Abstract
The term endothelial progenitor cell (EPC) was coined to refer to circulating cells that displayed the ability to display cell surface antigens similar to endothelial cells in vitro, to circulate and lodge in areas of ischemia or vascular injury, and to facilitate the repair of damaged blood vessels or augment development of new vessels as needed by a tissue. More than 10 years after the first report, the term EPC is used to refer to a host of circulating cells that display some or all of the qualities indicated above, however, essentially all of the cells are now known to be members of the hematopoietic lineage. The exception is a rare viable circulating endothelial cell with clonal proliferative potential that displays the ability to spontaneously form inosculating human blood vessels upon implantation into immunodeficient murine host tissues. This paper will review the current lineage relationships among all the cells called EPC and will propose that the term EPC be retired and that each of the circulating cell subsets be referred to according to the terms already existent for each subset. This article is part of a special issue entitled, "Cardiovascular Stem Cells Revisited".
Keywords: Endothelial progenitor cell (EPC), Endothelial cells
1. Introduction
The continuous endothelial lining of the blood vascular system has generally been reported to be a slow or non-replicative population of cells in the adult organism [1]. However, some evidence exists to support the role of a limited local endothelial cell replicative response to an endothelial denudation injury, leading to rapid endothelial restitution [2–5]. In areas of large injury, multiple circulating blood elements (platelets, neutrophils, monocytes, and other blood cells) are recruited to the exposed basement membrane in the area of injury with a more delayed recruitment of resident endothelium to reestablish vascular integrity and blood flow [6–9] (Fig. 1). In 1997, circulating blood cells displaying a variety of cell surface proteins thought to be endothelial specific upon in vitro culture and the ability to localize and promote vascular regeneration at sites of ischemia upon transplantation, were identified and called endothelial progenitor cells (EPC) [10]. Since the EPC were derived from the systemic circulation, the role of these cells in promoting new blood vessel formation was considered to be an example of postnatal vasculogenesis. Subsequently, many studies performed in a variety of animal model systems appeared to support the contention that bone marrow derived cells played an important role in vascular repair and regeneration and could facilitate tissue recovery after experimental ischemic injuries [11–14]. Indeed, recruitment of bone marrow-derived EPC was being promoted as a primary mechanism for endothelial replacement in areas of vascular damage. In parallel, numerous studies conducted in human subjects with a host of cardiovascular, metabolic, malignant, autoimmune, inflammatory, or other disorders were reported to display changes in the circulating concentration of EPC that correlated with the severity or risk of adverse outcome for that particular disease state [15–18]. The ability of human EPC to rescue diminished blood flow in preclinical experimental animal models combined with the known deficiency of circulating EPC in some patients with cardiovascular disorders provided the rationale to initiate clinical trials of the infusion of various bone marrow or EPC populations into human subjects with myocardial or systemic vascular disorders. The results of these studies have largely proven the infusion of marrow cells or EPC to be safe and beneficial in certain circumstances, though the effects on organ recovery in human subjects have been less robust than the results obtained in the preclinical rodent studies [19–21]. Thus, questions have arisen as to whether the preclinical rodent models are not as predictive for human cardiovascular disorders as might have been speculated or whether the EPC examined in the preclinical rodent models do not function in similar ways upon infusion into human subjects. It has become clear over the past 3–4 years that a whole host of different types of blood cells and endothelial cells are somewhat ambiguously being included within the single term EPC [22,23]. Thus, at present, the term EPC fails to refer to a distinct or unique cell type with definable characteristics. The most extreme differences in cellular functional subsets among those cells called EPC are most apparent in studies conducted with human blood cells. This review will attempt to examine the commonly used methods to identify putative EPC in human subjects and present the most recent data that provides clear distinction as to what types of cells may be isolated within each of the specific methods. Given the analysis of these results, we will make recommendations as to the most accurate nomenclature that may be applied to the cells involved in angiogenesis and postnatal vasculogenesis.
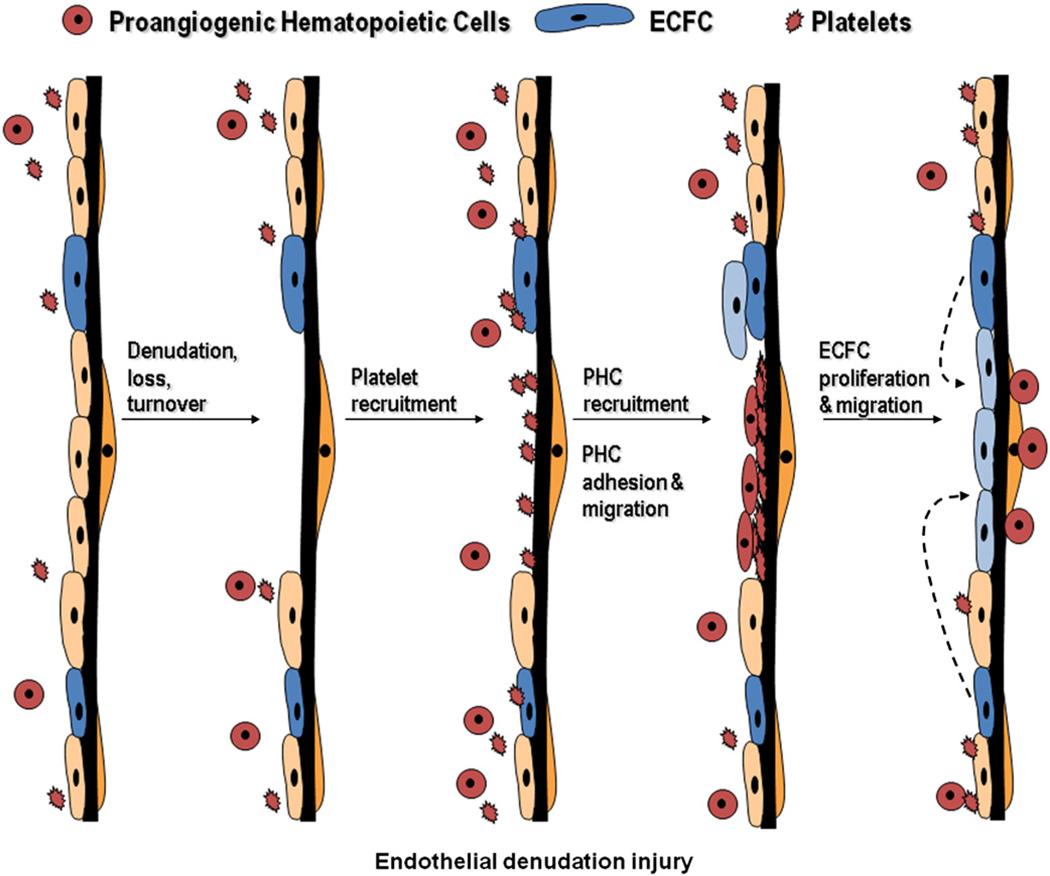
Fig. 1.
