Abstract
Background
Cardiac hypertrophy is an adaptive response to increased workload that, if unrelieved, leads to heart failure. It has been reported that cardiomyocyte apoptosis contributes to failure, and that vascular endothelial growth factor (VEGF) treatment of hypertrophied myocardium increases capillary density and improves myocardial perfusion. In this study we hypothesized that VEGF treatment reduces cardiomyocyte apoptosis and thereby preserves myocardial contractile function.
Methods and Results
Newborn rabbits underwent aortic banding. At 4 and 6 weeks of age, hypertrophied animals were treated with intrapericardial administration of recombinant VEGF protein. Three groups of animals were investigated: age-matched controls (C), untreated hypertrophied (H), and VEGF-treated hypertrophied hearts (T). Cardiomyocyte apoptosis was determined by TUNEL staining and PARP cleavage (immunoblotting of nuclear extracts) and cardiac function by transthoracic echocardiography. Death attributable to severe heart failure occurred in 14 of 43 untreated and 2 of 29 VEGF-treated animals (P<0.01). TUNEL-positive cardiomyocyte nuclei (n/1000 nuclei) were significantly increased in untreated hearts at 5 weeks (H: 10±1.8 versus T: 3±0.7) and at 7 weeks (H: 13±3.6 versus T: 5±1.5; P<0.05). Increased apoptosis in untreated hypertrophy was also confirmed by the presence of PARP cleavage (H: 74±7 versus T: 41±4 arbitrary densitometry units; P<0.05). VEGF treatment preserved left ventricular mass, prevented dilation (T: 1.01±0.06 versus H: 0.77±0.07; P<0.05), and preserved contractility indices compared with untreated hearts.
Conclusions
Lack of adaptive capillary growth impairs myocardial perfusion and substrate delivery in hypertrophying myocardium. VEGF treatment reduces myocardial apoptosis and prolongs survival in a model of severe progressive left ventricular hypertrophy. Promoting capillary growth with VEGF reduces apoptosis, preserves myocardial contractile function, and delays the onset of failure in pressure-loaded infant myocardium.
Keywords: angiogenesis, apoptosis, hypertrophy
In response to persistent elevations in wall stress attributable to sustained increase in workload on the ventricle, the heart attempts to compensate by remodeling. The process of remodeling relates to the progressive changes that occur in myocardial structure and function. During this dynamic process the ventricle is initially compensated with adequate contractile function, normal geometric shape, and supranormal ventricular mass-to-volume (M/V) ratio, and followed by decompensation with impaired contractile function, change in geometric shape, and disproportionate wall thickness relative to chamber volume. These alterations involve various components of the myocardium, particularly cardiomyocytes. Cardiomyocytes are considered terminally differentiated cells unable to proliferate. In response to mechanical stress, cardiomyocytes are enlarging through addition of sarcomeres and progressively increase in size as hypertrophy develops. Contractile dysfunction ensuing as hypertrophy progresses to failure may result from a shift in myocyte contractility in part because of re-expression of fetal isoforms involved in contraction and/or alterations in calcium homeostasis.1,2 Structural and functional alteration in the coronary circulation of the hypertrophied heart such as decrease in myocardial capillary density have also been demonstrated.3,4 As cardiomyocytes enlarge with hypertrophy, a mismatch develops between the number of capillaries and cardiomyocytes per unit area, resulting in an increase in diffusion distance. The area of myocardial tissue supplied by one capillary increases and oxygen as well as nutritional supply are impaired.5,6 During normal cardiac growth between childhood and young adulthood (physiological hypertrophy) coronary microvascular growth parallels the degree of cardiac myocyte growth.7 In pathologic hypertrophy this tight relationship appears to be lost. However, the ability of endothelial and nonmyocyte cells to respond to exogenous angiogenesis inducing growth factors such as vascular endothelial growth factor (VEGF) is preserved as we have previously shown.8 In late stages of heart failure, at the cellular level, the change in ventricular structure includes loss of functional cardiomyocytes via apoptosis. Cardiomyocyte apoptosis has been established in animal models and humans in end-stage heart failure but has not been explored during the compensated phase of hypertrophy. 9–11 Based on these observations we hypothesized that in hypertrophying myocardium, loss of cardiomyocytes to apoptotic cell death occurs at early stages of hypertrophy, concomitant with increased diffusion distance. Improving perfusion to hypertrophying cardiomyocytes by proangiogenic treatment with VEGF prevents cardiomyocyte apoptosis and delays onset of failure.
