Abstract
Epidemiological studies demonstrated that the number of emergency room visits for respiratory indications increase during periods of Florida Red Tides. The purpose of this study was to examine whether or not repeated brevetoxin inhalation, as may occur during a Florida Red Tide, affects pulmonary responses to influenza A. Male F344 rats were divided into 4 groups: (1) sham aerosol/no influenza; (2) sham aerosol/influenza; (3) brevetoxin/no influenza; and (4) brevetoxin/influenza. Animals were exposed by nose-only inhalation to vehicle or 50μg brevetoxin-3/m3, 2 hr/day for 12 days. On the sixth day of aerosol exposure, Groups 2 and 4 were administered 10,000 plaque forming units of influenza A, strain HKX-31 (H3N2) by intratracheal instillation. Subgroups were euthanized at 2, 4, and 7 days post influenza treatment. Lungs were evaluated for viral load, cytokine content and histopathologic changes. Influenza virus was cleared from the lungs over the 7 day period, however, there was significantly more virus remaining in the Group 4 lungs compared to Group 2. Influenza virus significantly increased interleukins-1α and 6 and monocyte chemotactic protein-1 in lung; brevetoxin exposure significantly enhanced the influenza-induced response. At 7 days, the severity of perivascular and peribronchiolar inflammatory cell infiltrates was greatest in Group 4. Bronchiolitis persisted, with low incidence and severity, only in Group 4 at day 7. These results suggest that repeated inhalation exposure to brevetoxin may delay virus particle clearance and recovery from influenza A infection in the rat lung.
INTRODUCTION
Harmful algal blooms (HAB) of Karenia brevis occur almost annually in the Gulf of Mexico (Heil and Steidinger, 2009). K. brevis produces a family of potent polyether neurotoxins, brevetoxins that are released into the water when the cells are disrupted by wind and surf. Brevetoxins reaching the water surface in breaking waves can be incorporated into marine aerosols that, with onshore winds, are transported as far as one mile inland (Kirkpatrick et al., 2010).
Recent epidemiological studies showed correlations between brevetoxin exposure at affected Florida beaches and symptoms including eye, nose and throat irritation, cough, shortness of breath, wheezing, and chest tightness that may last for several days after only 1 hr exposure, particularly among asthmatics (Backer et al., 2003, 2005; Fleming et al., 2005a, 2005b, 2007b, 2009; Kirkpatrick et al., 2009). Ambient brevetoxin concentrations of approximately 60 ng/m3, measured at Florida beaches during a Red Tide event, increased self reported symptoms of respiratory distress among asthmatics exposed for only 1 hr (Fleming et al., 2009). Studies analyzing visits to the emergency room at a coastal hospital for respiratory diagnoses during a 3-month period of Florida Red Tide (2001) compared to the same 3-month period when there was no Red Tide (2002) demonstrated an association between the occurrence of a Florida Red Tide and increased number of emergency room visits for pneumonia, bronchitis and asthma (Kirkpatrick et al., 2006). A subsequent assessment of emergency room visits for respiratory symptoms from 2001–2006 indicated that the presence of K. brevis blooms near Sarasota is a significant predictor of emergency room visits for upper airway disease and bronchitis (Hoagland et al., 2009).
Epidemic outbreaks of influenza often occur concurrently with K. brevis blooms (October through May) (Simonsen et al., 1997; Hoagland et al., 2009). The severity of influenza infection ranges from mild upper respiratory tract infections to severe lower respiratory tract infections involving pneumonia, bronchiolitis, and coincidental bacterial super-infections. No data are available on the effects of brevetoxin inhalation on the clearance and pulmonary responses to influenza.
The purpose of this study was to examine whether repeated inhalation of brevetoxin alters the pulmonary response to influenza A in male F344 rats. Brevetoxin-3 was chosen for these studies because it is a major component of the brevetoxin-containing marine aerosols measured during K. brevis blooms (Pierce et al., 2005). The 12 day exposure scenario employed was intended to mimic a prolonged bloom. Repeated inhalation exposure of F344 rats to brevetoxins has produced only minimal macrophage hyperplasia in rat lung (Benson et al., 2005). However the compounds accumulate in pulmonary, bronchus-associated lymphoid tissue and splenic macrophages, and suppress humoral-mediated immunity in rats upon repeated inhalation exposure (Benson et al., 2004a, 2004b, 2005). Therefore brevetoxin may alter the innate and/or acquired immune responses to inhaled pathogens, including viruses and bacteria.
Influenza A H1N1 is a common strain of human influenza virus. Wolf et al. (2009) established a model of non-rat adapted influenza A infection in F344 rats. In this model, viral particles replicate within 48 hr post administration, infect macrophages and epithelial cells, and produce pulmonary responses, including infiltration of inflammatory cells into perivascular and peribronchiolar regions, alveolitis, and bronchiolitis in a viral dose-dependent manner. While the virus is almost completely cleared in 7 days, pulmonary responses persist.
METHODS
Chemicals
Brevetoxin-3 was isolated and purified from K. brevis cultures at the Center for Marine Sciences, University of North Carolina, Wilmington, using established procedures (Baden et al., 1981). The material was > 99% pure by High Performance Liquid Chromatographic analysis.
