Abstract
Migraine is a common disabling brain disorder whose pathophysiology is now being better understood. The study of anatomy and physiology of pain producing structures in the cranium and the central nervous system modulation of the input have led to the conclusion that migraine involves alterations in the sub-cortical aminergic sensory modulatory systems that influence the brain widely.
Keywords: Brainstem, dyshabituation, migraine
Introduction
Migraine is likely to be a brain disorder involving altered regulation and control of afferents, with a particular focus on the cranium.[1] An understanding of the pathophysiology of migraine should be based upon the anatomy and physiology of the pain-producing structures of the cranium integrated with knowledge of their central nervous system modulation.[2] Current views concerning migraine will be reviewed concluding the disorder is a disturbance in the brain of the subcortical aminergic sensory modulatory systems, in addition to other brainstem, hypothalamic and thalamic structures.
Migraine: Explaining the Clinical Phenotype
Migraine is in essence a familial episodic disorder whose key marker is headache, with certain associated features [Tables 1 and 2]. It is these features that give crucial clues to its pathophysiology and, ultimately, will provide insights leading to new treatments.
Table 1.
Features of migraine as included in the International Classification of Headache Disorders – second edition[85] Repeated episodes of headache (4–72 h) with the following features

Table 2.
Neuroanatomical processing of vascular head pain
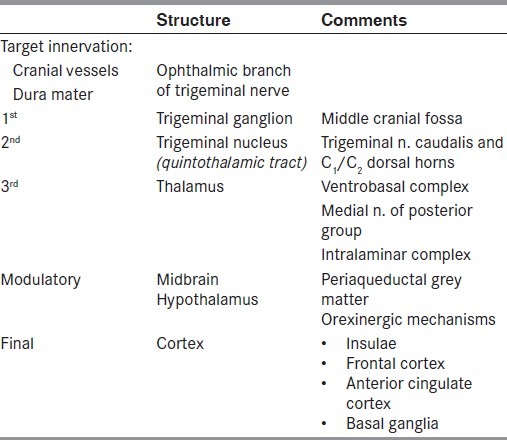
The essential elements to be considered are:
Genetics of migraine
Physiological basis for the aura
Anatomy of head pain, particularly that of the trigeminovascular system
Physiology and pharmacology of activation of the peripheral branches of the ophthalmic branch of the trigeminal nerve
Physiology and pharmacology of the trigeminal nucleus, in particular its caudal most part, the trigeminocervical complex
Brainstem and diencephalic modulatory systems that influence trigeminal pain transmission and other sensory modality processing
Migraine is a form of sensory processing disturbance with wide ramifications for central nervous system function, and while pain is used as the exemplar symptom, a brain-centered explanation provides a framework to understand all the manifestations of migraine.
Genetics of migraine
One of the most important aspects of the pathophysiology of migraine is the inherited nature of the disorder.[3] It is clear from clinical practice that many patients have first-degree relatives who also suffer from migraine. Transmission of migraine from parents to children has been reported as early as the seventeenth century, and numerous published studies have reported a positive family history.
Genetic epidemiology
Studies of twin pairs are the classical method to investigate the relative importance of genetic and environmental factors. A Danish study included 1,013 monozygotic and 1,667 dizygotic twin pairs of the same gender, obtained from a population-based twin register.[4] The pairwise concordance rate was significantly higher among monozygotic than among dizygotic twin pairs (P<0.05). Several studies have attempted to analyze the possible mode of inheritance in migraine families, and conflicting results have been obtained. Both twin studies and population-based epidemiological surveys strongly suggest that migraine without aura is a multifactorial disorder, caused by a combination of genetic and environmental factors. An unexplained but epidemiologically well-established predisposition relates to methyltetrahydrofolate reductase gene mutation C677T that is certainly overrepresented in migraine with aura.[5] The presence of aura seems to be associated, in rarer inherited cases, such as CADASIL or autosomal-dominant retinal vasculopathy with cerebral leukodystrophy, with structural protein dysfunction[6] and perhaps with an embryonic syndrome that includes patent foramen ovale.[7] Such a view makes the small excess stroke risk for young migraineurs unsurprising,[8] and suggests a common genetics as opposed to a pathophysiological link for migraine pain. Remarkably, and importantly for patients and clinicians, the most recent population-based epidemiological data suggest that migraine carries no excess risk for cognitive function compared with age- and sex-matched controls. In that French cohort, the presence or absent of changes in brain magnetic resonance imaging was also not predictive of cognitive decline.
Familial hemiplegic migraine
In approximately 50% of the reported families, Familial hemiplegic migraine (FHM) has been assigned to chromosome 19p13. Few clinical differences have been found between chromosome 19-linked and -unlinked FHM families.[3] Indeed, the clinical phenotype does not associate particularly with the known mutations. The most striking exception is cerebellar ataxia, which occurs in approximately 50% of the chromosome 19-linked, but in none of the unlinked families. Another less-striking difference includes the fact that patients from chromosome 19-linked families are more likely to have attacks that can be triggered by minor head trauma or are that associated with coma.[9]
The biological basis for the linkage to chromosome 19 is mutations involving the Cav2.1 (P/Q) type voltage-gated calcium channel CACNA1A gene.[10] Now known as FHM-I, this mutation is responsible for about 50% of the identified families. One consequence of this mutation may be enhanced glutamate release. Mutations in the ATP1A2 gene have been identified to be responsible for about 20% of the FHM families.[11] Interestingly, the phenotype of some FHM-II involves epilepsy. The gene codes for a Na+/K+ ATPase, and the mutation results in a smaller electrochemical gradient for Na+. One effect of this change is to reduce or inactivate astrocytic glutamate transporters, leading to a build-up of synaptic glutamate. A mis-sense mutation (Q1489K) in SCN1A has been reported as FHM-III.[12] This mutation affects a highly conserved amino acid in a part of the channel that contributes to its rapid closure after opening in response to membrane depolarization (fast inactivation). This represents a gain of function: instead of the channel rapidly closing, allowing the membrane to repolarize fully after an action potential, the mutated channel allows a persistent sodium influx.