Materials and Methods
Left Ventricular Hypertrophy Model
Pressure-overload hypertrophy was achieved by banding the descending aorta in 10-day-old New Zealand White rabbits (Millbrook Farms; Amherst, Mass). This model has been previously described by our group in more detail.6,8,12. One set of animals (n=6 to 8/group) was euthanized at 1-week intervals, starting at 3 weeks of age (early hypertrophy) until 8 weeks of age (severe heart failure). While still in the compensated phase of hypertrophy (4 weeks of age in this model), the animals were treated with an intrapericardial administration of either 2 µg/kg VEGF165 (R&D Systems Inc, Minneapolis, Minn) dissolved in phosphate-buffered saline (PBS)/0.1% rabbit serum or vehicle only via subxiphoid exposure of the heart. Two weeks later the animals were treated again with VEGF. In 1-week intervals starting at 5 weeks of age (1 week after the first treatment) until 8 weeks of age, animals were euthanized (n =6 to 8/group). All animals were euthanized by an intravenous injection of ketamine (100 mg/kg) and xylazine (5 mg) with heparin (500 IU) added. Blood was flushed out of the hearts and tissue was frozen in liquid nitrogen and stored at −80°C for further analysis.
In Vivo Myocardial Function Measurements by Transthoracic Echocardiography
Transthoracic echocardiography was performed using an Acuson 128 or Hewlett-Packard Sonos 1500 Cardiac Imager equipped with a 7 to 7.5 MHz transducer. Measurements of LV dimensions, wall thickness, and cavity volume were taken with transthoracic echocardiography as we have previously described.6,8,12 Measurements of left ventricular M/V ratio were used as an index for progression of hypertrophy. As a measure of cardiac performance, shortening fraction was determined with the formula (systolic diameter–diastolic diameter)/diastolic diameter and is expressed as percentage.
Determination of Apoptosis by TUNEL
Apoptotic nuclei were quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining. Left ventricular muscle paraffin sections from the midsection of the heart were deparaffinized, and then rehydrated with xylene and graded alcohol series. The sections were stained using the FragEL DNA fragmentation detection kit (EMD; Biosciences Inc, San Diego, Calif) per the manufacturer’s instructions. Briefly, the sections were incubated with terminal deoxynucleotidyl transferase and fluorescein-labeled dUTP. To identify the cardiomyocytes, sections were incubated with mouse desmin monoclonal antibody (Sigma-Aldrich, St. Louis, Mo), followed by incubation with a secondary anti-mouse immunoreagent conjugated to the red-fluorescent Alexa-594™ fluorophore at a concentration of 1:200 (Molecular Probes, Eugene Ore). Finally, to identify all nuclei (nonapoptotic and apoptotic), sections were stained with blue fluorescent DAPI nucleic acid stain (Molecular Probes). Cover-slips were applied to the sections with fluorescent mounting medium (Dako Corporation, Carpinteria, Calif). Slides were visualized using an Axiovert 35 Microscope with a Nikon 10× objective, NA =10×/0.25. Ten sections randomly picked from each heart were analyzed by a blinded microscopist. Cardiomyocyte nuclei were determined by automatic counting with the computer software/image analyzer using the MetaMorph® Imaging System software (Universal Imaging Corporation, West Chester, Pa). Apoptotic nuclei were identified manually to determine that only apoptotic cardiomyocyte nuclei were included. Data are expressed as apoptotic nuclei per 1000 nuclei.
Determination of Apoptosis by PARP Cleavage
Nuclear poly(ADP-ribose) polymerase (PARP) cleavage was measured by immunoblotting. Nuclear protein extracts were obtained from left ventricular myocardial tissue using a commercially available isolation kit (Active Motif, Carlsbad, Calif). Nuclear extracts were stored at −80°C until used for immunoblotting. The protein extracts were separated by gel electrophoresis with 10% SDS-PAGE gels. Proteins were electrophoretically transferred to nitrocellulose membranes, incubated in 5% nonfat dry milk in Tris-buffered saline with Triton X-100 for 30 minutes at room temperature to block nonspecific binding, and then incubated with primary antibody against PARP (BD Pharmingen, San Jose, Calif) at a dilution of 1:1000 overnight; this was followed by incubation with horseradish peroxidase-conjugated secondary antibody (Amersham Life Science, Arlington Heights, Ill) at a dilution of 1:1000. The bound antibody was detected by the enhanced chemiluminescence method according to the manufacturer’s instruction (Amersham Life Science). After exposure on films, quantitative protein analysis was conducted using laser densitometry.
Statistical Analysis
Data were analyzed using SPSS software package (version 11.0; SPSS Inc, Chicago, Ill) and are reported as mean±standard error of the mean. One-way ANOVA was used for comparison among and between groups, or Kruskal-Wallis test if normality was not passed, followed by Bonferroni or Dunn post-hoc analysis when appropriate. Fisher exact test was used for comparison of two proportions. A value of P≤0.05 was considered statistically significant.
Animal Care
All animals received humane care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 86-23, revised 1996). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston.
Statement of Responsibility
The authors had full access to the data and take full responsibility for their integrity. All authors have read and agree to the manuscript as written.