Role of various putative EPC in endothelial denudation injury. Following endothelial cell denudation injury, loss, or turnover, endothelial cells with proliferative potential (ECFC) divide and migrate to repair the injured area. This follows a series of events where platelets and proangiogenic hematopoietic cells are first recruited to the site of injury to facilitate repair.
2. Isolating putative EPC by cell adhesion to fibronectin-coated dishes with display of certain lectin and lipoprotein binding properties
In the original paper defining a progenitor endothelial cell, Asahara et al [10] reported that 15.7% of adult peripheral blood cells expressing CD34 could be isolated using immunomagnetic beads and culture of this population on fibronectin-coated tissue culture wells led to the emergence of spindle-shaped cells within 3 days. At 7 days of culture, 9–71% of the attached cells expressed CD45, CD34, CD31, Flk-1 (vascular endothelial growth factor 2 receptor), Tie-2, or E-selectin (some cell surface markers thought to identify cells of the endothelial lineage). In this same paper, the 7-day attached cultured cells also displayed the ability to take up the lectin Ulex Europeaus agglutinin-1 (UEA-1) and fluorescence labeled acetylated low density lipoprotein (acLDL). In other studies performed in that seminal paper, the adherent cell population recovered from the cultures expressing the above antigens was noted to co-localize with capillary vessels within the ischemic tissues of experimentally instrumented rabbits and mice and was associated with improved recovery of blood flow to ischemic limbs in these experimental animals. The ability of these cultured cells to enhance blood flow recovery, to co-localize with new vessels, and to display endothelial-like antigens in vitro gave confidence to the authors to proclaim this population as circulating progenitor cells for the endothelial lineage.
In a series of subsequent papers, EPC were defined as those cells that attached to fibronectin-coated culture dishes within 4–7 days and displayed the ability to take up UEA-1 and acLDL [24–26]. Use of this definition permitted the authors to isolate low-density peripheral blood mononuclear cells or bone marrow cells from rodents, rabbits, or human subjects and to compare their properties to rescue blood flow in ischemic states in animals with induced vascular injuries. Over time, this approach to an EPC definition has been utilized extensively in both human and rodent studies and has been translated to human clinical studies where the concentration of the adherent putative EPC circulating in the peripheral blood has been correlated with various clinical states [27–30].
If UEA-1 and acLDL uptake were unique to EPC and no other cells in this assay format, then this approach might lead to a viable definition. However, UEA-1, which recognizes L-fucosylated molecules on the surface of mammalian cells, is not restricted to binding to endothelial cells, but binds to many types of epithelial cells (transformed and non-transformed) and various hematopoietic cells including platelets [31–39]. This latter point is critical as Prokopi et al. [40] have recently reported that platelets are a common contaminant of peripheral blood mononuclear cells prepared for plating in the EPC adherence assay. The platelets were noted to readily disintegrate into microparticles and fuse with the adherent heterogenous mononuclear cells attached to the fibronectin-coated dishes. Of interest, many of the attached mononuclear cells displayed a variety of cell surface proteins that were contributed by the platelets (the attached mononuclear cells did not express the mRNA for the proteins being expressed on the cell membrane). Thus, the presence of any contaminating platelets in this adherence assay for EPC could lead to UEA-1 binding on the mononuclear cells, providing false-positive results [40]. In addition, peripheral blood monocytes are known to be highly enriched upon plating on fibronectin-coated dishes [41] and would be expected to display many proteins, including the scavenger receptors that bind acLDL, that are also expressed by endothelial cells (in the presence of the growth factors and serum used in the “EPC” assay culture medium) [42,43]. In a recent extensive mRNA expression profiling analysis of EPC derived from peripheral blood mononuclear cells, Medina et al. [44] reported that the adherent EPC displayed a pattern of mRNA expression that was enriched in hematopoietic specific pathways, particularly those that were immune related or inflammatory. In fact, proteomic comparison between the EPC and monocytes indicated that 77% of the proteins isolated by 2-D gels from EPC are also expressed by monocytes. In sum, neither acLDL nor UEA-1 binding are restricted in binding to a cell that could be called an EPC. Furthermore, platelets (or platelet microparticles) and monocytes would be expected to be bound to the fibronectin-coated dishes in this assay and would have to be depleted from the mononuclear cells under evaluation prior to defining any putative adherent cell as an EPC. Indeed, Prokopi et al. [40] reported that the highest correlation between any type of circulating blood cell and the concentration of in vitro adherent UEA-1+acLDL+ putative EPC was noted for monocytes and platelets in a large population based study of >500 human subjects. These findings call for significant scrutiny to be applied to papers in which putative EPC are being identified using UEA-1 and ac-LDL binding to adherent human mononuclear cells as sole definitive criteria.
