Abstract
Objectives
Recent epidemiological evidence suggests that genotypic and phenotypic characteristics that have typically distinguished community-associated methicillin-resistant Staphylococcus aureus (MRSA) and healthcare-associated MRSA strains may be evolving. The objective of this study was to examine the association between reduced vancomycin susceptibility (RVS) and staphylococcal cassette chromosome mec (SCCmec) type in MRSA bloodstream isolates.
Methods
A cohort study of patients who were hospitalized from 2007 to 2009 with S. aureus bacteraemia was conducted within a university health system. Bivariable analyses were conducted to determine the association between RVS and SCCmec type, as well as other microbiological characteristics including Panton–Valentine leucocidin, accessory gene regulator (agr) dysfunction and vancomycin heteroresistance.
Results
A total of 188 patients with MRSA bacteraemia were identified: 116 (61.7%) and 72 (38.3%) patients had infections due to healthcare-associated MRSA and community-associated MRSA, respectively. As defined by a vancomycin Etest MIC > 1.0 mg/L, the prevalence of RVS was 40.4%. Isolates with RVS were significantly more likely to be associated with SCCmec II compared with isolates without RVS (74.7% and 47.3%, respectively, P < 0.001), but not with Panton–Valentine leucocidin (P = 0.10), agr dysfunction (P = 0.19) or healthcare-associated infection (P = 0.36).
Conclusions
The results of our study demonstrate important microbiological characteristics among MRSA isolates characterized by RVS, including a significant association between SCCmec II and elevated vancomycin MIC. It is clear that the clinical and molecular epidemiology of MRSA is evolving, and further understanding of factors determining virulence will be important for the elucidation of optimal treatment approaches for associated infections.
Keywords: MRSA, virulence factors, antimicrobial resistance, epidemiology
Introduction
The increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in both the healthcare and community setting is of major public health concern.1 A recently recognized phenomenon of increasing vancomycin MICs over time has been described among vancomycin-susceptible S. aureus isolates.2 This reduced vancomycin susceptibility (RVS) has been associated with prior vancomycin exposure,3 as well as with increased mortality in the setting of MRSA bacteraemia.4
Community-associated MRSA (CA-MRSA) has usually referred to strains causing infections in patients without recent contact with the healthcare environment.1 CA-MRSA has typically been distinguished from healthcare-associated MRSA (HA-MRSA) by the staphylococcal cassette chromosome mec (SCCmec) element, with SCCmec IV and V predominating in CA-MRSA strains, and SCCmec I, II and III predominating in HA-MRSA strains. However, recent epidemiological evidence indicates that CA-MRSA and HA-MRSA strains are increasingly mixing in both community and healthcare settings.1 It is possible that the genotypic and phenotypic characteristics that have typically distinguished CA-MRSA and HA-MRSA strains may be evolving, and that selection pressure for RVS may be increasingly similar among CA-MRSA and HA-MRSA. However, to our knowledge, there are few studies that have primarily evaluated the association between RVS in MRSA and SCCmec type.5–7 Furthermore, these studies have focused on study populations characterized by persistent bacteraemia and enriched for vancomycin treatment failure,6 a low prevalence of bacteraemia5 or outside of the USA, where different SCCmec types predominate.7
We conducted this study to determine the association between RVS and SCCmec type in MRSA bloodstream isolates. We also sought to comprehensively characterize the genotypic and phenotypic characteristics of MRSA bloodstream isolates with and without RVS, including the presence of Panton–Valentine leucocidin (PVL), accessory gene regulator (agr) dysfunction and vancomycin heteroresistance.
