Abstract
BACKGROUND
Hematopoietic stem cells (HSCs) are routinely obtained from marrow, mobilized peripheral blood, and umbilical cord blood. Mesenchymal stem cells (MSCs) are traditionally isolated from marrow. Bone marrow–derived MSCs (BM-MSCs) have previously demonstrated their ability to act as a feeder layer in support of ex vivo cord blood expansion. However, the use of BM-MSCs to support the growth, differentiation, and engraftment of cord blood may not be ideal for transplant purposes. Therefore, the potential of MSCs from a novel source, the Wharton’s jelly of umbilical cords, to act as stromal support for the long-term culture of cord blood HSC was evaluated.
STUDY DESIGN AND METHODS
Umbilical cord–derived MSCs (UC-MSCs) were cultured from the Wharton’s jelly of umbilical cord segments. The UC-MSCs were then profiled for expression of 12 cell surface receptors and tested for their ability to support cord blood HSCs in a long-term culture-initiating cell (LTC-IC) assay.
RESULTS
Upon culture, UC-MSCs express a defined set of cell surface markers (CD29, CD44, CD73, CD90, CD105, CD166, and HLA-A) and lack other markers (CD45, CD34, CD38, CD117, and HLA-DR) similar to BM-MSCs. Like BM-MSCs, UC-MSCs effectively support the growth of CD34+ cord blood cells in LTC-IC assays.
CONCLUSION
These data suggest the potential therapeutic application of Wharton’s jelly–derived UC-MSCs to provide stromal support structure for the long-term culture of cord blood HSCs as well as the possibility of cotransplantation of genetically identical, HLA-matched, or unmatched cord blood HSCs and UC-MSCs in the setting of HSC transplantation.
Hematopoietic stem cells (HSCs) are routinely obtained from marrow, mobilized peripheral blood, and umbilical cord blood. Traditionally, adult marrow has been utilized as a source of mesenchymal stem cells (MSCs). Successful expansion of transplantable HSCs is thought to be possible by coculture of HSCs with cells believed to be representative of the stem cell “niche.” Contact with a stromal component or with MSCs1,2 may fulfill the requirement of the niche by preserving the necessary hematopoietic microenvironment to maintain stem cell function. Bone marrow–derived MSCs (BM-MSCs) have previously been shown to maintain the growth of HSCs obtained from cord blood and have been utilized for cord blood expansion purposes.3 Coculture with BM-MSCs has also been reported to promote engraftment of CD34+ defined cord blood hematopoietic stem and progenitor cells (HSCs/HPCs) into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice.4 However, it is possible that the use of BM-MSCs as a feeder layer to support the long-term culture of cord blood HSCs may not be ideal for the clinical transplant setting.
For clinical transplantation, it may be preferred that HSCs and MSCs be obtained from the same donor, thereby eliminating the potential for complications resulting from a HSC and MSC genetic mismatch. There may alternatively be an advantage of obtaining HLA-matched or unmatched donor MSC from a nonmarrow or nonadult tissue source. However, it has been previously reported that the numbers of MSCs obtainable from cord blood are small in comparison to marrow.5 As an alternative, the possibility of obtaining MSCs from placenta or from the umbilical cord is attractive. Others have shown that adherent cells from the placenta can be cultured in such a way that they proliferate and also show osteogenic and adipogenic differentiation potential.6
Isolation of fibroblastlike cells from the Wharton’s jelly of the umbilical cord was originally described in 1991.7 At the time these cells were not evaluated in the context of stem cell biology. More recently, putative MSCs were obtained from the umbilical cord itself using two different dissection methods, either from the subendothelial layer of the cord vein8 or from the Wharton’s jelly, the connective tissue of the umbilical cord.9–11 Importantly, MSCs isolated from the umbilical cord were shown to have the ability to differentiate down multiple lineages, including adipose,8 bone,8,10 and neuronal lineages,9,11 thereby suggesting that these cells are likely to have mesenchymal stem and/or progenitor cell potential.