3. Isolating putative EPC using the cell surface phenotype of CD34+CD133+KDR+
As noted in the first EPC description, both CD34 and vascular endothelial growth factor 2 receptor (KDR [human subjects] or Flk-1 [rodents]) expression on human peripheral blood mononuclear cells could be used to enrich for the putative EPC phenotype and function [10]. Other investigators extended these observations to suggest that a circulating population of endothelial precursors could be identified in human subjects by the phenotype CD34+AC133+KDR+ [45]. The authors noted that mature human umbilical vein endothelial cells did not express AC133 and thus, the ability of the AC133+KDR+ cells to give rise to adherent cells that bound acLDL and expressed KDR but not AC133 (a putative mature endothelial phenotype) confirmed the triple positive cells as an EPC. Further analysis of the cell surface proteins expressed on this subset included identification of CXCR4, CD31, CD13, and not CD14 or CD15 [45]. Surprisingly, the authors did not assay for expression of CD45, the common leukocyte antigen that is expressed on most nucleated circulating blood cells. Nonetheless, the cell surface phenotype CD34+AC133+KDR+ has gained wide use as a means to measure putative circulating EPC in healthy and diseased human subjects. However, the question of whether a putative EPC defined as a CD34+AC133+KDR+ cell, expresses CD45 has remained a controversial topic [23,46].
Several groups have subsequently examined the functional activities of the cells contained within the population of blood cell phenotype CD34+AC133+KDR+. While this population of cells is enriched 300- to 400-fold for hematopoietic stem and progenitor cells (defined by morphology, hematopoietic colony forming assays, and in vivo engraftment in immunodeficient mice), CD34+AC133+KDR+ cells do not spontaneously form capillary-like structures with lumens in vitro nor human blood vessels in vivo upon implantation in a collagen/fibronectin scaffold [47–49]. As noted by numerous authors, the CD34+AC133+KDR+ cells do facilitate the growth of tumor microvasculature and overall tumor growth [50–52]; however, there is no evidence that these cells directly form the long-lasting endothelial cells of the vessels. While these cells may be recruited to denuded vessels in ischemic sites early in the process of wound repair and thus occupy the position of an endothelial cell [22], the host endothelial cells comprise the long-lived vascular endothelium and are not derivatives of the hematopoietic system in the mouse [53–57]. It is more probable that the proangiogenic hematopoietic cells promote the process of angiogenesis via paracrine mechanisms [44,48] (Fig. 2).
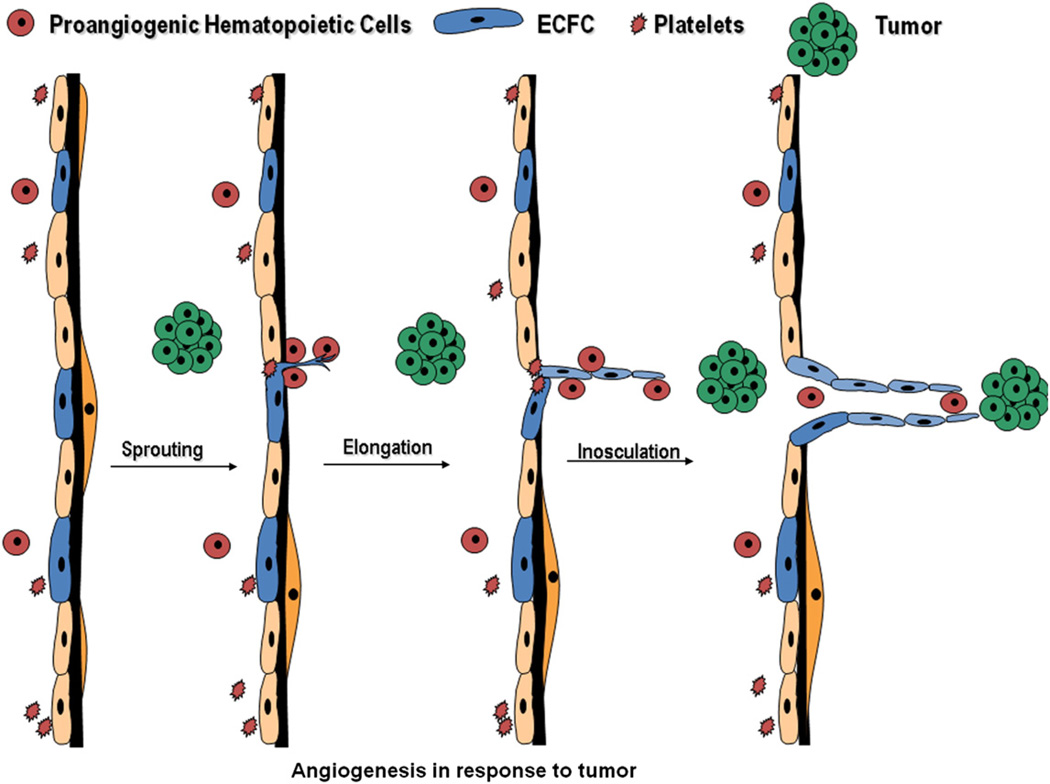
Fig. 2.