Taken together, the known mutations suggest that migraine, or at least the neurological manifestations currently called the aura, are ionopathies. Linking the channel disturbance for the first time to the aura process has demonstrated that human mutations expressed in a knock-in mouse produce a reduced threshold for cortical spreading depression.[13] Furthermore, studies of trigeminal dural-evoked nociceptive activation using Fos protein expression in these knock-in mice demonstrate reduced second-order neuronal activation compared with wild-type animals and enhanced fos protein expression in certain thalamic nuclei.[14] The data suggest that the brunt of the pathophysiological burden in this mutation may fall on thalamo-cortical mechanisms.
Migraine aura
Migraine aura is defined as a focal neurological disturbance manifest as visual, sensory or motor symptoms. It is seen in about 30% of patients, and it is clearly neurally driven. The case for the aura being the human equivalent of the cortical spreading depression (CSD) of Leao has been well made.[15] In humans, visual aura has been described as affecting the visual field, suggesting the visual cortex, and it starts at the center of the visual field, propagating to the periphery at a speed of 3 mm/min.[16] This is very similar to spreading depression described in rabbits.[17] Blood flow studies in patients have also shown that a focal hyperemia tends to precede the spreading oligemia, and again this is similar to what would be expected with spreading depression. After this passage of oligemia, the cerebrovascular response to hypercapnia in patients is blunted while autoregulation remains intact.[18] Again, this pattern is repeated with experimental spreading depression. An interesting recent study suggested that female mice are more susceptible generally to CSD than male mice,[19] which would be consistent with the excess risk of migraine in females after menarche that is still with them, on a population basis, into menopause and afterwards. Human observations, including a recent study showing that ketamine that is well known to block CSD in animals can ameliorate prolonged aura in patients,[20] have rendered the arguments reasonably sound that human aura has as equivalent in animals’ cortical spreading depression. An area of controversy surrounds whether aura in fact triggers the rest of the attack, and is indeed painful. The current data in humans, in particular the very well-recognised phenomenon of migraine aura without headache, suggest that it is indeed not painful.
Therapeutic manipulation of aura
Tonabersat is a CSD inhibitor has completed clinical trials in migraine. Tonabersat (SB-220453) inhibits CSD, CSD-induced nitric oxide (NO) release and cerebral vasodilation.[21] Tonabersat does not constrict isolated human blood vessels, but does inhibit trigeminally induced craniovascular effects.[22] Tonabersat has been shown to be ineffective in migraine when reduced attacks of pain are taken as the endpoint,[23] yet can reduce aura frequency.[24] Remarkably, topiramate, a proven preventive agent in migraine, also inhibits CSD in cat and rat,[25] and in the rat with prolonged dosing.[26] Topiramate inhibits trigeminal neurons activated by nociceptive intracranial afferents,[27,28] but not by a mechanism local to the trigeminocervical complex,[28] and thus CSD inhibition may be a model system to contribute to the development of preventive medicines, particularly agents to prevent aura. The model predicts that agents interacting with Na+-based mechanisms might be effective, as would glutamate–AMPA receptor mechanisms, but not GABAergic mechanisms, at least directly. Glutamate, NMDA-mediated effects have been reported to important in CSD, and in an active-controlled study of migraine with prolonged aura.[20] These may suggest some way forward for the management of at least the most disabled group who have persistent or prolonged aura.
Headache: Anatomy
The trigeminal innervation of pain-producing intracranial structures
Surrounding the large cerebral vessels, pial vessels, large venous sinuses and dura mater is a plexus of largely unmyelinated fibers that arise from the ophthalmic division of the trigeminal ganglion and in the posterior fossa from the upper cervical dorsal roots.[29] Trigeminal fibers innervating cerebral vessels arise from neurons in the trigeminal ganglion that contain substance P and calcitonin gene-related peptide (CGRP), both of which can be released when the trigeminal ganglion is stimulated either in humans or cats.[30] Stimulation of the cranial vessels, such as the superior sagittal sinus (SSS), is certainly painful in humans.[31,32] Human dural nerves that innervate the cranial vessels largely consist of small diameter myelinated and unmyelinated fibers that almost certainly subserve a nociceptive function.[31,32]
Headache Physiology: Peripheral Connections
Plasma protein extravasation
A series of laboratory experiments in the 1990s suggested that migraine pain may be due to a sterile neurogenically driven inflammation of the dura mater.[33] Neurogenic plasma extravasation can be seen during electrical stimulation of the trigeminal ganglion in the rat. Plasma extravasation can be blocked by ergot alkaloids, indomethacin, acetylsalicylic acid and the serotonin-5HT1B/1D agonist, sumatriptan.[33] Furthermore, preclinical studies have suggested that cortical spreading depression may be a sufficient stimulus to activate the trigeminal neurons,[34] although this has been a controversial area. In addition, there are structural changes in the dura mater that are observed after trigeminal ganglion stimulation. These include mast cell degranulation and changes in the postcapillary venules, including platelet aggregation.[35] While it is generally accepted that such changes, and particularly the initiation of a sterile inflammatory response, would cause pain, it is not clear whether this is sufficient in itself, or requires other stimulators, or promoters. What neurogenic dural plasma extravasation fails to predict is whether new targets when engaged are effective in either the acute or preventive treatment of migraine. Blockade of neurogenic Plasma protein extravasation (PPE) is not predictive of antimigraine efficacy in humans, as evidenced by the failure in clinical trials of substance P, neurokinin-1 receptor antagonists, specific PPE blockers, CP122,288 and 4991w93, an endothelin antagonist, a neurosteriod and an inhibitor of the inducible form of nitric oxide synthase (iNOS)-GW274150.[36]
Sensitization and migraine
While it is highly doubtful that there is a significant sterile inflammatory response in the dura mater during migraine, it is clear that some form of sensitization takes place during migraine. About two-thirds of the patients complain of pain from nonnoxious stimuli: Allodynia.[37] A particularly interesting aspect is the demonstration of allodynia in the upper limbs ipsilateral and contralateral to the pain. This finding is consistent with at least third-order neuronal involvement, such as sensitization of thalamic neurons, and firmly places the pathophysiololgy within the central nervous system.[38] Sensitization in migraine may have a peripheral component with local release of inflammatory markers, which would certainly activate the trigeminal nociceptors,[39] although a peripheral component is not necessary to explain the symptoms. More likely in migraine, there is a form of central sensitization that may be classical central sensitization, or a form of dysinhibitory sensitization with dysfunction of descending modulatory pathways. Interestingly, the presence or absence of allodynia does not predict outcome from acute therapy in randomized controlled trials.[40]
Just as dihydroergotamine (DHE) can block trigeminovascular nociceptive transmission, probably at least by a local effect in the trigeminocervical complex,[41] DHE can block central sensitization associated with dural stimulation by an inflammatory soup. Indeed, localization of DHE binding in the midbrain dorsal raphe nucleus and periaqueductal grey matter[42] and the antinociceptive effect of naratriptan when injected locally into PAG or sensory thalamus[43] offer challenges to the orthodoxy that acute antimigraine medicines are simply inhibitors of the trigeminovascular system.
