Abstract
Aims
Stem cell transplantation holds promise as a therapeutic approach for the repair of damaged myocardial tissue. One challenge of this approach is efficient delivery and long-term retention of the stem cells. Although several synthetic and natural biomaterials have been developed for this purpose, the ideal formulation has yet to be identified.
Materials & methods
Here we investigate the utility of a nondenatured, noncrosslinked, commercially available natural biomaterial (TissueMend® [TEI Biosciences, Boston, MA, USA]) for delivery of human mesenchymal stem cells (MSCs) to the murine heart.
Results
We found that MSCs attached, proliferated and migrated within and out of the TissueMend matrix in vitro. Human MSCs delivered to damaged murine myocardium via the matrix (2.3 × 104 ± 0.8 × 104 CD73+ cells/matrix) were maintained in vivo for 3 weeks and underwent at least three population doublings during that period (21.9 × 104 ± 14.4 × 104 CD73+ cells/matrix). In addition, collagen within the TissueMend matrix could be remodeled by MSCs in vivo, resulting in a significant decrease in the coefficient of alignment of fibers (0.12 ± 0.12) compared with the matrix alone (0.28 ± 0.07), and the MSCs were capable of migrating out of the matrix and into the host tissue.
Conclusion
Thus, TissueMend matrix offers a commercially available, biocompatible and malleable vehicle for the delivery and retention of stem cells to the heart.
Keywords: biomaterial, cardiac patch, cardiac regeneration, collagen, mesenchymal stem cells
Ischemic heart disease is the leading cause of death in the USA and worldwide [101,102]. Ischemic heart disease is the result of an imbalance between myocardial blood flow and the metabolic demand of the myocardium that may eventually lead to infarction(s) [1,2]. Following myocardial infarction, pharmacological therapies (e.g., vasodilators, thrombolytics and antihypertensives) and cardiovascular intervention therapies (e.g., angioplasty, atherectomy, stenting and coronary artery bypass grafts) can improve blood supply to the heart. Regenerative therapies (e.g., transplantation of stem cells or stem cell progeny) are under development to recover myocardial function [3–10]. This is a promising approach since stem cells can give rise not only to functional cardiomyocytes, but also to endothelial cells and smooth muscle cells that can promote revascularization of damaged tissue [11–15]. The success of this approach requires an effective means to deliver cells to the damaged heart in a manner that enhances donor cell retention and long-term engraftment. Several delivery modalities have been attempted including bolus intravascular injection [16–19], intramyocardial injection [20–28], and delivery via various synthetic [29–31] or natural biomaterials [32–34].
Direct stem cell injection (i.e., intravascular or intramyocardial) is typically associated with poor cell retention [35,36] and so biomaterial constructs have been developed to overcome this challenge (reviewed in [37–42]). These efforts have been met with varying levels of success and include the following approaches. Several polymer constructs have been attempted to date, including cell encapsulation in poly(ethylene glycol) (PEG) hydrogels [43]. PEG has been utilized due to the inert quality of the material as well as the relative ease with which mechanical and physical properties can be tailored. However, cells seeded in PEG hydrogels typically exhibited rounded morphology, even when adhesion peptides (e.g., RGD, YIGSR) are covalently linked to the polymer. In addition, because the polymer cannot be degraded by cellular enzymes, cells in PEG constructs cannot migrate and thus tend to preferentially undergo chondrogenesis [44–47]. While natural polymers, such as the extracellular matrix (ECM) proteins, may be less tunable, they provide native attachment, proliferation and differentiation signals. ECM proteins also contain matrix metalloproteinase binding sites, providing the opportunity for cellular remodeling of the matrix to mimic the tissue of interest. However, most commercially available ECM matrices are chemically crosslinked, which increases stiffness but can also mask matrix metalloproteinase binding sites [48–50]. In addition, ECM constructs are typically derived from tissues other than the heart and thus contain a mixture of extracellular proteins distinct from that of the myocardium.
We have recently identified an extracellular matrix-based material that is well poised for cardiac repair due to its protein composition and noncrosslinked state. This matrix, TissueMend® (TEI Biosciences, Boston, MA, USA), is derived from fetal bovine dermis and was initially created for tendon surgery to reinforce the soft tissue. Unlike mature dermis, this tissue matrix is comprised of relatively high concentrations of type III collagen (20–30%) in addition to type I collagen (70–80%; company communication [103]). This composition of fibrillar collagens is similar to that of the mature heart [51]. In addition, unlike most available animal-derived tissue matrices, TissueMend is not chemically crosslinked. This is an important feature because it may allow transplanted cells and cells of the host to remodel the network prior to and following transplantation to a form that is structurally favorable. Here we test the utility of TissueMend as a delivery vehicle for human mesenchymal stem cells (MSCs) in the heart. MSC-seeded matrices were probed for cell engraftment, migration and extended viability in vitro as well as for cell retention, remodeling and differentiation potential in vivo.
Material & methods
Cell isolation & maintenance
Mesenchymal stem cells derived from human embryonic stem cells (WA-09, a gift from Dr Peiman Hematti) were expanded and cultured as previously described [52]. Briefly, cells were cultured on a 0.1% gelatin (Sigma-Aldrich, St Louis, MO, USA) pretreated flask containing α-minimum essential media (MEM)-complete. α-MEM-complete consisted of α-MEM (Invitrogen, Carlsbad, CA, USA), 10% fetal bovine serum (Hyclone, Logan, UT, USA), 1% nonessential amino acids (Invitrogen) and 1% l-glutamine (Invitrogen). Cultures were allowed to grow to 60–70% confluency and were replated at a concentration of 1500 cells/cm2. MSCs cultured in this way express CD73, CD90 and CD105 (Supplementary Figure 1) (see online at www.futuremedicine.com/doi/suppl/10.2217/rme.11.48) and with proper stimulation are able to differentiate down the chondrogenic, osteogenic and adipogenic pathways. Experiments were performed using passages 7–10 and all cultures were maintained at 37°C in 5% CO2.
Preparation of the TissueMend matrix
For in vitro studies, pieces of TissueMend matrix (2 × 2 × 0.8 mm) were placed in wells of 24-well plates and hydrated with α-MEM-complete culture medium. H9MSCs were seeded on the TissueMend sections at a concentration of 1 × 106 cells/ml. The medium was changed every 3–4 days and cultures were maintained up to 3 weeks. For in vivo studies, TissueMend matrix was prepared in the same way and cultured with MSCs for 2 days prior to implantation (see Delivery of MSCs to the murine myocardium section) at which point the matrix contained approximately 2.3 × 104 total cells (see In vitro optical analysis below).
In vitro optical analysis
To determine the number of cells delivered, the degree of cell attachment and cell spreading, MSC-seeded TissueMend matrices were cultured for 2 days before staining with CellTracker™ Red (15 µM; Invitrogen), according to the manufacturer’s instructions. Cells of the matrix were imaged using multiphoton laser scanning microscopy (MPLSM) [53]. MPLSM allows for deep sectioning of 3D tissues, such as the TissueMend matrix, and affords noninvasive analysis of collagen fiber orientation via second harmonic generation (SHG) [54]. SHG signal is generated when two photons of incident light interact with the noncentrosymmetric structure of collagen fibers, such that the resulting photons are half the wavelength of the incident photons. For all multiphoton and second harmonic imaging, a custom multiphoton workstation at the University of Wisconsin Laboratory for Optical and Computational Instrumentation (LOCI) was used [55–57]. The tissue samples were imaged using a TE300 inverted microscope (Nikon, Tokyo, Japan) equipped with a Plan APO VC 20× (numerical aperture 0.75; Nikon Instruments, Tokyo, Japan) objective lens by using a mode-locked Ti:Sapphire laser (Spectra-Physics® Mai Tai®, Mountain View, CA, USA). Tuning the excitation wavelength to 890 nm, a 445/1 nm narrow band pass emission filter (Thin Film Imaging, Greenfield, MA, USA) was used to detect the SHG signal of collagen in the backscattered mode using a H7422P GaAsP photon counting photomultiplier tube (Hamamatsu Photonics KK, Shizuoka, Japan). For detection of CellTracker Red, a 580 nm long pass emission filter (Thin Film Imaging) was used. Images of 1024 × 1024 pixels were acquired using WiscScan under identical conditions. The power of the laser at the sample and gain were set to allow 5% or less saturation prior to data collection. The number of cells in the TissueMend matrix just prior to implantation was determined by first counting the number of cells in at least three fields of three different matrices at a 200 µm 3D volume (images taken at 5 µm intervals); the total number of cells were expressed per unit volume (total volume of each field was 0.08 mm3). Cell number per volume (mm3) was then multiplied by the total volume of the matrix (3.2 mm3) to yield total cell number per matrix. Quantitative analysis of spreading was conducted by outlining at least 16 cells from at least two matrices (3D) and one culture (2D). The area of outlined cells was determined using ImageJ (Fiji distribution, open source software). 3D reconstructions were generated using Imaris 7.2.3 software (Bitplane AG, Zurich, Switzerland).