Patients and methods
This study was conducted at two hospitals in the University of Pennsylvania Health System (UPHS) in Philadelphia.8 All inpatients with an episode of MRSA bacteraemia occurring between 1 December 2007 and 31 May 2009 were identified through the Hospital of the University of Pennsylvania Clinical Microbiology Laboratory. For patients with multiple episodes of MRSA bacteraemia, only the first episode was included. The study was approved by the institutional review board of the University of Pennsylvania and based on a database constructed for a prior study.8
Identification and susceptibility testing of S. aureus was performed and interpreted according to CLSI guidelines. The vancomycin MIC of the isolates was determined by the Etest and broth microdilution methods as previously described,8 with RVS defined as a vancomycin MIC > 1.0 and ≥1.0 mg/L, respectively.4,6 Vancomycin heteroresistance was screened for and confirmed as previously described.8 Detection of the genes encoding PVL was performed using real-time PCR.9 Isolates were evaluated for agr dysfunction by delta-haemolysin production as previously described.10 SCCmec typing was performed using previously described methods.11 Isolates were compared quarterly (six at 3 month intervals) to determine whether there were significant changes in the proportion of a given characteristic over the study period.
Baseline demographic and hospitalization data were abstracted from the Pennsylvania Integrated Clinical and Administrative Research Database, as previously described.8 Infections were classified as HA-MRSA if the date of the first positive blood culture was ≥48 h from the date of admission or if the patient was admitted as a transfer from another institution or had been hospitalized at UPHS in the 30 days prior to the culture date. Otherwise, the infection was classified as CA-MRSA.
Continuous variables were compared using the Wilcoxon rank-sum test, and categorical variables were compared using the χ2 or Fisher's exact test, including the χ2 test for trend to determine temporal changes. For all calculations, a two-tailed P-value <0.05 was considered significant. All statistical calculations were performed using commercially available software (STATA version 11.0; StataCorp LP, College Station, TX, USA).
Results
Study population
A total of 188 patients with MRSA bacteraemia were identified during the study period. The mean age of patients was 60 years (standard deviation, 17), 76 (40.4%) were female, with 116 (61.7%) and 72 (38.3%) patients classified as having infections due to HA-MRSA and CA-MRSA, respectively. Of 171 patients for whom race was indicated, 100 (58.5%) were white and 70 (40.9%) were African-American.
Microbiological characteristics
The distribution of vancomycin MICs among isolates as determined by Etest was as follows: 11 (5.9%) with MIC ≤ 0.5 mg/L, 34 (18.1%) with MIC = 0.75 mg/L, 67 (35.6%) with MIC = 1.0 mg/L, 73 (38.8%) with MIC = 1.5 mg/L and 3 (1.6%) with MIC = 2.0 mg/L. Accordingly, 40.4% of the MRSA bloodstream isolates demonstrated RVS.8 There was no significant change in the proportion of isolates with RVS over time during the study period (P = 0.31). Table 1 reports the microbiological characteristics of MRSA isolates with and without RVS.
Table 1.
Characteristics of MRSA isolates with and without RVS
| Characteristic | RVS (n = 76)a | No RVS (n = 112)a | P-value |
|---|---|---|---|
| SCCmec type | |||
| II | 56 (74.7) | 53 (47.3) | <0.001 |
| III | 1 (1.3) | 0 (0.0) | 0.40 |
| IV | 17 (22.7) | 58 (51.8) | <0.001 |
| V | 1 (1.3) | 0 (0.0) | 0.40 |
| VIII | 0 (0.0) | 1 (0.9) | >0.99 |
| Panton–Valentine leucocidin | 16 (21.1) | 36 (32.1) | 0.10 |
| agr dysfunction | 14 (18.4) | 13 (11.6) | 0.19 |
| hGISA | 11 (14.5) | 1 (0.9) | 0.001 |
hGISA, heteroresistant glycopeptide-intermediate S. aureus.
aData are presented as numbers (percentages).