Animals
Male F344 rats were purchased from Charles Rivers Laboratories (Wilmington, MA). Animals were approximately 6–8 weeks old, and on average weighing 200 g upon receipt. The animals were housed in shoebox cages with hardwood chip bedding and microisolator filter tops. The animal rooms were maintained at 21 ± 1° C with 20–60% relative humidity. A light/dark cycle of 12 hr was implemented with light starting at 0600. Food (Harlan Teklad certified rodent diet, Harlan Teklad, Madison, WI) and water were provided ad libitum, except during inhalation exposures. Prior to exposure, rats were randomized by weight into dose groups using a computerized data acquisition system (Path-Tox® Version 4.4.2; Xybion, Cedar Knolls, NJ). The rats were identified by unique alphanumeric number encoded onto a transponding microchip (Trovan®; Electronic ID Devices, Ltd., Santa Barbara, CA) injected subcutaneously (sc). All rats were conditioned to the nose-only inhalation restraint tubes (In-Tox Products, Moriarty, NM) on three separate days, for 0.5, 1 or 2.5 hr.
Experimental Design
The chronology of dosing in this experiment was intended to mimic development of an influenza infection during an ongoing K. brevis bloom. The experimental design is summarized in Table 1 and Figure 1. Groups of rats were exposed nose only to vehicle or brevetoxin-3 (50 μg/m3), 2 hr per day for 12 consecutive days. The brevetoxin exposure concentration was found to suppress humoral-mediated immunity in rats (Benson et al., 2004b). Influenza (104 plaque forming units/lung; PFU/lung), or vehicle instillation, occurred after the aerosol exposure on day 6. This influenza dose was chosen because Wolf et al. (2009) demonstrated that this provided a robust, but not maximal pulmonary inflammatory response, allowing for enhancement or attenuation of response in the presence of brevetoxin exposure.
TABLE 1.
Experimental Design
| Study Group | Inhalation Exposure | Instillation Treatment | Rats/Sacrifice at 48 hr, 96 hr and 7 day Post Instillation |
|---|---|---|---|
| 1 | Ethanol/saline | None - Sham instillations with culture medium | 4 |
| 2 | Ethanol/saline | Intratracheal instillation of 10,000 influenza particles on exposure day 6 | 8 |
| 3 | 50 μg/m3 brevetoxin, 2 hr/day for 12 days | None - Sham instillations with culture medium | 4 |
| 4 | 50 μg/m3 brevetoxin, 2 hr/day for 12 days | Intratracheal instillation of 10,000 influenza particles on exposure day 6 | 8 |
FIG. 1.
Experimental time line.
Subgroups of 4 animals (treatment Groups 1 and 3) or 8 animals (treatment Groups 2 and 4) were euthanized 2, 4, and 7 days post influenza instillation (study days 8, 10 and 13), spanning the time of expected virus clearance from the lung. Reduced numbers of animals were included in Groups 1 and 3 because previous studies in rats indicated minimal histopathological changes in lung were to be expected with repeated brevetoxin exposure at 50μg/m3 (Benson et al., 2004b, 2005).
Brevetoxin Inhalation Exposure System
The exposure system consisted of a 48-port cylindrical nose-only inhalation chamber (brevetoxin-3 aerosols) and a 96-port flat nose-only chamber (vehicle control aerosols; In-Tox Products, Moriarty, NM). Aerosols were generated by nebulization (Hospitak, Inc., Farmingdale, NY), and dried and diluted with supply air to achieve the desired chamber aerosol concentration. Total airflow through the chambers was 20 L/min. Chambers were operated at a slightly negative pressure with respect to the glove boxes in which they were enclosed. The chambers were housed in a glove box to minimize worker exposure to brevetoxin and influenza. Temperatures were monitored continuously with an acceptable range of 18–22° C. Chamber oxygen concentration was monitored with an action level at ≤ 18%.
Aerosol Generation and Characterization
Stock solutions of brevetoxin-3 were prepared at a concentration of 0.25 mg brevetoxin-3/ml 100% ethanol. Generator solutions were prepared daily by diluting the stock solutions in 0.9% saline to achieve a final concentration of 0.038 mg brevetoxin-3/ml (15% ethanol in saline). The ratio of brevetoxin mass to total mass in the solutions was 0.005.
Total aerosol concentrations in the exposure atmospheres were determined gravimetrically. Aerosol was collected from the breathing zone of the animals at a flow rate of 2 L/min onto pre-weighed 25-mm Zefluor filters (SKC Gulf Coast, Houston, TX). Sequential 20 min filter samples were collected from the brevetoxin-3 chamber and 40 min filter samples were collected from the vehicle control chamber throughout each exposure. Brevetoxin-3 aerosol concentrations were confirmed by ELISA analysis performed on a subset of filter samples extracted with PBS-Tween and 5% melted gelatin (Naar et al., 2002).
