Abstract
Purpose.
Age-Related Eye Disease Study 2 (AREDS2) is a randomized, placebo-controlled study designed to determine whether supplementation with 10 mg of lutein and 2 mg of zeaxanthin per day can slow the rate of progression of age-related macular degeneration (AMD). Although some biomarkers of response to carotenoid supplementation such as serum concentrations are part of the AREDS2 protocol, measurement of carotenoid concentrations in the eye and other tissues is not. In this approved ancillary study, macular pigment optical density (MPOD), macular pigment distributions, and skin carotenoid levels at enrollment and at each annual visit were measured to assess baseline carotenoid status and to monitor response to assigned interventions.
Methods.
All subjects enrolled at the Moran Eye Center had MPOD and macular pigment spatial distributions measured by dual-wavelength autofluorescence imaging and total skin carotenoids measured by resonance Raman spectroscopy.
Results.
Baseline MPOD in enrolled subjects was unusually high relative to an age-matched control group that did not consume carotenoid supplements regularly, consistent with the high rate of habitual lutein and zeaxanthin consumption in Utah AREDS2 subjects prior to enrollment. MPOD did not correlate with serum or skin carotenoid measurements.
Conclusions.
Useful information is provided through this ancillary study on the ocular carotenoid status of AREDS2 participants in the target tissue of lutein and zeaxanthin supplementation: The macula. When treatment assignments are unmasked at the conclusion of the study, unique tissue-based insights will be provided on the progression of AMD in response to long-term, high-dose carotenoid supplementation versus diet alone. (ClinicalTrials.gov number, NCT00345176.)
Baseline macular pigment measurements at the Utah AREDS2 site were unusually high relative to an age-matched control group that did not consume carotenoid supplements regularly, consistent with the high rate of habitual lutein and zeaxanthin consumption in Utah AREDS2 subjects prior to enrollment.
Introduction
Accumulating evidence suggests that supplementation with the dietary macular pigment carotenoids lutein and zeaxanthin may be an important public health intervention to prevent or slow visual loss from age-related macular degeneration (AMD), a common cause of irreversible visual disability and blindness in the elderly in developed countries.1,2 Lutein and zeaxanthin are nutrients that humans obtain from dark green leafy vegetables and from orange and yellow fruits and vegetables. The typical American diet provides 1 to 2 mg of these xanthophyll carotenoids per day, and they are concentrated specifically in the primate fovea at very high levels, where they are thought to act as antioxidants and as screening pigments to ameliorate the phototoxic effects of short-wavelength visible light.3 Several very large epidemiologic studies including the Eye Disease Case Control (EDCC) study,4 a follow-up study on a subset of EDCC patients by Seddon and colleagues,5 and the original Age-Related Eye Disease Study (AREDS)6 have demonstrated a significant protective effect of high levels of dietary consumption of lutein and zeaxanthin and of high blood concentrations of these carotenoids against AMD. Although these epidemiologic studies are very important, it must be considered that nutritional surveys and blood analyses are, at best, indirect measures of the status of the tissue of interest: the macula of the human eye.7 This is because uptake and stabilization of lutein and zeaxanthin in the macula are complex, biologically regulated processes mediated by specific binding and transport proteins.8 This means that macular uptake of lutein and zeaxanthin is subject to saturation effects and is dependent on protein expression levels and binding affinities of the proteins' various isoforms. Our laboratory has identified GSTP1 as the specific binding protein for zeaxanthin in the primate macula, and we have recently identified StARD3 as the specific lutein-binding protein.9,10 Other laboratories have contributed insights into the potential carotenoid transport proteins in the gut and the eye of various organisms such as the scavenger receptor class proteins SR-BI and CD36.11–14
Epidemiology studies and biochemical investigations of nutrient roles in complex degenerative diseases of aging such as AMD are very helpful in formulating clinical recommendations, but many clinicians still demand definitive results from at least one adequately powered randomized clinical trial (RCT) before altering clinical practice. The first RCT to have a major impact on AMD treatment and prevention strategies was the original AREDS study published in 2001.15 AREDS examined the incidence of advanced AMD and severe visual loss in patients with high-risk characteristics for AMD progression such as large drusen and pigmentary changes in one or both eyes and/or advanced AMD in one eye (choroidal neovascularization or geographic atrophy). Approximately 3600 subjects followed for at least 5 years were randomized into four treatment arms: high dose zinc + antioxidants (vitamin C, vitamin E, and β-carotene); high dose zinc alone; antioxidants alone; or placebo. The AREDS group reported significant reduction of AMD progression with either zinc or antioxidants versus placebo, and even better protection when the interventions were combined.
AREDS was a crucial first step in guiding nutritional interventions against AMD, but in the interval between its inception and publication of its results, it became clear that the formulation could be potentially improved.16 Supplementation with lutein and zeaxanthin appeared to be a better choice than β-carotene because they are abundantly and specifically concentrated in the macula, whereas β-carotene is not. Moreover, there were serious concerns about elevated risk of lung cancer in smokers consuming such a high dose of β-carotene.17–19 Additionally, several epidemiologic studies indicated a potential benefit of omega-3 fatty acids typically found in fish oil (EPA and DHA).20–22 Thus, the AREDS2 study was initiated in 2006 to evaluate this updated nutritional knowledge.23 Using a similar size sample and time period as AREDS, high-risk AMD patients have been randomized to receive 10 mg lutein and 2 mg zeaxanthin (L+Z arm), 1000 mg of fish oil (EPA+DHA arm), L+Z and EPA+DHA, or placebo. All participants were also encouraged to take either an AREDS supplement or a modified AREDS supplement with reduced zinc and β-carotene. Results are expected to be released in 2013.
