Abstract
As enhanced adipogenesis contributes to programmed obesity, adipogenic and lipogenic signaling pathways in intrauterine growth restricted (IUGR) offspring were examined. From 10 days to term gestation, rats received ad libitum food (control) or were 50% food-restricted (IUGR). Pups were nursed and weaned to ad libitum diet. mRNA and protein levels of adipogenic transcription factors and lipid enzymes (1 day and 9 month) and adipocyte cell size (3 weeks and 9 months) were determined. One-day-old IUGR males showed upregulation of peroxisome proliferator-activated receptor (PPARγ2), including upstream factors regulating PPARγ, and RXRα, with which PPARγ heterodimerizes. Intracellular lipolytic enzyme (hormone-sensitive lipase) was downregulated. Nine-month-old IUGR males showed upregulation of adipogenic and lipogenic (SREBP1c) transcription factors with upregulation of enzymes facilitating fatty acid uptake (lipoprotein lipase) and synthesis (fatty acid synthase), leading to hypertrophic adipocytes. Paradoxical upregulation of adipogenesis signaling cascade prior to the development of obesity in IUGR males suggests early changes in signaling mechanisms.
Keywords: Adipocyte differentiation, peroxisome proliferator-activated receptor γ, CCAAT enhancer-binding proteins, sterol regulatory element binding protein, lipid enzymes
Obesity has emerged as a preeminent public health problem.1,2 Furthermore, obesity is a major risk factor for diabetes and atherosclerosis, afflictions associated with a constellation of insulin resistance, hypertension, and lipid abnormalities.3 One of the underlying factors that links these disorders is a dysregulation of white adipose tissue mass or enhanced adipogenesis,4 resulting in alterations in adipocyte size and/or number.5,6 Adipogenesis, in turn, is triggered by the induction of adipocyte differentiation, which leads to lipogenesis and hence lipid accumulation within the adipocyte.5,7
Adipocyte differentiation is triggered by a set of interacting transcription factors.8,9 In particular, the peroxisome proliferator-activated receptor (PPAR) and CCAAT/enhancer binding protein (C/EBP) family members play a cooperative role in influencing adipocyte differentiation.10,11 The C/EBP family members (C/EBPβ, C/EBPδ, and C/EBPα) activate PPARγ2, forming a heterodimer with the retinoid X receptors (RXRα). Alternatively, PPARγ2 activation can directly induce the expression of C/EBPα and synergistically promote adipocyte differentiation.12,13
Lipogenesis entails induction of a lipogenic transcription factor, sterol regulatory element binding protein (SREBP1c), by PPARγ2.14 SREBP1c, in turn, induces expression of lipolytic and lipogenic enzymes, which modulate fatty acid uptake and synthesis and thus adipocyte lipid accumulation.15 The adipocyte lipid accumulation is regulated by an extracellular lipolytic enzyme, lipoprotein lipase,16 which acts on the triglyceride within lipoproteins in the blood stream, resulting in free fatty acid molecules that are taken up by adipocytes. Fatty acid synthase plays a central role in de novo lipogenesis by catalyzing the conversion of acetyl CoA and malonyl CoA to long-chain fatty acids.17 Furthermore, fatty acid synthase is recognized as the rate-limiting step for the overall lipogenesis, and inhibition of fatty acid synthase in rodents induces profound weight loss and reduced food intake.18 An intracellular lipolytic enzyme, hormone-sensitive lipase, acts on the stored triglyceride and releases free fatty acids from the adipocyte.19 Thus, dysregulation of these enzymes influences adipocyte lipid storage and release.
Human and animal models have highlighted the critical role of early nutrition in influencing adiposity and lipid metabolism. Paradoxically, both maternal nutrition deprivation20,21 and maternal obesity22 produce obese offspring though the underlying mechanisms remain unclear. Intrauterine growth-restricted (IUGR) newborns have increased risk of obesity, especially when it is associated with catch-up growth in the first few years of life. To reflect this scenario, we have established a rat model of maternal food restriction during pregnancy that results in IUGR newborns. When provided standard nursing and postweaning diet, IUGR newborns demonstrate rapid catch-up growth hyperphagia and adult obesity. In particular, they develop obesity as adults, with excess body fat and increased plasma levels of leptin, insulin, glucose, and triglycerides.23,24 Although increased food intake contributes, in part, to enhanced weight gain,25 the role of peripheral adipose tissue metabolism is unclear. The development of obesity is associated with increased adipocyte differentiation, adipocyte hypertrophy, and upregulation of lipogenic genes.26 As increased adiposity is one of the key features of programmed obesity, we hypothesized that maternal food restriction upregulates the adipogenic signaling pathway, including lipogenic transcription factor and enzymes, leading to adipocyte hypertrophy and lipid storage. We therefore sought to determine the expression (mRNA and protein) of adipogenic/lipogenic transcriptional factors and lipid enzymes in newborn and adult offspring. The present study demonstrates upregulation in the expression of the key adipogenic transcription factor PPARγ2, including upstream factors regulating PPARγ, in IUGR newborns’ adipose tissue. As adults, these offspring exhibit hypertrophic adipocytes with persistent upregulation of adipogenic and lipogenic transcription factors as well as enzymes that promote increased fatty acid uptake and synthesis by adipocytes. The upregulation of the adipogenesis signaling cascade prior to the development of obesity in IUGR males suggests that signaling mechanisms are changed very early in life.