Neuropeptide studies
Electrical stimulation of the trigeminal ganglion in both humans and cats leads to increases in extracerebral blood flow and local release of both CGRP and SP.[30] In the cat, trigeminal ganglion stimulation also increases cerebral blood flow by a pathway traversing the greater superficial petrosal branch of the facial nerve, again releasing a powerful vasodilator peptide, vasoactive intestinal polypeptide (VIP). Interestingly, the VIP-ergic innervation of the cerebral vessels is predominantly anterior rather than posterior, and this may contribute to this regions’ vulnerability to spreading depression, explaining why the aura is so very often seen to commence posteriorly. Stimulation of the more specifically pain-producing superior sagittal sinus increases cerebral blood flow and jugular vein CGRP levels.[44] Human evidence that CGRP is elevated in the headache phase of severe migraine,[45] although not in less-severe attacks, in cluster headache and chronic paroxysmal hemicrania, supports the view that the trigeminovascular system may be activated in a protective role in these conditions.[46] Moreover, NO-donor-triggered migraine, which is typical migraine in all regards, also results in increases in CGRP that are blocked by sumatriptan, just as in spontaneous migraine.[47] It is of interest in this regard that compounds that have not shown activity in migraine, notably the conformationally restricted analogue of sumatriptan, CP122,288, and the conformationally restricted analogue of zolmitriptan, 4991w93, were both ineffective inhibitors of CGRP release after superior sagittal sinus in the cat.[48,49] The development of nonpeptide highly specific CGRP receptor antagonists and the successful results of now at least four different CGRP receptor antagonists in acute migraine firmly establishes this as a novel and important new emerging principle for acute migraine.[50] Interestingly, given the variability, it will probably not provide a biomarker for migraine. At the same time, the lack of any effect of CGRP receptor antagonists on PPE explains in some part why that model has proved inadequate at translation into human therapeutic approaches.
Headache physiology: Central connections
The trigeminocervical complex
Fos immunohistochemistry is a method for looking at activated cells by plotting the expression of Fos protein. After meningeal irritation with blood, Fos expression is noted in the trigeminal nucleus caudalis, while after stimulation of the superior sagittal sinus, Fos-like immunoreactivity is seen in the trigeminal nucleus caudalis and in the dorsal horn at the C1 and C2 levels in the cat and monkey.[51] These latter findings are in accord with similar data using 2-deoxyglucose measurements with superior sagittal sinus stimulation.[52] Similarly, stimulation of a branch of C2, the greater occipital nerve, increases metabolic activity in the same regions, i.e. trigeminal nucleus caudalis and C1/2 dorsal horn. In experimental animals, one can record directly from trigeminal neurons with both supratentorial trigeminal input and input from the greater occipital nerve a branch of the C2 dorsal root. Stimulation of the greater occipital nerve for 5 min results in substantial increases in responses to supratentorial dural stimulation, which can last for over 1 h.[53] Conversely, stimulation of the middle meningeal artery dura mater with the C-fiber irritant mustard oil sensitizes responses to occipital muscle stimulation.[54] It can be shown, again using the Fos method, that this interaction is likely to involve at least activation of the NMDA-sub-type glutamate receptors.[55] Taken together, these data suggest convergence of cervical and ophthalmic inputs at the level of the second-order neuron[56] Moreover, stimulation of a lateralized structure, the middle meningeal artery, produces Fos expression bilaterally in both cat and monkey brain.[57] This group of neurons from the superficial laminae of trigeminal nucleus caudalis and C1/2 dorsal horns should be regarded functionally as the “trigeminocervical” complex.
These data demonstrate that trigeminovascular nociceptive information comes by way of the most caudal cells. This concept provides an anatomical explanation for the referral of pain to the back of the head in migraine. Moreover, experimental pharmacological evidence suggests that abortive antimigraine drugs, such as ergot derivatives, acetylsalicylic acid, sumatriptan, eletriptan, naratriptan, rizatriptan and zolmitriptan, and the novel approach of CGRP receptor antagonists can have actions at these second-order neurons reduce cell activity and suggest a further possible site for therapeutic intervention in migraine.[58] The triptan action can be dissected out to involve each of the 5-HT1B, 5-HT1D and 5-HT1F receptor subtypes, consistent with the localization of these receptors on peptidergic nociceptors. Interestingly, triptans also influence the CGRP promoter, regulate CGRP secretion from neurons in culture and may not access their receptors until the trigeminovascular system is activated. Furthermore, the demonstration that some part of this action is postsynaptic with either 5-HT1B or 5-HT1D receptors located non-presynatically offers a prospect of highly anatomically localized targets for treatment.[59] Certainly, the triptan, 5-HT1B/1D/1F, receptors are not localized peripherally as they can be identified at every level of sensory input from the trigeminal ganglion through the cervical, thoracic, lumbar and sacral dorsal root ganglia.[60]
Serotonin–5-HT1F receptor agonists and migraine
Some, but not all, of the triptans as well as begin 5-HT1B/1D receptor agonists are also potent 5-HT1F receptor agonists. A notable example is naratriptan, which is highly potent by the injectable route. With the same second messenger activity as the 5HT1B and 5-HT1D receptors, adenylate cyclase inhibition and no contractile effects on blood vessels so far identified, it is a good novel neural target for migraine treatment. It can be shown that 5-HT1F activation inhibits trigeminal nucleus fos activation and neuronal firing in response to dural stimulation, the latter without cranial vascular effects.[61] One early compound was found to be effective in the clinic but had toxicological problems unrelated to the mechanism, and another lasmiditan (COL-144) has now been shown to be an effective acute antimigraine treatment in a randomized controlled trial.[62,63] These data add further the concept that vascular mechanisms are not necessary for acute migraine treatments.