For migration studies, H9MSCs were seeded at a concentration of 1 × 106 cells/ml on the TissueMend matrix and allowed to attach for 24 h. The matrix was then rinsed twice with fresh medium to remove nonadherent cells and transferred to new gelatin-coated wells (as above) containing fresh culture media. Over the course of 62 h, brightfield images at 10× magnification were taken using an Axiovert 40 CFL inverted microscope (Zeiss, Thornwood, NY, USA). The distance between matrix border and the leading edge of cells exiting the matrix was determined at 12, 40 and 62 h. Migration data was acquired from three matrices at each time point; at least three distance measures were made per matrix per time point. Migration data were plotted as the distance traveled (µm) as a function of time (h). Migration speed was defined as the distance traveled (µm) per unit time (h) [58].
Proliferation was determined using a Click-it EdU assay (Invitrogen) according to the manufacturer’s instructions. Briefly, H9MSCs were seeded at a concentration of 1 × 106 cells/ml and allowed to adhere for 48 h on either 2D tissue culture substrate or 3D TissueMend matrix. Cell cultures (n = 3 each for 2D and 3D conditions) were incubated with 20 µM EdU for 24 h, followed by fixation with 4% paraformaldehyde (Acros Organics, Pittsburgh, PA, USA), permeabilization with 0.5% Triton-X (MP Biomedicals, Solon, OH, USA) and nuclear counter-stained with 10 µg/ml Hoechst 33342 (Invitrogen). Cell counts were taken from at least three fields per 2D culture or 3D matrix using an IX71 inverted deconvolution fluorescence microscope (Olympus, Center Valley, PA, USA).
Mechanical testing of TissueMend matrix
An ARES-LS2 rheometer (TA Instruments, New Castle, DE, USA) was used to measure the viscoelasticity of the hydrated TissueMend matrix. The matrix was cut with an 8 mm biopsy punch and submerged in phosphate-buffered saline (PBS) or 2 mg/ml collagenase II (Worthington Biochemical, Lakewood, NJ, USA) in PBS on a rocker for 24 h at room temperature. The upper plate (parallel plate, 8 mm diameter) was lowered until it was in conformal contact with the top surface of the matrix, corresponding to gap distances of 893 ± 80.22 mm and 453 ± 162.15 mm. Storage modulus and loss modulus were measured at 1.0% strain, the temperature was maintained in a chamber at 25°C and three independent matrices were measured for each condition.
Induction of myocardial infarction in mice
Myocardial infarction was induced in C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME, USA) by left coronary artery ligation as previously described [59] and as is routinely performed in the University of Wisconsin Cardiovascular Physiology Core Facility. All animal procedures were performed in accordance with the guidelines of the American Association for Laboratory Animal Science and the University of Wisconsin-Madison Animal Care and Use Committee. Briefly, an intercostal thoracotomy was performed to visualize the heart. A 7–0 or 8–0 Prolene suture (Ethicon, Somerville, NJ, USA) was placed through the myocardium in the anterolateral wall. The suture was secured and coronary artery entrapment was confirmed by observing blanching of the distal circulation (ventricular apex). The ribs, muscle layers and skin were closed with 6–0 Prolene (Ethicon).
Delivery of MSCs to the murine myocardium
A 2 × 2 × 0.8 mm TissueMend matrix containing approximately 2.3 × 104 H9MSCs or unseeded (control) was tacked to the myocardium at each corner of the matrix using 7–0 Prolene suture 2 days after infarction. Human MSCs were selected as the donor cell population to most directly assess the clinical utility of human cell delivery with the TissueMend matrix. Tacking of the matrix to the myocardium utilized a stitch similar to a mattress stitch and the area covered by the matrix included a portion of the infarct and peri-infarct regions.
Optical analysis of heart/tissue explants
Murine hearts were harvested 3 weeks after matrix implantation to assess engraftment and cell state within the scaffold (Figure 1). Hearts were bisected longitudinally through the scaffold. The tissues were immediately placed into 10% buffered formalin (pH = 7.2; Fisher Scientific, Forest Lawn, NJ, USA) for 24 h followed by 24 h of fresh 10% buffered formalin, and a final 24 h of 70% ethanol. Samples were further processed for histological analysis as previously described [60].
Figure 1. Ventricular section and associated TissueMend® (TEI Biosciences, Boston, MA, USA) matrix 3 weeks after in vivo transplantation.
(A) Representative excised heart containing the scaffold. (B) Representative left ventricular cross-section as prepared for histology. (C) Mosaic of images of the left ventricle (heart, red arrow), border region (border, orange arrow) and TissueMend matrix (TissueMend, black arrow). This low-magnification composite allows qualitative inspection of the morphology of the cells of the myocardium, border and matrix and the relationship between components. The rectangle, with the overlay of the fluorescence image of labeled endothelial cells from Supplementary Figure 2, indicates the region from which images of Figure 5B were taken.
(A) Scale bar = 1 cm; (B) Scale bar = 1 cm; (C) Scale bar = 100 µm.
For phenotypic analyses, 5 µm thick paraffin histological sections were probed for MSCs, and cardiac and vascular proteins using immunofluorescent staining. To identify MSCs, sections were probed with goat anti-CD73 (polyclonal antibody, V-20; Santa Cruz Biotech, Santa Cruz, CA, USA). The primary antibody was used at a 1:25 dilution in diluting buffer (5% bovine serum albumin [Fisher Scientific], 0.02% NaN3− [Acros Organics] in PBS [Fisher Scientific]) and incubated overnight at 4°C. Detection of CD73 was achieved by incubating with a secondary antibody, donkey anti-goat Alexa Fluor (AF488; Invitrogen), at a 1:200 dilution in preadsorption solution (90% diluting buffer, 5% human serum [Pelfreez, Brown Deer, WI, USA] and 5% mouse serum [Equitech-Bio, Inc, Kerrville, TX, USA]) for 45 min at room temperature. To identify mature cell types, sections were probed with primary antibodies for cardiomyocytes (mouse anti-troponin T cardiac isoform Ab-1, cardiac troponin T [cTnT], clone 13–11 [Fisher Scientific]); for endothelial cells (rabbit anti-von Willebrand Factor [vWF]; Sigma-Aldrich); and for fibroblast-like cells (rabbit anti-discoidin domain receptor [DDR]-2 (H-108); Santa Cruz Biotechnology). Following deparaffinization and rehydration, sections were subjected to antigen retrieval as recommended by the manufacturer: anti-vWF–1 mM ethylenediaminetetraacetic acid (Fisher Scientific), pH 8.0 + 0.01% Triton-X 100 (MP Biomedicals) for 30 min at 97°C; anti-cTnT and anti-DDR2, 10 mM sodium citrate (Sigma-Aldrich), pH 6.0 + 0.01% Triton-X for 15 min to 30 at 97°C. Following incubation for 30 min at room temperature in blocking solution (either 5% bovine serum albumin, 1% glycine [Fisher Scientific], 2% donkey serum [Sigma-Aldrich], 0.1% Triton-X 100 for anti-cTnT or with 5% bovine serum albumin, 2% normal goat serum [Invitrogen], 0.1% Triton-X 100 for anti-vWF and anti-DDR2) the sections were incubated in the primary antibodies, diluted at 1:20 (anti-DDR2) or 1:50 (anti-cTnT and anti-vWF) in blocking solution overnight at 4°C. Sections were incubated in secondary antibodies (donkey anti-mouse AF 546, goat anti-rabbit AF 588 or goat anti-rabbit AF 647 [Invitrogen]) for 1 h at room temperature. All stained sections were mounted in 1,4-diazabicyclo[2.2.2]octane/4´,6-diamidino-2-phenylindole mounting medium (2.5% 1,4-diazabicyclo[2.2.2]octane [Sigma-Aldrich], 50% glycerol [Fisher Scientific], and 0.005% 4´,6-diamidino-2-phenylindole [Sigma-Aldrich] in PBS). Fluorescence emission was detected on an IX71 inverted deconvolution fluorescence microscope (Olympus). Images were acquired with 20× UPlanFluor objective (numerical aperture = 0.5) and analyzed with Slidebook software (Intelligent Imaging Innovations, Denver, CO, USA) and with ImageJA (Fiji; open source software). Images were normalized using a secondary only control for each labeling procedure in order to minimize background fluorescence attributable to nonspecific secondary antibody labeling and autofluorescence. Positive events were reported as a percentage of the total cell number obtained from analysis of at least eight optical fields from multiple histological sections of at least three matrices seeded with MSCs and at least one unseeded matrix. For DDR2-labeled tissue, a total of 11 optical fields of the border region from three sections from each of two hearts with seeded matrices or a total of six optical fields from three sections from one heart with unseeded matrix were counted. The total number of CD73+ in the matrix was determined by counting the number of positive events in at least three optical fields from multiple histological sections of at least three matrices. Based on the dimensions of the field of view and assuming a tissue depth of 5 µm, a cell number per mm3 was determined. Cell number per volume (mm3) was then multiplied by the total volume (3.2 mm3) per matrix to yield the total cell number per matrix. Cell retention was defined as the calculated number of CD73+ cells in the seeded matrices minus the calculated number of CD73+ cells in the unseeded matrices.