MATERIAL AND METHODS
Maternal Rat Diets
Studies were approved by the Animal Research Committee of the Los Angeles BioMedical Research Institute at Harbor-UCLA and were in accordance with the American Association for Accreditation of Laboratory Care and National Institutes of Health guidelines. The rat model used for maternal food restriction during pregnancy and lactation has been previously described.24 Briefly, first time pregnant Sprague Dawley rats (Charles River Laboratories, Hollister, California) were housed in a facility with constant temperature and humidity and controlled in a 12:12 hour light/dark cycle. At 10 days of gestation (e10), rats were provided either an ad libitum (control; n = 12) diet of standard laboratory chow (Lab Diet 5001; PMI, Brentwood, Missouri) or a 50% food-restricted diet (IUGR; n = 6) determined by quantification of normal intake in ad libitum fed rats. These diets were provided from e10 to term (e21).The rationale for initiating the food restriction on e10 was based on studies showing that by this developmental time point the placenta is fully formed, and our primary interest was to study programming of the fetus rather than the placenta.27
Offspring
At day 1 of birth, litter size was culled to 8 offspring, maintaining a sex ratio of 1:1 to normalize rearing. Cross-fostering techniques were used to study the effects of maternal food restriction solely during pregnancy. Offspring from food-restricted dams were cross-fostered to rat dams fed ad libitum diet during pregnancy to eliminate the effect of maternal food restriction on lactation, as studies have shown that nutrition, especially during late gestation, can significantly affect mammary development and subsequent lactation potential.28,29 To control for the cross-fostering effects, control pups were similarly cross-fostered among ad libitum fed control dams. At 3 weeks of age, all offspring were housed individually and weaned to ad libitum standard laboratory chow till 9 months of age. Body weight, body composition as determined by dual-energy X-ray absorptiometry, and food intake have been previously reported.23
At 1 day of age, male pups were decapitated and, because of feasibility issues, the entire abdominal visceral fat was collected. At 3 weeks and 9 months of age, males were euthanized by an overdose of pentobarbital (100 mg/kg intraperitoneally) and retroperitoneal visceral adipose tissue was obtained. Adipose tissue was either snap frozen and stored at −80°C for mRNA and protein expression or fixed in 4% phosphate-buffered formalin for determination of cell size. We studied newborn animals at day 1 of age to demonstrate the proximate mechanisms as a result of in utero insult. Nine-month-old animals, which are mature adults, were studied to confirm that the changes observed were permanent. Furthermore, we have previously demonstrated that at 9 months of age IUGR offspring have increased body fat and metabolic syndrome. 23–25 Because of sectioning difficulty of adipose tissue from 1-day-old newborns, the earliest adipocyte cell size was determined at 3 weeks of age. We elected to study males as females would have required estrus assessment, because estrogen is known to affect adiposity and lipid metabolism.30
Analytical Methods
Primer
The primer sequence was designed using Primer 3.0 based on NCBI Sequence Viewer v2.0.Table 1 shows the primer sequence and Genbank accession numbers of PPARγ, C/EBPδ, C/EBPβ, C/EBPα, RXRα, SREBP1c, lipoprotein lipase, fatty acid synthase, and hormone-sensitive lipase.
Table 1.
Primer Sequences of Genes Used for Detection of mRNA
| Gene | Accession Number | Forward | Reverse |
|---|---|---|---|
| PPARγ | NM_013124 | cataaagtccttcccgctga | aagaaactggcacccttgaaaa |
| C/EBPδ | NM_013154 | agagcgccatcgacttcagc | ccaagctcaccactgtctgc |
| C/EBPβ | AY056052 | tacccaggacccattggata | ttcacttggccactcttcct |
| C/EBPα | NM_012524 | tgttggagttgaccagtgac | atccagcgaccctaaaccat |
| RXRα | NM_012805 | gatggcctgtgtggatcttt | aaccagcaaccagaacaagc |
| SREBP1c | AF286469 | caagtgctgcaggaaactga | catggccttgtcaatggaac |
| Lipoprotein lipase | NM_012598 | acaggtgcaattccaaggag | ctttcagccactgtgccata |
| Fatty acid synthase | NM_017332 | agaggccagtgcattaagga | aaacacgccctcttgcttta |
| Hormone-sensitive lipase | NM_012859 | cctcaaagtcaaaccctcca | cattgtgcgtaaatccatgc |
Abbreviations: PPAR, peroxisome proliferator-activated receptor; EBP, enhancer binding protein; RXR, retinoid X receptor; SREBP1c, sterol regulatory element binding protein 1c.
RNA extraction
Total RNA was extracted from adipose tissue using RNeasy lipid tissue kit (Cat No 74804; QIA-GEN Inc, Valencia, California).
Real-time polymerase chain reaction (PCR)
cDNA was generated with 1 µg of total RNA using Omniscrip reverse transcription (RT) reagent (QIAGEN). The RNA was incubated in 20 µL of a RT reaction mixture (1× RT buffer, 0.5 mM dNTP, 1 µM random hexamers, 10 U Rnasin [RNase inhibitor], and 4 U Omniscript reverse transcriptase) at 37°C for 1 hour. The RT was inactivated by heating at 95°C for 5 minutes. PCR was performed in 96-well optical reaction plates (Applied Biosystems, Foster City, California) on cDNA equivalent to 50 ng RNA in a volume of 25 µL, containing 1× reaction buffer (Eurogentec, San Diego, California), forward and reverse appropriate primers selected from Assay on Demand (Applied Biosystems). PCR was performed for respective gene and β-actin gene, using the ABI-Prism 7700 Sequence System (Applied Biosystems) at the following conditions: 10 minutes at 95°C for 1 cycle; and 15 seconds at 95°C, 1 minute at 60°C for 40 cycles. All samples were run in triplicate. Control PCR replaced cDNA with water and gave a threshold level (CT) value of 40, indicating no detectable PCR product under these cycle conditions.
ABI Sequence Detection System 1.6 software (Applied Biosystems) was used to select a threshold level of fluorescence that was in the linear phase of the PCR product accumulation. Results from the RT–PCR assay were determined as the difference between the CT for a specific mRNA gene and the CT for a reference, β-actin mRNA and expressed as fold change, using the formula 2−(ΔΔCT).
Antibodies
The antibodies were obtained from Santa Cruz Biotechnology Inc (Santa Cruz, California), Cayman Chemical (Ann Arbor, Michigan), or Sigma-Aldrich (St Louis, Missouri) and diluted 1:500, and the band density was analyzed as indicated: PPARγ2 (Cayman 101700; 57 kDa), C/EBPδ (SC-22; 34 kDa), C/EBPβ (SC-7962; 45 kDa),C/EBPα (SC-61; 42 kDa),RXRα (SC-553; 54 kDa), SREBP1 (SC-8984; 125 kDa), fatty acid synthase (SC-20140; 270 kDa), hormone-sensitive lipase (SC-25843; 84 kDa), and β-actin (Sigma, A-5441; 40 kDa).
Protein extraction
Protein was extracted in RIPA lysis buffer containing inhibitors. Supernatant protein concentration was determined by BCA solution (PIRCE, Rockford, Illinois).