Glutamatergic transmission in the trigeminocervical complex
A potential target for antimigraine drugs is the family of glutamate receptors (GluRs), which consist of the ionotropic (iGluRs): N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA), kainate; and the metabotropic glutamate receptors (mGluRs) 1-8.[64] NMDA receptor channel blockers have been shown to reduce nociceptive trigeminovascular transmission in vivo. The AMPA/kainate receptor antagonists 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzoquinoxaline-7-sulfonamide reduced Fos protein expression after activation of structures involved in nociceptive pathways, and direct application of CNQX in the trigeminocervical complex attenuated neurons with nociceptive trigeminovasccular inputs. Regarding the group III mGluR receptor, the agonist L-(+)-2-amino-4-phosphonobutyric acid decreased Fos protein expression in an animal model of trigeminovascular nociceptive processing. It is also notable that the group I mGluR5 modulator ADX10059 has been reported to be effective in the acute treatment of migraine.[65]
Kainate receptors are constituted by the “low affinity” iGluR5, iGluR6, iGluR7 and the “high affinity” KA1 and KA2 subunits, which form different homo- or heteromeric assemblies, giving rise to functional receptors. The presence of iGluR5 subunits in the trigeminal ganglion neurons and at the presynaptic sites of primary afferents indicates a possible role of kainate receptors in trigeminovascular physiology. Most recently, it has been shown that activation of the iGluR5 kainate receptors with the selective agonist iodowillardiine is able to inhibit neurogenic dural vasodilation probably by inhibition of prejunctional release of CGRP from trigeminal afferents.[66] Furthermore, in a double-blinded randomized placebo-controlled study in acute migraine, LY466195, an iGluR5 kainate receptor antagonist, was effective at the 2-h pain-free endpoint.[67] In a separate small study of acute migraine, intravenous application of the decahydroisoquinoline AMPA/iGluR5 antagonist LY293558 improved headache pain in two-thirds of migraineurs and relieved the associated symptoms of the attack.[68] Taken together, these studies suggest a strong basis to pursue glutamate targets, with some care to considering how to do this without attracting unwanted side-effects.
Higher Order Processing
Following transmission in the caudal brain stem and high cervical spinal cord, information is relayed rostrally.
Thalamus
0Processing of vascular nociceptive signals in the thalamus occurs in the ventroposteromedial (VPM) thalamus, medial nucleus of the posterior complex and in the intralaminar thalamus.[69] Application of capsaicin to the superior sagittal sinus activates trigeminal projections with a high degree of nociceptive input that are processed in neurons, particularly in the VPM thalamus and in its ventral periphery. These neurons in the VPM can be modulated by activation of GABAA inhibitory receptors, and perhaps of more direct clinical relevance by propranolol though a β1-adrenoceptor mechanism.[70] Remarkably, triptans through 5-HT1B/1D mechanisms can also inhibit VPM neurons locally, as demonstrated by microiontophoretic application, suggesting a hitherto unconsidered locus of action for triptans in acute migraine. Importantly, human imaging studies have confirmed activation of thalamus contralateral to pain in acute migraine, cluster headache, SUNCT (short-lasting unilateral neuralgiform headache with conjunctival injection and tearing) and hemicrania contniua.
Activation of modulatory regions
Stimulation of nociceptive afferents in the superior sagittal sinus in the cat activates neurons in the ventrolateral periaqueductal grey matter (PAG). PAG activation in turn feeds back to the trigeminocervical complex with an inhibitory influence.[71] PAG is clearly included in the area of activation seen in positron emission tomography (PET) studies in migraineurs, and may have a more generic antinociceptive role. This typical negative feedback system will be further considered below as a possible mechanism for the symptomatic manifestations of migraine.
Another potential modulatory region activated by stimulation of nociceptive trigeminovascular input is the posterior hypothalamic grey. This area is crucially involved in several primary headaches, notably cluster headache, SUNCT, paroxysmal hemicrania and hemicrania continua.[72] Moreover, the clinical features of the premonitory phase and other features of the disorder suggest dopamine neuron involvement in some part. It can be shown in the experimental animal that D2 family receptors are more often seen in rat trigeminocervical neurons than D1 family receptors, and that dopamine locally iontophoresed into the trigeminocervical complex, but not administered intravenously, inhibits trigeminovascular nociceptive transmission. Moreover, it seems plausible that this effect, at least in part, emanates from the dopamine-containing A11 neurons, which inhibit trigeminovascular nociceptive transmission through a D2 receptor-mediated mechanism, and after lesioning of this region, trigeminal nociceptive transmission is facilitated.[73] Orexinergic neurons in the posterior hypothalamus can have both pro- and antinociceptive downstream effects and are activated when trigeminovascular nociceptive afferents are stimulated. Orexin A activation of the OX1 receptor can both modulate dural–vascular responses to trigeminal afferent activation and inhibit second-order trigeminovascular neurons in the trigeminocervical complex. Orexinergic mechanisms may be an attractive component to the central matrix of neuronal systems that are dysfunctional in migraine.[74]
The “Vascular hypothesis”: A good story ruined by the facts
For much of the later part of the twentieth century, a rather straightforward concept dominated thinking about migraine; first proposed in some part by Willis and best articulated by Wolff, the theory explained the pain of migraine to be due to dilation of cranial vessels. By the later part of the nineteenth century, neuronal theories had been well articulated and, indeed, Gowers[75] seemed happy with that concept. It is now clear that the “Vascular Hypothesis” is untenable as an explanation for migraine pathophysiology and some of the data behind this view is covered here.