Most recently, umbilical cord–derived MSCs (UC-MSCs) were shown to secrete several important cytokines and growth factors, including granulocyte–colony-stimulating factor, granulocyte-macrophage–colony-stimulating factor (GM-CSF), interleukin (IL)-6, and IL-8 and that UC-MSCs–produced cytokines were capable of enhancing CFU-GM colony formation during a standard methylcellulose-based myeloid colony assay supplemented with 30 percent fetal bovine serum (FBS), stem cell factor, GM-CSF, IL-3, and erythropoietin (EPO).12 Cotransplantation of UC-MSCs along side CD34+ cord blood cells were also shown to increase the numbers of human CD45+ cells detectable in the marrow of a lethally irradiated NOD/SCID recipient 6 to 8 weeks after transplant.12
Having accepted the previous reports suggesting that cells isolated from the Wharton’s jelly showed characteristics of MSCs we sought to evaluate another important functional characteristic of MSCs, the ability to support the maintenance of blood. We therefore hypothesized that UC-MSCs would have the ability to act as stromal cells and support ex vivo the long-term growth and maintenance of cord blood–derived HSCs, similar to that previously reported for BM-MSCs. To test this hypothesis, MSCs were isolated from the Wharton’s jelly of umbilical cord segments and defined morphologically utilizing established cell surface markers.13 UC-MSCs were then tested for their ability to support the growth of CD34+ cord blood cells in bulk long-term culture-initiating cell (LTC-IC) assays.
MATERIALS AND METHODS
Isolation of umbilical cord: MSCs
Umbilical cord samples were obtained after the delivery of normal-term babies with institutional review board approval. A portion of the umbilical cord was then cut into approximately 3-cm-long segments. The segments were then placed immediately into 25 mL of phosphate-buffered saline without calcium and magnesium (PBS) supplemented with antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin, 0.025 µg/mL amphotericin B). Up to three separate segments collected from the same donor cord were labeled A, B, or C. The tubes were then brought to the laboratory for dissection within 6 hours. Each 3-cm umbilical cord segment was dissected longitudinally utilizing aseptic technique. The tissue was carefully undermined and the umbilical vein and both umbilical arteries were removed. The remaining segment was sutured inside out and incubated in 25 mL of PBS, 1× antibiotic, and 1 mg per mL collagenase at room temperature. After 16 to 18 hours, the remaining suture and connective tissue were removed and discarded. The cell suspension was separated equally into two tubes, and the cells were washed three times by diluting with PBS to yield a final volume of 50 mL per tube and then centrifuged. Red blood cells were then lysed using a hypotonic solution. Finally, cells were plated onto six-well plates (surface area, 9.6 cm2 per well) at a concentration of 5 × 106 to 20 × 106 cells in 1 mL of medium per well.
Culture of umbilical cord: MSCs
UC-MSCs were cultured in low-glucose Dulbecco’s modified Eagle medium (DMEM) with 10 percent FBS (Hyclone, Logan, UT), 2 mmol per L l-glutamine, and antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin, 0.025 µg/mL amphotericin B). Cells were washed 48 hours after the initial plating with PBS and given fresh medium. Cell culture medium was subsequently changed twice a week through half medium changes. After 7 to 10 days following the initial plating in six-well plates, or approximately 70 to 80 percent confluence, cells were then passed using nonmammalian dissociation solution (HyQTase, Hyclone) from each individual well into one 10-cm plate (surface area, 58.95 cm2). Cells were then regularly passaged with a 1:2 dilution every 7 to 10 days or upon reaching 80 percent confluence. Aliquots of cultures were frozen beginning with Passage 1 in medium supplemented with 10 percent DMSO and 50 percent FBS.
Cell morphology
Cellular morphology was observed and documented under an inverted microscope and images were captured with a digital camera. Initial documentation occurred following 7 days of culture after the initial plating and continued as cells were continually passaged and subsequently utilized for long-term culture assays. Cultures were documented at 400× magnification.