Putative EPC roles in angiogenesis. Resident endothelial cells with proliferative potential are likely to initiate sprouting, elongation, and inosculation leading to the formation of a new vessel in the presence of a stimulus such as a tumor. The circulating proangiogenic hematopoietic cells may play roles in disruption of the endothelial barrier properties, matrix degradation, promotion of endothelial cell sprouting, and endothelial capillary remodeling.
As to the question of whether or not the CD34+AC133+ cells also display CD45 expression, Estes et al. [48] have recently utilized polychromatic flow cytometry and functional assays to confirm that these double positive cells all express CD45. Since these cells also express the morphological and functional properties of hematopoietic stem and progenitor cells, it is apparent that CD34+AC133+ are proangiogenic hematopoietic stem and progenitor cells and not EPC [48]. Indeed, the lack of the ability of the CD34+AC133+ cells to directly form human blood vessels in vivo confirms that they do not possess the postnatal vasculogenic activity that was originally proposed for a cell with EPC properties [58].
4. Isolating putative EPC using colony forming assays
In the original EPC description, Asahara et al. [10] noted that the plated human peripheral blood CD34+ cells rapidly formed cellular clusters in vitro, particularly if re-exposed to the CD34− cells during the co-culture. These clusters were comprised of an aggregate of round cells with spindle-shaped cells radiating from beneath the aggregate and away from the cluster. The spindle-shaped cells readily bound acLDL. Asahara et al. [10] described these clusters as resembling the blood island structures that are observed in chick embryos and are known to contain both blood and endothelial cells. Thus, these clusters were reported as evidence that the CD34+ peripheral blood cells were turning into spindle-shaped acLDL binding putative endothelial cells.
Ito et al. [59] modified this original assay of Asahara et al. [10] for identification of EPC by attempting to deplete the human peripheral blood mononuclear cells of monocytes or circulating mature endothelial cells by allowing all of these mature cells to attach to the fibronectin-coated dishes for 24 h before removing the non-adherent cells. The non-adherent fraction was replated on the fibronectin-coated dishes and the clusters that emerged at 7 days were scored as EPC colony forming units. Subsequently, Hill et al. [60] further modified the assay to include a 48-h pre-plating period prior to replating the non-adherent cells. The colonies of putative EPC that emerged from the cultured non-adherent human peripheral blood mononuclear cells were called CFU-Hill. This assay was used to identify an inverse correlation of the concentration of CFU-Hill with cardiovascular risk status of human subjects.
Other investigators have identified a different type of colony of cells emerging from the plated peripheral blood mononuclear cells. Endothelial colony forming cells (ECFC), also called late outgrowth endothelial cells (OEC) or blood outgrowth endothelial cells (BOEC), typically emerge from an adult blood sample in 14–21 days [61,62]. In contrast, ECFC emerge as early as 6 days from time umbilical cord blood cells are plated on type 1 collagen or fibronectin-coated dishes [61,63]. The ECFC emerge as tightly adherent colonies with a typical cobblestone appearance and are rare in adult human blood samples with approximately 1 colony/108 mononuclear cells plated. ECFC possess clonal proliferative potential that can be observed in single cell cultures [61]. Umbilical cord blood contains a higher frequency of ECFC than adult peripheral blood and the circulating concentration appears to decrease with advancing age in human subjects. The human cord blood cells display much greater telomerase activity than the ECFC derived from human subjects [61]. While both cord blood and adult peripheral blood ECFC spontaneously form human blood vessels when implanted in matrix scaffolds in immunodeficient mice, cord blood ECFC display a greater density of vessels than the ECFC from adult blood samples [64,65]. An important aspect of the human blood vessel forming ability of ECFC is the property of these vessels to be connected to the host immunodeficient murine vessels and to become a part of the systemic circulation of the host animals [65,66]. This functional capacity of the ECFC is certainly indicative of postnatal vasculogenesis; the ability to form a vessel in the absence of a pre-existing vessel proposed to be uniquely displayed by circulating EPC (Fig. 3).