The stability of the total aerosol concentration during the exposure was monitored using a TSI DustTrak™ aerosol monitor (TSI Industries, Shoreview, MN). The mass median aerodynamic particle size of a 50 μ/m3 brevetoxin/saline aerosol measured by cascade impactor was 0.58 μm, with a geometric standard deviation of 2.13. Based on an assumed minute volume of 0.25 L/min and a total respiratory deposition fraction of 0.1 for a 0.5 μm particle (Schlesinger, 1989), daily brevetoxin deposition was 150 ng/rat/day (600 ng/kg/day).
Madin-Darby Canine Kidney Cell Culture
Madin-Darby Canine Kidney cell culture was performed in a manner similar to that described by Matsuoka et al. (2009). Madin-Darby Canine Kidney, MDCK NBL-2 cells (cat. number CCL-34) were purchased from the American Type Culture Collection (Manassas, VA) and were used for Influenza virus stock preparation and titration and also for titration of infectious virus loads in lungs of infected animals. The cell line was grown in Minimum Essential Medium Eagle (MEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine and penicillin/Streptomycin. After influenza infection, cells were maintained in MEM supplemented with 2 mM L-glutamine and penicillin/Streptomycin and 3 μg/ml TPCK-treated Trypsin (Worthington, Lakewood, NJ) but with no serum (IM).
Influenza A HKX-31 (H3N2) Instillation
Influenza A HKX-31(H3N2) purchased from American Type Culture Collection was cultured and tittered on MDCK cells using standard protocols. Virus was diluted in culture media to a concentration of 4 × 104 PFU/ml. Rats were anesthetized by inhalation of 4% isoflurane in oxygen. Once a deep plane of anesthesia was reached, the animal was placed on an instillation platform, and a catheter of appropriate length and diameter was placed transorally into the trachea. Then 0.25 ml of culture medium (vehicle) or 104 PFU influenza in 0.25 ml media (approximately 7.5 × 103 PFU/g lung) was instilled through the catheter into the lung. After instillation, the catheter was removed, and the animal was returned to its cage and observed until the rat was mobile.
In-Life Observations
All rats were observed for clinical signs of toxicity before each day’s exposure and body weights were recorded every other day.
Necropsy, Lung Harvest, and Tissue Processing
On the day of scheduled sacrifice, rats were euthanized by intraperitoneal (ip) injection of Euthasol® and the lung was harvested and weighed. The bronchus to the right lung was ligated and the left lung was infused with 10% neutral buffered formalin. The right lung was removed, weighed and frozen at −80° C for assessment of viral titer and cytokine analysis.
Right lung lobes were homogenized with sufficient cold MDCK Influenza Infection Media containing 0.5% BSA to make a final concentration of 10% homogenate by weight. The sample was clarified by spinning at 2,500 × g for 10 min and the supernatant was sterile filtered using a 0.22 μm pore size syringe filter. Samples of 0.1 ml each were aliquoted into individual vials and frozen (−80° C) for later analysis.
Plaque Assay for Viral Titer
The lung supernatants were serially diluted in sterile tubes with cold MDCK Infection Media and plated in triplicate on 6-well plates containing 100% confluent MDCK cells that had been washed twice with warm MDCK Infection Media. The plates were incubated for 1 hr at 37° C in an atmosphere containing 5% CO2. An overlay containing 50% 1.4% agarose and 50% 2× Influenza Overlay Media was added and allowed to harden at room temperature. The plates were incubated at 37° C/5% CO2 for 72 hr. The plates were fixed with 3% formaldehyde and 0.15 M NaCl for 1 hr. The agarose and fixing solution were removed and the plates were stained with crystal violet, washed with water and allowed to dry. Plaques were manually counted to determine the viral titer. Assays were repeated twice.
Inflammatory Cytokines
Lung homogenate supernatants were assayed for a panel of cytokines and chemokines (GMCSF, IL-1α, MCP-1, IL-4, IL-1β, IL-2, IL-6, IL-10, IL-12 (p70), IL-5, IFN-γ, IL-18, GRO-KC, and TNFα) using a Multiplex™ map kit (Millipore Corp., Billerica, MA, USA) according to manufacturer’s instructions. A seven point standard curve was run for each cytokine, with values ranging from 5 pg/ml to 20,000 pg/ml. The lowest quantifiable value reported was 24 pg/ml.
For the assays, 200 μl of assay buffer was added to a 96-well filter plate and shaken at room temperature for 10 min to pre-wet the plate and then removed. Twenty-five μl of each standard was added to appropriate wells accompanied by 25 μl of matrix solution (MDCK influenza infection media). Lung homogenate supernatants were thawed, centrifuged (1 min at 13,400 × g) and added to appropriate wells (25 μl/well). Assay buffer (25 μl) was added to the sample wells. Premixed antibody-immobilized beads were vortexed and 25 μl was added to each well. The plates were then incubated with agitation for 20 hr at 4° C. The fluid was removed by vacuum and the plate was washed (twice) with wash buffer. Detection antibodies were added to each well and the plate was incubated with agitation for 2 hr at room temperature (20–25° C). After incubation 25 μl of streptavidin-phycoerythrin was added into each well and incubated with agitation for 30 min at room temperature. The contents were removed by vacuum and the plates were washed (twice) with 200 μl of wash buffer. Sheath buffer was added (150 μl/well) and the plate was placed on a shaker for 5 min to re-suspend the beads, and read using a Luminex® 100™ IS (Austin, TX). Data was analyzed using the LDS 1.7 software. All samples were assayed in duplicate.