As with any well-designed RCT, the AREDS2 designers included as many relevant biomarkers as possible to ensure compliance with the treatment assignments and to assess physiologic response. In the AREDS2 protocol, all subjects fill out dietary surveys and have periodic pill counts, and a subset of participants has serum carotenoid levels measured by high-performance liquid chromatography (HPLC); however, as discussed earlier, these are, at best, indirect measures of response to L+Z supplementation in the tissue of interest: the subject's macula. A variety of noninvasive methods exist to quantify and image macular carotenoid pigment levels and spatial distributions,24 but due to budget and logistic limitations, they were not included in the final AREDS2 protocol. At the Moran Eye Center AREDS2 study site, we initiated an approved ancillary study to image macular pigment distributions at the initiation visit and yearly thereafter in our site's subjects, to understand more completely the time course and long-term tissue response to AREDS2 interventions and to provide insights into which AMD subjects are most likely to derive clinical benefits from carotenoid supplementation. We report here the baseline characteristics and correlations between carotenoid measurements in the subjects enrolled in our ancillary study.
Materials and Methods
Subjects
This study was performed under the approval of the Institutional Review Board of University of Utah School of Medicine as well as the AREDS2 executive committee. All subjects signed an informed consent form that complied with the tenets of the 1975 Declaration of Helsinki as revised in 1983. All Moran Eye Center patients enrolling in AREDS2 were offered enrollment in our ancillary study. In addition to standard AREDS2 procedures (dietary surveys, eye examinations, and fundus photography), our patients had macular pigment optical density (MPOD) measured by our dual-wavelength autofluorescence imaging (AFI) technology25; blood was drawn for baseline serum carotenoid concentrations, and skin carotenoid levels were measured by resonance Raman spectroscopy (RRS). As specified in the AREDS2 protocol, all enrolled subjects had to discontinue any supplements containing substantial amounts of any AREDS2 constituents (lutein, zeaxanthin, or omega-3 fatty acids) at least 30 days prior to the baseline visit. Subjects were encouraged to take an original AREDS supplement during the run-in period unless they were current or recent smokers.
Measurement of MPOD and Spatial Distribution
For the quantitative detection and spatial imaging of macular pigment (MP) we chose AFI as the most suitable MP detection method for AREDS2 subjects. The method implemented at our center has been described in detail in a prior publication25 and is particularly useful in subjects with the irregular lipofuscin patterns and moderate cataracts commonly encountered in AMD patients. This procedure is based on the principle that the visible absorption spectrum of the macular carotenoid pigment overlaps with the excitation spectrum of the intensely autofluorescent lipofuscin pigment of the retinal pigment epithelium (RPE) at an excitation wavelength of 488 nm (blue laser light) but not at 532 nm (green laser light).25,26 The emission spectrum of lipofuscin extends broadly into the red/near infrared wavelength range, and we restrict the imaging to emission wavelengths above 680 nm to avoid intrinsic lens fluorescence that occurs at lower wavelengths.25 When lipofuscin fluorescence is imaged in this way under blue light excitation at 488 nm, a dark image area centered on the fovea is present because the MP attenuates the excitation light, but not the long-wavelength lipofuscin fluorescence. When a similar lipofuscin fluorescence image is made with green laser excitation, the MP does not attenuate either the excitation or the emission light, so no dark area is seen. After use of suitable scaling factors to account for the absorption and fluorescence excitation and emission spectra at the wavelengths used and assignment of the MPOD to zero in the periphery, an image of the spatial macular pigment distribution and a value for the peak MPOD within the distribution are generated by digital subtraction of the green excitation lipofuscin fluorescence image from the blue excitation fluorescence image. Although visually significant cataracts may attenuate AFI quantification of MPOD in some situations,27 the presence of such advanced cataracts was grounds for exclusion from AREDS2 enrollment.