Results
Mortality
During a mean observation period of 38.4±1.3 days and median of 37.5 days after banding, 14 of 43 untreated hypertrophied animals died from heart failure or had to be euthanized because of incapacitating clinical symptoms for severe heart failure. In comparison, 2 of 29 animals had died in the group of hypertrophied hearts treated with VEGF (P<0.01).
Myocyte Apoptosis Measured by TUNEL Assay
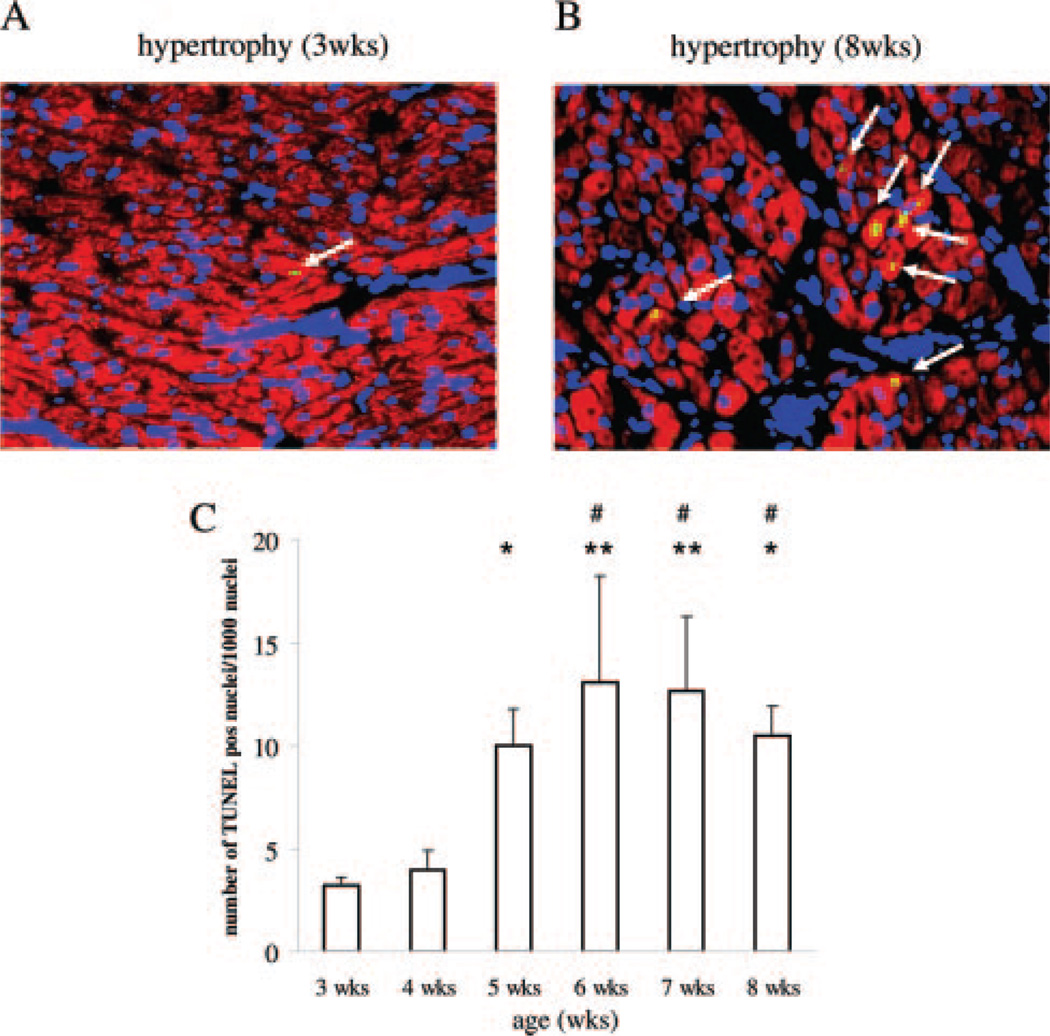
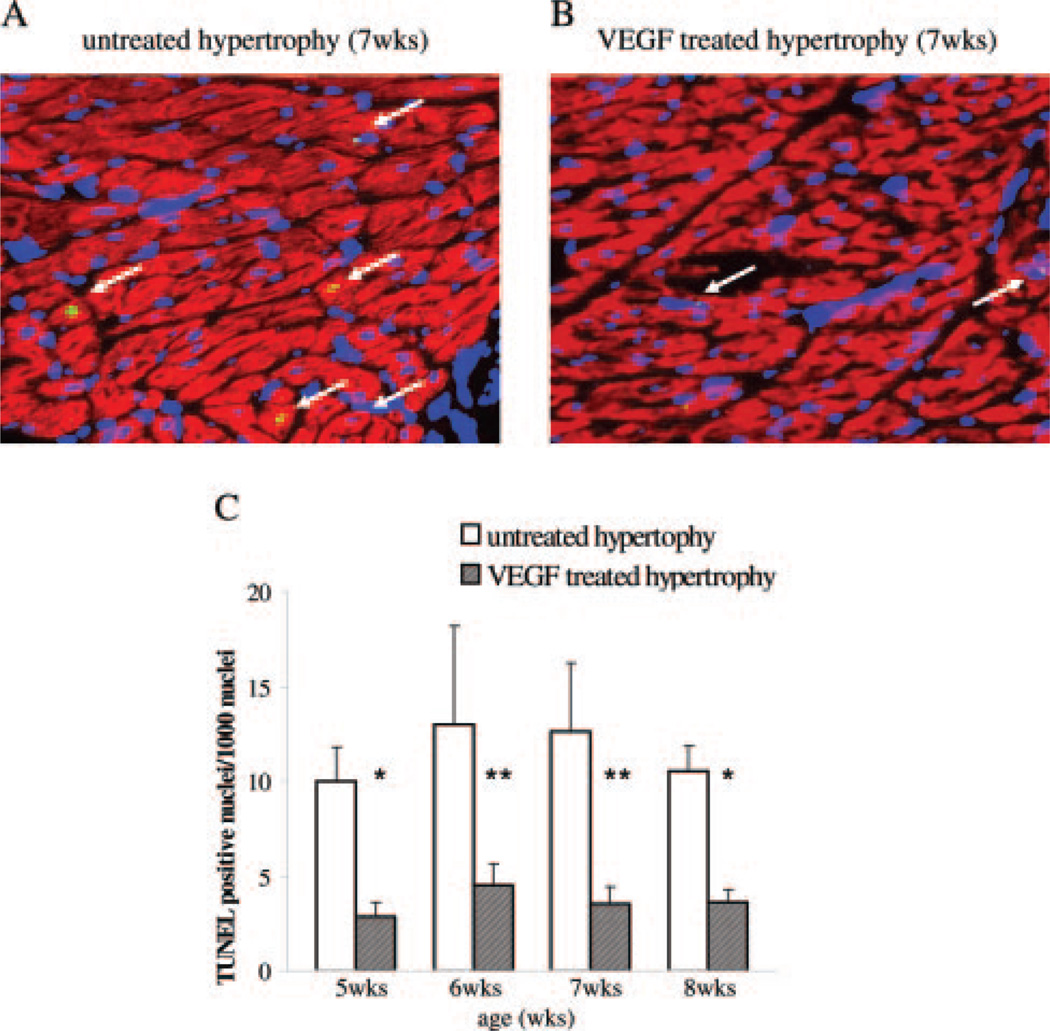
Aortic banding resulted in acute pressure overload that rapidly led to a dramatic increase of left ventricular mass. TUNEL staining experiments demonstrated a basal level of apoptosis in control animals (1±0.3 TUNEL positive nuclei per 1000 nuclei) with a strong increase after aortic banding starting at 3 weeks of age (3±0.4 TUNEL positive/1000 nuclei) when the first signs of compensatory increase in ventricular wall thickness can be detected echocardiographically. Concomitant with the increase in diffusion distance and impaired glucose uptake at 5 weeks of age,6 a significant increase in cardiomyocyte apoptosis can be detected (10±1.7 TUNEL positive/1000 nuclei) with continuous cell loss as hypertrophy progresses to failure (Figure 1). In hypertrophied hearts treated with VEGF, apoptotic cell loss (3±0.7 TUNEL positive/1000 nuclei) could be prevented (Figure 2).