To determine the origin of cells in the border region, a fluorescent in situ hybridization tissue digestion kit targeting all human centromeres (Kreatech, Amsterdam, The Netherlands) was performed. Samples were processed by the Cytogenetics Laboratory (WiCell Research Institute, Madison, WI, USA) according to manufacturer’s protocol. Briefly, paraffin-embedded slides were baked for 4 h at 56°C. Specimens were incubated with pepsin for 20 min for tissue digestion prior to probe application.
For in vivo remodeling studies, SHG imaging and analysis was conducted on histological sections of hearts containing the TissueMend matrix using MPLSM. Traditional Fourier-based analysis methods [61,62] are not particularly effective at detecting edges in images with overlapping fibers or curves, which are a major feature in images of the ECM. As an alternative, the curvelet transform, developed by Candes and Donoho [63], is specifically designed to determine a sparse representation of edges in images even in the presence of complex geometries such as those associated in the SHG images of the collagen. We used software from LOCI that utilizes the curvelet transform to interactively measure distribution of angles for a defined region of interest. Generated images were reconstructed using the LOCI curvelet software. The curvelet software was used to determine a ‘coefficient of alignment’ that corresponds to the relative distribution of angles of fibers in each sample. A coefficient that approaches 1 indicates that the orientation of fibers in the sample are grouped closely about the mean. Image analysis was conducted on at least three optical fields from one matrix seeded with MSCs and one unseeded matrix.
Statistical analysis
For comparison of collagen fiber alignment, MSC spreading and MSC proliferation in the TissueMend matrix versus controls, a normal distribution was assumed and one-way analyses of variance and Student’s t test were used. Data were analyzed with Microsoft Excel (Microsoft, Redmond, WA, USA).
Results
MSC attachment & proliferation in the TissueMend matrix
To determine whether MSCs could survive and be maintained for an extended period in the TissueMend matrix, we seeded the matrix with 2.3 × 104 MSCs and monitored the ability of MSCs to attach to the matrix and proliferate. MSCs were well adhered by 24 h but exhibited decreased spreading (496 ± 209 µm2), compared with those cultured on tissue culture polystyrene (2660 ± 861 µm2, p < 0.01; Table 1). In addition, MSCs were able to infiltrate the matrix (Figure 2 & Supplementary Movie 1) and could descend at least 400 µm into the 800 µm thick matrix. Furthermore, MSCs could proliferate in the TissueMend matrix at approximately the same rate as when grown on tissue culture polystyrene. Over a 24 h period, approximately 30 ± 17% of all cells in the matrix were undergoing proliferation while 53 ± 4% were proliferating on the polystyrene (p = 0.18; Table 1). The high variability of proliferation of MSCs seeded in the TissueMend matrix could be attributed, in part, to contact inhibition. We noted the MSCs of the matrix were more densely packed than those on culture plates, perhaps because initial seeding concentration assumed a solid 3D structure when, in fact, the matrix is porous.
Table 1.
Indication of cell state in 2D tissue culture versus 3D TissueMend® (TEI Biosciences, Boston, MA, USA) matrix.
| Cell state | 2D tissue culture | 3D TissueMend | p-value |
|---|---|---|---|
| Spreading | 2660 ± 861 mm2 | 496 ± 209 mm2 | <0.01 |
| Proliferation | 53 ± 4% of total cells | 30 ± 17% of total cells | 0.18 |
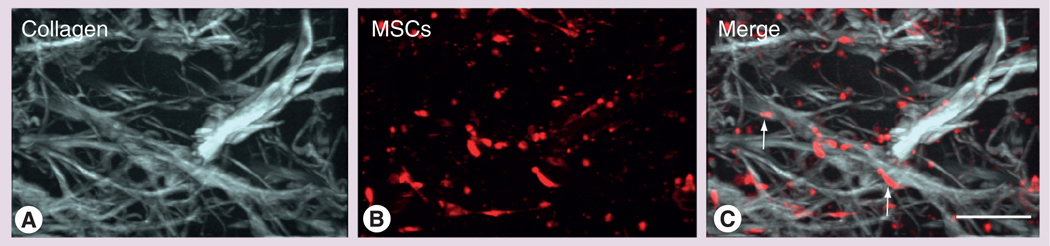
Figure 2. Optical analysis of mesenchymal stem cell–TissueMend® (TEI Biosciences, Boston, MA, USA) interactions in vitro.
MSCs were seeded at a concentration of 1 × 106 cells/ml on top of the TissueMend matrix. The matrix was imaged 48 h after seeding using multiphoton laser scanning microscopy. 3D reconstructions of (A) second harmonic generation optical sections showing collagen fibrils in the matrix (white) and (B) CellTracker™-labeled MSCs (red). Cells were detected as deep as 400 µm into the tissue; images shown are at a 160 µm thick. (C) MSCs appeared healthy and well spread in the matrix and grew in alignment with the matrix fibrils (arrows).
Scale bar = 150 µm.
MSC: Mesenchymal stem cell.
MSC migration from the TissueMend matrix
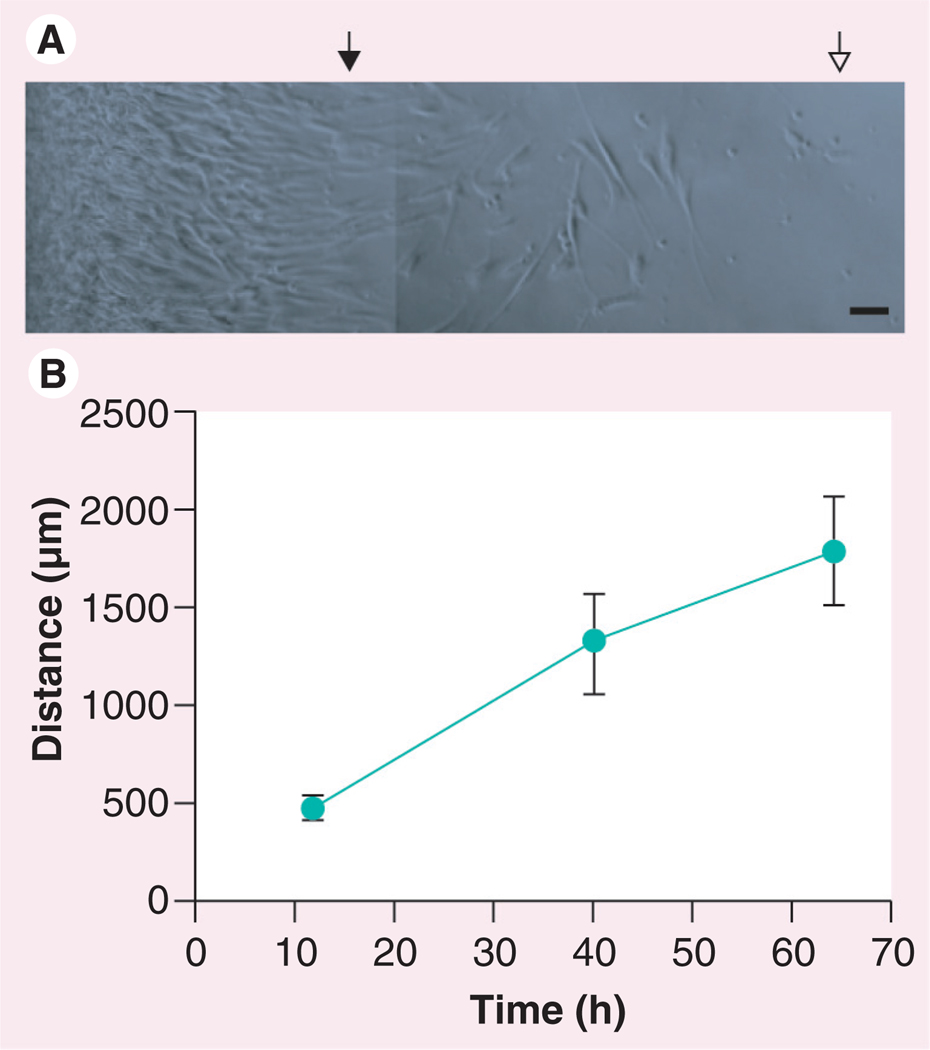
One goal of stem cell transfer to the heart is incorporation or interface with the myocardium. To determine whether MSCs were capable of migration from the TissueMend matrix, we transferred MSC-seeded TissueMend matrices to fresh tissue culture wells and measured the distance traveled at approximately 1, 2 and 3 days after transfer. The rate of departure was determined by measuring the distance between the TissueMend border and the leading edge of migrating cells over time. MSCs did migrate from the matrix at an average speed of migration of 33 ± 5 µm/h (Figure 3). Migration of many adherent cell types including MSCs can be facilitated by durotaxis, the tendency of cells to move up or down a rigidity gradient [64–66]. This cell behavior might prove adventitious for cardiac repair via the TissueMend matrix. In its current form, TissueMend compressive modulus is significantly stiffer than the native myocardium (~40 MPa vs ~40 KPa) or some previously used ECM-derived patches [67], however, future TissueMend formulations could be designed to be less stiff than diseased and fibrotic myocardium, which may promote repopulation of diseased myocardium over corresponding healthy tissue.