Measurement of Total Skin Carotenoids
We used RRS to quantify total skin carotenoids.28,29 This validated noninvasive optical method significantly correlates with total skin and serum carotenoid concentrations measured by HPLC of skin biopsies and blood samples in normal subjects, and it is an established biomarker for fruit and vegetable intake.29–31 It is based on the principle that certain wavelengths of light resonantly enhance vibrational stretch modes of the polyene backbone of carotenoid molecules. The resonance enhancement is so strong that carotenoids can be readily detected and quantified even in complex biological tissues.28 Blue argon laser light (488 nm, 10 mW) is delivered through a handheld light delivery and collection module to the palm of the subject's hand for 10 seconds in a 2 × 3-mm elliptical excitation spot. Backscattered light is routed via the optical fiber collection bundle to a Raman spectrograph coupled to a Peltier-cooled charge-coupled device camera that measures the intensity and spectrum of the backscattered light. The peak height at the carotenoid C=C stretch frequency of 1525 cm−1, which correlates linearly with carotenoid concentration, is quantified after digital subtraction of background dermal fluorescence.28
Serum Carotenoid Analysis
Carotenoids were analyzed by reverse-phase HPLC using photodiode array detection as previously described31 with recent modifications. Briefly, 200 μL of human serum was extracted with ethanol, ethyl acetate, and hexane in succession, and then the combined extracts were dried under inert gas. Subsequently, the dried film was cleaned by hexane/methanol/distilled water, and the supernatant was recovered and dried. The dried extract was dissolved in 250 μL mobile phase and filtered through a 0.2-μm filter before HPLC analysis. An HPLC-diode array detection (DAD) method has been developed to simultaneously determine the major carotenoids, including 3′-oxolutein, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene from patients' serum. The HPLC system is equipped with a liquid chromatograph (Surveyor Plus HPLC System; Thermo Fisher Scientific Inc., Waltham, MA) including vacuum degasser, quaternary pump, autosampler, column oven, and a DAD detector connected to a commercial software program (Xcalibur; Thermo Scientific, West Palm Beach, FL). A YMC-pack pro-C18 column (250 × 4.6 mm, 5 μm; Waters, Milford, MA) and a YMC guard column (20 × 4.0 mm, 4 μm; Waters) were used. The gradient mobile phase was composed of methanol and methyl tert-butyl ether. Flow rate was 1.0 mL/min, and the oven temperature was set at 40°. Detection was at 450 nm, and the injection volume of standards and extracts was 100 μL. Peak identities were confirmed by DAD spectra, by mass spectra (Thermo Scientific MSQ single-quadrupole liquid chromatography mass spectrometer [San Jose, CA], equipped with an atmospheric pressure chemical ionization [APCI] source under positive ionization mode), and by coelution with authentic standards as necessary. Standard solutions of each individual carotenoid of interest were prepared with known concentrations calculated spectroscopically using published extinction coefficients32,33 and then injected in different volumes so as to achieve final injected amounts ranging from 0.1 to 100 ng. Major serum carotenoids were quantified based on standard curves of known quantities of the authentic carotenoid standards injected into the HPLC system versus integrated UV/visible absorbance of their eluted peaks. We do not routinely use internal standards because they may interfere with low-level analytes in small biological samples.34 In our laboratory, typical extraction efficiencies from biological samples are >95%, and HPLC reproducibility with external standardization is ±5%.34,35
Statistics
Pearson correlation coefficients were first evaluated between right and left eye peak MPOD. Since right and left eye MPOD and MP distributions are tightly correlated, average peak MPOD was used whenever data from both eyes were available, and macular pigment distribution grading was based on examination of pictures from both eyes. General linear regression models along with the model r2 values were used to assess linear associations between continuous outcomes (peak MPOD, age, total serum carotenoids, serum lutein+zeaxanthin, and skin carotenoid levels). A value of P < 0.05 was considered statistically significant. Analysis was performed on two commercially available software programs (SAS 9.1.3; SAS Institute, Cary, NC; and SPSS 16.0; SPSS Inc., Chicago, IL).
Results
Patient Population
Fifty-five subjects enrolled in the AREDS2 study at the Moran Eye Center site. By definition, all of these subjects met the AREDS2 enrollment criteria of 50 to 85 years of age and intermediate AMD in both eyes (defined as one or more large drusen >125 μm in diameter and/or noncentral geographic atrophy) or advanced AMD in one eye (choroidal neovascularization or geographic atrophy involving the fovea) and intermediate AMD in the fellow eye. Of the 55 eligible Moran AREDS2 subjects, 53 (29 female and 24 male) agreed to enroll in this ancillary study. Mean age ± SD was 77.4 ± 7.7. Only one subject was a current smoker, and another had quit smoking within the past year. Forty (75.5%) had bilateral intermediate AMD, and 13 (24.5%) had advanced AMD in one eye at enrollment. Thirty-five (66%) of the participating subjects reported regular use of supplements containing high doses of lutein and/or zeaxanthin in the years prior to enrollment, although all discontinued use of these supplements for at least 1 month prior to the baseline visit as required by the AREDS2 protocol.