Flow cytometry
Flow cytometry was performed on putative UC-MSCs to characterize the cells phenotypically and to compare them to BM-MSCs. Monoclonal antibodies to specific human cell markers were utilized to stain cells and multivariate flow cytometry was utilized to analyze the surface expression. Cells were stained and analyzed in three antibody groups allowing for characterization based on the published cell surface receptor profile of MSCs derived by in vitro expansion from marrow. Group A consisted of anti-human CD45-fluorescein isothiocyanate (FITC), CD73-phycoerythrin (PE), CD34-peridinin chlorophyll protein (PerCP)-Cy5.5, and CD105-allophycocyanin (APC). Group B consisted of anti-human CD90-FITC, CD166-PE, CD117-PerCP-Cy5.5, and CD38-APC. Group C consisted of anti-human CD44-FITC, HLA-ABC-PE, HLA-DR-PerCP-Cy5.5, and CD29-APC. The staining protocol was as follows. The UC-MSCs were detached from the plate using 2 mL of nonmammalian dissociation solution (HyQTase, Hyclone) after a PBS wash and transferred to a 5-mL polystyrene tube. Cells were then washed with flow cytometry buffer composed of 1× PBS supplemented with antibiotic (100 U/mL penicillin/100 µg/mL streptomycin) and 1 percent bovine serum albumin and resuspended at a concentration of 1 × 106 cells per 100 µL buffer containing the appropriate antibodies. Samples were then mixed and incubated at 4°C in the dark for 40 minutes. The cells were then washed twice with flow cytometry buffer and fixed in PBS plus 1 percent paraformaldehyde for later flow cytometric analysis.
Isolation of umbilical cord: hematopoietic stem and progenitor cells
Umbilical cord blood samples were obtained with institutional review board approval after the delivery of normal-term babies. Mononuclear cells (MNCs) were obtained by density centrifugation (Ficoll-Paque PLUS, GE Healthcare Biosciences AB, Uppsala, Sweden). Phenotypically defined CD34+-enriched HSCs/HPCs were obtained from MNCs by CD34-positive magnetic bead enrichment to a purity of greater than 98 percent on a magnetic cell sorter (AutoMACS, Miltenyi Biotech, Auburn, CA).
LTC-IC assay
Bulk LTC-IC assays14–16 were performed to assess the ability of UC-MSCs to support the growth and maintenance of cord blood–derived clonogenic cells using a modification of the standard LTC-IC procedure,17 as described below. Confluent mitomycin C (200 µg/mL)-treated feeder layers of UC-MSCs or BM-MSCs were established in six-well plates from initial plating at concentrations of 1 × 105 cells per well. At 24 hours after mitomycin C treatment, each well was seeded with 1 × 105 pooled CD34+ cord blood cells and incubated at 37°C 5 percent CO2 for 35 days. The use of 1 × 105 CD34+ cells in bulk culture rather than the use of limiting dilution analysis17 was chosen for these experiments since the ability of UC-MSCs to support long-term hematopoietic culture was being evaluated by LTC-IC method as opposed to determining the LTC-IC cell frequency within the total hematopoietic population. LTC-IC medium consisted of Iscove’s modified Dulbecco’s medium, 20 percent FBS, 2 mmol/L l-glutamine, 1000 units per mL penicillin, 100 units/mL streptomycin, and 1 mcM hydrocortisone. Medium were changed three times per week by half-media replacements. After 35 days, nonadherent and adherent hematopoietic cells were harvested and assayed for colony formation. Cells collected from each well were then plated in triplicate in 3.5-cm plates for myeloid progenitor colony formation in 1 percent methylcellulose culture medium with 30 percent FBS, 1 U per mL of recombinant human EPO, 100 U per mL recombinant human GM-CSF, 100 U per mL recombinant human IL-3, and 50 ng per mL recombinant human stem cell factor (SCF, steel factor).18 Cells were scored after 14 days of incubation at 37°C, 5 percent CO2, and 5 percent O2 generated in a dual-gas incubator through the addition of compressed CO2 and N2 gas. Data are presented as the absolute numbers of colony-forming cells (CFCs) per 1 × 105 CD34+ cord blood cells originally plated in the LTC-IC assay.