Western blot
Equal amounts of protein (50 µg) were mixed with sodium dodecyl sulfate sample buffer, boiled for 3 minutes, and separated on a 10% polyacrylamide gel. The separated proteins were transferred electrophoretically to pure nitrocellulose membrane (Bio-Rad, Hercules, California). Nonspecific antibody binding was blocked by incubation for 1 hour at room temperature with 5% nonfat dry milk in TTBS (Tris-buffered saline, 0.1% Tween 20). The membrane was then incubated with the appropriate anti-antibody in 5% milk in TTBS overnight at 4°Cand washed 3 × 10 minutes with TTBS at room temperature. Antirabbit or antimouse IgG secondary antibody labeled with horseradish peroxidase (Bio-Rad; 1:2000) in 5% milk was added onto the membrane and incubated for 1 hour at room temperature. The membrane was washed 3 times. SuperSignal West Pico Chemiluminescent Substrate (PIRCE, Rockford, Illinois) was used to detect the targeted protein. The band density was analyzed by Alpha DigiDoc Gel Documentation & Image Analysis System (Alpha Innotech, San Leandro, California). Data presented are normalized to β-actin and expressed as fold change.
Cell size
Computer-assisted image analysis was used to determine the cross-sectional area of adipocytes. Briefly, the adipose tissue was fixed in 4% phosphate-buffered formalin and then processed for paraffin embedding and sectioning according to standard procedures. Five-micrometer sections were stained with hematoxylin–eosin stain, and the photomicrographs were captured at 20× magnification. Image PRO software (version 5.1) was used to determine the area of adipocytes (4 images per section, and 3 sections per animal).
Statistical Analysis
Differences between control and IUGR male offspring were compared using unpaired t test. Data for mRNA and protein expression were normalized to β-actin, which showed no change between the 2 groups at 1 day and 9 months of age. Values are expressed as means ± standard errors.
RESULTS
There was no difference in litter size between control and food-restricted pregnancies (11.9 ± 0.6 vs 12.3 ± 0.7 pups/litter). As previously reported, at 1 day of age, IUGR newborns had lower body weights (6.0 ± 0.3 g vs 7.1 ± 0.3 g; P < .01) when compared with controls. At 3 weeks of age, IUGR offspring exhibited catch-up growth (50 ± 1 g vs 45 ± 1 g; P< .01), and at 9 months of age, IUGR males were significantly heavier than controls (742 ± 17 g vs 647 ± 18 g; P < .01).23 Furthermore, at 1 day of age, IUGR newborns had lower blood glucose, plasma insulin, and plasma triglyceride levels, though at 9 months of age all were significantly higher when compared with controls.24
Adipogenic Transcription Factors
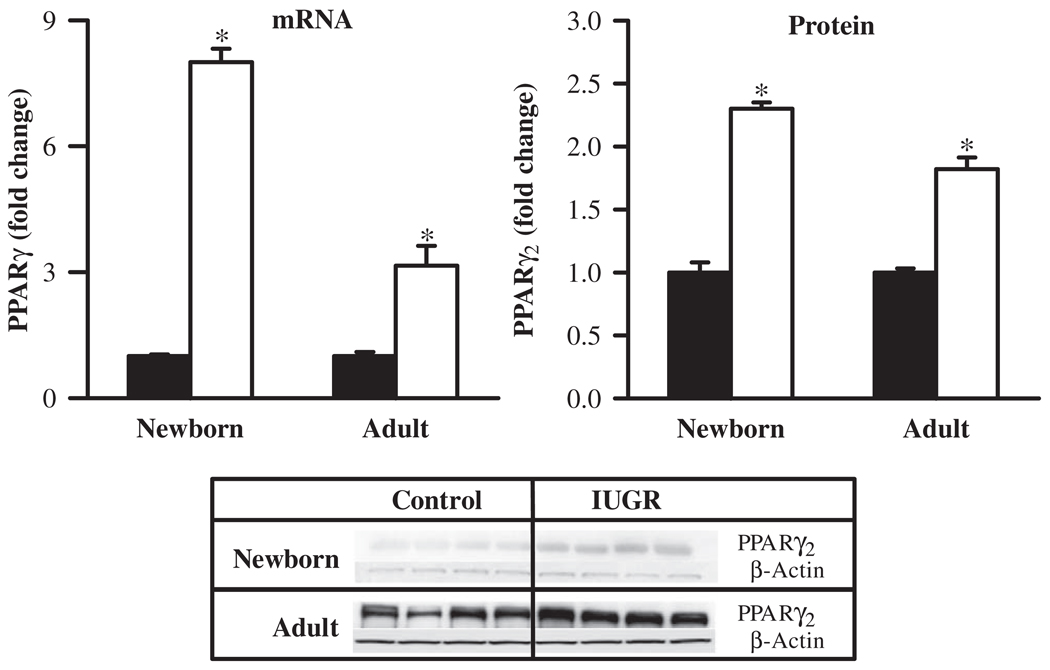
At 1 day of age, IUGR newborns showed significantly increased mRNA (8-fold) and protein (2-fold) expression of principal adipogenic transcription factor, PPARγ, when compared with the control newborns. At 9 months of age, adult IUGR offspring continued to express increased mRNA (3.2-fold) and protein (1.8-fold) levels of PPARγ (Figure 1).
Figure 1.
mRNA and protein levels of PPARγ in newborn and adult male offspring from control (■) and IUGR (□) groups. Data are normalized to β-actin and presented as fold difference. β-Actin was comparable between IUGR and control offspring at both ages. Number of animals studied per group per age was 6 males from 6 litters for mRNA and 4 males from 4 litters for protein. *P < .001 versus control offspring.
