series symphysis pubis diastasis and pubic ramus fracture are associated with PFAUI.3 However, our limited numbers preclude statistical analysis of the correlation of fracture patterns to EER success.
PFAUI is an uncommon yet debilitating injury resulting from significant blunt trauma to the pelvis. The mechanism of this injury involves signifi-cant shearing forces at the prostatomembranous junction, resulting in avulsion of the urethra from the fixed urogenital diaphragm.8 In the last 80 years debate has been ongoing in the urological and trauma literature regarding the safest method of treating these potentially catastrophic injuries, with the goal of treatment to produce unobstructed voiding while maintaining the highest rates of continence and potency. This debate has changed from immediate open surgical repair, to rudimentary forms of urethral realignment, to suprapubic drainage with delayed urethral stricture repair. In the last 30 years practice patterns have returned to favor early urethral realignment via endourological methods.1,9–11
Modern techniques of early endoscopic realignment were introduced in the late 1980s. These techniques evolved into a combination of transurethral and transvesical endourological procedures in conjunction with fluoroscopy.8 This technique is postulated to avoid further damage to erectile function since there is no manipulation of the periprostatic tissues and, thus, no additional trauma to the neurovascular bundles.8
Recent data on primary realignment are mixed with regard to subsequent urethral stricture development. Webster et al performed a comprehensive literature review encompassing 538 cases of urethral injuries associated with pelvic fracture dating from 1953 to 1995.12 Of the 508 patients treated with initial suprapubic cystostomy, stricture requiring repair developed in 97% vs 53% of the 326 patients who underwent mixed techniques of primary realignment. Other single institution series report rates of urethral stricture after primary realignment ranging from 14%7 to 45%.13 These published series combined with our 79% failure rate indicate that primary realignment after PFAUI is not often successful. Continued followup of these patients after initial catheter removal after primary realignment is critical as delayed urethroplasty will often be required. While some may advocate initiation of intermittent self-catheterization if primary realignment is unsuccessful, we do not support this palliative stricture management strategy as posterior urethroplasty has durable results to allow volitional voiding.14
In a comprehensive literature review published in 1983 Webster et al reported that the rate of impotence was double in the primary realignment group vs the suprapubic cystostomy group (36% vs 19%).12 A common criticism of this comparison is that the technique of primary realignment was not uniform, as the cohort was from multiple centers (15) and time periods (1961 to 1983). In addition, there was no standardization of realignment technique or subsequent followup duration in many of the studies. As previously stated it is also unfair to compare rates of impotence in patients after initial suprapubic cystostomy to rates in those who underwent primary realignment due to differential misclassification because those treated with primary realignment were likely less severely injured.
EER is thought to be less traumatic than other means of primary realignment after PFAUI.8 A report of 29 patients with blunt trauma and posterior urethral injury used EER as the sole method of primary relignment.15 The authors reported a subjective 86% potency rate after EER, which is much higher than in older reports using mixed means of primary realignment. These improved findings with EER alone compare more closely to our series. Assuming the patient lost to followup after EER in our cohort was impotent, our subjective potency rate was 14 of 19 (74%).
While cases of PFAUI are relatively rare, these findings have influenced our clinical practice in several ways. Despite limited numbers, we did not find that patients with complete urethral disruption had higher rates of failure after EER, as 2 of the 4 successes with EER alone had endoscopic evidence of complete disruption. Subjective assessment during cystoscopy was the only mechanism for diagnosis and endoscopic visibility could have impaired our visual assessment. However, we do not counsel patients on the eventual need for urethroplasty or internal urethrotomy based on the initial endoscopic assessment of injury severity. Instead, the high failure rate after EER (78% in our series) has reinforced the necessity for close followup after initial catheter removal. Setting appropriate expectations of outcomes and educating patients regarding the need for close followup due to the risks of obstructive voiding and complete retention are of paramount impor tance. As such, we schedule close followup with uroflowmetry and post-void residual in the first month after EER catheter removal. If EER fails then we will recommend 1 internal urethrotomy for short strictures (less than 1 to 2 cm) that are not obliterative. However, we do not repeat internal urethrotomy if the initial attempt is unsuccessful (success defined as no use of self-catheterization). Using this approach 50% of patients (3 of 6) who underwent internal urethrotomy were successfully treated with a single internal urethrotomy. We do not advocate self-catheterization unless the patient has severe medical comorbidities preventing open surgical repair. The injured cohort is often young (median age 38 years in our analysis). When faced with a decision of lifelong intermittent self-catheterization vs definitive repair, most patients will opt for the latter option. As such, if EER and possibly internal urethrotomy fail, we recommend posterior urethroplasty as it has been shown to be a durable treatment option for posterior urethral strictures.14 In our selective series of patients with blunt PFAUI and failed EER, all 11 patients were treated successfully (ie no need for self-catheterization).
All but 1 of the patients in our cohort undergoing posterior urethroplasty was treated via a perineal approach. Of the 11 patients 3 required urethral mobilization to perform the urethroplasty. While we do not have a case matched cohort of patients who underwent initial SPT with delayed urethroplasty at our institution, this rate of additional urethral lengthening maneuvers is consistent with the findings of Cooperberg et al in a series of 134 patients who underwent posterior urethroplasty.14 In this series 30% of patients required corporal splitting, 22% required partial pubectomy and 4% required a combined abdominoperineal approach.