RESULTS
UC-MSC derivation and cell morphology
UC-MSCs were derived in a low-glucose DMEM supplemented with serum and antibiotics. Under these conditions, UC-MSCs display an adherent cell morphology and grow in colonies. Cells can be detached and replated to form a monolayer (Fig. 1A) similar to BM-MSCs. We observed that UC-MSCs can be cultured under these conditions longer than BM-MSCs, having the ability to be divided greater than 13 passages in our hands. Three cord segments (A, B, and C) were obtained from three different donors (Donors 1, 2, and 3) for the experiments described here. UC-MSCs were successfully obtained from UC-MSC Samples 1A, 1B, 1C, 2B, 2C, 3A, and 3B. UC-MSC Samples 2A and 3C did not successfully culture out cells. Upon review it was noted that Samples 2A and 3C did not achieve a threshold of 5 × 106 cells per well initial plating density.
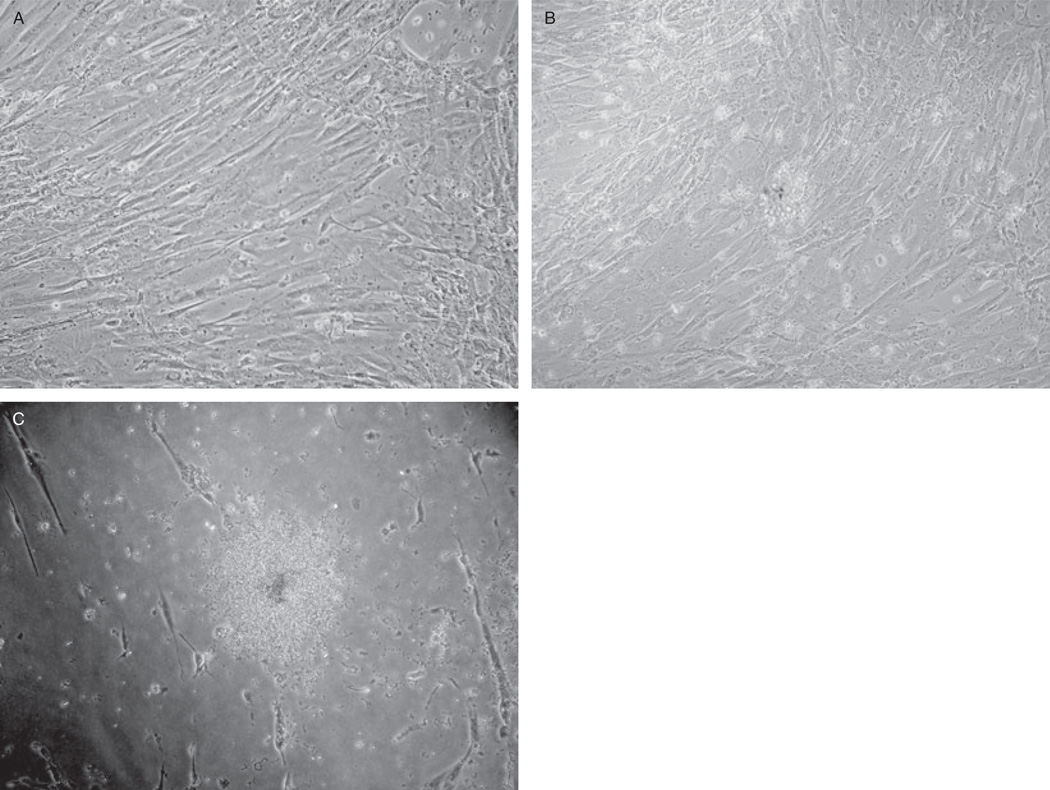
Fig. 1.
LTC-IC colony on UC-MSCs. UC-MSCs were observed to have the capacity to support the maintenance of hematopoietic colonies. (A) Shown is a typical monolayer of UC-MSCs (Passage 5) that was subsequently utilized for LTC-IC assays and (B) a typical LTC-IC colony formed when human cord blood CD34+ cells are grown on the UC-MSCs for 35 days. These colonies can then be detached using trypsin-ethylenediaminetetraacetate, placed in single-cell suspensions, and replated for myeloid progenitor colony formation in methylcellulose. Myeloid colonies (C) then form after an additional 14 days of methylcellulose culture. Magnification, 400×.