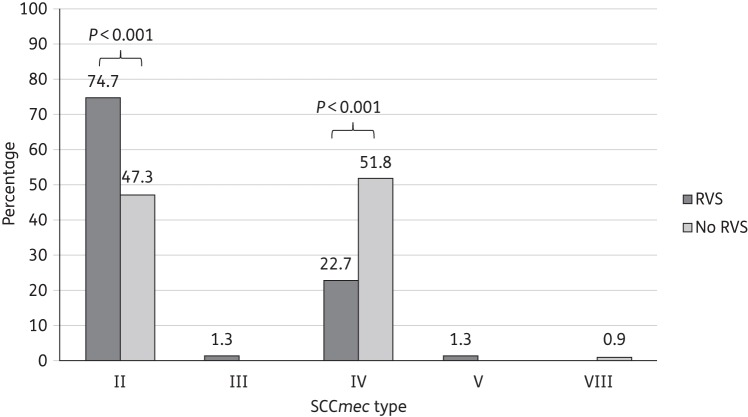
The distribution of SCCmec types among isolates was as follows: 109 (58.0%) with type II, 75 (40.0%) with type IV, 1 (0.5%) with type III, 1 (0.5%) with type V, 1 (0.5%) with type VIII and 1 (0.5%) was untypeable. The distribution of SCCmec types among isolates over time did not significantly change during the study period (P = 0.41). Figure 1 compares SCCmec type distribution among isolates with and without RVS. Isolates characterized as RVS-positive were significantly more likely to be associated with SCCmec II compared with isolates without RVS (74.7% and 47.3%, respectively; P < 0.001). Conversely, isolates without RVS were more likely to be associated with SCCmec type IV compared with isolates with RVS (51.8% and 22.7%, respectively; P < 0.001).
Figure 1.
Distribution of SCCmec types among MRSA isolates with and without RVS. Fisher's exact test was used for all comparisons.
On secondary analyses utilizing broth microdilution to determine RVS, 48 (25.5%) of isolates were characterized as demonstrating RVS. SCCmec type distribution among isolates was similar to that seen with Etest, with isolates characterized by RVS more likely to be associated with SCCmec II compared with isolates without RVS (80.9% and 50.7%, respectively; P < 0.001).
Finally, HA-MRSA isolates were significantly more likely than CA-MRSA isolates to be associated with SCCmec type II (66.1% versus 45.8%, respectively; P = 0.03) and absence of PVL (80.2% versus 59.7%, respectively; P = 0.004). However, there was no significant difference in agr dysfunction among HA-MRSA compared with CA-MRSA isolates (12.1% versus 18.1%, respectively; P = 0.29). There was no significant difference in the proportion of isolates classified as HA-MRSA, compared with CA-MRSA, that demonstrated RVS by Etest (43.1% versus 36.1%, respectively; P = 0.36).
Discussion
The prevalence of RVS among 188 MRSA bloodstream isolates in the present study was 40.4% as defined by Etest. MRSA isolates characterized by RVS were significantly more likely to be associated with SCCmec type II but not with agr dysfunction or absence of PVL.
Previous studies have implicated prior exposure to vancomycin as a risk factor for RVS,3 most likely as a result of antimicrobial selection pressure. However, the relationship between specific microbiological factors with RVS in MRSA is unclear, including microbial virulence determinants. Similarly to prior studies,5,6 we found that MRSA isolates with RVS were more likely to harbour SCCmec II. However, unlike previous studies, our patient population was not enriched for vancomycin failure or persistent bacteraemia,6 and the samples consisted solely of bloodstream isolates.5 Furthermore, MRSA isolates without RVS in the present study were associated with SCCmec IV. SCCmec II has been associated with increased mortality in MRSA bacteraemia.1 Similarly, studies suggest that RVS is associated with poor clinical outcomes in the setting of MRSA bacteraemia.4 Given this, the relatively high prevalence of RVS found in our study is of concern, and further research on optimal treatment strategies for infections due to MRSA with RVS is urgently needed.
SCCmec II has also typically been a marker for healthcare-associated infections due to MRSA. However, similarly to a previous study,5 there was no significant association between RVS and HA-MRSA in our study. While our definition of HA-MRSA differed from the CDC definition, this finding suggests that risk factors or antimicrobial selection pressure, apart from those seen with recent healthcare exposure, may contribute to the development of RVS. Finally, while elevated vancomycin MIC has been postulated to be a marker for an as yet unidentified organism virulence factor,4 the present study demonstrated no significant association between RVS and agr dysfunction or PVL.