Figure 3. Migration of mesenchymal stem cells from the TissueMend® (TEI Biosciences, Boston, MA, USA) matrix in vitro.
(A) Brightfield image of mesenchymal stem cells migrating from the TissueMend matrix. The left edge indicates the edge of the matrix, closed triangle indicates location of the leading edge after 12 h and the open triangle indicates the location of the leading edge after 40 h. (B) Distance traveled by mesenchymal stem cells from matrix as a function of time.
(A) Scale bar = 50 µm.
Oscillating rheometry of the TissueMend matrix
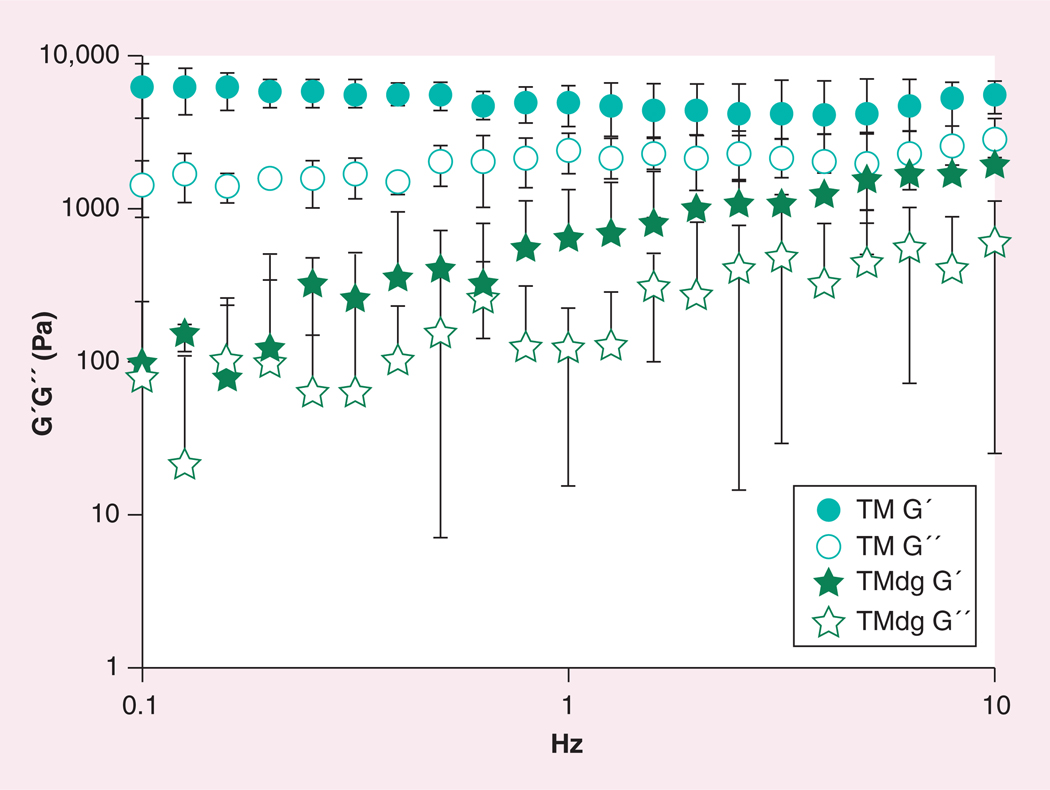
To test whether the mechanical properties of the TissueMend matrix could be altered, the storage modulus of the matrix in its current form (TM) and the matrix following digestion with collagenase (TMdg) were calculated with a rheometer. It was hypothesized that the storage modulus of the digested matrix would be lower than the undigested counterpart due to loss or partial degradation of collagen fibrils and fibers. After 24 h digestion, the thickness of the matrix and the storage modulus (TMdg G´) was decreased by at least twofold (Figure 4). The increase of the storage modulus (TMdg G´) with increasing Hz could be attributed to a faster dehydration of the membrane, as a consequence of the significantly decreased thickness of the matrix.
Figure 4. Oscillating rheometry of TissueMend® (TEI Biosciences, Boston, MA, USA) following collagenase treatment.
Oscillating rheometry was conducted on untreated matrix and digested TissueMend matrix; n = 3 for each condition.
TM: TissueMend matrix; TMdg: TissueMend matrix following digestion with collagenase.
Donor/recipient cell transfer after cardiac implantation of MSC-seeded TissueMend
To determine whether TissueMend could promote retention of MSCs for an extended period in vivo, seeded matrices were sutured to the left ventricle (spanning both the infarct and healthy tissue) of mice. It should be noted that wild-type mice were used as the recipient organism (as opposed to immunodeficient mice) to best mimic myocardial remodeling following infarction. The possibility that human cells could be rejected by the immune system of the mouse was considered, but extensive evidence exists to suggest MSCs avert immune responses via a variety of mechanisms [68–73]. Even so, rejection of transplanted cells is possible and thus the cell retention determined here may be lower than what could be attained in an allogeneic or autologous case.
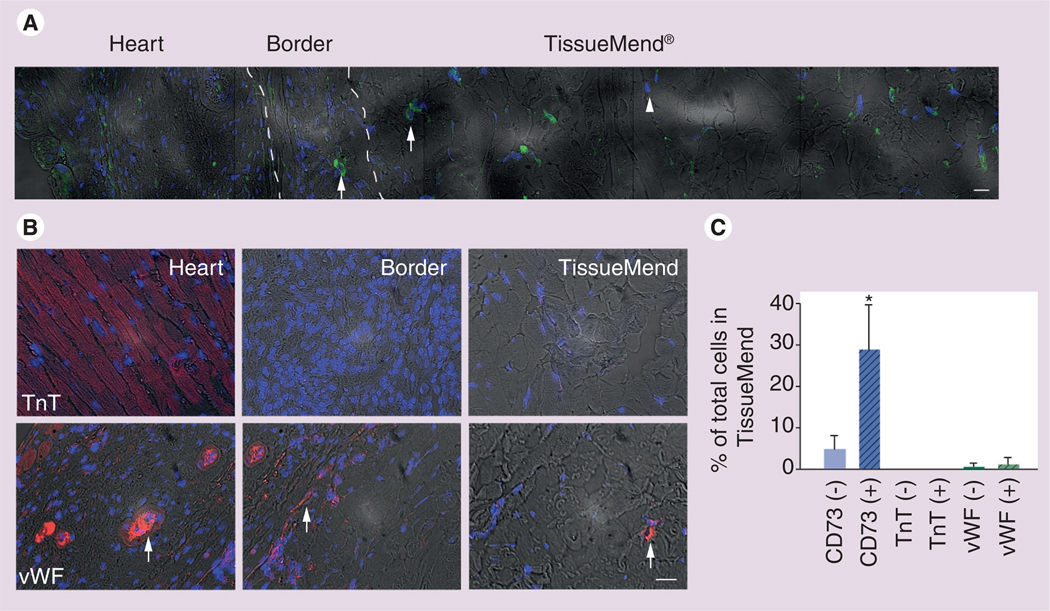
Hearts were removed and bisected laterally to include the matrix 3 weeks after transplantation. A gross view of the matrix and heart after excision and a representative mosaic of brightfield images taken from histological sections of heart tissue and TissueMend is displayed in Figure 1. Histological sections were probed for CD73, a marker indicative of MSC phenotype (Figure 5A) and cell retention was defined as the calculated number of CD73+ cells in the seeded matrices less the calculated number of CD73+ cells in the unseeded matrices (see Materials and methods). The total number of MSCs in the matrix was 21.9 × 104 ± 14.4 × 104 cells representing at least three population doublings during that period (initial number of cells present prior to implantation was 2.3 × 104 ± 0.8 × 104 CD73+ cells/matrix). MSCs were found within the matrix, representing 29 ± 9% of total cells in the matrix.
Figure 5. Retention of mesenchymal stem cells in the TissueMend® (TEI Biosciences, Boston, MA, USA) matrix in vivo.