Flow cytometric analysis
We determined that both UC-MSCs that have been cultured to Passage 5 and BM-MSCs are positive for CD29 (integrin β1), CD44, CD73, CD90 (Thy-1), CD105 (endoglin), CD166 (activated leukocyte cell adhesion molecule), and HLA-A. In addition, both UC-MSCs and BM-MSCs are negative for CD45, CD34, CD38, CD117 (c-kit), and HLA-DR expression (Table 1). This expression profile is indicative of MSC, based on examination of BM-MSC cultures. Earlier passages of UC-MSCs, however, have a slightly different profile (Table 1). Specifically, Passage 3 UC-MSCs within the same culture have variable CD45 expression, express CD38, and lack CD105 and CD90.
TABLE 1.
MSC surface expression (%) of phenotypic markers
| MSCs | CD29 | CD44 | CD73 | CD166 | HLA-ABC | CD90 | CD105 | CD45 | CD38 | CD34 | CD117 | HLA-DR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM-MSC p3 | 99.9 | 99.9 | 99.9 | 100.0 | 99.95 | 99.1 | 99.8 | 0.6 | 0.3 | 6.0 | 0.2 | 3.4 |
| UC-MSC p3 | 100.0 | 99.8 | 92.7 | 99.6 | 99.8 | 0.6 | 1.5 | 99.9 | 99.5 | 0.1 | 2.7 | 0.9 |
| UC-MSC p5 | 99.9 | 99.9 | 99.8 | 99.7 | 99.8 | 99.9 | 99.7 | 0.8 | 1.1 | 0.5 | 0.5 | 1.0 |
| UC-MSC p7 | 99.8 | 99.6 | 99.6 | 99.5 | 99.8 | 99.8 | 99.8 | 0.6 | 0.8 | 0.7 | 0.7 | 1.1 |
| UC-MSC p13 | 99.9 | 99.6 | 99.8 | 99.6 | 99.9 | 100.0 | 99.7 | 0.4 | 0.9 | 0.3 | 0.3 | 2.1 |
LTC-IC assay
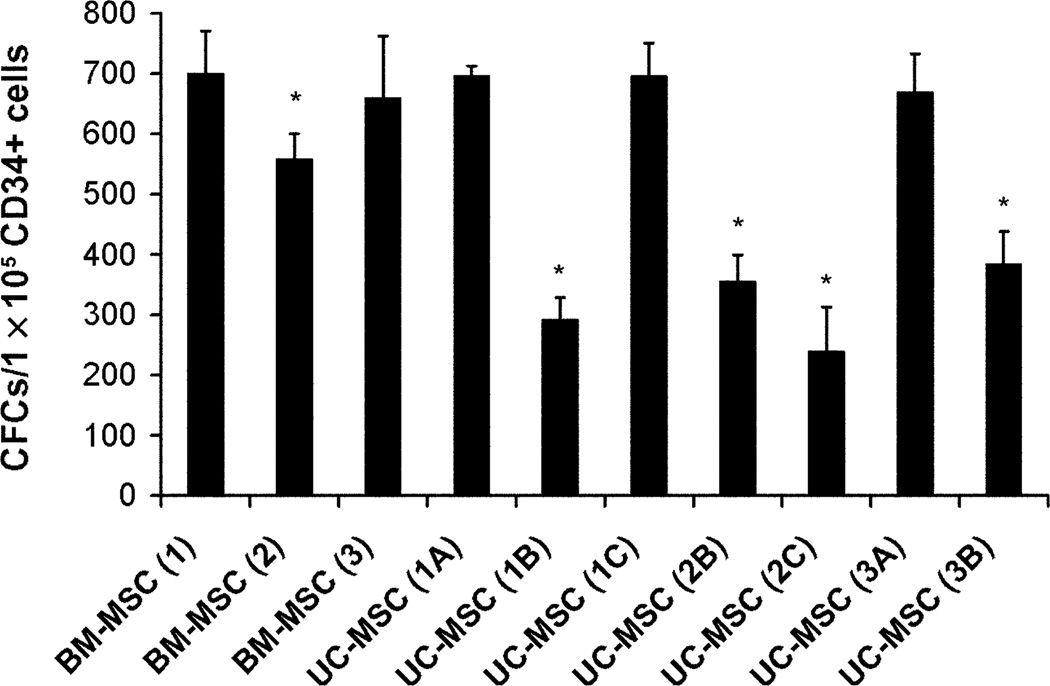
To test the UC-MSCs in a manner relevant to hematopoietic function, we chose to evaluate their capacity to act as a stromal layer by supporting the growth and maintenance of HSCs. As a component of this experiment, CD34+ cord blood cells were cultured on top of a UC-MSC feeder layer for 35 days and allowed to form colonies (Fig. 1B). The hematopoietic cells were then replated for an additional 14 days of culture in methylcellulose supplemented with growth factors for hematopoietic progenitor colony formation (Fig. 1C). UC-MSCs isolated from separate umbilical cord donors (Donors 1 through 3) from different sections of the cord (Sections A through C) support the growth of (1A) 696 ± 9.74, (2A) 292 ± 1 21.17, (3A) 696 ± 31.75, (2B) 356 ± 25.06, (2C) 240 ± 42.14, (3A) 670 ± 36.86, and (3B) 385 ± 30.87 CFCs per 1 × 105 CD34+ cord blood cells (mean ± SEM, n = 3 per segment; Fig. 2). BM-MSCs supported the growth of (1) 701 ± 40.60, (2) 559 ± 23.64, and (3) 660 ± 59.19 CFCs per 1 × 105 CD34+ cord blood cells (Fig. 2). UC-MSCs (1B) and (3C) are not included because no UC-MSCs were successfully cultured from these cord segments. When compared to BM-MSCs (1), there was no significant difference in the ability of BM-MSCs (3) and UC-MSCs (1A, 1C, 3A) to support LTC-IC growth. All other samples, including BM-MSCs (2) as well as UC-MSCs (1B, 2B, 2C, 3B), had a significant reduction in their ability to support the growth of LTC-ICs (t test, p ≤ 0.05, n = 3).