Histopathology
Left lungs were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at approximately 5 μm and stained with hematoxylin and eosin. Histological findings were divided into (1) effects in the peripheral lung region (perivascular infiltration of inflammatory cells, alveolitis, and alveolar macrophage hyperplasia), and (2) effects in the conducting airways (bronchus associated lymphoid tissue [BALT] hyperplasia, peribronchiolar infiltrates, and bronchiolitis). Findings in each animal were given a severity score of 0 to 4 (not present, minimal, mild, moderate, marked), and scores for each finding (including zeros) were averaged for each dose group. Mean severity scores are reported as a function of time following influenza administration.
Statistical Analysis
Group mean body weight and organ weight data were tested for statistical significance using Path-Tox® software. After testing for an overall trend among test groups by an analysis of variance, Bartlett’s test was used to establish the homogeneity of the data. If the data were homogeneous, group differences were evaluated using a modified Dunnett’s test. If data were non-homogeneous, group differences were assessed using a modified t-test. Significance levels were set at p ≤ 0.05.
For all other endpoints, group means and standard deviations were calculated using Microsoft Excel software. For cytokine and viral load endpoints, an analysis of variance for an unbalanced two-way design was performed on rank transformed data using the SAS general linear models procedure (PROC GLM, SAS Institute Inc., Cary, NC). The data were examined for treatment effects, time effects and combined effects (group*time). The analyses of variance were followed with multiple comparison analyses of the least square means for treatment groups and time groups. Significance levels were set at p ≤ 0.05.
RESULTS
Aerosol
The mean ± SD total aerosol concentrations achieved during the 12 days of exposure in the control and brevetoxin chambers were 10.40 ± 2.97 mg/m3 (n = 36) and 9.93 ± 1.56 mg/m3 (n = 72), respectively. The mean calculated brevetoxin aerosol concentration (based on mass fraction of brevetoxin in the total aerosol) was 49.6 μg ± 7.93 μg/m3 (n = 72), while the mean value measured by ELISA was 50.3 ± 8.54 μg/m3 (n = 24).
Body Weights and Clinical Signs
There were no marked effects of brevetoxin exposure, influenza exposure, or combination exposure on body weights (data not shown). No significant treatment regime resulted in clinical signs of toxicity.
Lung Weight
There was no marked treatment related effect on group mean lung weights of Groups 2, 3 or 4 compared to sham exposed (Group 1) lung weights at any sacrifice time.
Viral Clearance
At 2 days post influenza administration, the pulmonary influenza titers were 1.28 × 107 ± 2.81 × 106 PFU/g lung and 1.98 × 107 ± 7.38 × 106 PFU/g lung for Groups 2 and 4, respectively (Figure 2). These results indicated that virus had replicated in the lung since original instillation of 104 PFU and that brevetoxin inhalation exerted no significant effect on viral replication or clearance during the 2 day post viral infection. Viral titers were reduced by more than 95% between 2 and 4 days post infection, and brevetoxin treatment had no significant effect on clearance at that time. However, at 7 days post viral infection, there were significantly greater numbers of PFU/lung in the brevetoxin-exposed rats 2005 ± 744 PFU/lung than in rats administered influenza and sham exposed to air 142 ± 106 PFU/lung, suggesting that brevetoxin inhalation delayed overall clearance of viral particles.
FIG. 2.
Clearance of influenza A from the lungs of rats in the presence (Group 2) or absence (Group 4) of brevetoxin inhalation. Results are the mean ± SD of 8 values. “a” indicates means are statistically different (2 way ANOVA, p = 0.0032).