It has been shown that pituitary adenylate cyclase activating peptide (PACAP-38) infusion can produce cranial vasodilation and trigger a delayed migraine in sufferers but not in controls, and not in migraineurs when infused with placebo.[76] The same group using the same methods has shown vasoactive intestinal polypeptide (VIP); another member, with PACAP, of the secretin/glucagon peptide superfamily, can induce an equal craniovascular vasodilation but does not trigger migraine at all.[77] Therefore, it is not the dilation but the receptor site activated – put simply the vasodilation is an epiphenomenon neither sufficient nor necessary.[78] Another lynch-pin of the vascular argument came from the behavior of cranial vessels in migraine sufferers. It had been shown that ergotamine could produce vasoconstriction in line with its efficacy in migraine. When more closely examined, it was shown that vascular changes were unrelated to the phase of the attack; indeed, blood flow could be reduced or normal during the pain phase. Most recently, using high-resolution 3T magnetic resonance angiography, it has been reported that migraine triggered by nitroglycerin occurs without any continuing change in intracranial or extracranial vessels.[79] An important result for all of us, but more particularly for patients, is that the neuronal–vascular acute treatment debate is now full circle in favor of a neuronal approach. Triptans, serotonin 5-HT1B/1D receptor agonists, which are extremely effective treatments, and were developed initially as cranial vasconstrictors, have been for some time described to have effects on neuronal transmission in the brain. The most recent studies demonstrate that calcitonin gene-related peptide (CGRP) receptor antagonists, developed based on the elevation of CGRP in acute, severe migraine and its normalization with treatment, such as olcegepant and telcagepant, are both effective and without vascular effects. Similarly, a purely neurally acting 5-HT1F receptor agonist, lasmiditan, is effective and devoid of vasoconstrictor actions. Taken together, be it triggering, measuring or inducing vascular change with therapies, vascular change is neither necessary, sufficient nor needed in migraine – in short, an epiphenomenon of the neural substrates that are activated by the underlying pathophysiology of the disorder.
Central modulation of trigeminal pain
Brain imaging in humans
Functional brain imaging with PET has demonstrated activation of the dorsal midbrain, including the periaqueductal grey (PAG), and in the dorsal pons, near the locus coeruleus, in studies during migraine without aura.[80] Dorsolateral pontine activation is seen with PET in spontaneous episodic and chronic migraine, and with nitrogylcerin-triggered attacks. These areas are active immediately after successful treatment of the headache but are not active interictally. The activation corresponds with the brain regions reported to cause migraine-like headache when stimulated in patients with electrodes implanted for pain control. Similarly, excess iron has been noted in the PAG of patients with episodic and chronic migraine, and chronic migraine can develop after a bleed into a cavernoma in the region of the PAG, or with a lesion of the pons. What could dysfunction of these brain areas lead to?
Animal experimental studies of sensory modulation
It has been shown in the experimental animal that stimulation of nucleus locus coeruleus, the main central noradrenergic nucleus, reduces cerebral blood flow in a frequency-dependent manner through an a2-adrenoceptor-linked mechanism.[81] This reduction is maximal in the occipital cortex. While a 25% overall reduction in cerebral blood flow is seen, extracerebral vasodilatation occurs in parallel. In addition, the main serotonin-containing nucleus in the brain stem, the midbrain dorsal raphe nucleus can increase cerebral blood flow when activated.[82] Furthermore, stimulation of PAG will inhibit sagittal sinus-evoked trigeminal neuronal activity in cat, while blockade of P/Q-type voltage-gated Ca2+ channels in the PAG facilitates trigeminovascular nociceptive processing with the local GABAergic system in the PAG still intact.
Electrophysiology of migraine in humans
Studies of evoked potentials and event-related potentials provide some link between animal studies and human functional imaging. Authors have shown changes in neurophysiological measures of brain activation, but there is much discussion as to how to interpret such changes. Perhaps the most reliable theme is that the migrainous brain does not habituate to signals in a normal way, nor indeed do patients who have first-degree relatives with migraine. Similarly, contingent-negative variation (CNV), an event-related potential, is abnormal in migraineurs compared with controls. Changes in CNV predict attacks and preventive therapies alter, normalize, such changes and recent evidence suggests involvement of thalamo-cortical relays in these habituations deficits.[83] Attempts to correlate clinical phenotypes with electrophysiological changes may enhance further studies in this area.
Conclusion: What is Migraine?