Myocardium and matrix were removed 3 weeks after transplantation and analyzed via immunofluorescent staining for indicators of mesenchymal stem cells (MSCs) or cells of cardiac lineage. (A) A panorama showing a merge of CD73 expression (green), nuclei (blue) and brightfield (gray) in a representative region containing recipient heart tissue, border tissue and TissueMend matrix. CD73 is most prevalent in the matrix, but some rare cells can be found in the border region (arrows). Note, some nuclei of the matrix are unlabeled indicating CD73-negative cells are present (arrowhead) in the matrix. (B) Expression of phenotypic markers of cardiomyocytes (cardiac TnT) or endothelium (vWF) in representative fields from recipient heart tissue, border tissue and TissueMend matrix. Cardiac TnT was found only in heart tissue, however, vWF was found in heart tissue, in the border (arrows) and rarely in the TissueMend matrix (arrow). Full composite is shown in Supplementary Figure 2. (C) Relative contribution of MSCs, cardiomyocytes and endothelial cells to the total cell count in the TissueMend matrix. Solid bar corresponds to unseeded TissueMend matrix (−) and dashed bar corresponds to MSC-seeded TissueMend matrix (+).
(A) Scale bar = 50 µm; (B) Scale bar = 50 µm.
*p < 0.05.
TnT: Troponin T; vWF: von Willebrand Factor.
Notably, many cells in the matrix were CD73 negative. Because we are interested in improving cardiomyocyte repopulation, we sought to determine whether these CD73-negative cells could be cardiomyocytes by probing the same histological tissues for cTnT, a phenotypic marker of cardiomyocytes. We were unable to detect cardiac myocytes in either the border region or in the matrix, suggesting that, in this time frame, the MSCs are neither differentiating to cardiomyocytes nor are the host cardiomyocytes migrating into the matrix. Extended maintenance of cells in the matrix benefits from neovascularization. To examine the samples for indications of angiogenesis or vasculogenesis, we probed the histological samples with an antibody against vWF, a marker for endothelial cells. We detected endothelial cells rarely in the matrix (1 ± 1% of cells in the matrix) and in the region between the matrix and the heart (Figure 5B & C & Supplementary Figure 2). Cells of endothelial origin formed circular structures characteristic of capillaries; yet it is still unclear whether the endothelial cells present were of donor or recipient origin. At least a fraction of endothelial cells that infiltrated the matrix were of recipient origin since a matrix implanted without MSCs also showed evidence of endothelium (0.5 ± 0.7%).
Notably, a cellular ‘border region’ formed in many cases between the matrix and the heart tissue. MSCs (CD73+ cells) could be found in this region, but only accounted for 1 ± 1% of the total number of cells in the border. The border region appeared to consist mainly of mature mesenchymal or fibroblast-like cells (DDR2 [74,75], DDR-2+ in TM + cells, 73 ± 9%; in TM only, 65 ± 5%; p = 0.1; Figure 6A) with occasional macrophages and neutrophils (data not shown). To determine whether the fibroblast-like cells were of donor or recipient origin, we probed the border region for human cells using a human-specific centromeric probe. Human cells accounted for 29 ± 9% of the cells in the border region (Figure 6B), suggesting both donor and recipient cells contribute to the ‘border’ structure, strongly supporting the fact that MSCs can migrate out of the matrix in vivo, into the surrounding host tissue.
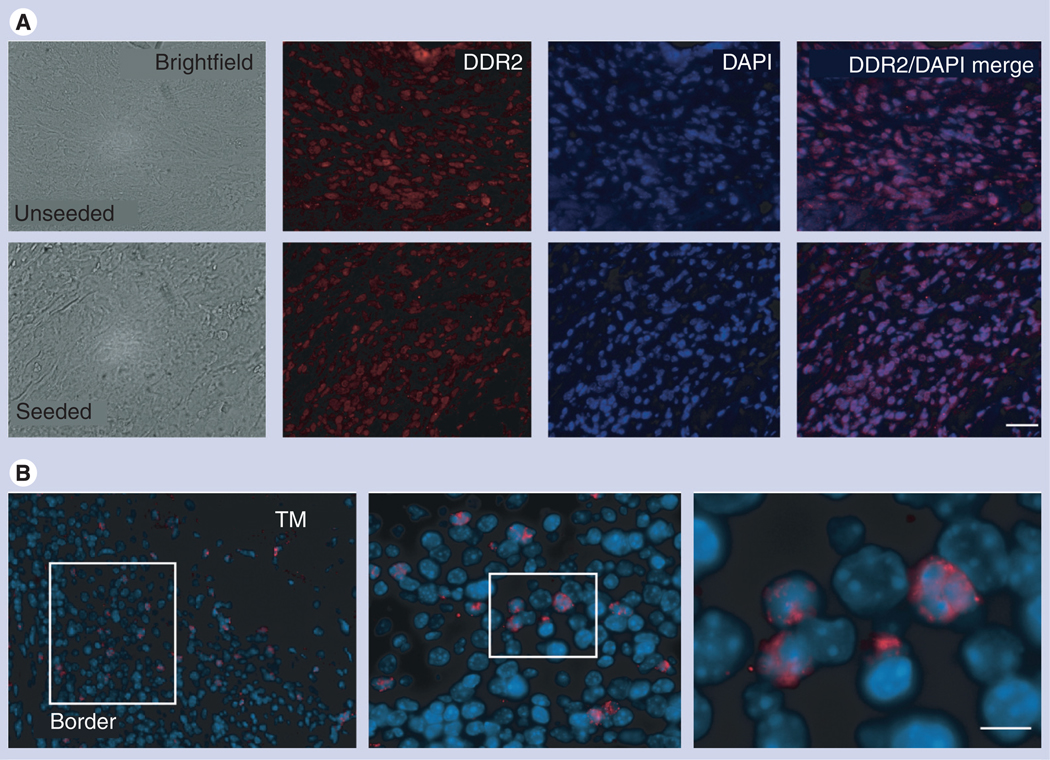
Figure 6. Fibroblast-like cells, expressing DDR2, populate the ‘border’ region.
(A) The majority of cells in the border region that often develops between the peri-infarct and infarct region of the heart following TissueMend® (TM; TEI Biosciences, Boston, MA, USA) placement are DDR2+. This result is consistent in the case of both TM only and TM + mesenchymal stem cells (MSCs). (B) Donor MSCs are capable of contributing to the ‘border’ region. Fluorescent in situ hybridization analysis using a human-specific centromeric probe of the border region. Human cells populated the ‘border’ region. Lowest magnification of a representative border region from a TM + MSCs heart sample is shown on the left, with subsequent magnifications of region outline in white. The relative contribution of human cells relative to the total number of cells in the border was 29 ± 9% in the case of TM + MSCs.
(A) Scale bar = 50 µm; (B) Scale bar = 5 µm.
DAPI: 4’,6-diamidino-2-phenylindole; DDR: Discoidin domain receptor; TM: TissueMend.
Remodeling of the TissueMend matrix after implantation
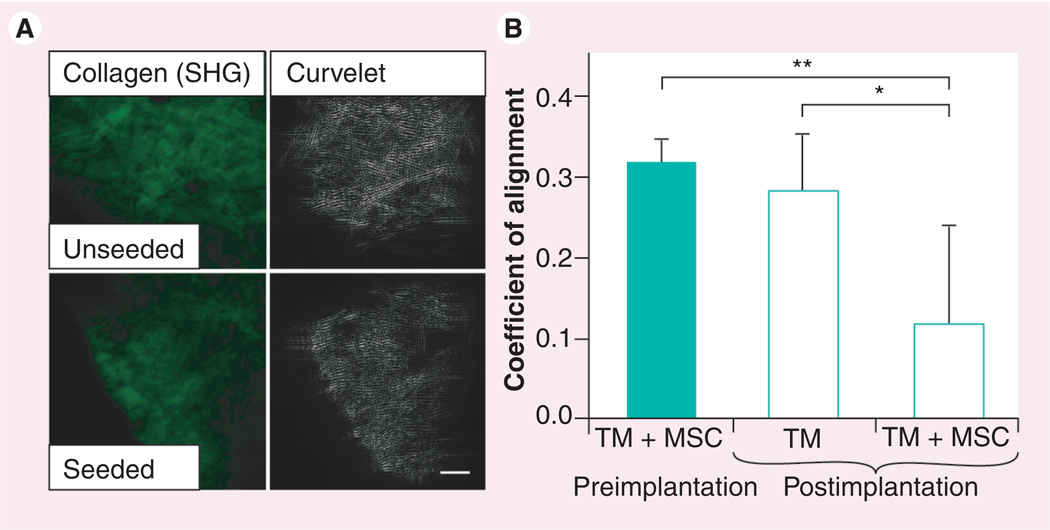
The utility of TissueMend for cell therapy in the heart would be augmented if the matrix could be remodeled to more closely resemble ventricular tissue. The ECM proteins of the TissueMend matrix (which include type I and type III collagen, but not elastin or glycosaminoglycans; company communication [103]) are not chemically crosslinked and so it should be possible for cells, such as MSCs, to secrete matrix metalloproteinases [76] in order to remodel the matrix (as added exogenously to degrade the matrix; Figure 4). In addition, the mechanical force generated by the heart with each beat could enhance MSC-directed remodeling. To measure remodeling of the TissueMend matrix, MPLSM was used to obtain images of the collagen fibers via SHG. Representative images were collected from the matrix 3 weeks after implantation with MSCs and without MSCs. A coefficient of collagen fiber alignment was determined using Curvelet software (Figure 7). A coefficient that approaches 1 indicates that fibers within a sample are largely parallel; a coefficient that approaches 0 indicates fibers within a sample are randomly aligned. Fibers of a matrix implanted with MSCs had a coefficient of alignment of 0.12 ± 0.12, while the matrix without cells had a coefficient of alignment of 0.28 ± 0.07 (p < 0.01) and the matrix prior to implantation had a coefficient of alignment of 0.31 ± 0.03 (p < 0.001). Despite the high variability of collagen alignment between samples and regions within each sample, it was clear that most regions of the MSC-seeded matrix were altered compared with the unseeded control matrix. Thus, TissueMend matrix is capable of being remodeled in vivo.