Inflammatory Cytokines
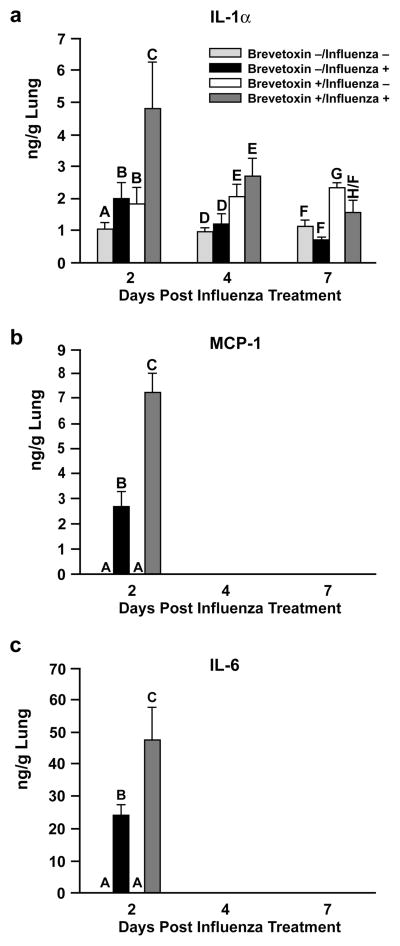
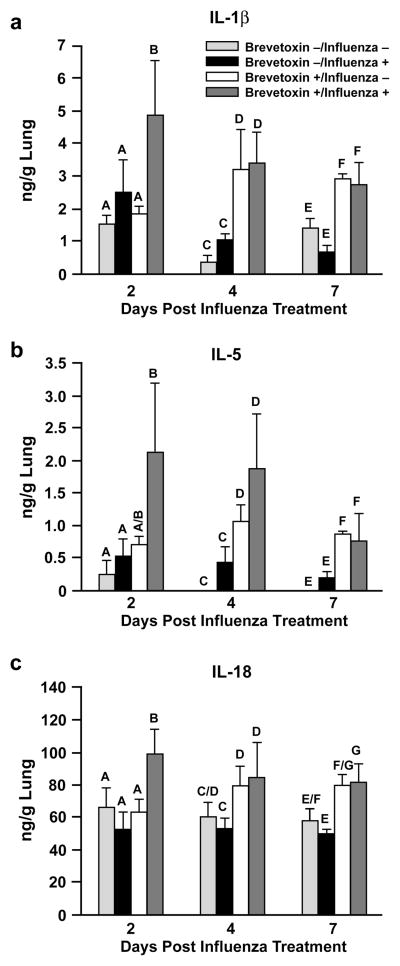
Influenza A significantly increased the lung content of IL-1α, MCP-1, and IL-6 compared to control rats (Figure 3), and only at day 2 post infection. Brevetoxin co-exposure at least doubled the influenza-induced rise in these cytokines at this time. While neither influenza A alone (Group 2) nor brevetoxin alone (Group 3) produced significant increases in IL-1β, IL-5, or IL-18 lung content at day 2 post infection, these inflammatory cytokines were significantly elevated by the virus-brevetoxin combined treatment (Figure 4). At later sacrifice times, the lung content of IL-1β, IL-5, and IL-18 was significantly increased in both Group 3 and 4 brevetoxin-exposed rats compared to Groups 1 and 2 rats, with no further enhanced response by combined virus and brevetoxin treatment.
FIG. 3.
Cytokines increased by influenza infection and the influence of brevetoxin inhalation; a) = IL-1α; b) = MCP-1; c) = IL-6. Results are the mean ± SD of 4 values for Groups 1 (Brevetoxin −/Influenza −) and 3 (Brevetoxin +/Influenza −) or the mean ± SD of 6–8 values for Groups 2 (Brevetoxin −/Influenza +, and 4–8 values for Group 4 (Brevetoxin +/Influenza +). Bars with different letter designations within each time point are significantly different from each other (p < 0.05).
FIG. 4.
Cytokine responses induced by brevetoxin inhalation and the impact of influenza infection. a) = IL-1β; b) = IL-5; c) = IL-18. Data presented are the mean ± SD of 3–4 values (Group 1), 2–4 values (Group 3), 6–8 values (Groups 2; except for IL-5 at 7 days where n = 3), and 4–8 (Group 4). Bars with different letter designations within each time point are significantly different from each other (p < 0.05).
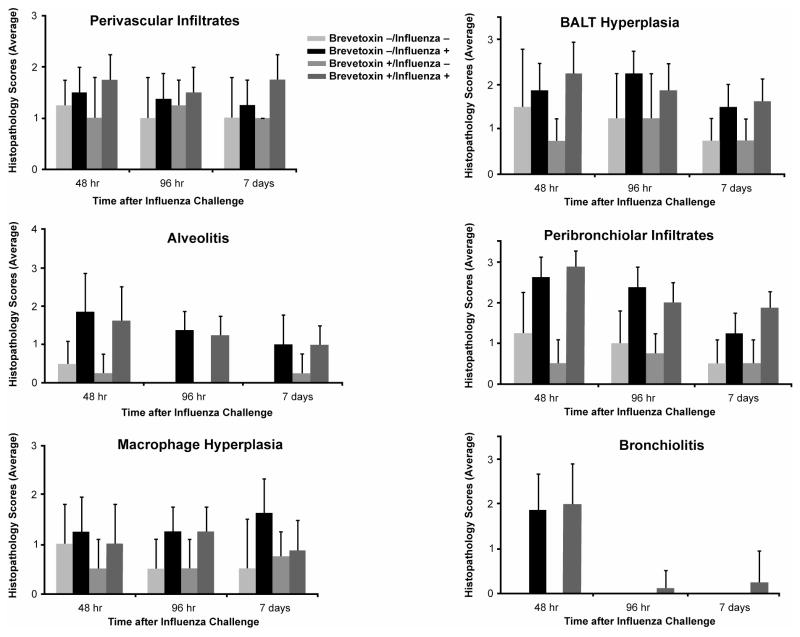
Histopathology
Influenza induced alveolitis (minimal to mild severity), peribronchiolar infiltration of inflammatory cells (mild to moderate severity), and bronchiolitis (mild severity). The severity of these responses peaked at day 2 post administration and then generally declined by day 7 post infection (Figure 5). At day 2 post infection, influenza produced relatively more modest increases in the severity of perivascular infiltrates of inflammatory cells, alveolar macrophage hyperplasia and BALT hyperplasia compared to findings in controls (Group 1). Influenza-induced alveolar macrophage hyperplasia and BALT hyperplasia peaked on days 7 and 2, respectively.
FIG. 5.