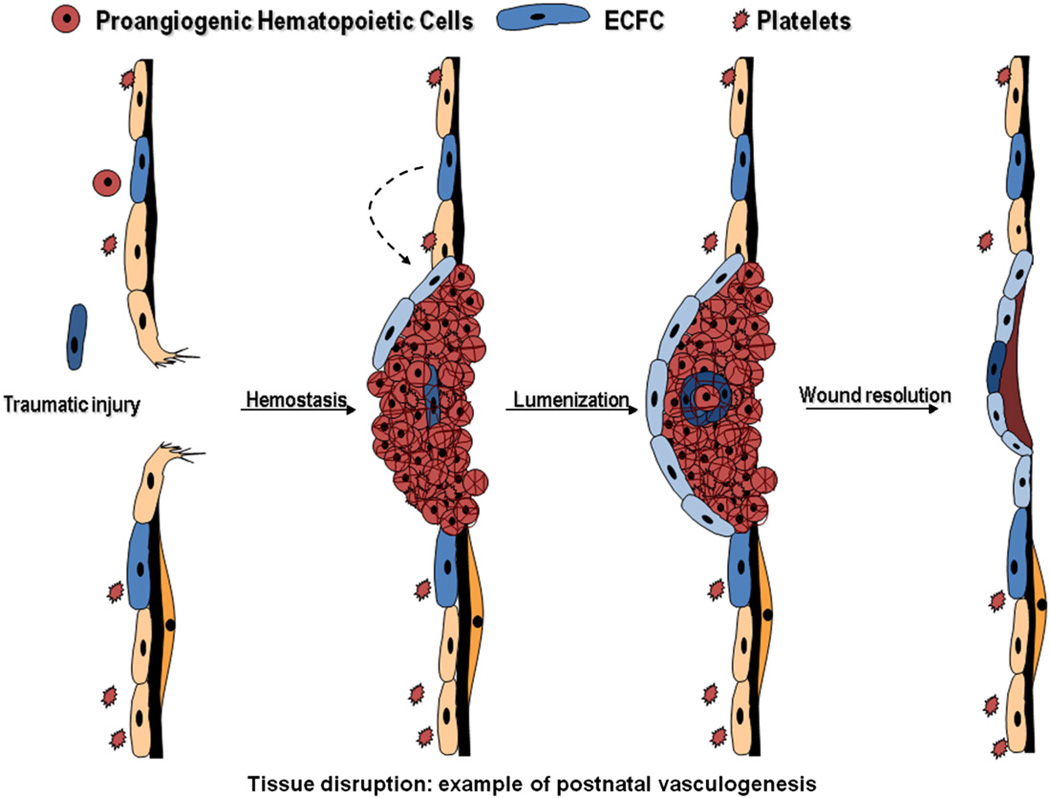
Fig. 3.
Circulating ECFC display postnatal vasculogenic activity. Following traumatic injury to a tissue vasculature, hemostasis is initiated to prevent extensive hemorrhage. Rare circulating ECFC may become trapped within the clot and initiate vasculogenesis as a first step toward recanalization of the thrombus with or without subsequent vessel remodeling.
While the cell surface phenotype of the ECFC progeny is nearly indistinguishable from the pattern of expression displayed by vascular endothelial cells, some progress has been made in enriching this population by first depleting the mononuclear cells of monocytes, red blood cells, dead cells, and CD45 expressing blood cells [48]. The highly expressing CD34+ population of cells co-expresses CD146, CD31, and CD105. The CD34hiCD45neg cells contain essentially all of the viable circulating endothelial cells and are enriched by 300- to 400-fold for ECFC activity [47]. At present, no specific antigen has been identified that can unequivocally discriminate the ECFC from other circulating blood elements. In fact, a recent comparison of the proteome of human peripheral blood OEC and dermal microvascular endothelial cells revealed a 90% overlap; though a few (8 indicated) protein spots on the gels of the OEC proteome remain as possible candidate OEC markers but must be sequenced for further verification [44].
Several research groups have directly compared the functional potential of the CFU-Hill with the ECFC derived from adult peripheral blood or cord blood. It is apparent that CFU-Hill is comprised of a variety of blood cells including monocytes, lymphocytes, and hematopoietic progenitor cells skewed toward the myeloid lineage [39,65,67–69]. The spindle-shaped cells emerging from the aggregate of hematopoietic cells are macrophages verified by the fact that the cells express the colony stimulating factor-1 receptor, display active phagocytosis of bacteria, express nonspecific esterase that is inhibited by sodium fluoride, and readily ingest acLDL. The macrophages in the CFU-Hill assay also express CD31, CD144, von Willebrand factor, endothelial nitric oxide synthase, CD105, and bind UEA-1 lectin (in these assay and culture medium conditions). Thus, by only searching for evidence of endothelial marker expression on adherent cells emerging from within the clusters and failing to exclude other potentially “contaminating” cell types, the original publications may have concluded they had identified a unique cell type, when in fact the cells belonged to already known hematopoietic lineages.
In contrast to the hematopoietic functions identified for the CFU-Hill, ECFC display functions such as in vitro clonal proliferative potential, in vitro self-renewal potential, in vitro incorporation into endothelial monolayers or capillary tube formation, in vivo human vessel formation with incorporation into the systemic circulation of immunodeficient mice, and in vivo chimeric vessel formation into areas of ischemia [65,66,70,71]. Thus, ECFC appear to function as a circulating precursor with in vivo human vessel forming ability and thereby among all current putative EPC, represent the cell displaying the most features of a human postnatal vasculogenic cell [22].