Peak MPOD and MP Spatial Distributions
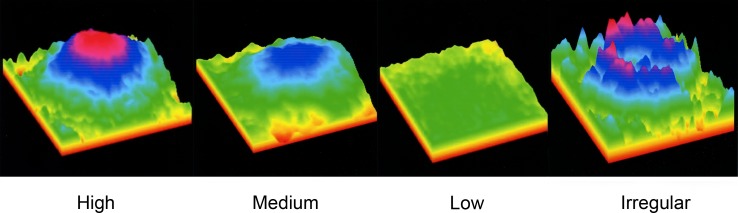
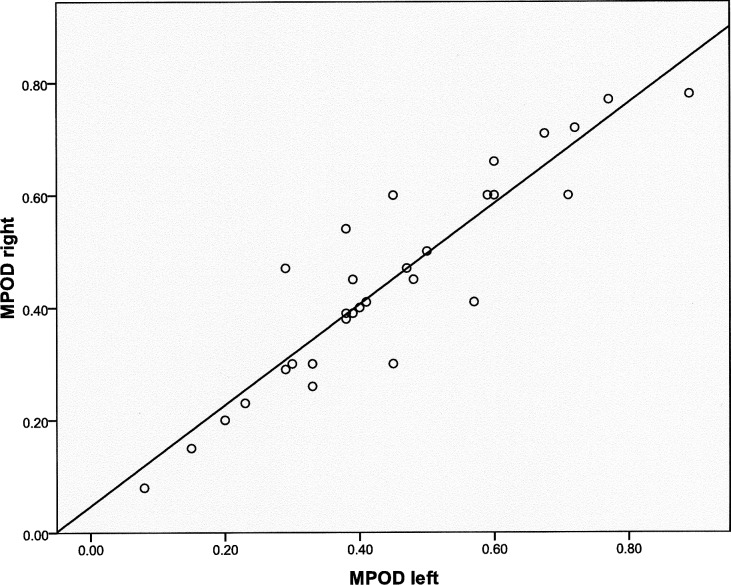
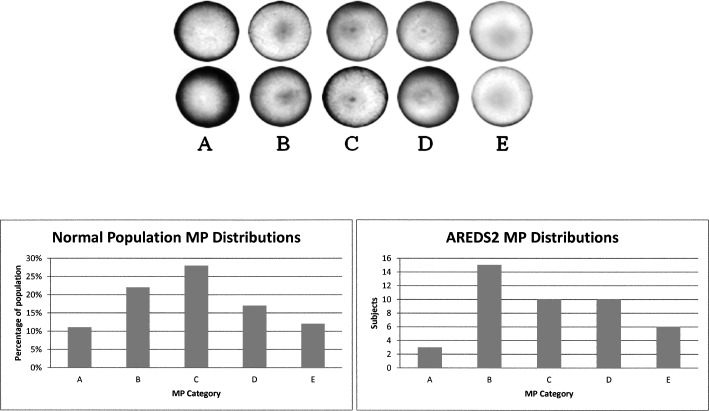
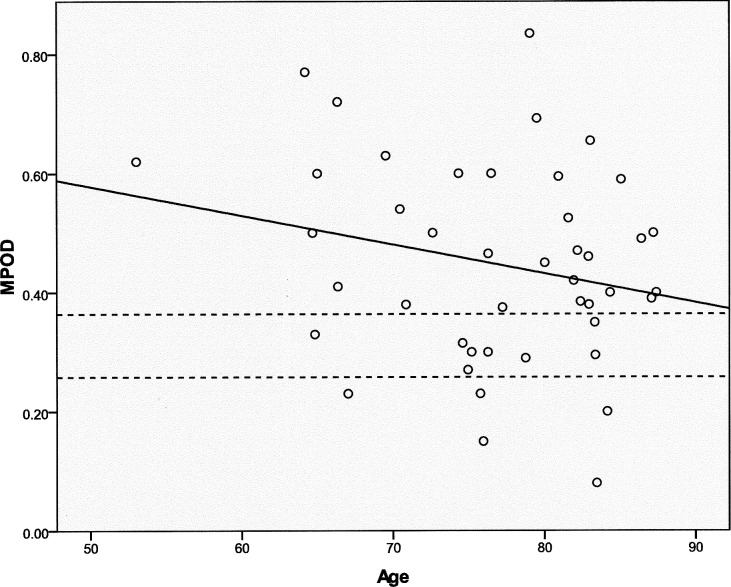
Macular carotenoid pigment optical densities and distributions were measured by autofluorescence imaging. Evaluable images could be obtained in at least one eye in 44 of the 53 enrolled subjects. Causes of unusable images that were excluded from further analysis included poor patient cooperation with the test, media opacities, and very irregular macular pigment distributions. Examples of high, medium, and low regular macular pigment distributions and an irregular distribution are shown in Figure 1. In subjects with bilaterally quantifiable images, the peak MPOD was tightly correlated between eyes (Fig. 2; n = 33; r = 0.9206; P < 0.0001). In a previously published study,25 we developed an AFI grading system in which MP distributions are divided into five categories (Fig. 3, top panel). In a normal population, the C-type distribution (sharp central peak with no shoulder of lower MP concentrations) is most common, whereas in the AREDS2 AMD population, a B-type distribution (sharp central peak surrounded by a shoulder of lower MP concentrations) is most common (Fig. 3, bottom panels). Peak MPOD declined with age insignificantly (Fig. 4; n = 44; r = −0.2268; P = 0.1388), and, as a whole, the AREDS2 population had considerably higher peak MPOD relative to our previously reported mean ± SD of 0.311 ± 0.053 for an age-matched clinic-based population (50–89 years of age; n = 70 eyes), measured with the same instrument, who did not regularly take high-dose lutein and/or zeaxanthin supplements in prior years and who did not have AMD or other significant macular pathology.25
Figure 1 .
Examples of macular pigment autofluorescence images obtained from AREDS2 participants. Most subjects have radially symmetric patterns, but a few have very low MP levels with fragmented distributions (scale is expanded 3-fold for clarity for fragmented distribution).
Figure 2 .
Correlation of peak macular pigment optical density (MPOD) in right and left eyes of subjects who had both eyes measured by autofluorescence imaging (AFI) (n = 33; r = 0.9206; P < 0.0001).