Figure 7. Remodeling of TissueMend® (TEI Biosciences, Boston, MA, USA) matrix by mesenchymal stem cells in vivo.
The second harmonic signal was used to determine the arrangement of the fibrillar collagen matrix and curvelet software was used to analyze the relative distribution of these fibers within the matrix. (A) Image analysis of matrix after implantation for 3 weeks. Top panels: second harmonic image of excised tissue that was seeded with MSCs prior to transplantation (TM + MSC, left) and corresponding curvelet analysis (TM, right). Lower panels: second harmonic image of excised tissue that was not seeded with MSCs prior to transplantation (left) and corresponding curvelet analysis (right). (B) Quantitative analysis of the coefficient of alignment of the matrix prior to and postimplantation. Matrices seeded with MSCs underwent remodeling, which corresponded to a significant increase in coefficient of alignment compared with matrices implanted without MSCs (*p < 0.01) and with matrices seeded with MSCs prior to implantation (**p < 0.001).
(A) Scale bar = 100 µm.
MSC: Mesenchymal stem cell; SHG: Second harmonic generation; TM: TissueMend.
Discussion
Recent evidence suggests transplanted MSCs promote improved cardiac function in the myocardium [77–79] . Although the effect is apparent, the mechanism is unclear and has been difficult to unravel, due in part to difficulty maintaining healthy MSCs in, or in close proximity to, the myocardium. Many synthetic and natural polymers have been proposed as delivery vehicles, each with limitations, including poor cell viability, local immune responsiveness and inability of the material to be remodeled (reviewed in [42,80]). Here we test the utility of TissueMend, a material available clinically for tendon repair, as a vehicle for MSC transplantation to the heart. Stem cells seeded on the TissueMend were capable of attachment, spreading, proliferation and migration in vitro. In vivo, stem cells delivered to the heart via TissueMend were maintained for an extended period and even underwent at least three population doublings. This represents a substantial improvement over retention reported for bolus injection of stem cells into the myocardium (<10% of total transplanted cells at up to 4 weeks) [16–19]. In addition, TissueMend underwent MSC-directed remodeling in vivo and supported survival of endothelial cells, which formed capillary-like structures, suggesting that long-term incorporation of the matrix into the cardiac microenvironment may be possible.
The primary limitation of the TissueMend matrix for delivery of cells to the heart is the mismatch of compliance between the healthy myocardium and TissueMend. The compressive modulus of the ventricle is 10–20 KPa [81–83] and can stiffen to 55–80 KPa due to scar formation following infarction [84,85]. The elastic modulus of TissueMend, in its current formulation, is approximately 3–15 MPa [86,87], as the matrix was originally fabricated to mimic tendon tissue. However, we show here that due to lack of chemical crosslinks, the mechanical properties of matrix can be modified.
Compliance mismatch can give rise to a labored and disrupted rhythm, which can, in turn, cause cell damage. Cell damage in this context can trigger immune responses that can drive local inflammation, foreign body response and/or activation of cardiac fibroblasts. We have observed a cellular infiltrate between the heart and the matrix, which could indicate a fibrotic response to the matrix. However, such a response could also be a consequence of the induced infarction, the placement of sutures or simply to opening of the chest cavity. The putative ‘fibrotic response’ might be reduced if the fabrication of the matrix was altered to more closely mimic the strength and compliance of cardiac tissue. In addition, since the matrix can be remodeled by MSCs and/or mechanical stimulation of the cardiac tissues, it may be possible for the fibrous layer to be replaced or at least mechanically supported in the long term. With support of this type, the myocardium might be less susceptible to the chronic effects of myocardial damage, including hypertrophy and failure. Alternatively, the cellular infiltrate might not be fibrotic in nature, but might instead correspond to the recruitment of progenitor and stem cells. Responses of this type have been reported following administration of ECM degradation products in a model of digit amputation [88]. Further studies are underway to determine the characteristics of this population of cells.
The primary advantage of the TissueMend matrix for the delivery of cells to the heart is improved cell survival, proliferation and retention. Cell retention might have been even greater if the model employed immunosuppressed mice to avoid immune-mediated rejection. We opted to use wild-type mice because MSCs have been reported to inhibit or at least limit immune responses including host-versus-graft responses [68–73]. In addition, immune function is necessary to facilitate myocardial remodeling following infarction. Here we observed that many cells in the matrix did not express CD73 (although more than 98% of transplanted cells expressed this protein). In addition, we found that very few cells in the matrix exhibit mature cardiac phenotypes. What is the identity of those cells that do not express CD73 and yet have not acquired mature phenotypes of cardiac cells? One possibility is that they are in the process of maturation and thus express only low or no levels of cardiac-specific markers. Alternatively, they may have adopted a phenotype responsive to the xenogeneic microenvironment and so are perhaps anti-inflammatory in nature. Finally, they may have undergone differentiation to an alternate mesenchymal cell type (i.e., osteogenic, chondrogenic or adipogenic cell types). These possibilities need to be investigated as well as the possibility that the remaining cells are of host hematopoietic origin. This last possibility seems less likely as nuclei size and cytoplasmic morphology are more consistent with a mesenchymal phenotype.
Conclusion
TissueMend can promote engraftment of MSCs in the heart for an extended period, and is both commercially available and clinically approved for tendon repair. These qualities support the utility of the TissueMend matrix for, at least, basic research into the mechanisms of MSC-dependent cardiac recovery and, at most, the development of this matrix for clinical applications in the heart. As one example, we and others are investigating the impact of stem cell fusion in the heart. Studies of this kind are challenging to conduct when the retention of stem cells in the heart is low. This delivery system will be used to promote stem cell–cardiomyocyte contact in vivo to assess whether fusion can promote stem cell programming toward a cardiomyocyte phenotype. The study of other mechanisms that may account for cardiac functional improvement with MSC transplantation might also be enhanced with the use of the TissueMend matrix or similar natural biomaterial matrices.
Future perspective
The ultimate goal of delivery of cells to the heart is to improve cardiac function. However, the design of delivery vehicles is dictated by unique criteria which depend, in large part, on the cell type to be delivered. In the case of MSCs, the primary goal is to deliver the cells in close proximity to the heart such that MSC-derived paracrine factors might promote vascularization and immune modulation. A secondary goal of MSC delivery is to enable ‘appropriate’ differentiation of MSCs (e.g., smooth muscle cell and endothelial cell differentiation) and incorporation of those cells into the functional heart. Thus, the mode of delivery must favor MSC attachment and growth without aberrant differentiation. This might be accomplished with commercially available ECM-based matrices (such as the one described here), but might be improved with synthetic technologies to mimic ECM in a ‘tunable’ manner. More elegant and well-defined matrices are already under development. In our view the most significant advance needed to further propel the field forward is a comprehensive understanding of the content and relative distribution of essential ECM proteins in the myocardium and epicardium. If we do not fill this crucial knowledge gap, we are in essence, designing matrices without the complete set of blueprints. The technology now exists to accurately and noninvasively evaluate the content and distribution of ECM proteins in tissue and so complete assessment should be possible in the next 5 years. Once this is accomplished, upcoming years are sure to bring matrices capable not only of delivery of stem cells, but of directing cell behavior in said matrices before and with transplantation.
Executive summary.
-
▪
TissueMend® (TEI Biosciences, Boston, MA, USA) is a decellularized dermal matrix composed primarily of type I and type III collagen.
-
▪
Mesenchymal stem cells (MSCs) can attach to the matrix and proliferate within the matrix at the same rate achieved in cell culture plates.
-
▪
MSCs migrate into the matrix and are capable of migrating out of the matrix both in vitro and in vivo.
-
▪
MSCs delivered to damaged murine myocardium via the matrix were maintained in vivo for 3 weeks and underwent at least two population doublings during that time.
-
▪
Extracellular matrix proteins of the matrix could be remodelled by MSCs in vivo.
-
▪
TissueMend matrix offers a commercially available, biocompatible and malleable vehicle for the delivery and retention of stem cells to the heart.