We did not evaluate our EER cohort with validated questionnaires to assess incontinence and potency. Instead, patients were questioned about continence and erectile function during followup visits. This lack of validated outcome measures is a limitation of our study. Data provided by the International Index of Erectile Function and the International Consultation on Incontinence Questionnaire have since been added to our clinical practice to provide improved quantitative outcome assessment. Our study is also limited by the small cohort size, reflecting the rarity of this injury pattern even at a Level 1 trauma center. Despite these limitations our study highlights the importance of close monitoring as the majority of patients undergoing EER after PFAUI will ultimately require complex posterior urethral reconstruction. Our series has shown that posterior urethroplasty after failed EER is durable.
CONCLUSIONS
The majority of patients who undergo early endoscopic realignment after pelvic fracture associated urethral injury will require subsequent posterior urethroplasty (11 of 19). One attempt at internal urethrotomy for focal strictures after EER failure may be useful for definitive repair. However, close followup is necessary to assess for delayed failure.
Abbreviations and Acronyms
- DVIU
direct vision internal urethrotomy
- EER
early endoscopic realignment
- PFAUI
pelvic fracture associated urethral injury
- SPT
suprapubic tube
- SUI
stress urinary incontinence
REFERENCES
- 1.Pokorny M, Pontes JE, Pierce JM., Jr Urological injuries associated with pelvic trauma. J Urol. 1979;121:455. doi: 10.1016/s0022-5347(17)56822-6. [DOI] [PubMed] [Google Scholar]
- 2.Guillé F, Cippola B, el Khader K, et al. Early endoscopic realignment for complete traumatic rupture of the posterior urethra–21 patients. Acta Urol Belg. 1998;66:55. [PubMed] [Google Scholar]
- 3.Basta AM, Blackmore CC, Wessells H. Predicting urethral injury from pelvic fracture patterns in male patients with blunt trauma. J Urol. 2007;177:571. doi: 10.1016/j.juro.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Bjurlin MA, Fantus RJ, Mellett MM, et al. Genitourinary injuries in pelvic fracture morbidity and mortality using the National Trauma Data Bank. J Trauma. 2009;67:1033. doi: 10.1097/TA.0b013e3181bb8d6c. [DOI] [PubMed] [Google Scholar]
- 5.Mouraviev VB, Coburn M, Santucci RA. The treatment of posterior urethral disruption associated with pelvic fractures: comparative experience of early realignment versus delayed urethroplasty. J Urol. 2005;173:873. doi: 10.1097/01.ju.0000152145.33215.36. [DOI] [PubMed] [Google Scholar]
- 6.Ku JH, Kim ME, Jeon YS, et al. Management of bulbous urethral disruption by blunt external trauma: the sooner, the better? Urology. 2002;60:579. doi: 10.1016/s0090-4295(02)01834-4. [DOI] [PubMed] [Google Scholar]
- 7.Hadjizacharia P, Inaba K, Teixeira PG, et al. Evaluation of immediate endoscopic realignment as a treatment modality for traumatic urethral injuries. J Trauma. 2008;64:1443. doi: 10.1097/TA.0b013e318174f126. [DOI] [PubMed] [Google Scholar]
- 8.Koraitim MM. Pelvic fracture urethral injuries: the unresolved controversy. J Urol. 1999;161:1433. [PubMed] [Google Scholar]
- 9.Clark SS, Prudencio RF. Lower urinary tract injuries associated with pelvic fractures. Diagnosis and management. Surg Clin North Am. 1972;52:183. doi: 10.1016/s0039-6109(16)39642-6. [DOI] [PubMed] [Google Scholar]
- 10.McRoberts JW, Ragde H. The severed canine posterior urethra: a study of two distinct methods of repair. J Urol. 1970;104:724. doi: 10.1016/s0022-5347(17)61821-4. [DOI] [PubMed] [Google Scholar]
- 11.Ragde H, McInnes GF. Transpubic repair of the severed prostatomembranous urethra. J Urol. 1969;101:335. doi: 10.1016/s0022-5347(17)62338-3. [DOI] [PubMed] [Google Scholar]
- 12.Webster GD, Mathes GL, Selli C. Pros-tatomembranous urethral injuries: a review of the literature and a rational approach to their management. J Urol. 1983;130:898. doi: 10.1016/s0022-5347(17)51561-x. [DOI] [PubMed] [Google Scholar]
- 13.Sofer M, Mabjeesh NJ, Ben-Chaim J, et al. Long-term results of early endoscopic realignment of complete posterior urethral disruption. J Endourol. 2010;24:1117. doi: 10.1089/end.2010.0069. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, McAninch JW, Alsikafi NF, et al. Urethral reconstruction for traumatic posterior urethral disruption: outcomes of a 25-year experience. J Urol. 2007;178:2006. doi: 10.1016/j.juro.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Moudouni SM, Patard JJ, Manunta A, et al. Early endoscopic realignment of post-traumatic posterior urethral disruption. Urology. 2001;57:628. doi: 10.1016/s0090-4295(00)01068-2. [DOI] [PubMed] [Google Scholar]