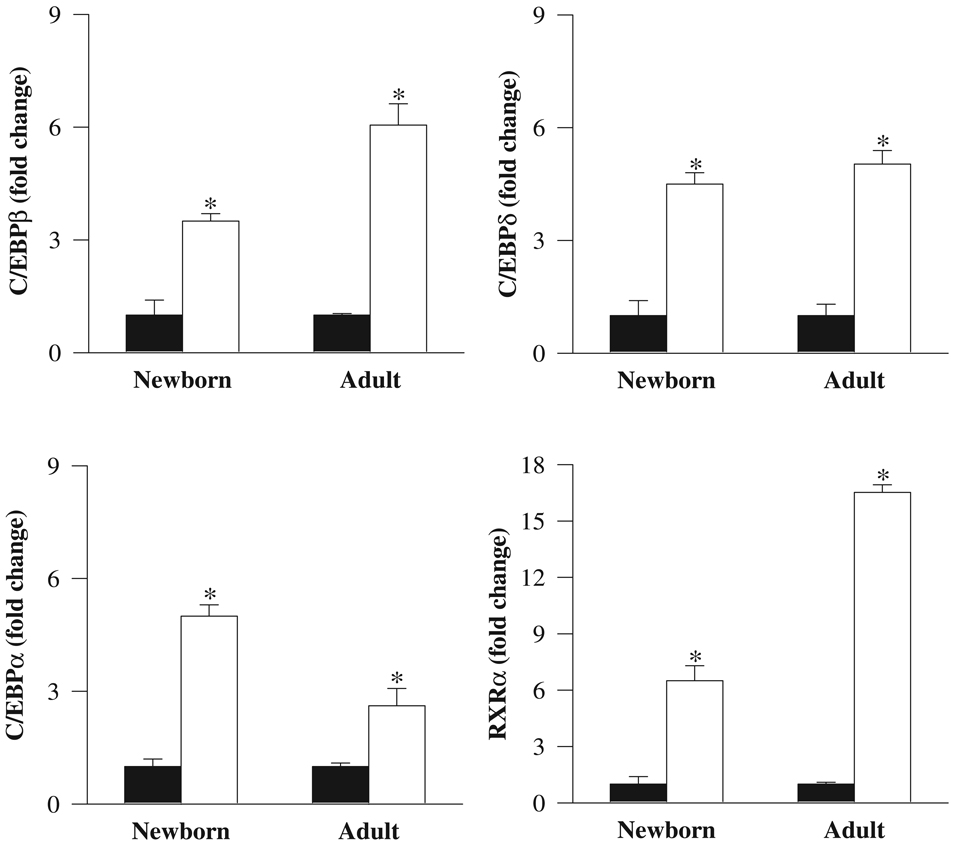
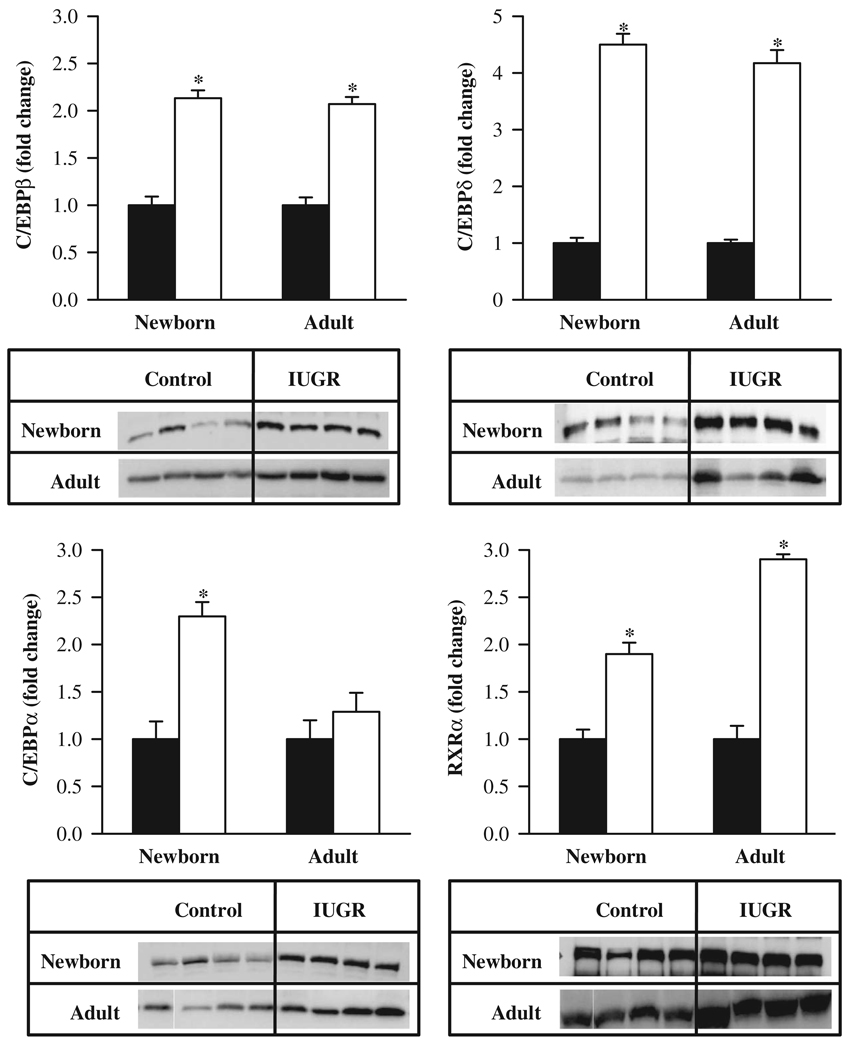
Furthermore, the mRNA expression of upstream factors that activate PPARγ were similarly upregulated (C/EBPβ, 3.5-fold; C/EBPδ, 4.5-fold; C/EBPα, 5-fold) in IUGR newborns. RXRα, with which PPARγ heterodimerizes, also showed increased expression (6.5-fold). At 9 months of age, IUGR offspring showed persistently increased mRNA levels of adipogenic transcription factors. Nevertheless, there were subtle differences between 1-day-old and adult IUGR offspring. For example, all 4 factors had increased mRNA expression in newborn and adult IUGR. However, the expression of mRNA for C/EBPβ and RXRα was further enhanced in adults, whereas mRNA expression of C/EBPδ was unchanged between adult and newborn measurements. mRNA expression of C/EBPα was still higher in IUGR adults, but the increase was less than that displayed in newborns (Figure 2).The protein expression of these adipogenic transcription factors in IUGR newborns mirrored a comparable pattern as that of mRNA, with all factors being upregulated. At 9 months of age, adult IUGR offspring showed persistently increased protein levels of C/EBPβ, C/EBPδ, PPARγ2, and RXRα. However, C/EBPα protein levels were comparable with the control offspring (Figure 3).
Figure 2.
mRNA levels of C/EBPβ, C/EBPδ, C/EBPα, and RXRα in newborn and adult male offspring from control (■) and IUGR (□) groups. Data are normalized to β-actin and presented as fold difference. β-Actin was comparable between IUGR and control offspring at both ages. Number of animals studied per group per age was 6 males from 6 litters. *P < .001 versus control offspring.
Figure 3.