Acknowledgements
The authors thank J Schaefer and A Sagstetter of the Department of Biomedical Engineering, G Song and Jill Koch of the University of Wisconsin Cardiovascular Physiology Core Facility and K Lien of the Mayo Clinic College of Medicine for technical assistance; J Fong of LOCI for image analysis assistance; R Sullivan of the University of Wisconsin Research Animal Resources Center and G Lyons of the University of Wisconsin-Madison Department of Anatomy for reading of histological slides; and P Hematti and J Kim of the University of Wisconsin-Madison Department of Medicine for providing human mesenchymal stem cells.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Orsini E, Zito GB. Matching pathophysiology and evidence-based medicine for optimal management of ischemic heart disease. J. Cardiovasc. Med. (Hagerstown) 2010;11(6):469–479. doi: 10.2459/jcm.0b013e328336ecde. [DOI] [PubMed] [Google Scholar]
- 2.Baig MK, Mahon N, McKenna WJ, et al. The pathophysiology of advanced heart failure. Am. Heart J. 1998;135(6 Pt 2 Su):S216–S230. doi: 10.1016/s0002-8703(98)70252-2. [DOI] [PubMed] [Google Scholar]
- 3.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc. Res. 2008;77(1):134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, Fan GC, Zhou X, et al. Overexpression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J. Mol. Cell. Cardiol. 2008;44(2):281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf D, Reinhard A, Seckinger A, Gross L, Katus HA, Hansen A. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium – complicated by myocardial tumor formation. Scand. Cardiovasc. J. 2009;43(1):39–45. doi: 10.1080/14017430802100280. [DOI] [PubMed] [Google Scholar]
- 6.Xu YL, Gao YH, Liu Z, et al. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int. J. Cardiol. 2010;138(2):182–195. doi: 10.1016/j.ijcard.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 7.Guddati AK, Otero JJ, Kessler E, et al. Embryonic stem cells overexpressing Pitx2c engraft in infarcted myocardium and improve cardiac function. Int. Heart J. 2009;50(6):783–799. doi: 10.1536/ihj.50.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider C, Jaquet K, Geidel S, et al. Transplantation of bone marrow-derived stem cells improves myocardial diastolic function: strain rate imaging in a model of hibernating myocardium. J. Am. Soc. Echocardiogr. 2009;22(10):1180–1189. doi: 10.1016/j.echo.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Qiao H, Surti S, Choi SR, et al. Death and proliferation time course of stem cells transplanted in the myocardium. Mol. Imaging Biol. 2009;11(6):408–414. doi: 10.1007/s11307-009-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25(9):2350–2357. doi: 10.1634/stemcells.2007-0132.. ▪ Describes a significant improvement in cardiac function following delivery of progenitor cells with an extracellular matrix-derived patch. Interestingly, transplanted cells were not detected after 1 week, but improvement was sustained several weeks beyond this point.
- 11.Li M, Yu J, Li Y, Li D, Yan D, Ruan Q. CXCR4+ progenitors derived from bone mesenchymal stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Cell. Reprogram. 2010;12(4):405–415. doi: 10.1089/cell.2009.0088. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty S, Kang B, Huang F, Guo YL. Mouse embryonic stem cells lacking p38α and p38δ can differentiate to endothelial cells, smooth muscle cells, and epithelial cells. Differentiation. 2009;78(2–3):143–150. doi: 10.1016/j.diff.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo YL, Ye J, Huang F. p38α MAP kinase-deficient mouse embryonic stem cells can differentiate to endothelial cells, smooth muscle cells, and neurons. Dev. Dyn. 2007;236(12):3383–3392. doi: 10.1002/dvdy.21374. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Q, Zeng L, Zhang Z, et al. Sca-1+ progenitors derived from embryonic stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Arterioscler. Thromb. Vasc. Biol. 2006;26(10):2244–2251. doi: 10.1161/01.ATV.0000240251.50215.50. [DOI] [PubMed] [Google Scholar]
- 15. Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45.. ▪ Documents an early, comprehensive report of mesenchymal stem cell differentiation to cardiac cell types in a large animal model of chronic ischemia.
- 16.Dawn B, Stein AB, Urbanek K, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl Acad. Sci. USA. 2005;102(10):3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J. Mol. Cell. Cardiol. 2001;33(5):907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Murtuza B, Beauchamp JR, et al. Dynamics and mediators of acute graft attrition after myoblast transplantation to the heart. FASEB J. 2004;18(10):1153–1155. doi: 10.1096/fj.03-1308fje. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda T, Weisel RD, Kiani C, Mickle DA, Maganti M, Li RK. Quantitative analysis of survival of transplanted smooth muscle cells with real-time polymerase chain reaction. J. Thorac. Cardiovasc. Surg. 2005;129(4):904–911. doi: 10.1016/j.jtcvs.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Mazo M, Planat-Benard V, Abizanda G, et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur. J. Heart Fail. 2008;10(5):454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Schneider C, Krause K, Jaquet K, et al. Intramyocardial transplantation of bone marrow-derived stem cells: ultrasonic strain rate imaging in a model of hibernating myocardium. J. Card. Fail. 2008;14(10):861–872. doi: 10.1016/j.cardfail.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Tang W, Sun S, et al. Mesenchymal stem cells improve outcomes of cardiopulmonary resuscitation in myocardial infarcted rats. J. Mol. Cell. Cardiol. 2009;46(3):378–384. doi: 10.1016/j.yjmcc.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Lee J, Stagg MA, Fukushima S, et al. Adult progenitor cell transplantation influences contractile performance and calcium handling of recipient cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2009;296(4):H927–H936. doi: 10.1152/ajpheart.00931.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1α delivery and endogenous cytokine signaling. Am. J. Physiol. Heart Circ. Physiol. 2009;296(4):H976–H986. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krause K, Jaquet K, Schneider C, et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart. 2009;95(14):1145–1152. doi: 10.1136/hrt.2008.155077. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Jiang XJ, Lin T, et al. The inhibition of postinfarct ventricle remodeling without polycythaemia following local sustained intramyocardial delivery of erythropoietin within a supramolecular hydrogel. Biomaterials. 2009;30(25):4161–4167. doi: 10.1016/j.biomaterials.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 27.Makela J, Anttila V, Ylitalo K, et al. Acute homing of bone marrow-derived mononuclear cells in intramyocardial vs. intracoronary transplantation. Scand. Cardiovasc. J. 2009;43(6):366–373. doi: 10.1080/14017430903045350. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Wang S, Yu Z, et al. Direct injection of autologous mesenchymal stromal cells improves myocardial function. Biochem. Biophys. Res. Commun. 2009;390(3):902–907. doi: 10.1016/j.bbrc.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenar H, Kose GT, Hasirci V. Design of a 3D aligned myocardial tissue construct from biodegradable polyesters. J. Mater. Sci. Mater. Med. 2010;21(3):989–997. doi: 10.1007/s10856-009-3917-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Zhou JY, Zheng Z, Zhang H, Hu SS. A novel vascularized patch enhances cell survival and modifies ventricular remodeling in a rat myocardial infarction model. J. Thorac. Cardiovasc. Surg. 2010;140(6):1388–1396. e1–e3. doi: 10.1016/j.jtcvs.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 31. Bursac N, Loo Y, Leong K, Tung L. Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem. Biophys. Res. Commun. 2007;361(4):847–853. doi: 10.1016/j.bbrc.2007.07.138.. ▪▪ Utilized anisotropic polymer scaffolds made via leaching of sucrose templates and subsequent seeding with neonatal rat cardiomyocytes. Cells aligned and interconnected in the scaffold representing the first successful effort to engineer a centimeter scale, functional anisotropic cardiac tissue patch.
- 32. Kochupura PV, Azeloglu EU, Kelly DJ, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112(Suppl. 9):I144–I149. doi: 10.1161/CIRCULATIONAHA.104.524355.. ▪ Investigated the utility of an extracellular matrix-derived cardiac patch for the repair of a full-thickness defect in the heart. The extracellular matrix-derived patch was found to support cardiomyocyte survival 8 weeks after implantation and the ventricle maintained better mechanical stability than that seen with a Dacron patch.
- 33.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed. Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 34.Potapova IA, Doronin SV, Kelly DJ, et al. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am. J. Physiol. Heart Circ. Physiol. 2008;295(6):H2257–H2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(Suppl. 9):I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749.. ▪▪ One of the first compelling reports to suggest cell retention is limited following cell delivery to the heart: <10% retention, 1 h following cell delivery in all cases.