Figure 3 .
MP spatial distributions in normal and AREDS2 subjects. In the top panel we show examples of five different radially symmetric patterns seen when performing autofluorescence imaging: Category (A) has very low MP levels (peak MPOD <0.05). (B) Enhanced central MP levels and lower eccentric levels. (C) Has only a sharp, central MP distribution. (D) Has a central MP concentration surrounded by lower amounts arranged as a ring or shoulder. (E) Has both central and parafoveal MP levels. Bottom left panel is the MP distribution in a normal population.25 Bottom right panel is the MP distribution in the Moran Eye Center AREDS2 population.
Figure 4 .
Peak MPOD versus age in the study population. The solid line is the regression line of peak MPOD versus age (n = 44; r = −0.2268; P = 0.1388). The dashed lines denote the mean ± SD of an age-matched unsupplemented control population measured with the same instrumentation.25
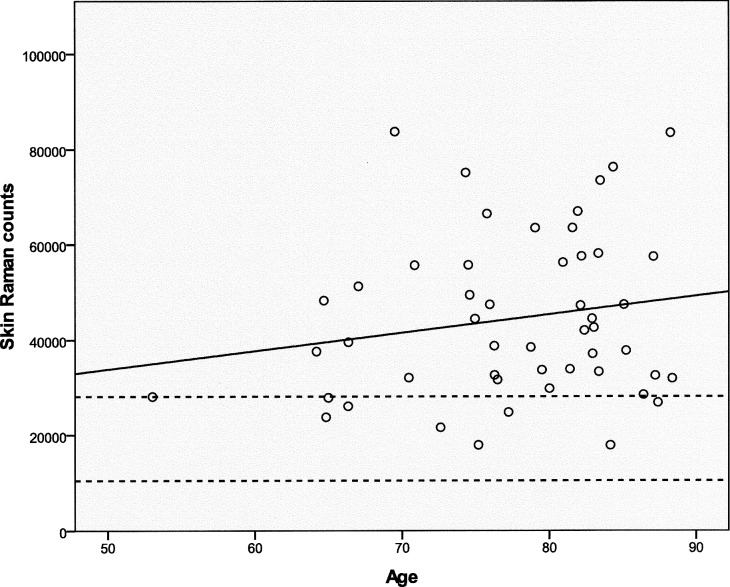
Skin and Serum Carotenoids
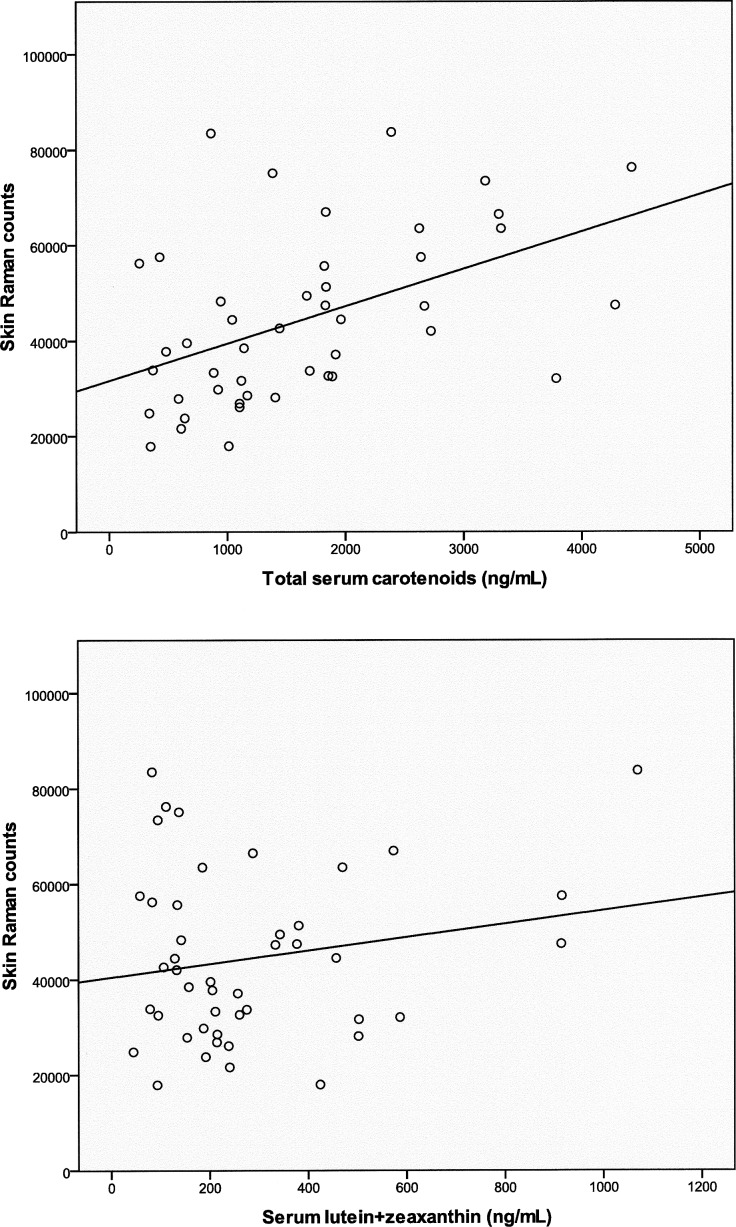