Protein levels of C/EBPβ, C/EBPδ, C/EBPα, and RXRα in newborn and adult male offspring from control (■) and IUGR (□) groups. Data are normalized to β-actin and presented as fold difference. β-Actin was comparable between IUGR and control offspring at both ages. Number of animals studied per group per age was 4 males from 4 litters. *P < .001 versus control offspring.
Adipocyte Lipid Metabolism
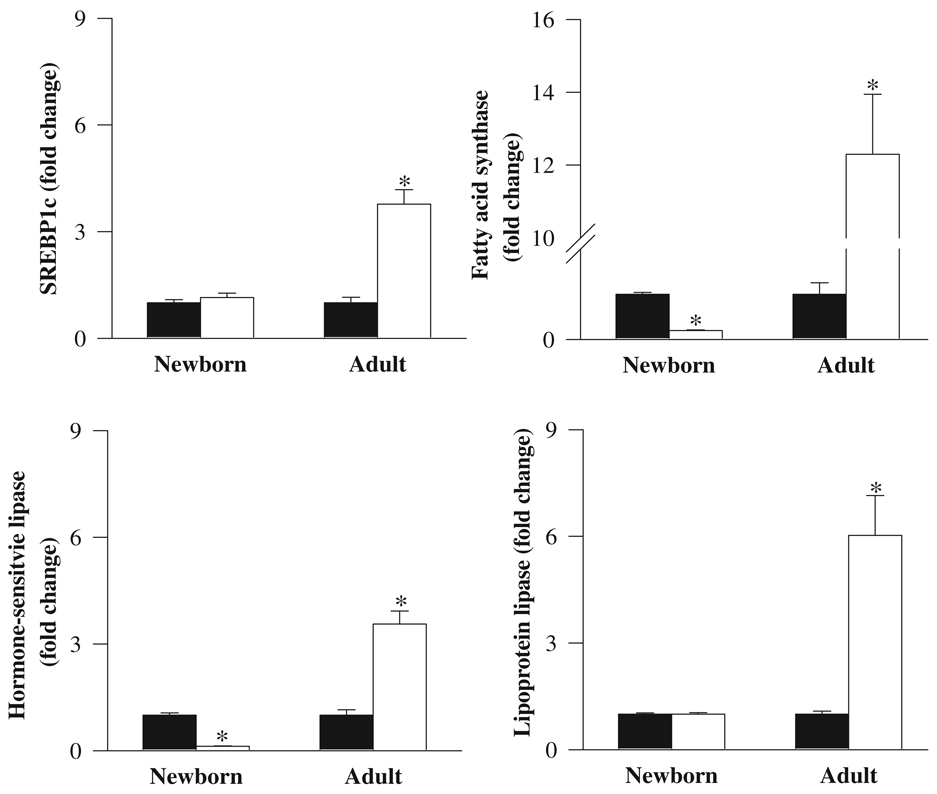
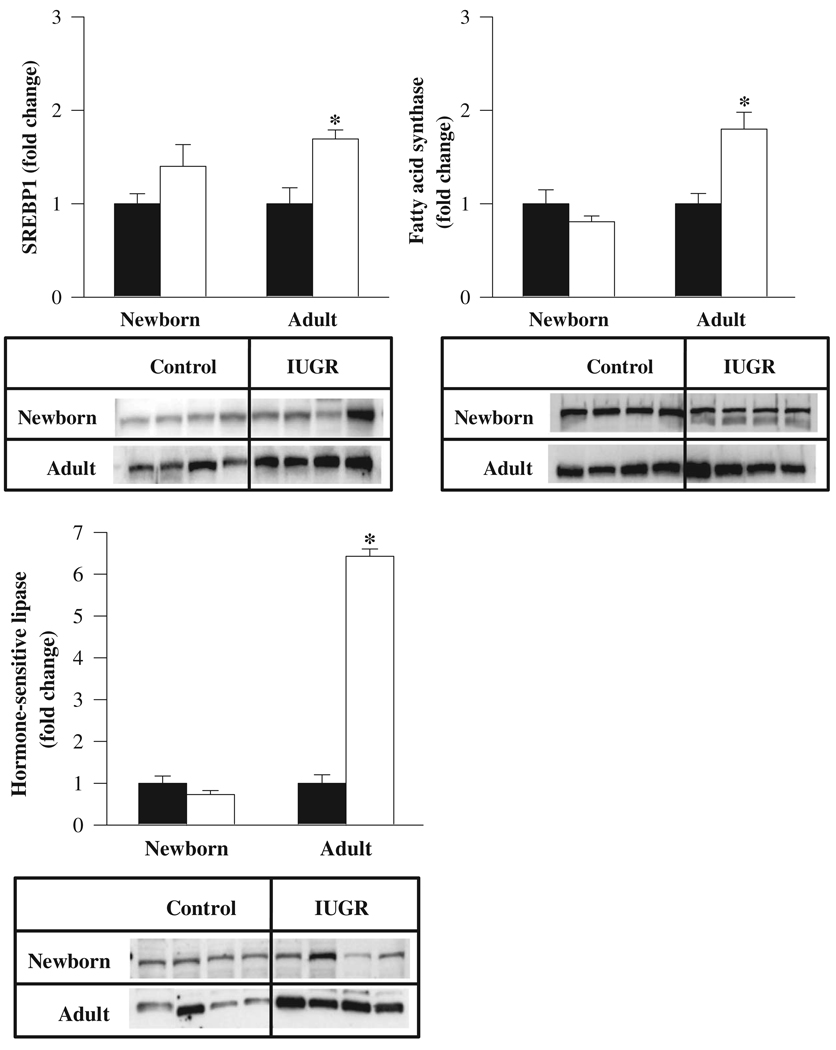
At 1 day of age, IUGR newborns showed no change in the adipose mRNA expression of the lipogenic transcription factor SREBP1c. Furthermore, there were no changes evident in the mRNA levels of the extracellular lipolytic enzyme lipoprotein lipase. However, the expressions of intracellular lipolytic enzyme, hormone-sensitive lipase, and lipogenic enzyme, fatty acid synthase, were significantly reduced in IUGR when compared with control newborns. At 9 months of age, the adult IUGR offspring demonstrated a transition, whereby there was now a significant upregulation of both lipogenic transcription factor (SREBP1c, 4-fold) as well as lipolytic and lipogenic enzymes—lipoprotein lipase (6-fold), hormone-sensitive lipase (3.5-fold), and fatty acid synthase (12-fold; Figure 4).