Figure 1.
TUNEL staining of untreated hypertrophy. A and B, Representative immunohistochemical sections are shown. Cardiomyocytes are stained with desmin in red, nuclei with DAPI in blue, and apoptotic nuclei in green fluorescence. Apoptotic cardiomyocyte nuclei are emphasized by white arrows. Hearts at 3 weeks of age (early, developing hypertrophy) and 8 weeks of age (heart failure) are depicted. C, The summarized results are expressed as TUNEL positive cardiomyocyte nuclei per 1000 nuclei indicating continuous apoptotic cell death starting at 5 weeks of age. *P<0.05 and ** P<0.01 vs 3 weeks hypertrophy; #P<0.05 vs 4 weeks hypertrophy.
Figure 2.
TUNEL staining of untreated and VEGF-treated hypertrophy. A and B, Representative immunohistochemical sections showing TUNEL staining are shown. Apoptotic nuclei (arrow) are shown by green fluorescence and the localization of nuclei is documented by DAPI counterstaining and labeling of cardiomyocytes with red fluorescent desmin. Hearts at 7 weeks of age from untreated hypertrophied animals and 1 week after the second VEGF administration are depicted. All sections are shown at the same magnification. C, The summarized results are expressed as TUNEL positive cardiomyocyte nuclei per 1000 nuclei. VEGF treatment prevented apoptotic cell death in progressive hypertrophy. *P<0.05 and **P<0.01 vs untreated hypertrophy.