Migraine is an inherited, episodic disorder involving sensory sensitivity. Patients complain of pain in the head that is throbbing, but there is no reliable relationship between vessel diameter and the pain, or its treatment. They complain of discomfort from normal lights and the unpleasantness of routine sounds. Some mention that otherwise pleasant odors are unpleasant. Normal movement of the head causes pain, and many mention a sense of unsteadiness as if they have just stepped off a boat, having been nowhere near the water. The anatomical connections of, for example, the pain pathways are clear, the ophthalmic division of the trigeminal nerve subserves sensation within the cranium and perhaps underpins why the top of the head is headache, and the maxillary division is “facial pain.” The convergence of cervical and trigeminal afferents explains why neck stiffness or pain is so common in primary headache. The genetics of channelopathies is opening up a plausible way to think about the episodic nature of migraine. Perhaps, electrophysiological changes in the brain have been mislabelled as “hyperexcitability,” whereas dyshabituation might be a simpler explanation. If migraine was basically a sensory attentional problem with changes in cortical synchronization, “hypersynchronisation,”[84] all its manifestations could be accounted for in a single overarching pathophysiological hypothesis of a disturbance of the subcortical sensory modulation systems. While it seems likely that the trigeminovascular system and its cranial autonomic reflex connections, the trigeminal–autonomic reflex, act as a feed-forward system to facilitate the acute attack, the fundamental problem in migraine is in the brain.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine- current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161:327–41. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 3.van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: Gene mutations and functional consequences. Curr Opin Neurol. 2007;20:299–305. doi: 10.1097/WCO.0b013e3281338d1f. [DOI] [PubMed] [Google Scholar]
- 4.Russell MB, Iselius L, Olesen J. Investigation of the inheritance of migraine by complex segregation analysis. Hum Genet. 1995;96:726–30. doi: 10.1007/BF00210307. [DOI] [PubMed] [Google Scholar]
- 5.Scher AI, Terwindt GM, Verschuren WM, Kruit MC, Blom HJ, Kowa H, et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol. 2006;59:372–5. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 6.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 7.Diener HC, Kurth T, Dodick D. Patent foramen ovale and migraine. Curr Pain Headache Rep. 2007;11:236–40. doi: 10.1007/s11916-007-0196-2. [DOI] [PubMed] [Google Scholar]
- 8.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: Systematic review and meta-analysis. Br Med J. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kors EE, Terwindt GM, Vermeulen FL, Fitzsimons RB, Jardine PE, Heywood P, et al. Delayed cerebral edema and fatal coma after minor head trauma: Role of CACNA1A calcium channel subunit gene and relationship with familial hemiplegic migraine. Ann Neurol. 2001;49:753–60. doi: 10.1002/ana.1031. [DOI] [PubMed] [Google Scholar]
- 10.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–52. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 11.De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump a2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33:192–6. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 12.Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A causes familial hemiplegic migraine. Lancet. 2005;366:371–7. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 13.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, et al. A Cacna1a knock-in migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–10. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Moon HS, Akerman S, Holland PR, Lasalandra M, Andreou AP, et al. Blunted trigeminovascular nociceptive responses in mice with the CACNA1A mutation. Headache. 2010;50(Suppl 1):61. [Google Scholar]
- 15.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain. 1994;117:199–210. doi: 10.1093/brain/117.1.199. [DOI] [PubMed] [Google Scholar]
- 16.Lashley KS. Patterns of cerebral integration indicated by the scotomas of migraine. Arch Neurol Psychiatry. 1941;46:331–9. [Google Scholar]
- 17.Leao AA. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–90. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 18.Olesen J. Cerebral and extracranial circulatory disturbances in migraine: Pathophysiological implications. Cerebrovasc Brain Metab Rev. 1991;3:1–28. [PubMed] [Google Scholar]
- 19.Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goadsby PJ, Giffin N, Kaube H, Afridi S. Ketamine reduces the severity of prolonged migraine aura: Support for the glutamate hypothesis of aura. Neurology. 2011;76(Suppl 4):A443. [Google Scholar]
- 21.Smith MI, Read SJ, Chan WN, Thompson M, Hunter AJ, Upton N, et al. Repetitive cortical spreading depression in a gyrencephalic feline brain: Inhibition by the novel benzoylamino-benzopyran SB-220453. Cephalalgia. 2000;20:546–53. doi: 10.1046/j.1468-2982.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- 22.Read SJ, Smith MI, Hunter AJ, Upton N, Parsons AA. SB-220453, a potential novel antimigraine compound, inhibits nitric oxide release following induction of cortical spreading depression in the anaesthetized cat. Cephalalgia. 1999;20:92–9. doi: 10.1046/j.1468-2982.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 23.Goadsby PJ, Ferrari MD, Csanyi A, Olesen J, Mills JG. Randomized double blind, placebo-controlled proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia. 2009;29:742–50. doi: 10.1111/j.1468-2982.2008.01804.x. [DOI] [PubMed] [Google Scholar]
- 24.Hauge AW, Asghar MS, Schytz HW, Christensen K, Olesen J. Effects of tonabersat on migraine with aura: A randomised, double-blind, placebo-controlled crossover study. Lancet Neurol. 2009;8:718–23. doi: 10.1016/S1474-4422(09)70135-8. [DOI] [PubMed] [Google Scholar]
- 25.Akerman S, Goadsby PJ. Topiramate inhibits cortical spreading depression in rat and cat: Impact in migraine aura. Neuro Report. 2005;16:1383–7. doi: 10.1097/01.wnr.0000175250.33159.a9. [DOI] [PubMed] [Google Scholar]