Figure 4.
mRNA levels of SREBP1c, lipoprotein lipase, fatty acid synthase, and hormone-sensitive lipase in newborn and adult male offspring from control (■) and IUGR (□) groups. Data are normalized to β-actin and presented as fold difference. β-Actin was comparable between IUGR and control offspring at both ages. Number of animals studied per group per age was 6 males from 6 litters. *P < .01 versus control offspring.
In contrast, at 1 day of age, the protein levels of SREBP1, fatty acid synthase, and hormone-sensitive lipase were similar in IUGR and control newborns. However, at 9 months of age, adult IUGR offspring showed increased protein expression of SREBP1, hormone-sensitive lipase, and fatty acid synthase enzymes (Figure 5).We were unable to determine lipoprotein lipase protein expression because of unavailability of suitable antibody.
Figure 5.
Protein levels of SREBP1, fatty acid synthase, and hormone-sensitive lipase in newborn and adult old male offspring from control (■) and IUGR (□) groups. Data are normalized to β-actin and presented as fold difference. β-Actin was comparable between IUGR and control offspring at both ages. Number of animals studied per group per age was 4 males from 4 litters. *P < .01 versus control offspring.
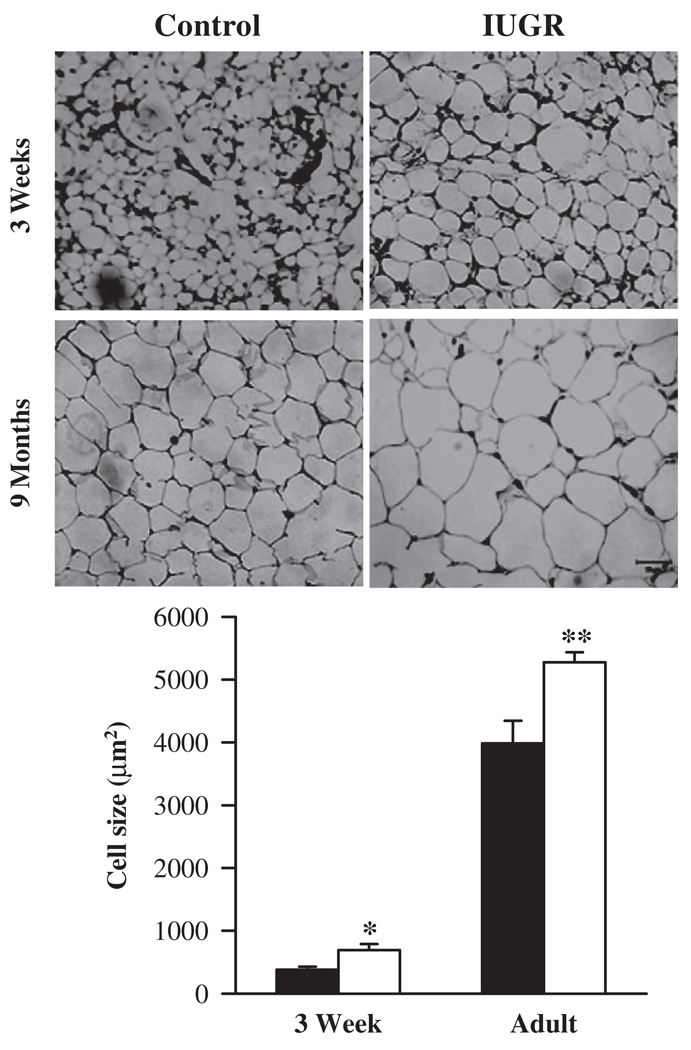
Adipocyte Cell Size
At 3 weeks of age, IUGR offspring exhibited 45% increase in adipocyte cell size when compared with controls. At 9 months of age, IUGR adipocyte cell size continued to remain significantly greater than controls (Figure 6).
Figure 6.
Adipocyte cell size in 3 week and 9 month male offspring from control (■) and IUGR (□) groups. Number of animals studied per group per age was 6 males from 6 litters. Each animal had 3 sections taken and each section had 4 images.*P < .05, **P < .01 versus control offspring.
DISCUSSION
We have previously demonstrated that exposure to maternal food restriction in utero results in IUGR newborns with reduced plasma triglyceride levels. When nursed by ad libitum fed dams, IUGR newborns exhibit rapid catch-up growth and develop relative obesity with increased percentage body fat and plasma triglyceride levels as adults.23,24 In view of the fact that programmed alterations in adipocyte gene expression may contribute to body fat accrual, the present study determined the mRNA and protein expression of transcriptional factors regulating adipocyte differentiation and lipogenesis in 1-day-old and 9-month-old control and IUGR offspring. The findings of this study provide a potential mechanism underlying gestational programming of adipogenesis in IUGR newborns nursed normally.
Adipocyte differentiation is principally regulated by PPARγ2 and hence, potentially, an important target of adipocyte programming. The present study has demonstrated for the first time that IUGR offspring at 1 day of age exhibit significant upregulation in gene transcription of the key adipogenic factors PPARγ2 and its upstream regulatory transcriptional factors (C/EBPβ, C/EBPδ, and C/EBPα). Moreover, activated PPARγ2, which heterodimerizes with RXRα, is also upregulated. The increased mRNA expression of these transcription factors in IUGR adipose tissue was accompanied by correspondingly increased protein levels. Most significant of these observations is the persistent increase in expression of the adipogenic transcription factors in the adult IUGR offspring with the exception of C/EBPα. Despite increased mRNA expression of C/EBPα in adult IUGR offspring, the protein expression remained unchanged, suggesting posttranslational regulation, perhaps in phosphorylation.
The lipogenic transcription factor, SREBP1c, a potential downstream target of PPARγ2, showed no change in mRNA and protein expression in 1-day-old IUGR offspring. SREBP1c expression during adipocyte differentiation induces gene expression of both lipolytic and lipogenic enzymes, contributing to increased uptake and fatty acid synthesis and promoting lipid accumulation within the adipocyte. In IUGR newborns, lack of change in extracellular lipolytic enzyme, lipoprotein lipase, and a reduction in intracellular lipolytic enzyme, hormone-sensitive lipase, suggests a propensity to fat accumulation in low-birth-weight offspring. The decreased expression of fatty acid synthase may reflect lack of fatty acid supply in IUGR newborns, and indeed, we have previously demonstrated that 1-day-old IUGR offspring have decreased plasma triglyceride levels.24 The reduced fatty acid synthase and lack of change in SREBP1c, in the presence of elevated PPARγ2, suggest a programmed downregulation of PPARγ2 induction of transcription following maternal food restriction. The change in mRNA and protein expression of lipid enzymes showed discordance, suggesting a posttranscriptional modification, which could include effects on mRNA stability, transcription efficiency, or other mechanisms. In IUGR adults, there was a consistent increase in mRNA and protein expression of SREBP1c, fatty acid synthase, and hormone-sensitive lipase.