Myocyte Apoptosis Determined by Detection of Cleaved PARP
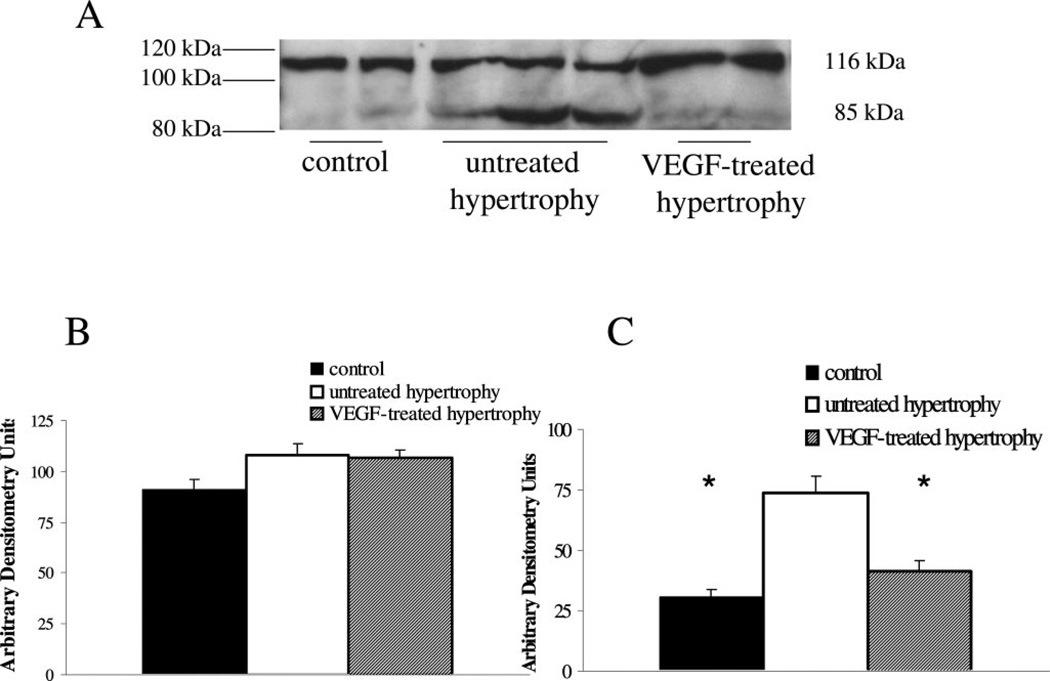
The validity of the technique detecting apoptotic cell death was confirmed by detection of cleaved nuclear PARP in the same samples as used for TUNEL staining. A representative immunoblot with total and cleaved PARP is show in Figure 3A and summary of densitometry data are provided in Figure 3B and 3C.
Figure 3.
Apoptotic cell death by PARP cleavage. A, To confirm apoptotic cell death by TUNEL, PARP cleavage was determined by immunoblot analysis with an antibody identifying total and cleaved PARP. A representative immunoblot is shown for nuclear extracts obtained from left ventricular muscle extracts from controls, untreated hypertrophied hearts and VEGF-treated hypertrophied hearts. A distinct immunoreactive band could be localized at 116 kDa, indicative of total PARP, and a second one at 85 kDa, which represents the cleaved form. Quantification of protein content of total (B) and cleaved (C) PARP was performed by laser densitometry and values are expressed as arbitrary densitometry units. There is no difference of total PARP protein content between controls (black bar), untreated hypertrophied hearts (white bar) and VEGF-treated hypertrophied hearts (shaded bar), but cleaved PARP levels were significantly higher in untreated hypertrophied hearts indicative of apoptosis (*P<0.05; vs control and VEGF-treated hypertrophy).