There are potential limitations of our study. Selection bias is a potential concern; however, patients were identified through the Clinical Microbiology Laboratory, which processed and cultured all specimens obtained during the study period, thereby minimizing the likelihood of excluding potential isolates. The present study was conducted in a single healthcare system, and these results may not be generalizable to other institutions, for example, those with differences in MRSA prevalence.
In conclusion, the results of our study demonstrate important microbiological characteristics among MRSA isolates characterized by RVS, including a significant association between SCCmec II and elevated vancomycin MIC. It is clear that the clinical and molecular epidemiology of MRSA is evolving, and further understanding of organism factors determining virulence and fitness will be important for elucidation of optimal treatment and preventive approaches for associated infections.
Funding
This work was supported by the National Institutes of Health (K24 AI080942 to E. L.), a Commonwealth Universal Research Enhancement Program grant from the Pennsylvania State Department of Health (to E. L.) and the Centers for Disease Control and Prevention Epicenters Program (U54-CK000163 to E. L.). The dataset on which this study was based was constructed as part of a study originally funded by Cubist Pharmaceuticals. However, Cubist had no role in the present study. The funding agencies had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data, or preparation, review or approval of the manuscript.
Transparency declarations
E. L. has received research grant support from Merck, AstraZeneca, Cubist and 3M. J. H. H. and P. H. E.: none to declare.
Acknowledgements
We thank Martha Edelstein for performing SCCmec typing and agr testing, Andrew Baltus for PVL testing and antimicrobial susceptibility testing, Baofeng Hu for assistance with SCCmec typing and Jose Mediavilla and the Kreiswirth laboratory at the University of Medicine and Dentistry of New Jersey for their assistance with SCCmec typing of some isolates.
References
- 1.Davis SL, Rybak MJ, Amjad M, et al. Characteristics of patients with healthcare-associated infection due to SCCmec type IV methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2006;27:1025–31. doi: 10.1086/507918. [DOI] [PubMed] [Google Scholar]
- 2.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 3.Moise PA, Smyth DS, El-Fawal N, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61:85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 4.Holland TL, Fowler VG., Jr Vancomycin minimum inhibitory concentration and outcome in patients with Staphylococcus aureus bacteremia: pearl or pellet? J Infect Dis. 2011;204:329–31. doi: 10.1093/infdis/jir275. [DOI] [PubMed] [Google Scholar]
- 5.Yamaki J, Lee M, Shriner KA, et al. Can clinical and molecular epidemiologic parameters guide empiric treatment with vancomycin for methicillin-resistant Staphylococcus aureus infections? Diagn Microbiol Infect Dis. 2011;70:124–30. doi: 10.1016/j.diagmicrobio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Moise PA, Smyth DS, Robinson DA, et al. Genotypic and phenotypic relationships among methicillin-resistant Staphylococcus aureus from three multicentre bacteraemia studies. J Antimicrob Chemother. 2009;63:873–6. doi: 10.1093/jac/dkp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang JL, Wang JT, Sheng WH, et al. Nosocomial methicillin-resistant Staphylococcus aureus (MRSA) bacteremia in Taiwan: mortality analyses and the impact of vancomycin, MIC = 2 mg/L, by the broth microdilution method. BMC Infect Dis. 2010;10:159. doi: 10.1186/1471-2334-10-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascitti KB, Edelstein PH, Fishman NO, et al. Prior vancomycin use is a risk factor for reduced vancomycin susceptibility in methicillin-susceptible but not methicillin-resistant Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol. 2012;33:160–6. doi: 10.1086/663708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 10.Schweizer ML, Furuno JP, Sakoulas G, et al. Increased mortality with accessory gene regulator (agr) dysfunction in Staphylococcus aureus among bacteremic patients. Antimicrob Agents Chemother. 2011;55:1082–7. doi: 10.1128/AAC.00918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Mediavilla JR, Oliveira DC, et al. Multiplex real-time PCR for rapid staphylococcal cassette chromosome mec typing. J Clin Microbiol. 2009;47:3692–706. doi: 10.1128/JCM.00766-09. [DOI] [PMC free article] [PubMed] [Google Scholar]