5. Quo vadis?
While numerous papers have been published discussing the role of EPC in various human disorders and in examining the role of various putative EPC in rescuing the blood flow to experimentally induce vascular lesions in rodents or in human clinical trials of cell therapy for cardiovascular disease, the field continues to struggle in defining the EPC. These changes in the field led us to add the Latin phrase, Quo vadis, which translates, “Whither goest thou” to the title of this review. A concise summary of all the above overview would be to consider that the cells originally identified as EPC in the various assays were and are in fact hematopoietic lineage cells (progenitors, monocytes, and/or platelets) that display proangiogenic properties (Table 1). The one cell type that displays postnatal vasculogenic activity upon transplantation in a matrix scaffold is the rare circulating ECFC or OEC (Table 1). Thus, the field may wish to delimit the use of the term EPC and simply describe the cells involved in vascular repair and regeneration with the specific terms already in existence; i.e. primary human cells promoting angiogenesis via paracrine effects include proangiogenic hematopoietic stem and/or progenitor cells, monocytes, macrophages, or platelets (or other blood cells) whereas the rare circulating cells that display vessel-forming ability are ECFC or OEC (Table 1). Because several different types of blood cells are implicated as proangiogenic, future studies will be required to determine the exact role that each cell plays in the process of vascular repair or regeneration, the mechanism of interaction with the host endothelium, and the molecular pathways engaged. For example, are the monocytes involved in angiogenesis uniquely specified from circulating monocyte precursors or do they exist as a distinctly separate differentiated subset [72,73]? Are the vascular anastomotic chaperoning properties displayed by tissue macrophages at sites of angiogenesis restricted to the Tie2 subset and do they involve direct contact with the endothelium [74]? Likewise, little is known of the in vivo functions of ECFC in the many preclinical models of human cardiovascular disease and increased studies in this area may be illuminating; for example, Dubois et al. [75] have reported that infusion of OEC into pigs following experimentally induced acute myocardial infarction resulted in significant improvement in myocardial infarct remodeling and heart function via direct incorporation of the cells into the host endothelium and Medina et al. [76] using a murine model of retinal ischaemia, reported that human OECs directly incorporate into the host murine vasculature, significantly decreasing avascular areas, concomitantly increasing normovascular areas and preventing pathologic pre-retinal neovascularisation. In the end, by better defining the cells that display functional roles in vessel repair, we may be better able to (1) consider cellular replacement of that subset if found to be dysfunctional (requires development of appropriate in vitro functional assay to assess the function of autologous cells) and (2) identification of the molecular mechanisms underlying the functions of the various hematopoietic and endothelial subsets to repair the vasculature and (3) development of small molecule effectors to mimic the beneficial effects of the efficacious cellular subsets (rescue the angiogenic functions of host cell subsets and augment and/or restore innate repair mechanisms).
Table 1.
Summary of hallmark studies in defining a human EPC.
| Year | Lead author |
Masker | Method Used to Isolate | Marker to define | Term | Weaknesses of the study | Proposed term |
|---|---|---|---|---|---|---|---|
| 1997 | Asahara | CD34 or KDR | Immunomagnetic bead separation of CD34 or KDR positive adult peripheral blood cells | CD45, CD34, CD31, Flk-1, Tie-2 E-selectin, UEA-1, acLDL | EPC | Failed to exclude the presence of hematopoietic cells and no direct demonstration of in vivo vessel forming ability | Proangiogenic hematopoietic cell |
| 2000 | Peichev | CD34, AC133, and KDR | Immunomagnetic bead separation of CD34 positive adult peripheral blood cells | CXCR4, CD31, CD13, acLDL, and not CD14 or CD15 | CEP | Did not assay for CD45 expression – cells neither form vessels in vitro nor in vivo | Proangiogenic hematopoietic cell |
| 2001 | Lin | CD146 (P1H12) | Plating of mononuclear cell pellet or immunomagnetic bead separation of CD146 positive adult peripheral blood cells | CD34, CD144, vWF, Flk-1, and not CD14 | BOEC | No evidence for clonal proliferative potential and no direct demonstration of vessel forming ability | BOEC |
| 2003 | Hill | Mononuclear cells | 48 h pre-plating period prior to replating non-adherent blood mononuclear cells and colony formation 4–9 days later | CD31, Tie2, and KDR | CFU-Hill | Failed to exclude the presence of hematopoietic cells in the colonies and no direct demonstration of vessel forming ability | Pro-angiogenic hematopoietic cell |
| 2004 | Ingram | Mononuclear cells | In vitro colony emergence and clonal proliferative potential | CD34, CD146, CD31, CD105, and not CD45 and direct demonstration of vessel formation in vivo | ECFC | Lacks antigen to distinguish ECFC from other circulating EC | ECFC |
| 2007 | Rohde | Rohde Mononuclear cells | 48 h pre-plating period prior to replating non-adherent blood mononuclear cells and colony formation 4–9 days later | (VEGF-R1, 2, and 3), CD31, CD34, CD146, vWF, and not CD14 CD45 | CFU-EC | Could not identify all of the proangiogenic activity related to cultured hematopoietic cells | Proangiogenic hematopoietic cell |
| 2007 | Yoder | Mononuclear cells | In vitro colony emergence and clonal proliferative potential and 48 h pre-plating period prior to replating non-adherent blood mononuclear cells and colony formation 4–9 days later | CD34, CD146, CD31, CD105, and not CD45 and direct demonstration of vessel formation in vivo | ECFC Vs. CFUHill | Lacks antigen to distinguish ECFC from other circulating EC | ECFC and Proangiogenic hematopoietic cell |
| 2009 | Prokopi | Mononuclear cells | Plating of mononuclear cell pellet on fibronectin-coated dishes and at 72 h removed nonadherent cells | CD31, vWF, UEA-1, CD4, and acLDL | EPC | Lack detailed method for determining the extent of the contaminating platelet proteome to the adherent blood cells | Putative EPC and platelets |
| 2010 | Estes | CD34, AC133, CD45, CD31 | Polychromatic flow cytometry and cell sorting | CD34, AC133, CD45, CD31 | Proangiogenic hematopoietic cell | Lacked detailed examination of contribution of proangiogenic cells to the tumor microvasculature | Proangiogenic hematopoietic cell |
UEA-1, Ulex Europeaus agglutinin-1; acLDL, acetylated low density lipoprotein; CEP, circulating endothelial precursor; ECFC, endothelial colony forming cells; BOEC, blood outgrowth endothelial cells; vWF, von Willebrand factor; CFU-EC, colony forming unit-endothelial cell; CFU-Hill, colony forming unit-Hill.