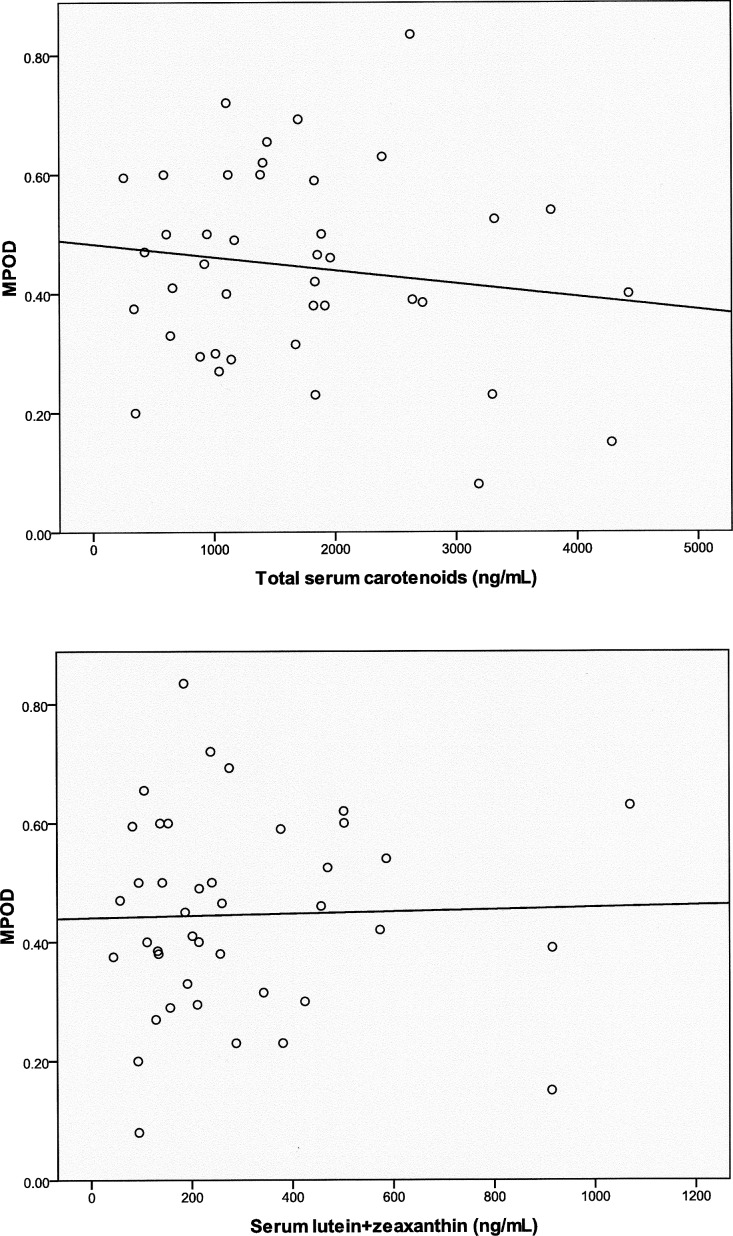
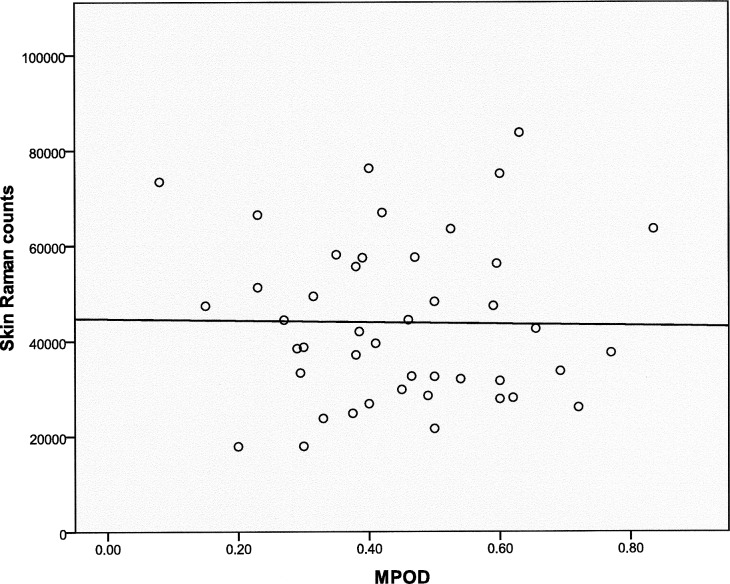
Skin carotenoid levels were measured in the enrolled subjects using resonance Raman spectroscopy. Skin Raman counts rose with age, but this trend was not significant (Fig. 5; n = 50; r = 0.1758; P = 0.22219), and our AREDS population's skin Raman counts were generally much higher than our laboratory's typical levels of 19,330 ± 8800 (mean ± SD; n = 1375) in an unsupplemented normal population measured with the same device.36 Skin carotenoid levels linearly correlated with total serum carotenoids (Fig. 6, top panel; n = 45; r = 0.4727; P = 0.0010) but not with serum lutein + zeaxanthin (Fig. 6, bottom panel; n = 45; r = 0.1843; P = 0.2256). There was no significant correlation of peak MPOD with either total serum carotenoids (Fig. 7, top panel; n = 41; r = −0.1436; P = 0.3705) or with serum lutein + zeaxanthin (Fig. 7, bottom panel; n = 41; r = 0.0261; P = 0.8714). There was no significant correlation between peak MPOD and skin Raman counts (Fig. 8; n = 44; r = −0.0167; P = 0.9144).