Cardiac Function
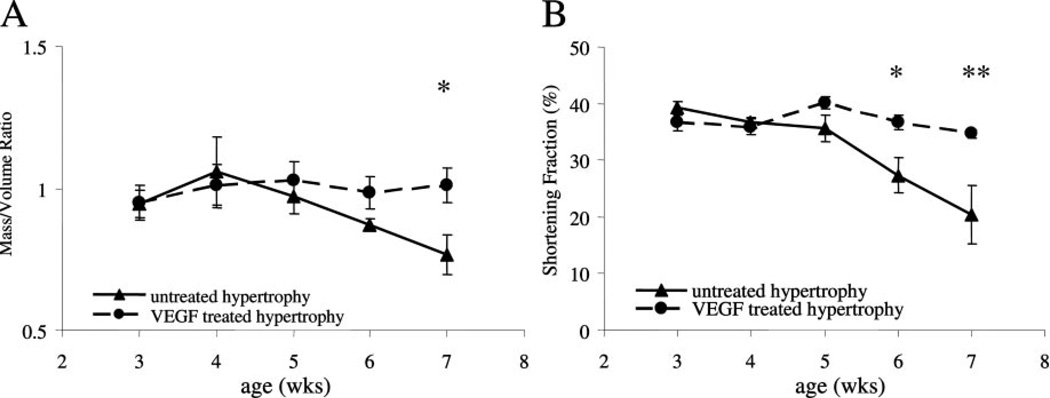
Cardiac hypertrophy is a compensatory response to sustained elevations in left ventricular wall stress. In this model left ventricular M/V ratio increased, reaching a plateau by 4 weeks of age. Because the pressure load remains unrelieved, the increase in muscle mass could no longer compensate to reduce peak systolic stress and the left ventricle began to dilate, as indicated by a decline in M/V ratio after week 4 (Figure 4A). In comparison, serial treatment with VEGF at maximal hypertrophy maintained hypertrophic growth and delayed the onset of ventricular dilation (7 weeks: 1.01±0.06) beyond the time point when untreated hypertrophied hearts (7 weeks: 0.77±0.07; P<0.05) had already shown signs of severe dilatation, failure, and death. Concomitant with severe ventricular dilatation, shortening fraction declined as hypertrophy progresses to failure (Figure 4B), whereas in the serial VEGF-treated group contractility remained within normal range and was significantly higher than the untreated group at 6 weeks of age (P<0.01) and 7 weeks of age (P<0.001).
Figure 4.
Left ventricular M/Vratio and shortening fraction. A, Left ventricular mass to left ventricular cavity volume measurement is an indicator of hypertrophic growth. VEGF-treated hearts maintained a higher ratio of left ventricular mass to cavity volume over an extended period compared with untreated hypertrophied hearts, which showed signs of severe ventricular dilatation (=decline in M/V ratio) (*P<0.05; vs untreated hypertrophied hearts). B, Shortening fraction was calculated based on echocardiographic measurements of untreated hypertrophied hearts and VEGF-treated hypertrophied hearts. VEGF treatment prevented myocardial dysfunction seen in the untreated hypertrophied hearts (*P<0.01 and **P<0.001; vs untreated hypertrophied hearts).
Discussion
In this study we show that apoptotic cardiomyocyte loss starts at early stages of pressure-overload hypertrophy in this infant aortic banding model. The timing of increase in myocyte apoptosis coincides with the time point when diffusion distance has increased and nutrient supply and uptake are impaired. Maladaptive remodeling with ventricular dilation of the hypertrophied left ventricle likely develops in response to focal loss of functional cardiac units from apoptosis. Furthermore, we established that improving myocardial perfusion and nutrient supply to hypertrophying cardiomyocytes by increasing capillary density prevented ongoing cardiomyocyte loss from apoptosis, delaying the onset of ventricular dilatation, and preserving contractile function.
Pressure loading on the heart produces a sustained abnormal elevation in myocardial wall stress, which in response initiates a complex progressive ventricular remodeling process that ultimately results in cardiac decompensation and heart failure. There is no further increase in left ventricular weight once the heart decompensates and significant thinning of the ventricular wall occurs because of the marked increase in left ventricular chamber size. Throughout this process, the left ventricular mass to volume ratio is decreasing, indicating an inability of the myocardium to normalize the elevated wall stress. Ventricular remodeling implies the hypertrophy of cardiomyocytes and proliferation of nonmuscle cells. Given the limited ability of cardiomyocytes for self-renewal, raises the possibility that inadequate sustained increase of muscle mass is caused by the continuous loss of functional contractile units, the cardiomyocytes. Excessive cell loss has been suggested to be linked to a variety of cardiac diseases including hypoxia, ischemia/reperfusion, myocardial infarction, chemotherapy-induced cardiomyopathy, and end-stage heart failure13–15.
Apoptosis is defined as an energy-dependent form of cell death characterized by distinct morphological and biochemical changes to the cell, like nucleosomal DNA fragmentation, cellular shrinking without the loss of membrane integrity, followed by break-up of the nucleus and cellular budding with the formation of apoptotic bodies which are rapidly phagocytosed by neighboring cells.16 Apoptosis can be initiated by internal and external triggers, both leading to activation of caspases in mammalian cells. There are 2 pathways leading to caspase activation, the intrinsic (mitochondrial) pathway, which involves cytochrome c release from mitochondria, and the extrinsic (death receptor) pathway. Caspases are aspartate-specific cysteine proteases, which can be divided into upstream or initiator caspases, and downstream or effector caspases. Caspases exist as inactive zymogens that are activated by either auto-proteolysis or by the proteolytic actions of other caspases. The majority of death receptors belong to the tumor necrosis factor receptor family. A pro-death stimulus triggers caspase activation resulting in caspase-activated endonucleases that digest DNA into oligonucleosome-sized fragments17. Downstream caspases also cleave proteins such as nuclear proteins (PARP), proteins involved in signal transduction, and cytoskeletal proteins.