Fig. 2.
LTC-IC assay. The numbers of CFCs per 1 × 105 CD34+ cord blood cells were assessed after 35 days of culture on either BM-MSCs or UC-MSCs followed by 14 days of myeloid progenitor colony formation in methylcellulose medium. Different MSC donors are represented by numbers (1–3) and different segments of the cord from the same donor are represented by letters (A–C). Some UC-MSC samples were observed to be capable of supporting cord blood LTC-IC formation at levels comparable to BM-MSCs (UC-MSC 1A, 1C, 3A) while other UC-MSCs were not (UC-MSC 1B, 2B, 2C, 3B). The ability to support LTC-IC cultures for all donor samples was statistically compared to BM-MSCs (1A) utilizing a t test (*p ≤ 0.05, n = 3 for each sample).
DISCUSSION
Cord blood has been used as a transplantable source of HSCs/HPCs since it was first introduced for hematopoietic stem cell transplantation (HSCT) in 1988.19–21 Given that cord blood is readily available, has a lower histocompatibility requirement,22,23 and carries a reduced risk of graft-versus-host disease,22,23 there are advantages to utilizing cord blood for allogeneic HSCT, especially when a matched marrow or peripheral blood stem cell donor is not available. However, the amount of cord blood collected is a limiting factor, in most cases only yielding sufficient quantities for a child recipient. This problem could possibly be overcome in part through ex vivo expansion of the donor HSC population in a manner that supports not only HSC maintenance but also subsequent homing and engraftment potential.
The use of MSCs as a feeder layer is an attractive alternative to cytokine based ex vivo expansion. This is in part due to reports that have described the potential of MSCs to promote engraftment of CD34+ HSCs/HPCs into NOD/SCID mice.4 UC-MSCs derived from the Wharton’s jelly of umbilical cords have been described by others as fibroblastlike cells,7 candidate MSC-like cells,8 matrix cells,9 or human umbilical cord perivascular cells.10 These cells, or similar cells derived from the umbilical cord, have been previously shown to have the ability to differentiate in vitro into adipocytes, chondrocytes, osteocytes, osteoblasts, neurons, and myocytes.8,9,24–27 This ability to differentiate down multiple lineages is supportive of the notion that these cells have MSC potential.