Figure 5 .
Skin Raman counts versus age in the study population. The solid line is the regression line of skin Raman counts versus age (n = 50; r = 0.1758; P = 0.2219). The dashed lines denote the mean ± SD of a normal unsupplemented control population measured with the same instrumentation.36
Figure 6 .
Skin Raman counts versus total serum carotenoids (top panel: n = 45; r = 0.4727; P = 0.0010) and serum lutein + zeaxanthin (bottom panel: n = 45; r = 0.1843; P = 0.2256) in the study population.
Figure 7 .
Peak MPOD versus total serum carotenoids (top panel: n = 41; r = −0.1436; P = 0.3705) and serum lutein + zeaxanthin (bottom panel: n = 41; r = 0.0261; P = 0.8714).
Figure 8 .
Skin Raman counts versus peak MPOD in the study population (n = 44; r = −0.0167; P = 0.9144).
Discussion
This baseline observational study provides a number of important insights into the AREDS2 cohort enrolled at the Moran Eye Center. First, it demonstrates that a large majority of patients with significant AMD can still have macular pigment levels and distributions measured by AFI with excellent correlations between imaged eyes. Second, the peak MPOD levels were unusually high relative to a control cohort of age-matched individuals who did not take lutein or zeaxanthin supplements regularly. Because 66% of our AREDS2 participants had been taking such supplements for years, this suggests that their maculae were still well saturated with lutein and zeaxanthin at the baseline visit. Clinical studies in humans and laboratory studies in monkeys indicate that the macular pigment is remarkably stable and can take many months or even years to decline in response to a substantial decrease in lutein and zeaxanthin consumption,37–40 so it is not surprising to observe such high levels in the macula even after the required 1-month washout period.
Although other cross-sectional studies have reported modest, but significant, correlations between MPOD and serum carotenoids,41,42 we did not observe significant associations. This may have been due to our small sample size and recent changes in lutein and zeaxanthin consumption. Skin carotenoid levels, on the other hand, were significantly associated with serum carotenoid concentrations, confirming our previously published findings in younger cohorts that did not consume any carotenoid supplements,30,36 although the correlation in the current study is noticeably smaller relative to our previous studies. Moreover, average skin Raman counts were substantially higher relative to previously published unsupplemented normal populations.28,36 This is not surprising because all enrolled nonsmoker subjects were taking a daily AREDS supplement containing 15 mg of β-carotene during the 30-day run-in period prior to the baseline visit. Skin carotenoid measurement is much easier and more convenient to implement in an elderly population relative to AFI measurement of MPOD, and we had hoped it would be a usable biomarker of MPOD, but unfortunately there was absolutely no correlation between skin Raman counts and peak MPOD. This result emphasizes that the deposition of lutein and zeaxanthin in the human macula is a specific high-affinity process mediated by binding proteins such as GSTP1 and StARD3, whereas skin deposition of carotenoids is a much less specific process that tends to reflect serum carotenoid concentrations with a preference for the nonpolar carotenes and lycopene relative to the more polar xanthophylls lutein and zeaxanthin.8–10,31,36
The major limitation of this study is the relatively small number of subjects, which decreases the power to perform statistical analyses beyond simple linear regressions. Moreover, our Utah cohort may not be representative of the entire AREDS2 population. Specifically, our cohort's prior usage of lutein and zeaxanthin supplements was exceedingly high, suggesting that we enrolled a very nutritionally aware cohort from a region of the country with a high prevalence of nutritional supplement use.
Despite the limitations described earlier, our ongoing ancillary study will provide considerable value to the AREDS2 study as a whole. We will be uniquely able to follow MP optical densities and distributions in a well-characterized cohort of subjects yearly during the 5-year period in which they are randomized to oral supplements containing 10 mg of lutein and 2 mg of zeaxanthin or to placebo. Since our population started with high average MPOD, we would expect subjects randomized to placebo might experience considerable declines in MPOD to a level closer to our age-matched unsupplemented normal population. We will also be able to learn the time course and variability of macular pigment response to long-term carotenoid supplementation and whether EPA/DHA cosupplementation enhances these effects. Although we probably do not have statistical power to draw definitive conclusions, we may be able to provide insights at the time of unmasking into which baseline macular pigment distributions may predispose to progression to advanced AMD and if any of these distributions are indicative of good response to supplementation.
Footnotes
Supported by Foundation Fighting Blindness (Columbia, MD); Research to Prevent Blindness (New York, NY); and National Eye Institute Grant EY-11600 (PSB).