Although IUGR offspring demonstrate salient changes in adipose tissue at the molecular level, which are commonly associated with obesity as well as starvation, they display profound changes unique to gestational programmed obesity. Human obesity as well as diet-induced obesity are characterized by hypertrophic adipocytes with increased expression of PPARγ2, C/EBPα, lipoprotein lipase, and fatty acid synthase31–33 and decreased expression of hormone-sensitive lipase.34,35 Conversely, studies on SREBP1c provide conflicting results, with some studies indicating increased expression and others showing decreased expression.36 These alterations differ greatly from food restriction/fasting, which is generally characterized by marked reduction in adipocyte size, decreased PPARγ2, fatty acid synthase, and lipoprotein lipase and increased hormone sensitive lipase and SREBP1c.37–39 Surprisingly, the change in transcription factors of IUGR newborns is consistent with obesity rather than starvation. Akin to obesity, the expression of PPARγ2 and C/EBPα is upregulated in IUGR newborn adipose tissue. This suggests that in IUGR newborns the consequence of activating the transcription factors, which coordinate adipocyte differentiation, may serve initially as an adaptation for storage that enables survival through periods of food shortages. This would be consistent with hypertrophic adipocytes seen in 3-week and adult IUGR males. In contrast to adipocyte differentiation, the changes in lipid regulation of IUGR newborns share some commonality with obesity as well as starvation. For example, the decreased expression of hormone-sensitive lipase in IUGR newborns is consistent with obesity, whereas decreased fatty acid synthase expression is analogous to starvation. This again emphasizes the propensity to fat accumulation within the IUGR adipocyte despite growth restriction.
The adult IUGR adipose phenotype resembles that of obesity, with the exception of hormone-sensitive lipase, which is now upregulated in adult IUGR offspring and also evident under conditions of starvation. The increased expression of hormone-sensitive lipase suggests increased release of fatty acids from IUGR adipocyte, likely contributing to elevated plasma levels. We have previously demonstrated a transition from hypotriglyceridemia in 1-day-old pups to hypertriglyceridemia in adult IUGR.24
In addition to its adipogenic role, PPARγ2 is also linked to insulin sensitivity.32,40We have previously shown that IUGR offspring show a shift from increased insulin sensitivity (lower glucose and insulin levels) in growth-restricted newborns to increased adiposity and insulin resistance (higher glucose and insulin levels) as adults.23,24 Notably, the paradoxical early upregulation of PPARγ2 in growth-restricted IUGR newborns corresponds with the glucose homeostasis of these offspring. It is likely that upregulation of PPARγ2 in IUGR may facilitate increased glucose and fatty acid uptake by adipocytes, thus contributing to increased insulin sensitivity. Although the persistent upregulation of PPARγ2 is consistent with obesity, as seen in the IUGR adults, it is in contrast to the development of insulin resistance in these offspring. This apparent dichotomy probably could be attributed to increased adiposity and the resultant reduced adipocyte responses or increased production of insulin antagonist.41 For example, the upregulated hormone-sensitive lipase seen in the IUGR adults may occur because of ineffective inhibition by insulin and thus contributes to increased lipolysis and release of free fatty acids from the adipose tissue.34,42 Furthermore, larger adipocytes are known to have altered and increasingly dysregulated cellular homeostasis and secretory profile when compared with adipocytes of smaller size.7,9,32 This phenomenon would be consistent with the elevated plasma triglycerides, insulin resistance, and hypertrophic adipocytes seen in the IUGR adults.
The findings of this study are consistent with the concept proposed by Barker et al20 that IUGR offspring develop a “thrifty phenotype,” which predisposes them to increased weight gain when allowed access to ad libitum diet postnatally. In particular, the development of adult obesity and hyperinsulinemia seen in IUGR offspring subjected to 50% maternal food restriction parallels the findings reported by Vickers et al43 in their rat undernutrition (30% of ad libitum) model. The potential for these adult onset metabolic abnormalities has been demonstrated in humans and a variety of animal models.20–23 Majority of programming studies have manipulated maternal nutrient availability to produce IUGR newborns. Maternal nutrition and parity are known to influence birth weight in a number of species,44,45 though the effects of both maternal parity and nutrient restriction on the offspring obesity are unknown. Nonetheless, studies in sheep demonstrate that 50% maternal food restriction in the final month of gestation causes changes in the neonatal liver, kidney, and lung that are unrelated to the effects of maternal parity.46
In summary, our studies indicate that low-birth-weight offspring have altered adipose development. Paradoxically, these low-birth-weight 1-day-old offspring already exhibit changes consistent with obesity rather than starvation. In particular, the increased expression of adipogenic transcription factors, mainly PPARγ2, with reduced intracellular lipolytic enzyme serves to increase adipocyte differentiation and lipid accretion within the adipocytes. Furthermore, when such obesity-prone offspring are provided appropriate postnatal substrate, enhanced lipid storage occurs, promoting hypertrophic adipocytes and, ultimately, programmed obesity in IUGR adult offspring. We speculate that enhanced storage of triglycerides occurring in thrifty phenotype offspring provides a competitive advantage in preparation for a nutrient-deprived environment.
ACKNOWLEDGMENTS
The authors acknowledge Linda Day, Stacy Behare, and Glenda Calvario for technical assistance.The authors are grateful to Professor Michael G. Ross for advice and assistance in manuscript preparation.
This work was supported by the National Institutes of Health K01 DK 063994 and the March of Dimes.