Mean severity scores for lesions in the peripheral lung and in the conducting airways. Results are the mean ± SD of 4 values for Groups 1 and 3 and 8 values for Groups 2 and 4. Note that in the case where the lesion was absent, a zero severity score was included in the calculation of the mean.
As expected, brevetoxin inhalation alone produced no notable histological changes. However, brevetoxin inhalation appeared to quantitatively increase the severity of perivascular infiltrates (mean ± SD score of 1.3 ± 0.5 and 1.8 ± 0.5 for Groups 2 and 4, respectively) and for peribronchiolar infiltrates (1.3 ± 0.5 versus 1.9 ± 0.4 for Group 2 and 4, respectively) in influenza-treated animals at day 7 post infection. Further, while bronchiolitis was no longer present in the influenza-treated animals at 4 and 7 days post infection, one of the 8 Group 4 animals had minimal to mild bronchiolitis at these later times.
DISCUSSION
The purpose of this study was to determine whether repeated inhalation of brevetoxin 3 alters the pulmonary response to influenza A in rat lung. Florida Red Tides generally occur during the late fall and winter months; these are also months associated with seasonal flu. Epidemiological studies demonstrated an association between period of Florida Red Tides and number of emergency room visits for respiratory symptoms including pneumonia (a complication of flu infection), bronchitis, and asthma (Kirkpatrick et al., 2006; Hoagland et al., 2009). It was postulated that brevetoxin inhalation may influence pulmonary responses to influenza infection because inhaled brevetoxins in rats were reported to suppress humoral immune responses in rats (Benson et al., 2004b, 2005), and because recent studies showed that brevetoxins, at μM concentrations, are cytotoxic to lymphocytes in vitro (Sayer et al., 2005). The results of this study indicated the predominant effect of brevetoxin inhalation in influenza A-infected rats was a delay in clearance of influenza and enhancement of the severity of perivascular and peribronchiolar inflammation 7 days post infection.
Multiple cell types participate in the immune response to influenza. Activated macrophages produce IFN-α and IFN-β, and contribute to clearance as phagocytic cells and in antigen presentation and processing (Lebrec and Burleson, 1994). Natural killer (NK) cells, whose activity is augmented by influenza infection, play a major role in early viral clearance (Lebrec and Burleson, 1994). Cytotoxic T lymphocytes (CTL) respond to influenza infection by destroying virus-infected cells via cytolytic granules containing lytic proteins (Mintern et al., 2007). After virus is cleared memory CTL reside in lymphoid organs to maintain immune function against subsequent influenza infection.
Humoral immune responses also contribute to influenza clearance. The production of antibody to the influenza surface glycoprotein hemagglutinin neutralizes viral infectivity while antibodies to neuraminidase reduce the severity of infection by reducing virus yield and dissemination (Lebrec and Burleson, 1994). In mice infected with influenza, virus-specific IgM antibody-secreting cells were detected 3 days post infection in spleen and 5 days post infection in lung. IgG-secreting cells appeared in spleen on day 6 and in lung on day 10 (Ada and Jones, 1986).
Toxin-induced delay in viral clearance in a rat model of influenza was reported to occur following phosgene or tetrachlorodibenzo-p-dioxin (TCDD) treatment. In the case of phosgene, exposures of F344 rats to 1 ppm inhibited viral clearance by one thousand- to one million-fold on day 3 and 4 post infection, respectively (Ehrlich and Burleson, 1991). Viral particle clearance was complete by day 5 in the phosgene-treated and infected rats. The period of impaired clearance coincided with phosgene-induced inhibition of NK cell activity (Burleson and Keyes, 1989). In the case of TCDD, a single ip dose of 10 μg/kg impaired influenza viral particle clearance on days 2–4 post infection. Delayed clearance coincided with toxin-induced impairment of influenza-induced augmentation of NK cell activity on days 2 and 3 post infection (Yang et al., 1994). By 4 days post infection NK activity returned to baseline levels in non-toxin-treated animals, and no further significant differences in viral particle clearance between toxin-treated and control animals were observed. Yang et al. (1994) concluded that NK cells were important for viral clearance up to 4 days post infection, after which CTL cells and humoral responses mediated clearance.
With brevetoxin inhalation deposition of 600 ng/kg/day, delayed viral clearance was only observed 7 days post infection, suggesting effects on clearance may have been mediated by effects on CTL or on the humoral immune responses, rather than an effect on NK cell function. While the effects of brevetoxin on pulmonary humoral or cell-mediated immune responses were not measured, Benson et al. (2004b) previously demonstrated that inhalation of 50 μg brevetoxin-3/m3 for 5 days (deposition of 600 ng/kg/day) significantly suppressed splenic humoral immune function. Exposures for 7 days exerted no marked effect of splenic CD4+ or CD8+ lymphocytes populations, but cell function was not assessed (Benson et al., personal communication). Brevetoxins affect T cell proliferation in a dose-dependent manner as well as induce apoptosis damage in vitro (Murrell and Gibson, 2009). It is possible that delayed clearance may be mediated through suppression of pulmonary humoral immune responses, pulmonary cytolytic T cell responses, or both. Further experiments are needed to characterize the effects of brevetoxin on pulmonary immune function in response to influenza infection.