References
- 1.Schwartz SM, Gajdusek CM, Selden SC., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- 2.Kunz J, Schreiter B, Schubert B, Voss K, Krieg K. Experimental investigations on the regeneration of aortic endothelial cells. Automatic and visual evaluation of autoradiograms (author's transl) Acta Histochem. 1978;61(1):53–63. [PubMed] [Google Scholar]
- 3.Malczak HT, Buck RC. Regeneration of endothelium in rat aorta after local freezing. A scanning electron microscopic study. Am J Pathol. 1977 Jan;86(1):133–148. [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz SM, Gajdusek CM, Reidy MA, Selden SC, 3rd, Haudenschild CC. Maintenance of integrity in aortic endothelium. Fed Proc. 1980 Jul;39(9):2618–2625. [PubMed] [Google Scholar]
- 5.Schwartz SM, Stemerman MB, Benditt EP. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975 Oct;81(1):15–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Caplan BA, Schwartz CJ. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973 May-Jun;17(3):401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- 7.Haudenschild C, Studer A. Early interactions between blood cells and severely damaged rabbit aorta. Eur J Clin Invest. 1971 Nov;2(1):1–7. doi: 10.1111/j.1365-2362.1971.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 8.Haudenschild CC, Schwartz SM. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- 9.Taylor RG, Lewis JC. Endothelial cell proliferation and monocyte adhesion to atherosclerotic lesions of white carneau pigeons. Am J Pathol. 1986 Oct;125(1):152–160. [PMC free article] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb 14;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009 Oct 9;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno PR, Sanz J, Fuster V. Promoting mechanisms of vascular health: circulating progenitor cells, angiogenesis, and reverse cholesterol transport. J Am Coll Cardiol. 2009 Jun 23;53(25):2315–2323. doi: 10.1016/j.jacc.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 13.Pacilli A, Faggioli G, Stella A, Pasquinelli G. An update on therapeutic angiogenesis for peripheral vascular disease. Ann Vasc Surg. 2010 Feb;24(2):258–268. doi: 10.1016/j.avsg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010 May;79(3):200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008 Oct;45(4):530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekiguchi H, Ii M, Losordo DW. The relative potency and safety of endothelial progenitor cells and unselected mononuclear cells for recovery from myocardial infarction and ischemia. J Cell Physiol. 2009 May;219(2):235–242. doi: 10.1002/jcp.21672. [DOI] [PubMed] [Google Scholar]
- 17.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005 Sep 8;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 18.Zeoli A, Dentelli P, Brizzi MF. Endothelial progenitor cells and their potential clinical implication in cardiovascular disorders. J Endocrinol Invest. 2009 Apr;32(4):370–382. doi: 10.1007/BF03345729. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Zeiher AM. Cell therapy of acute myocardial infarction: open questions. Cardiology. 2009;113(3):155–160. doi: 10.1159/000187652. [DOI] [PubMed] [Google Scholar]
- 20.George JC. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Transl Res. 2010 Jan;155(1):10–19. doi: 10.1016/j.trsl.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008 Aug;29(15):1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 22.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells: an expanding universe. Hypertension. 2010 Mar;55(3):593–599. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 23.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009 Jul;16(4):269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 24.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000 Mar 28;97(7):3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999 Apr;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 26.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001 Jul 6;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 27.Assmus B, Rolf A, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010 Jan;3(1):89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 28.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008 Oct;45(4):514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Kovacic JC, Harvey RP, Dimmeler S. Cardiovascular regenerative medicine: digging in for the long haul. Cell Stem Cell. 2007 Dec 13;1(6):628–633. doi: 10.1016/j.stem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Urbich C, Rossig L, Dimmeler S. Restoration of cardiac function with progenitor cells. Novartis Found Symp. 2006;274:214–223. discussion 23-7, 72-6. [PubMed] [Google Scholar]
- 31.Abdul-Salam F, Mansour MH, Al-Shemary T. The selective expression of distinct fucosylated glycoproteins on murine T and B lymphocyte subsets. Immunobiology. 2005;210(9):695–708. doi: 10.1016/j.imbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Baldus SE, Thiele J, Charles A, Hanisch FG, Fischer R. Carbohydrate antigens of human megakaryocytes and platelet glycoproteins: a comparative study. Histochemistry. 1994 Sep;102(3):205–211. doi: 10.1007/BF00268897. [DOI] [PubMed] [Google Scholar]
- 33.Chionh YT, Wee JL, Every AL, Ng GZ, Sutton P. M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens. Infect Immun. 2009 Jul;77(7):2962–2970. doi: 10.1128/IAI.01522-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, et al. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int J Pharm. 2007 Aug 1;340(1–2):207–215. doi: 10.1016/j.ijpharm.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Georgiou S, Pasmatzi E, Monastirli A, Sakkis T, Alachioti S, Tsambaos D. Age-related alterations in the carbohydrate residue composition of the cell surface in the unexposed normal human epidermis. Gerontology. 2005 May-Jun;51(3):155–160. doi: 10.1159/000083986. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi N, Matsuzaki O, Shirai S, Aoki I, Yao M, Nagashima Y. Collecting duct carcinoma of the kidney: an immunohistochemical evaluation of the use of antibodies for differential diagnosis. Hum Pathol. 2008 Sep;39(9):1350–1359. doi: 10.1016/j.humpath.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz B, Thiele J, Otto F, Theile-Ochel S, Heedt T, Zensen U, et al. Interactions between endogeneous lectins and fucosylated oligosaccharides in megakaryocyte- dependent fibroblast growth of the normal bone marrow. Leukemia. 1996 Oct;10(10):1604–1614. [PubMed] [Google Scholar]