REFERENCES
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–2102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Plagemann A, Harder T. The changing face and implications of childhood obesity. N Engl J Med. 2004;350:2414–2416. [PubMed] [Google Scholar]
- 3.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 4.Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. doi: 10.1016/s0092-8674(00)81359-8. [DOI] [PubMed] [Google Scholar]
- 5.Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- 6.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 7.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 8.Morrison RF, Farmer SR. Insights into the transcriptional control of adipocyte differentiation. J Cell Biochem. 1999:32–33. 59–67. doi: 10.1002/(sici)1097-4644(1999)75:32+<59::aid-jcb8>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 10.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 12.Lane MD, Lin FT, MacDougald OA, Vasseur-Cognet M. Control of adipocyte differentiation by CCAAT/enhancer binding protein alpha (C/EBP alpha) Int J Obes Relat Metab Disord. 1996;20 suppl 3:S91–S96. [PubMed] [Google Scholar]
- 13.Rosen ED, Hsu CH, Wang X, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fajas L, Schoonjans K, Gelman L, et al. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999;19:5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 16.Maggio CA, Greenwood MR. Adipose tissue lipoprotein lipase (LPL) and triglyceride uptake in zucker rats. Physiol Behav. 1982;29:1147–1152. doi: 10.1016/0031-9384(82)90312-2. [DOI] [PubMed] [Google Scholar]
- 17.Berndt J, Kovacs P, Ruschke K, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50:1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 18.Mobbs CV, Makimura H. Block the FAS, lose the fat. Nat Med. 2002;8:335–336. doi: 10.1038/nm0402-335. [DOI] [PubMed] [Google Scholar]
- 19.Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 20.Barker M, Robinson S, Osmond C, Barker DJ. Birth weight and body fat distribution in adolescent girls. Arch Dis Child. 1997;77:381–383. doi: 10.1136/adc.77.5.381. [DOI] [PubMed] [Google Scholar]
- 21.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 22.Levin BE, Govek E. Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol. 1998;275:R1374–R1379. doi: 10.1152/ajpregu.1998.275.4.R1374. [DOI] [PubMed] [Google Scholar]
- 23.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 24.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196(555):e1–e7. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai M, Gayle D, Gaung H, Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J Biol Chem. 1993;268:6823–2686. [PubMed] [Google Scholar]
- 27.De Rijk E. Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol. 2002;30:271–282. doi: 10.1080/019262302753559614. [DOI] [PubMed] [Google Scholar]
- 28.Park CS. Role of compensatory mammary growth in epigenetic control of gene expression. FASEB J. 2005;19:1586–1591. doi: 10.1096/fj.05-3816hyp. [DOI] [PubMed] [Google Scholar]
- 29.McGuire MK, Littleton AW, Schulze KJ, Rasmussen KM. Pre-and postweaning food restrictions interact to determine reproductive success and milk volume in rats. J Nutr. 1995;125:2400–2406. doi: 10.1093/jn/125.9.2400. [DOI] [PubMed] [Google Scholar]
- 30.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med. 2004;229:127–135. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 31.Hotta K, Gustafson TA, Yoshioka S, Ortmeyer HK, Bodkin NL, Hansen BC. Relationships of PPARgamma and PPARgamma2 mRNA levels to obesity, diabetes and hyperinsulinaemia in rhesus monkeys. Int J Obes Relat Metab Disord. 1998;22:1000–1010. doi: 10.1038/sj.ijo.0800718. [DOI] [PubMed] [Google Scholar]
- 32.Kubota N, Terauchi Y, Miki H, et al. PPAR gamma mediates high fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 33.Llado I, Pons A, Palou A. Effects of fasting on lipoprotein lipase activity in different depots of white and brown adipose tissues in diet-induced overweight rats. J Nutr Biochem. 1999;10:609–614. doi: 10.1016/s0955-2863(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 34.Arner P, Bolinder J, Engfeldt P, Ostman J. The antilipolytic effect of insulin in human adipose tissue in obesity, diabetes mellitus, hyperinsulinemia, and starvation. Metabolism. 1981;30:753–760. doi: 10.1016/0026-0495(81)90020-2. [DOI] [PubMed] [Google Scholar]
- 35.Large V, Reynisdottir S, Langin D, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40:2059–2066. [PubMed] [Google Scholar]
- 36.Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferre P, Dugail I. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem. 1998;273:29164–29171. doi: 10.1074/jbc.273.44.29164. [DOI] [PubMed] [Google Scholar]
- 37.Bergo M, Wu G, Ruge T, Olivecrona T. Down-regulation of adipose tissue lipoprotein lipase during fasting requires that a gene, separate from the lipase gene, is switched on. J Biol Chem. 2002;277:11927–11932. doi: 10.1074/jbc.M200325200. [DOI] [PubMed] [Google Scholar]
- 38.Samra JS, Clark ML, Humphreys SM, Macdonald IA, Frayn KN. Regulation of lipid metabolism in adipose tissue during early starvation. Am J Physiol. 1996;271:E541–E546. doi: 10.1152/ajpendo.1996.271.3.E541. [DOI] [PubMed] [Google Scholar]
- 39.Sztalryd C, Kraemer FB. Regulation of hormone-sensitive lipase during fasting. Am J Physiol. 1994;266:E179–E185. doi: 10.1152/ajpendo.1994.266.2.E179. [DOI] [PubMed] [Google Scholar]
- 40.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Kopecky J, Flachs P, Bardova K, Brauner P, Prazak T, Sponarova J. Modulation of lipid metabolism by energy status of adipocytes: implications for insulin sensitivity. Ann NY Acad Sci. 2002;967:88–101. doi: 10.1111/j.1749-6632.2002.tb04267.x. [DOI] [PubMed] [Google Scholar]
- 42.Jocken JW, Langin D, Smit E, et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 2007;92:2292–2299. doi: 10.1210/jc.2006-1318. [DOI] [PubMed] [Google Scholar]
- 43.Vickers MH, Gluckman PD, Coveny AH, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 44.Xue F, Willett WC, Rosner BA, Forman MR, Michels KB. Parental characteristics as predictors of birthweight. Hum Reprod. 2008;23:168–177. doi: 10.1093/humrep/dem316. [DOI] [PubMed] [Google Scholar]
- 45.Gardner DS, Buttery PJ, Daniel Z, Symonds ME. Factors affecting birth weight in sheep: maternal environment. Reproduction. 2007;133:297–307. doi: 10.1530/REP-06-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yakubu DP, Mostyn A, Wilson V, et al. Different effects of maternal parity, cold exposure and nutrient restriction in late pregnancy on the abundance of mitochondrial proteins in the kidney, liver and lung of postnatal sheep. Reproduction. 2007;133:1241–1252. doi: 10.1530/REP-06-0211. [DOI] [PubMed] [Google Scholar]