Our interest in cellular therapy and stem cell transplantation biology led us to evaluate whether UC-MSCs can act as MSCs in the context of supporting hematopoiesis. To perform this task, we first set out to isolate UC-MSCs from umbilical cord segments utilizing established method for the derivation of MSCs using serum containing low-glucose medium. We obtained samples from three different BM-MSC donors and three different UC-MSC donors. We attempted to culture UC-MSCs from three segments from each UC-MSC donor individually. Our results show that we were not able to successfully culture adequate UC-MSCs from all segments but that we were able to culture UC-MSCs from all donors. We also successfully froze the UC-MSCs cryogenically and thawed for later use throughout these studies. Of interest was that although the segments were all 3 cm in length, the umbilical cord segments that did not grow UC-MSCs successfully were plated at concentrations of less than 5 × 106 cells per well. Clearly there is a relationship between plating density and the successful recovery of UC-MSCs. The reasons for the differences in the cell numbers obtained from the different segments could be related to the biology of the cord segments or to variations in the efficiency of collagenase treatment to disrupt the tissue from the segments. Our data suggest that further research into more effective methods to obtain cells from umbilical cord segments that subsequently culture as UC-MSCs is warranted. The data also confirm that it is possible to derive MSCs from umbilical cord Wharton’s jelly that share the same phenotypic markers as BM-MSCs but that early passages, 3 or less, may be phenotypically and possibly functionally different than later passages. Interestingly, we also observed that UC-MSCs may have an extended culture capacity compared to BM-MSCs, specifically greater than 13 passages.
To validate that we had succeeded in deriving cells that were phenotypically similar to the MSCs that have been previously described by other laboratories we profiled the cell surface expression of 12 receptors. We have observed that cultures of early passages of Wharton’s jelly–derived UC-MSCs expressed cell surface receptors not observed on BM-MSCs (CD45 and CD38) and are missing cell surface receptors present on BM-MSCs (CD90 and CD105). Eventually as the UC-MSCs were passaged the cell surface profile changed to meet the definition of MSCs that was originally described by BM-MSCs (positive for CD29 [integrin β1], CD44, CD73, CD90 [Thy-1], CD105 [endoglin], CD166 [activated leukocyte cell adhesion molecule], and HLA-A; negative for CD45, CD34, CD38, CD117 [c-kit], and HLA-DR). It is possible that his interesting observation is a result of the derivation process that likely involves selection of a particular subpopulation of the total cell population in culture resulting in one population that has become dominant in culture. It is also possible that the cells are simply phenotypically different because the cells are being derived from a more primitive fetal tissue source. Fundamentally, MSCs derived from various tissue sources may not initially have an identical cell surface receptor profile because the mixture of cells in different tissues is inherently different. Further investigation of umbilical cord–, placental-, other fetal tissue–, and other adult non–marrow-derived MSCs should elucidate the answer to this question.
Finally, we evaluated the capacity of UC-MSCs to support the growth and maintenance of HSCs/HPCs in long-term culture. The results of our experiments suggest that UC-MSCs do indeed have the capacity to support long-term maintenance of HSCs, as defined by the LTC-IC assay. Significant variation in the ability of BM-MSCs to support long-term maintenance of cord blood CD34+ cells in the LTC-IC assay was observed. It was also observed that UC-MSC cultures from some segments had an equal ability to support LTC-IC maintenance when compared to BM-MSC and UC-MSC cultures from other segments that had a significant reduction in their ability to support the growth of LTC-IC. Sometimes the UC-MSC cultures that had a reduction in their support of cord blood maintenance came from the same donor as a UC-MSC culture from a segment that performed equally well as compared to BM-MSCs. This suggests that the ability of UC-MSCs to support long-term culture of cord blood HSCs/HPCs may be independent of the specific donor. Of great importance is that all BM-MSC cultures and all UC-MSC cultures tested had the capacity to support the long-term ex vivo culture of cord blood HSCs/HPCs.
We believe these findings to have potential therapeutic application with respect to ex vivo stem cell expansion of cord blood HSCs utilizing a UC-MSC feeder layer. In addition, these data suggest the possibility of cotransplantation of matched mesenchymal and HSCs from the same umbilical cord and cord blood donor source or from HLA-matched umbilical cord and cord blood donors. Finally, these results describe a novel model system for future study of the interaction between cord blood HSCs and the appropriate supportive microenvironment represented by the UC-MSCs. Future experiments involving the transplantation of cord blood HSCs/HPCs cultured on UC-MSCs into immunodeficient mouse models will likely reveal more information about the potential future therapeutic application of UC-MSCs in the transplant setting.