- 36.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 37.Sui R, Liao X, Zhou X, Tan Q. The current status of engineering myocardial tissue. Stem Cell Rev. 2011;7(1):172–180. doi: 10.1007/s12015-010-9131-8. [DOI] [PubMed] [Google Scholar]
- 38.Shapira K, Dikovsky D, Habib M, Gepstein L, Seliktar D. Hydrogels for cardiac tissue regeneration. Biomed. Mater. Eng. 2008;18(4–5):309–314. [PubMed] [Google Scholar]
- 39.Akhyari P, Kamiya H, Haverich A, Karck M, Lichtenberg A. Myocardial tissue engineering: the extracellular matrix. Eur. J. Cardiothorac. Surg. 2008;34(2):229–241. doi: 10.1016/j.ejcts.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 40.Horton RE, Millman JR, Colton CK, Auguste DT. Engineering microenvironments for embryonic stem cell differentiation to cardiomyocytes. Regen. Med. 2009;4(5):721–732. doi: 10.2217/rme.09.48. [DOI] [PubMed] [Google Scholar]
- 41.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. J. Tissue Eng. Regen. Med. 2007;1(5):327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Guan J. Cellular cardiomyoplasty and cardiac tissue engineering for myocardial therapy. Adv. Drug Deliv. Rev. 2010;62(7–8):784–797. doi: 10.1016/j.addr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Wang T, Jiang XJ, Tang QZ, et al. Bone marrow stem cells implantation with α-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5(8):2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 44.Bryant SJ, Nicodemus GD, Villanueva I. Designing 3D photopolymer hydrogels to regulate biomechanical cues and tissue growth for cartilage tissue engineering. Pharm. Res. 2008;25(10):2379–2386. doi: 10.1007/s11095-008-9619-y. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J, Tang C, Kottke-Marchant K, Marchant RE. Design and synthesis of biomimetic hydrogel scaffolds with controlled organization of cyclic RGD peptides. Bioconjug. Chem. 2009;20(2):333–339. doi: 10.1021/bc800441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benton JA, Fairbanks BD, Anseth KS. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials. 2009;30(34):6593–6603. doi: 10.1016/j.biomaterials.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varghese S, Hwang NS, Canver AC, Theprungsirikul P, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27(1):12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Roche S, Ronziere MC, Herbage D, Freyria AM. Native and DPPA cross-linked collagen sponges seeded with fetal bovine epiphyseal chondrocytes used for cartilage tissue engineering. Biomaterials. 2001;22(1):9–18. doi: 10.1016/s0142-9612(00)00084-3. [DOI] [PubMed] [Google Scholar]
- 49.Ma L, Gao C, Mao Z, et al. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials. 2003;24(26):4833–4841. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 50.Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002;23(4):1205–1212. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 51.Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J. Am. Coll. Cardiol. 1995;25(6):1263–1272. doi: 10.1016/0735-1097(94)00557-7. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp. Hematol. 2008;36(3):350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 54.Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29(1):97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- 55.Kenealey JD, Subramanian L, Van Ginkel PR, et al. Resveratrol metabolites do not elicit early pro-apoptotic mechanisms in neuroblastoma cells. J. Agric. Food Chem. 2011;59(9):4979–4986. doi: 10.1021/jf104901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung KE, Yang N, Pehlke C, et al. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr. Biol. (Camb.) 2011;3(4):439–450. doi: 10.1039/c0ib00063a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28(49):4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbi S, Ueda H, Zheng C, et al. Regulation of focal adhesion kinase by a novel protein inhibitor FIP200. Mol. Biol. Cell. 2002;13(9):3178–3191. doi: 10.1091/mbc.E02-05-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michael LH, Entman ML, Hartley CJ, et al. Myocardial ischemia and reperfusion: a murine model. Am. J. Physiol. 1995;269(6 Pt 2):H2147–H2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 60.Ogle BM, Butters KA, Plummer TB, et al. Spontaneous fusion of cells between species yields transdifferentiation and retroviral transfer in vivo. FASEB J. 2004;18(3):548–550. doi: 10.1096/fj.03-0962fje. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhuri S, Nguyen H, Rangayyan RM, Walsh S, Frank CB. A Fourier domain directional filtering method for analysis of collagen alignment in ligaments. IEEE Trans. Biomed. Eng. 1987;34(7):509–518. doi: 10.1109/tbme.1987.325980. [DOI] [PubMed] [Google Scholar]
- 62.Marquez JP. Fourier analysis and automated measurement of cell and fiber angular orientation distributions. Int. J. Solids Struct. 2006;43(21):6413–6423. [Google Scholar]
- 63.Candes EJ, Donoho DL. Curvelets – a surprisingly effective nonadaptive representation for objects with edges. In: Cohen A, Rabut C, Schumaker LL, editors. Curves and Surface Fitting: St-Malo 1999. Nashville, TN, USA: Vanderbilt University Press; 2000. [Google Scholar]
- 64.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS One. 2011;6(1):e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through αvβ3 integrins and RPTPα. Biophys. J. 2006;90(5):1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79(1):144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Eitan Y, Sarig U, Dahan N, Machluf M. Acellular cardiac extracellular matrix as a scaffold for tissue engineering: in vitro cell support, remodeling, and biocompatibility. Tissue Eng. Part C Methods. 2010;16(4):671–683. doi: 10.1089/ten.TEC.2009.0111.. ▪ A compelling in vitro study of the effects of cell (cardiomyocyte, fibroblast and mesenchymal stem cell) survival and infiltration on the remodeling of and associated mechanical properties of an extracellular matrix-derived scaffold.
- 68.Choi EW, Shin IS, Lee HW, et al. Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J. Gene Med. 2011;13(1):3–16. doi: 10.1002/jgm.1531. [DOI] [PubMed] [Google Scholar]
- 69.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res. Ther. 2010;1(5):34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haddad E, Chen C, Greenberger E. The role of important non-parental adults (VIPs) in the lives of older adolescents: a comparison of three ethnic groups. J. Youth Adolesc. 2011;40(3):310–319. doi: 10.1007/s10964-010-9543-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li FR, Wang XG, Deng CY, Qi H, Ren LL, Zhou HX. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin. Exp. Immunol. 2010;161(2):357–363. doi: 10.1111/j.1365-2249.2010.04178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann. NY Acad. Sci. 2007;1106:272–278. doi: 10.1196/annals.1392.025. [DOI] [PubMed] [Google Scholar]
- 73. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559.. ▪▪ Provides one of the first systematic evaluations of the impact of mesenchymal stem cell behavior on immune responses. The data offer insights into the mechanisms involved in mesenchymal stem cell-induced tolerance in vivo.
- 74.Goldsmith EC, Hoffman A, Morales MO, et al. Organization of fibroblasts in the heart. Dev. Dyn. 2004;230(4):787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 75.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 76.Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol. Int. 2010;34(6):601–605. doi: 10.1042/CBI20090386. [DOI] [PubMed] [Google Scholar]
- 77.Ma J, Ge J, Zhang S, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res. Cardiol. 2005;100(3):217–223. doi: 10.1007/s00395-005-0521-z. [DOI] [PubMed] [Google Scholar]
- 78.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann. Thorac. Surg. 2002;73(6):1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 79.Nagaya N, Fujii T, Iwase T, et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am. J. Physiol. Heart Circ. Physiol. 2004;287(6):H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 80.Vunjak-Novakovic G, Tandon N, Godier A, et al. Challenges in cardiac tissue engineering. Tissue Eng. Part B Rev. 2010;16(2):169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieber SC, Aubry N, Pain J, Diaz G, Kim SJ, Vatner SF. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am. J. Physiol. Heart Circ. Physiol. 2004;287(2):H645–H651. doi: 10.1152/ajpheart.00564.2003. [DOI] [PubMed] [Google Scholar]
- 82. Bhana B, Iyer RK, Chen WL, et al. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010;105(6):1148–1160. doi: 10.1002/bit.22647.. ▪▪ Provides a thorough analysis of matrix stiffness in the heart at the monolayer or tissue level and the impact of stiffness on heart cell phenotype and functional properties.
- 83.van der Loo JJ, Jakot J, Bovendeerd PHM. BMTE 08.45, internal report. Technische Universiteit Eindhoven; 2008. The development in cardiac stiffness in embryonic, neonatal and adult mice evaluated with atomic force microscopy. [Google Scholar]
- 84.Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006;290(6):H2196–H2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 85.Zhang S, Sun A, Ma H, et al. Infarcted myocardium-like stiffness contributes to endothelial progenitor lineage commitment of bone marrow mononuclear cells. J. Cell. Mol. Med. 2010 doi: 10.1111/j.1582-4934.2010.01217.x. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aurora A, McCarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J. Shoulder Elbow Surg. 2007;16(Suppl. 5):S171–S178. doi: 10.1016/j.jse.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J. Bone Joint Surg. Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 88.Agrawal V, Johnson SA, Reing J, et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc. Natl Acad. Sci. USA. 2010;107(8):3351–3355. doi: 10.1073/pnas.0905851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Mayo Clinic: definition of heart disease by Mayo Clinic staff. www.mayoclinic.com/health/heart-disease/DS01120.
- 102.American Heart Association. www.americanheart.org.
- 103.Stryker: TissueMend®. www.stryker.com/en-us/products/Orthopaedics/SoftTissueRepair/index.htm.