The morphological changes of apoptosis represent only the end stage of various subsequent phases. As indicated by transgenic mouse experiments, cardiac hypertrophy renders cardiomyocytes more sensitive to apoptosis.18 Hypertrophied hearts are exposed to several pro-apoptotic triggers including mechanical, such as extensive stretch, elevated concentration of neurohumoral factors, or multiple intracellular and extracellular alterations such as tumor necrosis factor (TNF)-α upregulation, oxidative stress, or hypoxia/ischemia. In the failing heart, increasing concentrations of TNF-α correlate with an increasingly compromised heart implying that TNF-α may induce apoptosis in cardiomyocytes.19,20 However, in cardiomyocytes apoptosis predominantly proceeds via the mitochondria.21 Mitochondria mediated mechanisms for cardiomyocyte apoptosis include that the endogenous capillary network cannot provide the compensatory increase in perfusion required for the survival of hypertrophying cardiomyocytes. Diffusion distance between cardiomyocytes and capillaries increases, restricting the availability of nutrient substrates and oxygen. This potentially results in increased oxidative stress, free radical production, and mitochondrial dysfunction, as we and others have previously reported.22,23 Activation of components of the mitochondrial pathway might be the primary apoptotic pathway associated with oxidative stress in the heart.24,25 If ventricular dilatation in the initial phase of remodeling is prevented, the ventricle’s ability to compensate for the increased stress is greatly enhanced. We had previously shown that repetitive VEGF administration enhances microvascularity in this rabbit model of left ventricular hypertrophy and that adaptive microvascular growth normalizes substrate delivery to myocytes.8,26 Preventing the ongoing loss of muscle mass by apoptotic cardiomyocyte death is another key element of VEGF protection on the hypertrophied myocardium. We could show in this study that VEGF treatment facilitated appropriate adaptation of increase in ventricular muscle mass to compensate for increased workload, preserving myocardial function. Susceptibility of a cell to undergo apoptosis is regulated in part, by the relative levels of Bcl-2 family members.24 One potential protective mechanism provided by VEGF involves the balance of pro-apoptotic versus anti-apoptotic proteins of the Bcl-2 family or other intracellular signaling pathways that include Akt-1. These targets of VEGF action, however, remain to be determined.
Whereas the rate of apoptosis found in this study seems low in comparison to studies in which ischemia/reperfusion injury is the trigger for apoptosis, it is important to point out that the conditions for triggering programmed cell death in pressure-overload hypertrophy are not nearly as severe as in ischemia/reperfusion. However, the ongoing nature of the insult and continuous cell loss may accrue a substantial loss of functioning cardiomyocytes, explaining the effect on contractile function, and further adding to the workload of the remaining muscle mass, potentially creating a viscous cycle resulting in heart failure.
Our results indicate that apoptosis may be the initial dysregulatory mechanism involved in the maladaptive cardiac response to pressure overload. Apoptotic cardiomyocyte loss starts early at the stage of compensated hypertrophy and continuously progresses as heart failure develops. Improving perfusion to hypertrophying cardiomyocytes by VEGF treatment prevents cardiomyocyte apoptosis and prolongs survival in a model of severe progressive left ventricular hypertrophy. Promoting angiogenesis protects cardiomyocyte cell mass preserves myocardial contractile function, and delays the onset of failure.
Acknowledgments
Sources of Funding
This work was supported by National Heart, Lung, and Blood Institute grants HL-075430 (I.F.) and HL-063095 (P.J.d.N.).
Footnotes
Presented at the American Heart Association Scientific Sessions, Dallas, Tex, November 13–16, 2005.
Disclosures
None.