Acknowledgments
KWC was supported during these projects by grants from the American Association for Cancer Research, the Leukemia & Lymphoma Society, and the National Blood Foundation/AABB.
ABBREVIATIONS
- APC
allophycocyanin
- BM-MSC(s)
bone marrow–derived mesenchymal stem cells
- CFC(s)
colony-forming cell(s)
- HSC(s)
hematopoietic stem cells
- HSCs/HPCs
hematopoietic stem and progenitor cell(s)
- HSCT
hematopoietic stem cell transplantation
- LTC-IC(s)
long-term culture-initiating cell(s)
- MSC(s)
mesenchymal stem cell(s)
- NOD/SCID
nonobese diabetic/severe combined immunodeficient
- PBS
phosphate-buffered saline without calcium and magnesium
- PerCP
peridinin chlorophyll protein
- UC-MSCs
umbilical cord–derived mesenchymal stem cells
REFERENCES
- 1.Breems DA, Blokland EA, Siebel KE, Mayen AE, Engels LJ, Ploemacher RE. Stroma-contact prevents loss of hematopoietic stem cell quality during ex vivo expansion of CD34+ mobilized peripheral blood stem cells. Blood. 1998;91:111–117. [PubMed] [Google Scholar]
- 2.McNiece I, Harrington J, Turney J, Kellner J, Shpall EJ. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 3.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, Messinger R, Flagge F, de Lima M, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noort WA, Kruisselbrink AB, in’t Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870–878. doi: 10.1016/s0301-472x(02)00820-2. [DOI] [PubMed] [Google Scholar]
- 5.Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 7.McElreavey KD, Irvine AI, Ennis KT, McLean WH. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19:29S. doi: 10.1042/bst019029s. [DOI] [PubMed] [Google Scholar]
- 8.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 10.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 11.Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 2005;118:1987–1993. [PubMed] [Google Scholar]
- 12.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–1486. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Zandstra PW, Conneally E, Petzer AL, Piret JM, Eaves CJ. Cytokine manipulation of primitive human hematopoietic cell self-renewal. Proc Natl Acad Sci U S A. 1997;94:4698–4703. doi: 10.1073/pnas.94.9.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller CL, Eaves CJ. Long-term culture-initiating cell assays for human and murine cells. In: Klug CA, Jordan CT, editors. Methods in molecular medicine, 63. xi, 332. Totowa (NJ): Humana Press; 2002. pp. 123–142. [DOI] [PubMed] [Google Scholar]
- 16.Manchester M, Smith KA, Eto DS, Perkin HB, Torbett BE. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol. 2002;76:6636–6642. doi: 10.1128/JVI.76.13.6636-6642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland HJ, Lansdorp PM, Henkelman DH, Eaves AC, Eaves CJ. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci U S A. 1990;87:3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 19.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 21.Broxmeyer H, Smith F. Cord blood stem cell transplantation. In: Forman SJ, Blume KG, Thomas ED, editors. Hematopoietic cell transplantation. Oxford, Malden (MA): Blackwell Science; 1999. pp. 431–443. [Google Scholar]
- 22.Harris DT. Cord blood transplantation: implications for graft vs. host disease and graft vs. leukemia. Blood Cells. 1994;20:560–564. discussion 4–5. [PubMed] [Google Scholar]
- 23.Wagner JE. Umbilical cord blood transplantation: overview of the clinical experience. Blood Cells. 1994;20:227–233. discussion 33-4. [PubMed] [Google Scholar]
- 24.Covas DT, Siufi JL, Silva AR, Orellana MD. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36:1179–1183. doi: 10.1590/s0100-879x2003000900006. [DOI] [PubMed] [Google Scholar]
- 25.Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 26.Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 27.Conconi MT, Burra P, Di Liddo R, Calore C, Turetta M, Bellini S, Bo P, Nussdorfer GG, Parnigotto PP. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089–1096. [PubMed] [Google Scholar]