- 38.Thom I, Schult-Kronefeld O, Burkholder I, Goern M, Andritzky B, Blonski K, et al. Lectin histochemistry of metastatic adenocarcinomas of the lung. Lung Cancer. 2007 Jun;56(3):391–397. doi: 10.1016/j.lungcan.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, et al. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006 Jun;16(6):577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]
- 40.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, et al. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009 Jul 16;114(3):723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 41.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatincoated surfaces. J Immunol Methods. 1986 Dec 24;95(2):273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 42.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001 Feb 16;49(3):671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 43.Schmeisser A, Graffy C, Daniel WG, Strasser RH. Phenotypic overlap between monocytes and vascular endothelial cells. Adv Exp Med Biol. 2003;522:59–74. doi: 10.1007/978-1-4615-0169-5_7. [DOI] [PubMed] [Google Scholar]
- 44.Medina RJ, O'Neill CL, Sweeney M, Guduric-Fuchs J, Gardiner TA, Simpson DA, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3(1):18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000 Feb 1;95(3):952–958. [PubMed] [Google Scholar]
- 46.Bertolini F, Mancuso P, Braidotti P, Shaked Y, Kerbel RS. The multiple personality disorder phenotype(s) of circulating endothelial cells in cancer. Biochim Biophys Acta. 2009 Aug;1796(1):27–32. doi: 10.1016/j.bbcan.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007 Jul;35(7):1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Chapter 9: Unit 9 33. Curr Protoc Cytom. 2010 Apr;:1–11. doi: 10.1002/0471142956.cy0933s52. [DOI] [PubMed] [Google Scholar]
- 49.Timmermans F, Van Hauwermeiren F, De Smedt M, Raedt R, Plasschaert F, De Buyzere ML, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007 Jul;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 50.Bertolini F, Mancuso P, Kerbel RS. Circulating endothelial progenitor cells. N Engl J Med. 2005 Dec 15;353(24):2613–2616. author reply -6. [PubMed] [Google Scholar]
- 51.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008 Jan 11;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 52.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007 Jun 15;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007 Mar 2;100(4):581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 54.Hagensen MK, Shim J, Thim T, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to plaque endothelium in murine atherosclerosis. Circulation. 2010 Feb 23;121(7):898–905. doi: 10.1161/CIRCULATIONAHA.109.885459. [DOI] [PubMed] [Google Scholar]
- 55.Hillebrands JL, Klatter FA, van Dijk WD, Rozing J. Bone marrow does not contribute substantially to endothelial-cell replacement in transplant arteriosclerosis. Nat Med. 2002 Mar;8(3):194–195. doi: 10.1038/nm0302-194. [DOI] [PubMed] [Google Scholar]
- 56.Perry TE, Song M, Despres DJ, Kim SM, San H, Yu ZX, et al. Bone marrow-derived cells do not repair endothelium in a mouse model of chronic endothelial cell dysfunction. Cardiovasc Res. 2009 Nov 1;84(2):317–325. doi: 10.1093/cvr/cvp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008 May 6;105(18):6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytom A. 2010 May 25; doi: 10.1002/cyto.a.20921. [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito H, Miyake M, Nishitani E, Mori K, Hatano T, Okuda T, et al. Anti-tumor promoting activity of polyphenols from Cowania mexicana and Coleogyne ramosissima. Cancer Lett. 1999 Aug 23;143(1):5–13. doi: 10.1016/s0304-3835(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 60.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003 Feb 13;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 61.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004 Nov 1;104(9):2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 62.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000 Jan;105(1):71–77. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005 Apr 1;105(7):2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 64.Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008 Feb 1;111(3):1302–1305. doi: 10.1182/blood-2007-06-094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007 Mar 1;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007 Jun 1;109(11):4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 67.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007 Jul;25(7):1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 68.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006 Feb;24(2):357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 69.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008 Feb 12;51(6):660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 70.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004 Feb;24(2):288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 71.Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res. 2008 Feb 1;314(3):430–440. doi: 10.1016/j.yexcr.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, De Palma M. Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol. 2010 Apr;176(4):1564–1576. doi: 10.2353/ajpath.2010.090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood "resident" monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009 Jul 23;114(4):901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 74.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010 Apr 19; doi: 10.1182/blood-2009-12-257832. [ePub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dubois C, Liu X, Claus P, Marsboom G, Pokreisz P, Vandenwijngaert S, et al. Differential effects of progenitor cell populations on left ventricular remodeling and myocardial neovascularization after myocardial infarction. J Am Coll Cardiol. 2010 May 18;55(20):2232–2243. doi: 10.1016/j.jacc.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 76.Medina R, O'Neill CL, Humphreys MW, Gardiner TA, Stitt AW. Outgrowth endothelial cells: characterization and their potential for reversing ischaemic retinopathy. Invest Opthalmol Vis Sci. 2010 Jun 16; doi: 10.1167/iovs.09-4951. [epub ahead of print]. [DOI] [PubMed] [Google Scholar]