References
- 1.Morgan JP, Erny RE, Allen PD, Grossman W, Gwathmey JK. Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation. 1990;81(Suppl 2):III21–III32. [PubMed] [Google Scholar]
- 2.Litten RZ, Low BJ, Alpert NR. Altered myosin isozyme patterns from pressure overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982;50:856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- 3.Marcus ML, Harrison DG, Chilian WM, Koyanagi S, Inou T, Tomanek RJ, Martins JB, Eastham CL, Hiratzka LF. Alterations in the coronary circulation in hypertrophied ventricles. Circulation. 1987;75(Suppl I):I19–I25. [PubMed] [Google Scholar]
- 4.Hittinger L, Shannon RP, Bishop SP, Gelpi RJ, Vatner SF. Subendomyocardial exhaustion of blood flow reserve and increased fibrosis in conscious dogs with heart failure. Circ Res. 1989;65:971–980. doi: 10.1161/01.res.65.4.971. [DOI] [PubMed] [Google Scholar]
- 5.Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am J Physiol. 1989;256(1 Pt 2):H126–H131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- 6.Friehs I, Moran AM, Stamm C, Colan SD, Takeuchi K, Cao-Danh H, Rader CM, McGowan FX, del Nido PJ. Impaired glucose transporter activity in pressure-overload hypertrophy is an early indicator of progression to failure. Circulation. 1999;100(19 Suppl):II187–II193. doi: 10.1161/01.cir.100.suppl_2.ii-187. [DOI] [PubMed] [Google Scholar]
- 7.Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–2010. doi: 10.1016/j.athoracsur.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Narula J, Hajjar RJ, Dec GW. Apoptosis in the failing heart. Cardiol Clin. 1998;16:691–710. doi: 10.1016/s0733-8651(05)70045-x. [DOI] [PubMed] [Google Scholar]
- 10.Wencker D, Chandra M, Nguyen K, Miao W, Garantziotis S, Factor SM, Shirani J, Armstrong RC, Kitsis RN. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teiger E, Than VD, Richard L, Wisnewsky C, Tea BS, Gaboury L, Tremblay J, Schwartz K, Hamet P. Apoptosis in pressure overload-induced heart hypertrophy in the rat. J Clin Invest. 1996;97:2891–2897. doi: 10.1172/JCI118747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran AM, Friehs I, Takeuchi K, Stamm C, Hammer PE, McGowan FX, del Nido PJ, Colan SD. Non-invasive serial evaluation of myocardial mechanics in pressure overload hypertrophy of rabbit myocardium. Herz. 2003;28:52–62. doi: 10.1007/s00059-003-2392-0. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson AB, Gottlieb RA. Mechanisms of apoptosis in the heart. J Clin Immunol. 2003;23:447–459. doi: 10.1023/b:joci.0000010421.56035.60. [DOI] [PubMed] [Google Scholar]
- 14.de Moissac D, Gurevich RM, Zheng H, Singal PK, Kirshenbaum LA. Caspase activation and mitochondrial cytochrome C release during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol. 2000;32:53–63. doi: 10.1006/jmcc.1999.1057. [DOI] [PubMed] [Google Scholar]
- 15.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 16.Borgers M, Voipio-Pulkki L, Izumo S. Apoptosis. Cardiovasc Res. 2000;45:525–527. doi: 10.1016/s0008-6363(99)00404-6. [DOI] [PubMed] [Google Scholar]
- 17.van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–497. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- 18.Adams JW, Sakata Y, Davis MG, Sah VP, Wang Y, Liggett SB, Chien KR, Brown JH, Dorn GW., 2nd Enhances Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci U S A. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanou K, Kremastinos DT. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am J Cardiol. 2004;94:1326–1328. doi: 10.1016/j.amjcard.2004.07.127. [DOI] [PubMed] [Google Scholar]
- 20.Stamm C, Friehs I, Cowan DB, Moran AM, Cao-Danh H, Duebener LF, del Nido PJ, McGowan FX., Jr Inhibition of tumor necrosis factor-alpha improves postischemic recovery of hypertrophied hearts. Circulation. 2001;104(12 Suppl 1):I350–I355. doi: 10.1161/hc37t1.094851. [DOI] [PubMed] [Google Scholar]
- 21.Date T, Mochizuki S, Belanger AJ, Yamakawa M, Luo Z, Vincent KA, Cheng SH, Gregory RJ, Jiang C. Differential effects of membrane and soluble Fas ligand on cardiomyocytes: role in ischemia/reperfusion injury. J Mol Cell Cardiol. 2003;35:811–821. doi: 10.1016/s0022-2828(03)00139-1. [DOI] [PubMed] [Google Scholar]
- 22.Nathan M, Friehs I, Choi Y, Cao-Danh H, Cowan D, McGowan FX, del Nido PJ. Subsarcolemmal mitochondrial function is impaired in severe cardiac hypertrophy and failure. J Am Coll Surg. 2002;195(3 Suppl):S17. [Google Scholar]
- 23.Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O’Neill L, Cirielli C, Lakatta EG, Crow MT. p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes. J Clin Invest. 1997;99:2635–2643. doi: 10.1172/JCI119452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latif N, Khan MA, Birks E, O’Farrell A, Westbrook J, Dunn MJ, Yacoub MH. Upregulation of the Bcl-2 family of proteins in end stage heart failure. J Am Coll Cardiol. 2000;35:1769–1777. doi: 10.1016/s0735-1097(00)00647-1. [DOI] [PubMed] [Google Scholar]
- 25.Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vass. 2003;96:214–221. [PubMed] [Google Scholar]
- 26.Friehs I, Margossian RE, Moran AM, Cao-Danh H, Moses MA, del Nido PJ. Vascular endothelial growth factor delays onset of failure in pressure-overload hypertrophy through matrix metalloproteinase activation and angiogenesis. Basic Res Cardiol. 2006;101:204–213. doi: 10.1007/s00395-005-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]