- 26.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–61. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 27.Storer RJ, Goadsby PJ. Topiramate inhibits trigeminovascular neurons in the cat. Cephalalgia. 2004;24:1049–56. doi: 10.1111/j.1468-2982.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- 28.Andreou AP, Goadsby PJ. Topiramate in the treatment of migraine: A kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia. 2011;31:1343–1358. doi: 10.1177/0333102411418259. [DOI] [PubMed] [Google Scholar]
- 29.Petersen KA, Nilsson E, Olesen J, Edvinsson L. Presence and function of the calcitonin gene-related peptide receptor on rat pial arteries investigated in vitro and in vivo. Cephalalgia. 2005;25:424–32. doi: 10.1111/j.1468-2982.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- 30.Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of man and the cat during activation of the trigeminovascular system. Ann Neurol. 1988;23:193–6. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- 31.Feindel W, Penfield W, McNaughton F. The tentorial nerves and localization of intracranial pain in man. Neurology. 1960;10:555–63. doi: 10.1212/wnl.10.6.555. [DOI] [PubMed] [Google Scholar]
- 32.McNaughton FL, Feindel WH. Innervation of intracranial structures: A reappraisal. In: Rose FC, editor. Physiological aspects of Clinical Neurology. Oxford: Blackwell Scientific Publications; 1977. pp. 279–93. [Google Scholar]
- 33.Moskowitz MA, Cutrer FM. SUMATRIPTAN: A receptor-targeted treatment for migraine. Ann Rev Med. 1993;44:145–54. doi: 10.1146/annurev.me.44.020193.001045. [DOI] [PubMed] [Google Scholar]
- 34.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–42. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 35.Dimitriadou V, Buzzi MG, Moskowitz MA, Theoharides TC. Trigeminal sensory fiber stimulation induces morphological changes reflecting secretion in rat dura mater mast cells. Neuroscience. 1991;44:97–112. doi: 10.1016/0306-4522(91)90253-k. [DOI] [PubMed] [Google Scholar]
- 36.Peroutka SJ. Neurogenic inflammation and migraine: Implications for therapeutics. Mol Interv. 2005;5:306–13. doi: 10.1124/mi.5.5.10. [DOI] [PubMed] [Google Scholar]
- 37.Selby G, Lance JW. Observations on 500 cases of migraine and allied vascular headache. J Neurol Neurosurg Psychiatr. 1960;23:23–32. doi: 10.1136/jnnp.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack. Brain. 2000;123:1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 39.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–3. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 40.Diaz Insa S, Goadsby PJ, Fortea J, Falques M, Zanchin G, Vila C. Allodynia does not affect the efficacy of almotriptan when given early in migraine: Data from the ‘Act when Mild’ study. Int J Neurosci. 2011;121:655–661. doi: 10.3109/00207454.2011.605191. [DOI] [PubMed] [Google Scholar]
- 41.Hoskin KL, Kaube H, Goadsby PJ. Central activation of the trigeminovascular pathway in the cat is inhibited by dihydroergotamine. A c-Fos and electrophysiology study. Brain. 1996;119:249–56. doi: 10.1093/brain/119.1.249. [DOI] [PubMed] [Google Scholar]
- 42.Goadsby PJ, Gundlach AL. Localization of [3H]-dihydroergotamine binding sites in the cat central nervous system: Relevance to migraine. Ann Neurol. 1991;29:91–4. doi: 10.1002/ana.410290116. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch T, Knight YE, Goadsby PJ. Activation of 5-HT1B/1D receptors in the periaqueductal grey inhibits meningeal nociception. Ann Neurol. 2004;56:371–81. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- 44.Zagami AS, Goadsby PJ, Edvinsson L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]
- 45.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–7. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 46.Edvinsson L, Ekman R, Goadsby PJ. Measurement of vasoactive neuropeptides in biological materials: Problems and pitfalls from 30 years of experience and novel future approaches. Cephalalgia. 2010;30:761–6. doi: 10.1177/0333102409351807. [DOI] [PubMed] [Google Scholar]
- 47.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: Studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 48.Knight YE, Edvinsson L, Goadsby PJ. Blockade of CGRP release after superior sagittal sinus stimulation in cat: A comparison of avitriptan and CP122,288. Neuropeptides. 1999;33:41–6. doi: 10.1054/npep.1999.0009. [DOI] [PubMed] [Google Scholar]
- 49.Knight YE, Edvinsson L, Goadsby PJ. 4991W93 inhibits release of calcitonin gene-related peptide in the cat but only at doses with 5HT1B/1D receptor agonist activity. Neuropharmacol. 2001;40:520–5. doi: 10.1016/s0028-3908(00)00187-8. [DOI] [PubMed] [Google Scholar]
- 50.Ho TW, Edvinsson L, Goadsby PJ. CGRP and its receptors provide new insights into migraine pathophysiology. Nat Rev Neurol. 2010;6:761–6. doi: 10.1038/nrneurol.2010.127. [DOI] [PubMed] [Google Scholar]
- 51.Goadsby PJ, Hoskin KL. The distribution of trigeminovascular afferents in the nonhuman primate brain Macaca nemestrina: A c-fos immunocytochemical study. J Anat. 1997;190:367–75. doi: 10.1046/j.1469-7580.1997.19030367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain. 1991;114:1001–11. doi: 10.1093/brain/114.2.1001. [DOI] [PubMed] [Google Scholar]
- 53.Bartsch T, Goadsby PJ. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain. 2002;125:1496–509. doi: 10.1093/brain/awf166. [DOI] [PubMed] [Google Scholar]
- 54.Bartsch T, Goadsby PJ. Increased responses in trigeminocervical nociceptive neurones to cervical input after stimulation of the dura mater. Brain. 2003;126:1801–13. doi: 10.1093/brain/awg190. [DOI] [PubMed] [Google Scholar]
- 55.Le Doare K, Akerman S, Holland PR, Lasalandra MP, Classey JD, Knight YE, et al. Occipital afferent activation of second order neurons in the trigeminocervical complex in rat. Neurosci Lett. 2006;403:73–7. doi: 10.1016/j.neulet.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 56.Bartsch T, Goadsby PJ. Anatomy and physiology of pain referral in primary and cervicogenic headache disorders. Headache Curr. 2005;2:42–8. [Google Scholar]
- 57.Hoskin KL, Zagami A, Goadsby PJ. Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: A comparative study of monkey and cat. J Anat. 1999;194:579–88. doi: 10.1046/j.1469-7580.1999.19440579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andreou AP, Summ O, Charbit AR, Romero Reyes M, Goadsby PJ. Animal models of headache- From bedside to bench and back to beside. Expert Rev Neurother. 2010;10:389–411. doi: 10.1586/ern.10.16. [DOI] [PubMed] [Google Scholar]