Disclosure: P.S. Bernstein, None; F. Ahmed, None; A. Liu, None; S. Allman, None; X. Sheng, None; M. Sharifzadeh, None; I. Ermakov, None; W. Gellermann, None
References
- 1.Kokotas H, Grigoriadou M, Petersen MB. Age-related macular degeneration: genetic and clinical findings. Clin Chem Lab Med. 2010;49:601–616 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein PS. Nutritional interventions against age-related macular degeneration. Acta Hortic. 2009;841:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 4.Eye Disease Case-Control Study Group Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993;111:104–109 [DOI] [PubMed] [Google Scholar]
- 5.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–1420 [PubMed] [Google Scholar]
- 6.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report no. 22. Arch Ophthalmol. 2007;125:1225–1232 [DOI] [PubMed] [Google Scholar]
- 7.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr. 2003;133:992–998 [DOI] [PubMed] [Google Scholar]
- 8.Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;9:1418–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem. 2004;279:49447–49454 [DOI] [PubMed] [Google Scholar]
- 10.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry. 2011;50:2541–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A. 2002;99:10581–10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333 [PubMed] [Google Scholar]
- 14.Sakudoh T, Iizuka T, Narukawa J, et al. A CD36-related transmembrane protein is coordinated with an intracellular lipid-binding protein in selective carotenoid transport for cocoon coloration. J Biol Chem. 2010;285:7739–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jampol LM. Antioxidants, zinc, and age-related macular degeneration: results and recommendations. Arch Ophthalmol. 2001;119:1533–1534 [DOI] [PubMed] [Google Scholar]
- 17.Satia JA, Littman A, Slatore CG, Galanko JA, White E. Long-term use of beta-carotene, retinol, lycopene, and lutein supplements and lung cancer risk: results from the VITamins And Lifestyle (VITAL) study. Am J Epidemiol. 2009;169:815–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanvetyanon T, Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008;113:150–157 [DOI] [PubMed] [Google Scholar]
- 19.Touvier M, Kesse E, Clavel-Chapelon F, Boutron-Ruault MC. Dual association of beta-carotene with risk of tobacco-related cancers in a cohort of French women. J Natl Cancer Inst. 2005;97:1338–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127:656–665 [DOI] [PubMed] [Google Scholar]
- 21.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88:398–406 [DOI] [PubMed] [Google Scholar]
- 22.Chiu CJ, Klein R, Milton RC, Gensler G, Taylor A. Does eating particular diets alter the risk of age-related macular degeneration in users of the Age-Related Eye Disease Study supplements? Br J Ophthalmol. 2009;93:1241–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Age-Related-Eye-Disease-Study-2/(AREDS2) Current version January 21, 2009. May be accessed at http://clinicaltrials.gov/ct2/show/NCT00345176?term=AREDS2&rank=1 (accessed May 27, 2011) [Google Scholar]
- 24.Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010;50:716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006;23:2373–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delori FC, Goger DG, Hammond BR, Snodderly DM, Burns SA. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis. 2001;18:1212–1230 [DOI] [PubMed] [Google Scholar]
- 27.Sasamoto Y, Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Nishida K. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci. 2011;52:927–932 [DOI] [PubMed] [Google Scholar]
- 28.Ermakov IV, Ermakova MR, McClane RW, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett. 2001;26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 29.Ermakov IV, Sharifzadeh M, Bernstein PS, Gellermann W. Application of resonance Raman spectroscopy to the detection of carotenoids in vivo. In: Landrum JT.ed Carotenoids: Physical, Chemical, and Biological Functions and Properties. Atlanta, GA: CRC Press; 2009:87–109 [Google Scholar]
- 30.Ermakov IV, Gellermann W. Validation model for Raman based skin carotenoid detection. Arch Biochem Biophys. 2010;504:40–49 [DOI] [PubMed] [Google Scholar]
- 31.Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britton G. UV/visible spectroscopy. In: Britton G, Liaaen-Jensen S, Pfander H.eds Carotenoids. Basel, Switzerland: Birkhaeuser; 1995:13–62 [Google Scholar]
- 33.Zechmeister L. Cis-trans Isomeric Carotenoids, Vitamins A, and Arylpolyenes. 1st ed. New York: Academic Press; 1962 [Google Scholar]
- 34.Bhosale P, Zhao da Y, Serban B, Bernstein PS. Identification of 3-methoxyzeaxanthin as a novel age-related carotenoid metabolite in the human macula. Invest Ophthalmol Vis Sci. 2007;48:1435–1440 [DOI] [PubMed] [Google Scholar]
- 35.Bhosale P, Serban B, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys. 2009;483:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ermakov IV, Sharifzadeh M, Ermakova M, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005;10:064028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res. 1997;65:57–62 [DOI] [PubMed] [Google Scholar]
- 38.Berendschot TT, Plat J, de Jong A, Mensink RP. Long-term plant stanol and sterol ester-enriched functional food consumption, serum lutein/zeaxanthin concentration and macular pigment optical density. Br J Nutr. 2009;101:1607–1610 [DOI] [PubMed] [Google Scholar]
- 39.Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and co-antioxidants. The LUNA Study [in German]. Ophthalmologe. 2009;106:29–36 [DOI] [PubMed] [Google Scholar]
- 40.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci. 1980;19:857–863 [PubMed] [Google Scholar]
- 41.Sandberg MA, Johnson EJ, Berson EL. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010;51:1086–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008;87:1521–1529 [DOI] [PubMed] [Google Scholar]