In our study, instillation of 104 PFU influenza A/lung resulted in alveolitis, alveolar macrophage hyperplasia, bronchus associated lymphoid tissue hyperplasia, peribronchiolar and perivascular infiltration with inflammatory cells, and bronchiolitis that persisted through 7 days post exposure, when a majority of the viral particles had been cleared from the lungs. These pulmonary changes are consistent with those observed in the cotton rat intranasally administered influenza A H3N2 (Ottolini et al., 2005). The inflammatory changes in our study were associated with significant increases in the concentrations of the inflammatory cytokines IL-1α and IL-6 and in the chemokine MCP-1 in Group 2 (influenza-infected rats) at day 2 post infection. While brevetoxin co-exposure doubled these influenza-induced cytokine responses at day 2, only modest increases in perivascular and peribronchiolar infiltrates, BALT hyperplasia, and bronchiolitis in Group 4 versus Group 2 and reduction in alveolitis and alveolar macrophage hyperplasia were observed. Therefore the brevetoxin-induced enhancement of cytokine response at day 2 appears to have had no notable association with pulmonary inflammatory responses observed histologically. Further, because statistically significant increases in IL-1α, IL-1β, IL-5, and IL-18 occurred in brevetoxin-treated lungs in the absence of pulmonary histological responses, it appears these changes may not be biologically significant.
Pneumonia is a recognized complication of influenza infection, especially among the elderly. Brevetoxin-induced delayed influenza particle clearance and enhancement of perivascular and peribronchiolar inflammation suggest that exposure of individuals during a Florida Red Tide event, especially among the compromised may prolong their recovery, or enhance susceptibility to other pulmonary infections. Future studies will focus on examining the effects of brevetoxin on the pulmonary immune system and how these may mediate responses to viral infection.
Acknowledgments
Research conducted under NIEHS P01 ES10594. The authors are grateful for the technical and administrative assistance of the LRRI and Center for Marine Sciences staff that made this study possible. Special thanks go to Glenna Chavez, Susan Core, Dolores Esparza, Fred Kleinschnitz, Chris Ynostroza for technical support, Drs. JeanClare Seagrave and Frederick Koster for helpful discussions, and Drs. Laura Fleming and William Abraham for careful manuscript review.
Contributor Information
Janet M. Benson, Email: jbenson@lrri.org.
Molly L. Wolf, Email: mwolf@lrri.org.
Adriana Kajon, Email: akajon@lrri.org.
Brad M. Tibbetts, Email: btibbett@lrri.org.
Andrea J. Bourdelais, Email: bourdelaisa@uncw.edu.
Daniel G. Baden, Email: badend@uncw.edu.
Thomas H. March, Email: tmarch@lrri.org.
References
- Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D. Recreational exposure to aerosolized brevetoxins during Florida Red Tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Zhou Y, Baden D. Occupational exposure to aerosolized brevetoxins during Florida Red Tide events: Effects on a healthy worker population. Environ Health Perspect. 2005;113:644–649. doi: 10.1289/ehp.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden DG, Mende JJ, Lichter W, Wellham L. Crystallization and toxicology of T34: A major toxin from Florida’s Red Tide organism (Ptychodiscus brevis) Toxicon. 1981;19:455–462. doi: 10.1016/0041-0101(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, Tibbetts BM, Bowen LE, March TH, Langley RJ, Murray TF, Bourdelais AJ, Naar J, Zaias J, Baden DG. Florida Red Tide: Inhalation toxicity of Karenia breve extract in rats. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002, Proceedings of the Xth International Conference on Harmful Algae. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO; 2004a. pp. 502–504. [PMC free article] [PubMed] [Google Scholar]
- Benson J, Hahn F, March T, McDonald J, Sopori M, Seagrave J, Gomez A, Bourdelais A, Naar J, Zaias J, Bossart G, Baden D. Inhalation toxicity of brevetoxin 3 in rats exposed for 5 days. J Toxicol Environ Health A. 2004b;67:1443–1456. doi: 10.1080/15287390490483809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori ML, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days. Environ Health Perspect. 2005;113:626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleson GR, Keyes LL. Natural killer activity in Fischer-344 rat lungs as a method to assess pulmonary immunocompetence: Immunosuppression by phosgene inhalation. Immunopharmacol Immunotoxicol. 1989;11:421–443. doi: 10.3109/08923978909005378. [DOI] [PubMed] [Google Scholar]