- 59.Goadsby PJ, Akerman S, Storer RJ. Evidence for postjunctional serotonin (5-HT1) receptors in the trigeminocervical complex. Ann Neurol. 2001;50:804–7. doi: 10.1002/ana.10066. [DOI] [PubMed] [Google Scholar]
- 60.Classey JD, Bartsch T, Goadsby PJ. Distribution of 5-HT1B, 5-HT1D and 5-HT1F receptor expression in rat trigeminal and dorsal root ganglia neurons: Relevance to the selective anti-migraine effect of triptans. Brain Res. 2010;1361:76–85. doi: 10.1016/j.brainres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Shepheard S, Edvinsson L, Cumberbatch M, Williamson D, Mason G, Webb J, et al. Possible antimigraine mechanisms of action of the 5HT1F receptor agonist LY334370. Cephalalgia. 1999;19:851–8. doi: 10.1046/j.1468-2982.1999.1910851.x. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari MD, Farkkila M, Reuter U, Pilgrim A, Davis C, Krauss M, et al. Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan - A randomised proof-of-concept trial. Cephalalgia. 2010;30:1170–8. doi: 10.1177/0333102410375512. [DOI] [PubMed] [Google Scholar]
- 63.Farkkila M, Diener H-C, Lainez JM, Schoenen J, Pilgrim AJ. Lasmiditan (COL-144), a selective 5HT1F agonist, is a rapid and effective oral treatment for acute migraine. J Headache Pain. 2010;11(Suupl 1):209. [Google Scholar]
- 64.Andreou AP, Goadsby PJ. Therapeutic potential of novel glutamate receptor antagonists in migraine. Expert Opin Investig Drugs. 2009;18:789–803. doi: 10.1517/13543780902913792. [DOI] [PubMed] [Google Scholar]
- 65.Marin JC, Goadsby PJ. Glutamatergic fine tuning with ADX-10059- a novel therapeutic approach for migraine? Expert Opin Investig Drugs. 2010;19:555–61. doi: 10.1517/13543781003691832. [DOI] [PubMed] [Google Scholar]
- 66.Andreou AP, Holland PR, Goadsby PJ. Activation of GluR5 kainate receptors inhibits neurogenic dural vasodilation in animal model of trigeminovascular activation. Br J Pharmacol. 2009;157:464–73. doi: 10.1111/j.1476-5381.2009.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss B, Alt A, Ogden AM, Gates M, Dieckman DK, Clemens-Smith A, et al. Pharmacological characterization of the competitive GLUK5 receptor antagonist decahydroisoquinoline LY466195 in vitro and in vivo. J Pharmacol Exp Ther. 2006;318:772–81. doi: 10.1124/jpet.106.101428. [DOI] [PubMed] [Google Scholar]
- 68.Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, et al. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia. 2004;24:596–602. doi: 10.1111/j.1468-2982.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 69.Zagami AS, Lambert GA. Stimulation of cranial vessels excites nociceptive neurones in several thalamic nuclei of the cat. Exp Brain Res. 1990;81:552–66. doi: 10.1007/BF02423504. [DOI] [PubMed] [Google Scholar]
- 70.Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: A role in migraine? Brain. 2005;128:86–97. doi: 10.1093/brain/awh298. [DOI] [PubMed] [Google Scholar]
- 71.Knight YE, Bartsch T, Kaube H, Goadsby PJ. P/Q-type calcium channel blockade in the PAG facilitates trigeminal nociception: A functional genetic link for migraine? J Neurosci. 2002;22(RC213):1–6. doi: 10.1523/JNEUROSCI.22-05-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goadsby PJ, Cittadini E, Cohen AS. Trigeminal autonomic cephalalgias: Paroxysmal hemicrania, SUNCT/SUNA and hemicrania continua. Sem Neurol. 2010;30:186–91. doi: 10.1055/s-0030-1249227. [DOI] [PubMed] [Google Scholar]
- 73.Charbit AR, Akerman S, Holland PR, Goadsby PJ. Neurons of the dopaminergic/CGRP A11 cell group modulate neuronal firing in the trigeminocervical complex: An electrophysiological and immunohistochemical study. J Neurosci. 2009;29:12532–41. doi: 10.1523/JNEUROSCI.2887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holland PR, Goadsby PJ. The hypothalamic orexinergic system: Pain and primary headaches. Headache. 2007;47:951–62. doi: 10.1111/j.1526-4610.2007.00842.x. [DOI] [PubMed] [Google Scholar]
- 75.Gowers WR. A Manual of Diseases of the Nervous System. Philadelphia: P. Blakiston, Son and Co; 1888. [Google Scholar]
- 76.Henrik S, Steffen B, Wienecke T, Kruuse C, Olesen J, Messoud A. PACAP38 induces migraine-like attacks and vasodilatation- a causative role in migraine pathogenesis? Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 77.Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilatation but does not induce migraine. Cephalalgia. 2008;28:226–36. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 78.Goadsby PJ. The Vascular Theory of Migraine-a great story wrecked by the facts. Brain. 2009;132:6–7. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- 79.Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation–a 3T magnetic resonance angiography study. Brain. 2008;131:2192–200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- 80.Sprenger T, Goadsby PJ. What has functional neuroimaging done for primary headache and for the clinical neurologist? J Clin Neurosci. 2010;17:547–53. doi: 10.1016/j.jocn.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 81.Goadsby PJ, Lambert GA, Lance JW. Differential effects on the internal and external carotid circulation of the monkey evoked by locus coeruleus stimulation. Brain Res. 1982;249:247–54. doi: 10.1016/0006-8993(82)90058-0. [DOI] [PubMed] [Google Scholar]
- 82.Goadsby PJ, Piper RD, Lambert GA, Lance JW. The effect of activation of the nucleus raphe dorsalis (DRN) on carotid blood flow. I The Monkey. Am J Physiol. 1985;248:R257–62. doi: 10.1152/ajpregu.1985.248.2.R257. [DOI] [PubMed] [Google Scholar]
- 83.Schoenen J, Ambrosini A, Sandor PS, de Noordhout A Maertens. Evoked potentials and transcranial magnetic stimulation in migraine: Published data and viewpoint on their pathophysiologic significance. Clin Neurophysiol. 2003;114:955–72. doi: 10.1016/s1388-2457(03)00024-5. [DOI] [PubMed] [Google Scholar]
- 84.Angelini L, de Tommaso M, Guido M, Hu K, Ivanov P, Marinazzo D, et al. Steady-state visual evoked potentials and phase synchronization in migraine patients. Phys Rev Lett. 2004;93:038103. doi: 10.1103/PhysRevLett.93.038103. [DOI] [PubMed] [Google Scholar]
- 85.Headache Classification Committee of The International Headache Society. The International Classification of Headache Disorders (second edition) Cephalalgia. 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]