- Ehrlich JP, Burleson GR. Enhanced and prolonged pulmonary influenza virus infection following phosgene inhalation. J Toxicol Environ Health. 1991;34:259–273. doi: 10.1080/15287399109531565. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Backer LC, Baden DG. Overview of aerosolized Florida Red Tide toxins: Exposures and effects. Environ Health Perspect. 2005a;113:618–620. doi: 10.1289/ehp.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierce R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida Red Tide toxins (brevetoxins) in persons with asthma. Environ Health Perspect. 2005b;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Reich A, Zaias J, Cheng YS, Pierce R, Naar J, Abraham WM, Baden DG. Aerosolized red tide toxins (brevetoxins) and asthma. Chest. 2007;131:187–194. doi: 10.1378/chest.06-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming LE, Bean JA, Kirkpatrick B, Cheng YS, Pierce R, Naar J, Nierenberg K, Backer LC, Wanner A, Reich A, Zhou Y, Watkins S, Henry M, Zaias J, Abraham WM, Benson J, Cassedy A, Hollenbeck J, Kirkpatrick G, Clarke T, Baden DG. Exposure and effect assessment of aerosolized red tide toxins (brevetoxins) and asthma. Environ Health Perspect. 2009;117:1095–1100. doi: 10.1289/ehp.0900673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil CA, Steidinger KA. Monitoring, management and mitigation of Karenia blooms in the eastern Gulf of Mexico. Harmful Algae. 2009;8:611–617. [Google Scholar]
- Hoagland P, Jin D, Polansky LY, Kirkpatrick B, Kirkpatrick G, Fleming LE, Reich A, Watkins SM, Ullmann SG, Backer LC. The costs of respiratory illnesses arising from Florida gulf coast Karenia brevis blooms. Environ Health Perspect. 2009;117:1239–1243. doi: 10.1289/ehp.0900645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Environmental exposures to Florida Red Tides: Effects on emergency room respiratory diagnoses admissions. Harmful Algae. 2006;5:526–533. doi: 10.1016/j.hal.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Bean JA, Fleming LE, Backer LC, Akers R, Wanner A, Dalpra D, Nierenberg K, Reich A, Baden DG. Aerosolized Red Tide toxins (brevetoxins) and asthma: A 10 day follow up after 1 hour acute beach exposure. In: Moestrup Ø, et al., editors. Proceedings of the 12th International Conference on Harmful Algae. Copenhagen: International Society for Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO; 2009. pp. 297–299. [Google Scholar]
- Kirkpatrick B, Pierce R, Cheng YS, Henry MS, Blum P, Osborn S, Nierenberg K, Pederson BA, Fleming LE, Reich A, Naar J, Kirkpatrick G, Backer LC, Baden D. Inland transport of aerosolized Florida Red Tide toxins. Harmful Algae. 2010;9:186–189. doi: 10.1016/j.hal.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrec H, Burleson GR. Influenza virus host resistance models in mice and rats: Utilization for immune function assessment and immunotoxicology. Toxicology. 1994;91:179–188. doi: 10.1016/0300-483x(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Lamirande EW, Subbarao K. The mouse model for influenza. Curr Protoc Microbiol. 2009;Chapter 15:15G.3.1–15G.3.30. doi: 10.1002/9780471729259.mc15g03s13. [DOI] [PubMed] [Google Scholar]
- Mintern JD, Guillonneau C, Carbone FR, Doherty PC, Turner SJ. Cutting edge. Tissue-resident memory CTL down regulate cytolytic molecule expression following virus clearance. J Immunol. 2007;179:7220–7224. doi: 10.4049/jimmunol.179.11.7220. [DOI] [PubMed] [Google Scholar]
- Murrell RN, Gibson JE. Brevetoxins 2, 3, 6, and 9 show variability in potency and cause significant induction of DNA damage and apoptosis in Jurkat E6-1 cells. Arch Toxicol. 2009;83:1009–1019. doi: 10.1007/s00204-009-0443-x. [DOI] [PubMed] [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini MG, Blanco JCG, Eichelberger MC, Porter DD, Pletneva L, Richardson JY, Prince GA. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J Gen Virol. 2005;86:2823–2830. doi: 10.1099/vir.0.81145-0. [DOI] [PubMed] [Google Scholar]
- Pierce RH, Henry MS, Bloom PC, Hamel SL, Kirkpatrick B, Cheng YS, Zhou Y, Irvin CM, Naar J, Weidner A, Fleming LE, Backer LC, Baden D. Brevetoxin composition in water and marine aerosol along a Florida beach: Assessing the potential human exposure to marine biotoxins. Harmful Algae. 2005;4:965–972. [Google Scholar]
- Sayer A, Hu Q, Bourdelais AJ, Baden DG, Gibson JE. The effect of brevenal on brevetoxin-induced DNA damage in human lymphocytes. Arch Toxicol. 2005;79(11):683–688. doi: 10.1007/s00204-005-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RB. Deposition and clearance of inhaled particles. In: McClellan RO, Henderson RF, editors. Concepts in Inhalation Toxicology. New York: Hemisphere Publishing Co; 1989. pp. 163–192. [Google Scholar]
- Simonsen L, Clarke MJ, Williamson GD, Stroup DF, Arden NA, Schonberger LB. The impact of influenza epidemics on mortality: Introducing a severity index. Am J Public Health. 1997;87:1944–1950. doi: 10.2105/ajph.87.12.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YG, Lebrec H, Burleson GR. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on pulmonary influenza virus titer and natural killer (NK) activity in rats. Fundam Appl Toxicol. 1994;23:125–131. doi: 10.1006/faat.1994.1088. [DOI] [PubMed] [Google Scholar]
- Wolf M, Kajon A, March T, Baden D, Benson J. Development of a rodent model of influenza A infection in F344 rats. The Toxicologist. 2009;108:Abstract 1417. [Google Scholar]